Abstract
Background and objectives
Overproduction of oxalate in patients with primary hyperoxaluria (PH) leads to calcium oxalate deposition in the kidney and ESRD in a substantial number of cases. However, the key determinants for renal outcome remain unclear. Thus, we performed a retrospective analysis to identify predictors for renal outcome among patients with PH participating in the Rare Kidney Stone Consortium (RKSC) PH Registry.
Design, setting, participants, & measurements
We characterized clinical and laboratory features of patients enrolled in the RKSC PH Registry. We assessed correlation between urinary measures and eGFR at diagnosis by Spearman rank correlation and estimated renal survival using the Kaplan–Meier method. We determined factors associated with renal survival by Cox proportional hazard models.
Results
Of 409 patients enrolled in the RKSC Registry as of March 2014, we excluded 112 patients who had ESRD at PH diagnosis from analysis. Among the remaining 297 patients, 65% had PH type 1, 12% had type 2, 13% had type 3, and 11% had unclassified PH. Median (25th, 75th percentile) age at PH diagnosis was 8.1 (4.0, 18.2) years with an eGFR of 73.0 (56.4, 97.5) ml/min per 1.73 m2 and urinary oxalate excretion rate of 1.64 (1.11, 2.44) mmol/1.73 m2 per 24 hours. During a median follow-up of 3.9 (1.0, 12.8) years, 59 (20%) patients developed ESRD. Urinary oxalate excretion at diagnosis stratified by quartile was strongly associated with incident ESRD (hazard ratio [HR], 3.4; 95% confidence interval [95% CI], 1.4 to 7.9). During follow-up there was a significant association between urinary oxalate quartile (Q) and incident ESRD (Q4 versus Q1: HR, 3.3; 95% CI, 1.2 to 9.3). This association remained even when adjusted for sex, age, and baseline eGFR (HR, 4.2; 95% CI, 1.6 to 10.8).
Conclusions
Among patients with PH, higher urinary oxalate excretion is predictive of poor renal outcome.
Keywords: genetic renal disease; kidney stones; mineral metabolism; calcium oxalate; follow-up studies; humans; hyperoxaluria, primary; kidney failure, chronic; oxalates; registries
Introduction
The primary hyperoxalurias (PHs) are autosomal recessive disorders characterized by enzymatic defects in glyoxylate metabolism, which ultimately result in overproduction of oxalate (1–4). Three types of PH have been identified: primary hyperoxaluria type 1 (PH1) is caused by a deficiency of the liver-specific peroxisomal enzyme alanine-glyoxylate aminotransferase (AGT), which is encoded by the AGXT gene; primary hyperoxaluria type 2 (PH2) is secondary to glyoxylate reductase/hydroxypyruvate reductase (GRHPR) deficiency and caused by mutations in the GRHPR gene; and primary hyperoxaluria type 3 (PH3) is caused by mutations in the HOGA1 gene, which encodes the mitochondrial 4-hydroxy-2-oxoglutarate aldolase (HOGA) enzyme. Humans cannot degrade oxalate, and it is primarily eliminated by the kidneys. Hence, endogenous overproduction leads to substantial elevations in urinary oxalate excretion, typically >1 mmol (>88 mg)/1.73 m2 per 24 hours (normal <0.5 mmol [<45 mg]/1.73 m2 per 24 hours; conversion factor for oxalate: 1 mmol=88 mg) (5), which results in calcium oxalate (CaOx) supersaturation in the urine and subsequent urolithiasis and/or nephrocalcinosis. Progressive crystal deposition, accompanied by parenchymal inflammation and fibrosis, may eventually progress to ESRD (6).
Clinical manifestations of PH are heterogenous with respect to age at onset, type of presentation, severity of hyperoxaluria, and rate of progression to renal insufficiency (7). PH1 outcome appears, in part, to be associated with genotype, with the pGly170Arg mutation in particular associated with better renal survival (8,9). Nevertheless, patients with the same genotype can present with very disparate symptoms and disease course (10). Therefore, factors that determine renal outcome remain incompletely defined.
The Rare Kidney Stone Consortium (RKSC) PH Registry was developed in 2002 to advance understanding of the natural history of PH and to elucidate factors that influence outcomes (11). In the current study we used the RKSC PH Registry to evaluate PH type and the effect of urinary constituents that influence CaOx crystal formation (oxalate, calcium, citrate, phosphorus, volume) on renal outcomes and determine whether there are cut points that stratify risk.
Materials and Methods
Study Population
Clinical and laboratory information were collected from patients with PH enrolled in the RKSC PH registry (11). The first patient was enrolled in 2003. As of March 2014, 13 patients had died. Total person-years of follow-up was 2483. General information and clinical manifestations were abstracted from registry data. Symptoms included stone-related pain, stone passage, hematuria, or failure to thrive. PH1 was confirmed by mutations of the AGXT gene, liver biopsy confirming deficiency of AGT, or by marked hyperoxaluria in combination with hyperglycolic aciduria in a patient with no identifiable secondary causes. PH2 was established by mutations of GRHPR, liver biopsy confirming deficiency of GRHPR enzyme, or hyperoxaluria in combination with hyperglyceric aciduria without identifiable secondary cause. PH3 was diagnosed by mutations of HOGA1. Patients considered to have PH of unclassified type met the same clinical criteria as those with other types; had no identifiable enteric hyperoxaluria; and were negative on testing for mutations of AGXT, GRHPR, and HOGA1.
Laboratory results, including 24-hour urine volume, oxalate, calcium, citrate, and phosphorus and serum creatinine, were extracted from registry data. All 24-hour urine values were from baseline (0–60 days after diagnosis) or during follow-up (>60 days until last available before ESRD). Renal function was assessed by serum creatinine values to estimate GFR using the Schwartz formula (12) in children <18 years and the Modification of Diet in Renal Disease formula (13) in adults. ESRD or renal failure was defined as an eGFR<15 ml/min per 1.73 m2 or start of dialysis or renal transplantation. A cohort of nonstone-forming adults in good general health without any kidney disease or diabetes (52 women and 49 men; age 18–83 years) who completed 24-hour urine collections on a free-choice diet were used for a reference range population.
Statistical Analyses
Results were expressed in terms of the median (25th, 75th percentiles) for continuous variables and as percentages for categorical variables. Spearman rank correlation was used to analyze correlations between baseline clinical and laboratory variables and eGFR at diagnosis. To maximize available data, the diagnosis reading was defined as the closest reading within 3 years before PH diagnosis and up to 60 days after diagnosis.
The percentage of patients who were free of renal failure after PH diagnosis was estimated using the Kaplan–Meier method. Factors associated with renal survival were estimated by univariate analyses using the Cox proportional hazard model with log-rank tests and trend tests used for comparison between subgroups. The outcome of interest was time to ESRD and was censored on death or loss to follow-up. To display the effect of quantitative factors on ESRD, data were split into four groups based on quartiles of urine oxalate. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were presented. We further used a time-dependent Cox model to explore the effect of urinary oxalate excretion level on renal outcome during follow-up. Oxalate was first evaluated as a continuous factor and then as quartiles derived from all follow-up readings. Times to ESRD by oxalate quartile were estimated by dividing individual-patient follow-up time into intervals based on the quartile of latest oxalate reading and noting whether the interval ended in ESRD. Person-time and ESRD events were summed within oxalate quartiles with the rate=100×(events/person-time). P values <0.05 were considered to indicate statistically significant differences. All calculations were done using SAS software, version 9.
Results
There were 409 patients enrolled in the RKSC PH Registry as of March 2014. Most (n=297 [72.6%]) had PH1, 39 (9.5%) had PH2, 38 (9.3%) had PH3, and 35 (8.6%) had unclassified PH. ESRD was present at or before PH diagnosis in 112 (27.4%) patients (Table 1). Patients with ESRD at baseline were more likely to have PH1 than patients without ESRD at baseline (P<0.001), which indicates that PH type is a major factor with respect to ESRD status at time of diagnosis. Patients without ESRD were diagnosed at an earlier age than those with ESRD at diagnosis (median age, 8.1 versus 25.3 years; P<0.001) and had an earlier age at symptom onset (median age, 4.5 versus 9.4 years; P=0.02). The time between initial symptoms and diagnosis was shorter among patients without than among those with ESRD at diagnosis (median, 1.4 versus 3.5 years; P<0.001). Median eGFR among those without ESRD at diagnosis was 73.0 (56.4, 97.5) ml/min per 1.73 m2.
Table 1.
Clinical characteristics of patients with primary hyperoxaluria who had or did not have ESRD at or before diagnosis
| Characteristics | Without ESRD (n=297) | With ESRD (n=112) | P Value |
|---|---|---|---|
| Type of PH, % (n) | <0.001 | ||
| PH1 | 64.6 (192) | 93.8 (105) | |
| PH2 | 11.8 (35) | 3.6 (4) | |
| PH3 | 12.8 (38) | 0 (0) | |
| Unclassified | 10.8 (32) | 2.7 (3) | |
| Sex, % (n) | 0.05 | ||
| Female | 42.8 (127) | 53.6 (60) | |
| Male | 57.2 (170) | 46.4 (52) | |
| Median age, y | |||
| At diagnosis | 8.1 (4.0, 18.2) | 25.3 (6.8, 42.0) | <0.001 |
| At symptom onset | 4.5 (1.7, 11.3) | 9.4 (2.0, 21.6) | 0.02 |
| Median time between initial symptoms and diagnosis, y | 1.4 (0.1, 7.5) | 3.5 (0.3, 21.0) | <0.001 |
| Median eGFR, ml/min per 1.73 m2 | 73.0 (56.4, 97.5) | 6.6 (3.9, 13.8) | <0.001 |
Median values are expressed with 25th, 75th percentiles. PH, primary hyperoxaluria; PH1, primary hyperoxaluria type 1; PH2, primary hyperoxaluria type 2; PH3, primary hyperoxaluria type 3.
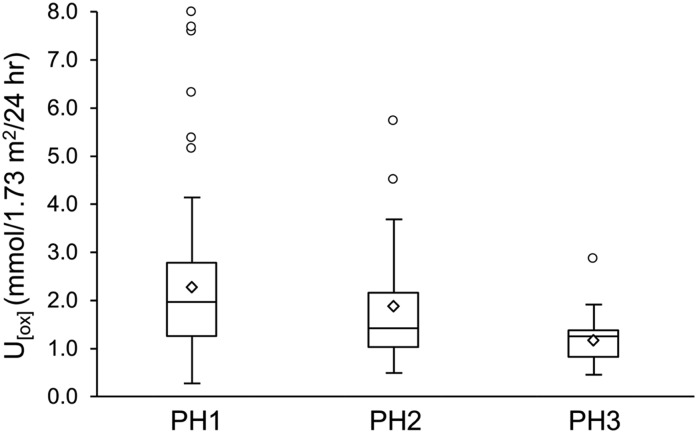
After 112 patients with ESRD at PH diagnosis were excluded, 297 patients remained for analysis of risk factors for renal function loss. Baseline urinary variables are shown in Table 2. Because the study population includes both pediatric and adult participants, results are normalized to 1.73 m2 body surface area. Values for these urine chemistries in the adults with PH are also shown without body-surface-area normalization for comparison with a group of healthy adult nonstone-forming volunteers. As expected, the median 24-hour urine oxalate excretion was higher compared with controls, as was urine volume; in contrast, urine calcium and citrate excretions were relatively lower. Urinary oxalate excretion at diagnosis was higher in PH1 compared with PH2 and PH3 (P trend <0.001) (Figure 1). At PH diagnosis, the median eGFR for patients with PH1, PH2, and PH3 were 69.2 (54.8, 86.7), 77.4 (69.7, 96.0), and 89.0 (66.2, 126.9) ml/min per 1.73 m2, respectively. Urinary volume at diagnosis correlated negatively with eGFR (r=−0.28; P=0.001) (Table 3), while urinary citrate at diagnosis correlated in a positive direction (r=0.27; P=0.01) (Table 3). Urinary oxalate excretion had an inverse association with eGFR at diagnosis, although it was not statistically significant (r=−0.13; P=0.12). Neither calcium excretion nor phosphorus excretion was significantly associated with eGFR at PH diagnosis (r=0.10 [P=0.29]; r=0.19 [P=0.08], respectively) (Table 3).
Table 2.
Baseline urinary measures of patients with primary hyperoxaluria without ESRD at or before diagnosis
| Variable | All Participants with PHa | Adult Participants with PHb | Adult Reference Populationc |
|---|---|---|---|
| U[vol], ml/24 h | 2193 (1605, 3031); 151 | 2740 (2200, 3095); 53 | 1319 (1046, 1889); 101 |
| U[ox], mmol/24 h | 1.64 (1.11, 2.44); 168 | 1.51 (0.89, 1.93); 56 | 0.27 (0.22, 0.32); 101 |
| U[Ca], mg/24 h | 72.9 (43.4, 134.2); 130 | 100.5 (57.5, 177.5); 44 | 191.0 (130.0, 250.0); 101 |
| U[Citrate], mg/24 h | 398 (197, 698); 120 | 375 (168, 520); 41 | 732 (522, 963); 101 |
| U[Phos], mg/24 h | 824 (552, 1044); 98 | 1012 (636, 1218); 36 | 975 (756, 1183); 101 |
Values are expressed as median (25th, 75th percentiles), followed by number of patients. U[vol], urinary volume; U[ox], urinary oxalate; U[Ca], urinary calcium; U[Citrate], urinary citrate; U[Phos], urinary phosphate; PH, primary hyperoxaluria.
Includes both adults and children and thus normalized to 1.73 m2 body surface area.
Includes only those aged ≥18 years at the time of collection and are not normalized to body surface area.
Values from 101 individuals aged 18–83 years without kidney stones or kidney disease are not normalized to 1.73 m2 body surface area.
Figure 1.
Urinary oxalate (U[ox]) excretion by primary hyperoxaluria (PH) type in patients without ESRD at diagnosis. Urinary oxalate was greatest in primary hyperoxaluria type 1 (PH1) and lowest in primary hyperoxaluria type 3 (PH3) (P<0.001 for trend). Boxes represent the interquartile range (25th, 75th percentiles) while the line inside each box indicates the median value; upper bars indicate the maximum values while the lower bars indicate the minimum values. Diamond symbols indicate the mean values; circles indicate outliners. PH2, primary hyperoxaluria type 2.
Table 3.
Correlations of eGFR at diagnosis with laboratory measures among patients with primary hyperoxaluria without ESRD at or before diagnosis
| Variable | r Value | P Value | Patients, n |
|---|---|---|---|
| U[vol], ml/1.73 m2 per 24 h | −0.28 | 0.001 | 128 |
| U[ox], mmol/1.73 m2 per 24 h | −0.13 | 0.12 | 140 |
| U[Citrate], mg/1.73 m2 per 24 h | 0.27 | 0.01 | 104 |
| U[Ca], mg/1.73 m2 per 24 h | 0.10 | 0.29 | 114 |
| U[Phos], mg/1.73 m2 per 24 h | 0.19 | 0.08 | 83 |
U[vol], urinary volume; U[ox], urinary oxalate; U[Citrate], urinary citrate; U[Ca], urinary calcium; U[Phos], urinary phosphate.
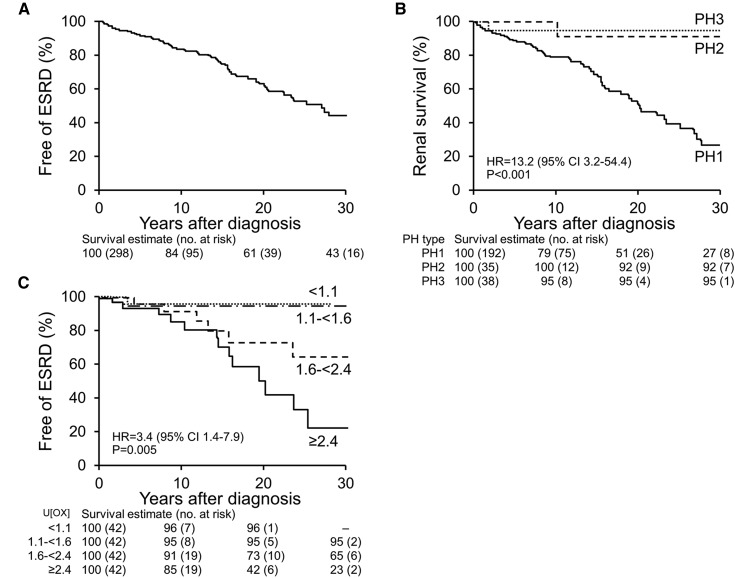
Among 297 patients with preserved renal function at PH diagnosis, median follow-up was 3.9 (1.0, 12.8) years and 59 (20%) patients developed ESRD, with a cumulative rate of renal survival of 84%, 61%, and 43% at 10, 20, and 30 years after PH diagnosis, respectively (Figure 2A). Univariate analyses showed that PH1, older age at diagnosis, baseline urinary oxalate excretion by quartile, and eGFR at diagnosis were associated with incident ESRD (Table 4). Type of PH was a significant predictor of renal outcome; the ESRD HR for PH1 versus PH2 and PH3 was 13.2 (95% CI, 3.2 to 54.4). At 30 years after PH diagnosis, the renal survival rate was 27% for PH1, 92% for PH2, and 95% for PH3 (Figure 2B). Renal outcome correlated with baseline urinary oxalate excretion when stratified by quartile, with an ESRD HR for quartile (Q) 4 versus Q1–Q3 of 3.4 (95% CI, 1.4 to 7.9). The 20-year renal survival was 96% for patients whose oxalate excretion rate was <1.11 mmol/1.73 m2 per 24 hours at PH diagnosis, 95% for those whose rate was 1.11–1.64 mmol/1.73 m2 per 24 hours, 73% for those whose rate was >1.64 and <2.45 mmol/1.73 m2 per 24 hours, and 42% for those whose rate was ≥2.45 mmol/1.73 m2 per 24 hours (Figure 2C). The association of urinary oxalate with ESRD risk was not modified by baseline eGFR level (>60 versus <60 ml/min per 1.73 m2; interaction term P=0.87). Sex, age at PH symptom onset, urinary volume, citrate, calcium, and phosphorous excretions at diagnosis did not significantly correlate with renal outcome (Table 4).
Figure 2.
Kaplan–Meier plots of renal survival. (A) Among all patients with primary hyperoxaluria (PH) who did not have ESRD at diagnosis, renal survival estimates at 10, 20, and 30 years after diagnosis were 84%, 61%, and 43%, respectively. (B) Among patients with PH who did not have ESRD at diagnosis, renal survival estimates at 10, 20, and 30 years after diagnosis were lowest for primary hyperoxaluria type 1 (PH1) (hazard ratio [HR], 13.2 for PH1 versus others; 95% confidence interval [95% CI], 3.2 to 54.4). (C) Renal survival was examined by quartile of urine oxalate (U[ox]) excretion (mmol/1.73 m2/24 hours) at diagnosis. Among patients with PH who did not have ESRD at diagnosis, renal survival estimates at 10, 20, and 30 years were lowest for those with a U[ox] excretion ≥2.4 mmol/1.73 m2 per 24 hours (HR, 3.4 for quartile Q4 versus quartiles Q1–Q3; 95% CI, 1.4 to 7.9). PH2, primary hyperoxaluria type 2; PH3, primary hyperoxaluria type 3.
Table 4.
Factors univariately associated with incident ESRD among patients with primary hyperoxaluria without ESRD at diagnosis
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| PH1 | 13.17 (3.19 to 54.38) | <0.001 |
| Male | 1.39 (0.82 to 2.37) | 0.22 |
| Age at symptoms | 1.00 (0.97 to 1.03) | 0.98 |
| Age at diagnosis | 1.02 (1.00 to 1.04) | 0.02 |
| U[vol] , ml/1.73m2 per 24h | 1.00 (1.00 to 1.00) | 0.31 |
| U[ox] , mmol/1.73m2 per 24h | 1.13 (0.94 to 1.37) | 0.20 |
| U[ox] , mmol/1.73m2 per 24h (Q4) | 3.40 (1.40 to 7.90) | 0.01 |
| U[Citrate] , mg/1.73m2 per 24h | 1.00 (1.00 to 1.00) | 0.24 |
| U[Ca] , mg/1.73m2 per 24h | 1.00 (0.99 to 1.00) | 0.29 |
| U[Phos] , mg/1.73m2 per 24h | 1.00 (1.00 to 1.00) | 0.59 |
| eGFR, ml/min per 1.73m2 | 0.96 (0.94 to 0.99) | 0.002 |
PH1, primary hyperoxaluria type 1; U[vol], urinary volume; U[ox], urinary oxalate; Q4, quintile 4; U[Citrate], urinary citrate; U[Ca], urinary calcium; U[Phos], urinary phosphate; 95% CI, 95% confidence interval.
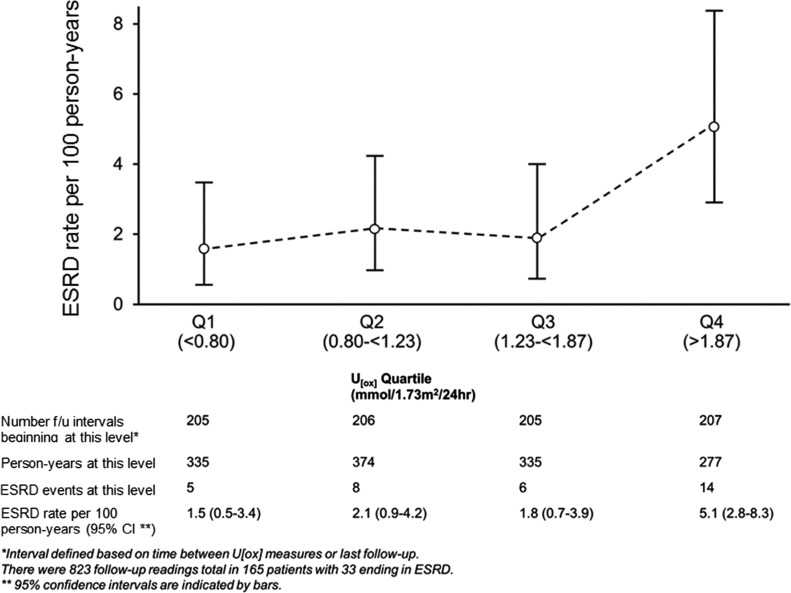
Oxalate excretion could potentially vary over time: for example, in patients with PH1 who received pyridoxine after diagnosis and who were responsive to this medication (14,15). There were 823 follow-up urine oxalate readings (>60 days after PH diagnosis) in 165 patients, of whom 33 developed ESRD. With use of follow-up urinary oxalate levels as a continuous time-dependent covariate, risk of ESRD was higher with higher urinary oxalate levels, yielding an HR of 1.8 (95% CI, 1.2 to 2.5) per 1 mmol/1.73 m2 per 24 hours higher. When examined by follow-up oxalate quartile (cut-off points of 0.80, 1.23, and 1.87), the highest quartile was at higher ESRD risk than the lowest quartile 1 (HR, 3.3; 95% CI, 1.2 to 9.3). ESRD rates per 100 person-years were 1.5, 2.1, 1.8, and 5.1 by urinary oxalate quartile, respectively (Figure 3). Comparing the highest oxalate quartile to the lower three, the HR for ESRD was 3.2 (95% CI, 1.5 to 6.56), which remained elevated and significant when adjusted for sex, age, and baseline eGFR (HR, 4.2; 95% CI, 1.6 to 10.8) (Supplemental Table 1).
Figure 3.
ESRD rate by urinary oxalate (U[ox]) quartile during follow-up (f/u). ESRD rates were similar for the lower three quartiles (Q) but increased for a urinary oxalate level >1.87 mmol/1.73 m2 per 24 hours (hazard ratio, 3.30; 95% confidence interval, 1.17 to 9.33; P=0.02).
Citrate, a commonly prescribed crystallization inhibitor, was used by 26% (76 of 297) of the cohort. However, citrate administration was not associated with baseline eGFR or progression to ESRD. Only three patients received thiazide medication.
Discussion
Earlier PH diagnosis has been possible during the last two decades with the advent of more readily available genetic testing. Thus, earlier treatment is possible. Nevertheless, in our cohort 27% of patients presented with ESRD at the time of or before PH diagnosis, highlighting the persisting need for earlier recognition. Importantly, an additional 20% of patients with PH who had preserved renal function at diagnosis went on to develop ESRD during follow-up, and the overall renal survival was only 43% at 30 years after PH diagnosis. Thus, improved understanding of risk factors for ESRD and prognosis are important to identify treatment targets and guide interventions for patients at highest risk.
The current study strongly suggests that the magnitude of hyperoxaluria is a primary determinant of the risk of renal function loss both at diagnosis and during follow-up. It is well known that the high rate of oxalate excretion among patients with PH results in supersaturation of CaOx in the urine, favoring CaOx crystal formation (16). CaOx crystals not only aggregate in the urinary space, producing stones (urolithiasis), but they also interact with the renal tubule epithelium and deposit in the renal interstitium (nephrocalcinosis), where they can induce an inflammatory response and progressive interstitial fibrosis that can compromise kidney function (17–20). In this cohort, higher urinary oxalate excretion at both diagnosis and follow-up were associated with poorer renal outcome. These findings confirm the critical importance of urinary oxalate excretion as a predictor of renal survival. Our data also suggest that the degree of hyperoxaluria at baseline is associated with renal outcome. Patients in the highest quartile of oxalate excretion at diagnosis (≥2.45 mmol/1.73 m2 per 24 hours) had a worse renal outcome, whereas those in the lower quartiles trended toward substantially better prognosis (Figure 2C).
Degree of oxalate excretion during follow-up was also associated with outcomes. By quartile, oxalate excretions >1.87 mmol/1.73 m2 per 24 hours were associated with higher ESRD rates. The significant association of higher follow-up oxalate with onset of ESRD remained even when adjusted for sex, age, and baseline eGFR (HR, 4.2; 95% CI, 1.6 to 10.8). Interestingly, the HR for ESRD compared with the lowest quartile (<0.80 mmol/1.73 m2 per 24 hours) was significantly higher only in the highest urinary oxalate excretion quartile (>1.87 mmol/1.73 m2 per 24 hours) (Figure 3). Altogether, these observations suggest that efforts to reduce urine oxalate excretion could favorably affect renal outcomes, even if oxalate levels are not entirely normalized.
Other urinary features, including 24-hour urine volume and excretions of citrate, calcium, and phosphate, considered associated with kidney stone risk and nephrocalcinosis could potentially influence kidney function outcome as well (21). Of note, baseline urine volume was increased in this PH cohort, and higher urine volume was associated with lower baseline eGFR (P=0.001). However, urine volume was not a significant predictor for ESRD in the long term. Higher volume might reflect recommendations to increase fluid intake in patients who were treated for stones before PH diagnosis. Alternatively, we speculate that the increased urine volume at PH diagnosis we observed could reflect decreased urinary-concentrating ability among patients with PH who have established tubulointerstitial injury. Increased CaOx supersaturation in proximal tubules resulting from high oxalate concentration might lead to increased crystallization, subsequent cellular response, and interstitial fibrosis, which in turn might reduce urinary-concentrating capacity. Indeed, decreased urinary-concentrating ability is a long-established consequence of CKD (22), and proper function of the countercurrent mechanism in the loop of Henle is essential for developing maximal urine osmolality (23). Thus, ongoing crystallization, injury, and fibrosis in the corticomedullary junction could potentially affect urinary-concentrating capacity. This evidence suggests that increased urine volume might be an early result of tubulointerstitial injury among patients with PH. However, further studies need to be performed to validate our findings and to clarify their cause.
In this study, patients with ESRD at the time of PH diagnosis were excluded from the analysis. The remaining patients with PH had relatively well preserved renal function. We hypothesize several reasons for this observation. First, the age of this cohort was relatively young (median, 8.1 years), and the patients might not yet have had time to develop renal damage. Alternatively, those without ESRD at diagnosis might have a relatively milder disease, with some degree of protection from renal damage (mechanisms to be determined). Finally, decline in GFR is not necessarily linear over a lifetime, and we have observed many patients with PH who experienced a rapid decline precipitated by acute events.
Urinary citrate is generally accepted to be an inhibitor of CaOx stone formation and is associated with growth inhibition of CaOx crystals (24–26). In our study, a significant association was observed between higher urinary citrate excretion and better renal function at PH diagnosis. However, our data did not demonstrate that citrate was a risk predictor for ESRD during long-term follow-up. Studies in other non-PH populations have demonstrated that urinary citrate levels decrease with advancing CKD (27). Thus, as for urine volume, the lower urine citrate at baseline may be a consequence of renal injury. Higher urinary calcium, meanwhile, is a common risk factor for CaOx nephrolithiasis, and previous studies suggest that urinary calcium concentration is of at least equal importance to urinary oxalate for increasing CaOx supersaturation (16). However, urinary calcium excretion was not associated with baseline renal function or longer-term renal outcome in our cohort of patients with PH. Indeed, average urine calcium was somewhat lower than expected compared with that in a healthy reference population, for reasons yet to be explained. Thiazide effect did not account for this observation because just three patients were receiving thiazide medication at the time of baseline urine measurements.
Our data also suggest that PH type is associated with ESRD. Patients with PH1 were more likely to present with ESRD at diagnosis (36% compared with ≤10% for other types and unclassified) and also more likely to develop ESRD during follow-up. In the PH1 group, renal survival was only 27% after 30 years of follow-up, compared with 92% and 95% for PH2 and PH3, respectively. These differences might reflect the higher average oxalate excretion rates in PH1 compared with the other types. The current data are also consistent with reports from other investigations demonstrating that genotypes with lower oxalate excretion, such as patients homozygous or heterozygous for the G170R mutation of AGXT (which also confers complete or partial pyridoxine responsiveness), have better long-term renal outcomes (8,28–31).
Our study has certain limitations. Because the RKSC PH Registry is a voluntary retrospective registry, this cohort may not represent all patients with PH. Moreover, comprehensive data at regular time points were not available for all participants; thus, multivariable analyses were somewhat limited because of missing data. The number of patients and ESRD events also limited the number of variables that could be included in models.
In conclusion, ESRD is a major and devastating consequence of PH. Patients with PH1 had higher risk for ESRD than other types of PH. The degree of urinary oxalate excretion appears to be the major predictor of renal outcome and probably explains the poor renal survival of PH1 compared with PH2 and PH3, though as-yet unknown factors could also play a role.
Disclosures
This study was partially supported by a grant from OxThera Pharmaceuticals. The investigators had full responsibility for study design, data collection, data interpretation, writing, and preparation of the manuscript. D.S.M. and J.C.L. have provided consulting services to OxThera AB (Stockholm, Sweden) and Alnylam (Cambridge, Massachusetts) pharmaceutical companies; D.S.M., to Alexion (Cheshire, Connecticut); and J.C.L., to Dicerna (Cambridge, Massachusetts). All services were provided under contract to Mayo Clinic.
Supplementary Material
Acknowledgments
We thank the patients and their families for their gracious participation. We are grateful to the investigators of the RKSC coordinating sites and many additional individual contributors to the PH Registry for generously providing clinical data.
This study was supported by the Rare Kidney Stone Consortium (grant U54KD083908), a member of the National Institutes of Health Rare Diseases Clinical Research Network, and funded by the National Institute of Diabetes and Digestive and Kidney Diseases, the National Center for Advancing Translational Sciences, the Oxalosis and Hyperoxaluria Foundation, and OxThera Inc.
Investigators of the RKSC coordinating sites with contributions to the PH Registry include Dean Assimos (University of Alabama, Birmingham), Michelle Baum and Michael Somers (Children’s Hospital, Harvard Medical School), Lawrence Copelovitch (Children’s Hospital of Philadelphia), Prasad Devarajan (Cincinnati Children’s Hospital Medical Center), David Goldfarb (New York University), Elizabeth Harvey and Lisa Robinson (The Hospital for Sick Children [Sickkids], Toronto), William Haley (Mayo Clinic, Jacksonville), and Craig Langman (Ann & Robert H. Lurie Children’s Memorial Hospital, Chicago).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02810315/-/DCSupplemental.
References
- 1.Danpure CJ, Jennings PR: Peroxisomal alanine:glyoxylate aminotransferase deficiency in primary hyperoxaluria type I. FEBS Lett 201: 20–24, 1986 [DOI] [PubMed] [Google Scholar]
- 2.Cregeen DP, Williams EL, Hulton S, Rumsby G: Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum Mutat 22: 497, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Belostotsky R, Seboun E, Idelson GH, Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D, Weissman I, Charon C, Frishberg Y: Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet 87: 392–399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cochat P, Rumsby G: Primary hyperoxaluria. N Engl J Med 369: 649–658, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Hoppe B, Beck BB, Milliner DS: The primary hyperoxalurias. Kidney Int 75: 1264–1271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoppe B, Kemper MJ, Bökenkamp A, Portale AA, Cohn RA, Langman CB: Plasma calcium oxalate supersaturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int 56: 268–274, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Danpure CJ, Jennings PR, Fryer P, Purdue PE, Allsop J: Primary hyperoxaluria type 1: Genotypic and phenotypic heterogeneity. J Inherit Metab Dis 17: 487–499, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Harambat J, Fargue S, Acquaviva C, Gagnadoux MF, Janssen F, Liutkus A, Mourani C, Macher MA, Abramowicz D, Legendre C, Durrbach A, Tsimaratos M, Nivet H, Girardin E, Schott AM, Rolland MO, Cochat P: Genotype-phenotype correlation in primary hyperoxaluria type 1: The p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int 77: 443–449, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Mandrile G, van Woerden CS, Berchialla P, Beck BB, Acquaviva Bourdain C, Hulton SA, Rumsby G, OxalEurope Consortium : Data from a large European study indicate that the outcome of primary hyperoxaluria type 1 correlates with the AGXT mutation type. Kidney Int 86: 1197–1204, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Hoppe B, Danpure CJ, Rumsby G, Fryer P, Jennings PR, Blau N, Schubiger G, Neuhaus T, Leumann E: A vertical (pseudodominant) pattern of inheritance in the autosomal recessive disease primary hyperoxaluria type 1: lack of relationship between genotype, enzymic phenotype, and disease severity. Am J Kidney Dis 29: 36–44, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Lieske JC, Monico CG, Holmes WS, Bergstralh EJ, Slezak JM, Rohlinger AL, Olson JB, Milliner DS: International registry for primary hyperoxaluria. Am J Nephrol 25: 290–296, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Lemley KV: Pediatric nephrology: Estimating GFR in children: Schwartz redux. Nat Rev Nephrol 5: 310–311, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Cochat P, Hulton SA, Acquaviva C, Danpure CJ, Daudon M, De Marchi M, Fargue S, Groothoff J, Harambat J, Hoppe B, Jamieson NV, Kemper MJ, Mandrile G, Marangella M, Picca S, Rumsby G, Salido E, Straub M, van Woerden CS, OxalEurope : Primary hyperoxaluria type 1:Iindications for screening and guidance for diagnosis and treatment. Nephrol Dial Transplant 27: 1729–1736, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Hopp K, Cogal AG, Bergstralh EJ, Seide BM, Olson JB, Meek AM, Lieske JC, Milliner DS, Harris PC, on behalf of the Rare Kidney Stone Consortium : Phenotype-genotype correlations and estimated carrier frequencies of primary hyperoxaluria. J Am Soc Nephrol 26: 2559–2570, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pak CY, Adams-Huet B, Poindexter JR, Pearle MS, Peterson RD, Moe OW: Rapid Communication: Relative effect of urinary calcium and oxalate on saturation of calcium oxalate. Kidney Int 66: 2032–2037, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Verhulst A, Asselman M, Persy VP, Schepers MS, Helbert MF, Verkoelen CF, De Broe ME: Crystal retention capacity of cells in the human nephron: Involvement of CD44 and its ligands hyaluronic acid and osteopontin in the transition of a crystal binding—Into a nonadherent epithelium. J Am Soc Nephrol 14: 107–115, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF: Crystals cause acute necrotic cell death in renal proximal tubule cells, but not in collecting tubule cells. Kidney Int 68: 1543–1553, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Schepers MS, van Ballegooijen ES, Bangma CH, Verkoelen CF: Oxalate is toxic to renal tubular cells only at supraphysiologic concentrations. Kidney Int 68: 1660–1669, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Salido E, Pey AL, Rodriguez R, Lorenzo V: Primary hyperoxalurias: Disorders of glyoxylate detoxification. Biochim Biophys Acta 1822: 1453–1464, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Tang X, Bergstralh EJ, Mehta RA, et al. : Nephrocalcinosis is a risk factor for kidney failure in primary hyperoxaluria. Kidney Int 87: 623–631, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perucca J, Bouby N, Valeix P, Bankir L: Sex difference in urine concentration across differing ages, sodium intake, and level of kidney disease. Am J Physiol Regul Integr Comp Physiol 292: R700–R705, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Sands JM, Kokko JP: Current concepts of the countercurrent multiplication system. Kidney Int Suppl 57: S93–S99, 1996 [PubMed] [Google Scholar]
- 24.Hess B, Jordi S, Zipperle L, Ettinger E, Giovanoli R: Citrate determines calcium oxalate crystallization kinetics and crystal morphology-studies in the presence of Tamm-Horsfall protein of a healthy subject and a severely recurrent calcium stone former. Nephrol Dial Transplant 15: 366–374, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Goldberg H, Grass L, Vogl R, Rapoport A, Oreopoulos DG: Urine citrate and renal stone disease. CMAJ 141: 217–221, 1989 [PMC free article] [PubMed] [Google Scholar]
- 26.Tiselius HG, Berg C, Fornander AM, Nilsson MA: Effects of citrate on the different phases of calcium oxalate crystallization. Scanning Microsc 7: 381–389, discussion 389–390, 1993 [PubMed] [Google Scholar]
- 27.Posada-Ayala M, Zubiri I, Martin-Lorenzo M, Sanz-Maroto A, Molero D, Gonzalez-Calero L, Fernandez-Fernandez B, de la Cuesta F, Laborde CM, Barderas MG, Ortiz A, Vivanco F, Alvarez-Llamas G: Identification of a urine metabolomic signature in patients with advanced-stage chronic kidney disease. Kidney Int 85: 103–111, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Hoppe B: Evidence of true genotype-phenotype correlation in primary hyperoxaluria type 1. Kidney Int 77: 383–385, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Monico CG, Olson JB, Milliner DS: Implications of genotype and enzyme phenotype in pyridoxine response of patients with type I primary hyperoxaluria. Am J Nephrol 25: 183–188, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Monico CG, Rossetti S, Olson JB, Milliner DS: Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int 67: 1704–1709, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Fargue S, Rumsby G, Danpure CJ: Multiple mechanisms of action of pyridoxine in primary hyperoxaluria type 1. Biochim Biophys Acta 1832: 1776–1783, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.