Abstract
Background and objectives
The quality and age of donor organs are known to have a major effect on patient and graft outcomes, but it is uncertain whether this association is uniform for all recipients. We aimed to determine whether the use of expanded criteria deceased donor (ECD) kidneys for transplantation compared with standard criteria deceased donor (SCD) kidneys has a different association with survival in younger (age <60 years old) compared with older (age ≥60 years old) recipients.
Design, setting, participants, & measurements
Using data from the Australian and New Zealand Dialysis and Transplant Registry (1997–2009), we compared the risk of all-cause mortality and death with functioning graft among younger and older recipients who had received either an SCD or an ECD kidney using the adjusted Cox proportional hazard models.
Results
In total, 3822 patients were transplanted between 1997 and 2009. Over a follow-up period of 21,249 person-years (a median duration of 5.3 years [interquartile range, 2.22–8.6 years]), 567 recipients (n=385 for those age <60 years old; n=182 for those age ≥60 years old) died. Recipient age was an effect modifier between donor types, all-cause mortality, and death with functioning graft (P values for interaction were 0.05 and 0.04, respectively). In younger recipients, there was an excess risk of all-cause mortality (adjusted hazard ratio [HR], 1.55; 95% confidence interval [95% CI], 1.23 to 1.97) and death with functioning graft (adjusted HR, 1.72; 95% CI, 1.28 to 2.29) after transplantation with ECD kidneys compared with SCD kidneys, but there was no statistically significant association among older recipients (adjusted HR, 1.11; 95% CI, 0.80 to 1.54 and adjusted HR, 1.30; 95% CI, 0.89 to 1.89, respectively). This excess risk was largely caused by death from cardiovascular disease.
Conclusions
There was an excess risk of all-cause mortality and death with functioning graft when younger recipients were transplanted with ECD kidneys compared with SCD kidneys. These findings suggest that caution is needed in allocating ECD kidneys to younger recipients.
Keywords: renal transplantation, mortality, kidney donation, cardiovascular diseases, death, follow-up studies, humans, renal dialysis, risk, tissue donors
Introduction
The increasing disparities between organ supply and demand of deceased donor kidneys have led to the growing use of expanded criteria deceased donor (ECD) kidneys over the past decade. In Australia, the proportion of ECD kidneys in the deceased donor kidney pool has risen from 20.7% in 2005 to 33.6% in 2011 (1), with a similar trend being observed in the United States (2,3). Compared with standard criteria deceased donor (SCD) kidney transplants, ECD kidneys transplants are associated with at least a 70% increased risk of graft failure (4). Despite the poorer expected graft survival associated with ECD kidneys, a relative survival advantage exists compared with being on dialysis, particularly for older patients with diabetes and other comorbidities (5–7).
Conversely, there are growing concerns with the use of ECD kidneys in younger recipients because of the longer projected life expectancy compared with older recipients as well as the likelihood of future retransplantation (6). Observational studies have reported conflicting results regarding the association between donor types and the risk of death among younger patients. Understanding the longer-term outcomes of recipients transplanted with the various types of deceased donor organs may provide guidance for patients and clinicians on the acceptance and use of donor kidneys, with the ultimate goal of maximizing patient survival after transplantation. The objective of the study was to determine whether the use of ECD kidneys, compared with SCD kidneys has a different association with survival in younger (age <60 years old) compared with older (age ≥60 years old) recipients.
Materials and Methods
Study Population
Using the Australian and New Zealand Dialysis and Transplant (ANZDATA) Registry, we included all adult recipients of kidney transplants (age >18 years old) who have received their first deceased donor kidney transplants between 1997 and 2009 in Australia and New Zealand. Recipients with prior grafts and those who had received multiple organ grafts were excluded.
Recipients of kidney transplant were stratified according to recipient age and deceased donor types: (1) recipients age <60 years old who have received SCD kidneys (younger SCD), (2) recipients age <60 years old who have received ECD kidneys (younger ECD), (3) recipients age ≥60 years old who have received SCD kidneys (older SCD), and (4) recipients age ≥60 years old who have received ECD kidneys (older ECD). ECDs are defined as deceased donors age >60 years old or deceased donors age >50 years old with at least two of three criteria: hypertension, death attributed to cerebrovascular accident, or terminal serum creatinine of >1.50 mg/dl (8). Deceased donors age between 50 and 60 years old who had fewer than two of the above criteria or donors age <50 years old were defined as SCD.
Data Collection
Recorded baseline data included recipient characteristics, such as age, race, sex, causes of ESRD, duration of waiting time on dialysis, and comorbidities at time of transplantation (smoking history, diabetes, coronary artery disease, and peripheral vascular disease) and transplant-related characteristics, such as peak panel reactive antibody (categorized as <50% and ≥50%), number of HLA mismatches, and era. Transplant era was categorized into four groups for analysis (1997–99, 2000–2002, 2003–2005, and 2006–2009). eGFR was estimated using the using the four–variable abbreviated Modification of Diet in Renal Disease equation (9).
Study Outcomes
Diagnoses of cause-specific death were coded in the ANZDATA Registry. Deaths were assigned to one of five broad categories (cardiac, vascular, infection, malignancy, and others) according to the International Classification of Disease for death (10). Death–censored graft loss was considered as the secondary outcome.
Statistical Analyses
Primary Analyses.
Comparisons of baseline characteristics between study groups were performed by chi-squared and t tests for categorical and continuous variables, respectively. Mortality rates (all-cause mortality and death with functioning graft) and death–censored graft loss were determined using the Kaplan–Meier method. Cox proportional hazard models were used to assess the association between the exposure (recipient’s age and donor types) and all-cause mortality, death with functioning graft, and death–censored graft loss. The covariates included in the Cox regression models were recipients’ characteristics, such as age, race, sex, and smoking history, comorbidities at time of transplant (coronary artery disease, peripheral vascular disease, cerebrovascular disease, and diabetes), duration of waiting time, and transplant era. For death–censored graft loss, the covariates included recipients’ characteristics, such as sex, age, race, and time on dialysis, and other immunologic factors, such as the number of HLA mismatches. Results were expressed as hazard ratio (HRs) with 95% confidence intervals (95% CIs). Potential effect modification was tested between the study factor and also, other covariates using two–way interaction terms. All analyses were performed using SPSS, version 16.0 (SPSS Inc., Chicago, IL), with P values of <0.05 considered statistically significant.
Competing Risk Analyses.
We conducted a competing risk regression for cause-specific mortality taking into account the informative nature of censoring because of competing risk using the method of Fine and Gray (11). The stratified proportional subdistribution HRs were calculated to estimate the covariate effects on the cumulative incidence function. Other causes of death were considered as competing events. Other covariates included in the competing risk models were recipients’ characteristics, such as age, race, sex, and smoking history; comorbidities at time of transplant, such as coronary artery disease, peripheral vascular disease, and diabetes; dialysis duration; and transplant era. Competing risk analyses were performed using R statistical software 3.0.2 (R Foundation for Statistical Computing).
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. This is a registry analysis using deidentified data. As such, ethics approval for this study was not required.
Results
Study Population
In total, 3822 patients were included over a follow-up of 21,249 person-years (median duration, 5.3 years; interquartile range, 5.3–8.6 years). Of these, >1873 (49%) patients were followed for >5 years; 2432 (63.6%) were men, 1800 (47.1%) were previous or current smokers, and 3015 (78.9%) were of white race (Table 1). In total, 2411 (63.1%) younger recipients received an SCD kidney, and 650 (17.0%) received an ECD kidney; 539 (14.1%) older recipients received an SCD kidney, and 222 (5.8%) received an ECD kidney. The mean±SD ages of younger recipients with SCD kidneys and younger recipients with ECD kidneys were 44.2±10.2 and 45.1±10.1 years old, respectively (P=0.04). For older recipients, the mean±SD age for those who received an ECD kidney was 64.4±3.4 years old compared with 64.2±3.5 years old for those who received an SCD kidney (P=0.35). Younger recipients with ECD kidneys experienced a longer waiting time on dialysis compared with younger recipients of SCD allografts (dialysis >5 years: 20.8% [younger ECD] versus 17.3% [younger SCD]; P<0.01). The mean±SD preretrieval serum creatinine among younger recipients with ECD allograft was significantly higher than in younger recipients with SCD kidneys (1.08±0.85 mg/dl [younger ECD] versus 0.99±0.58 mg/dl [younger SCD]; P<0.01). There were no statistically significant differences in other comorbidities, such as diabetes mellitus, coronary artery disease, and peripheral vascular disease, between recipients of SCD and ECD kidneys. The allograft functions at 1 and 5 years were significantly lower in recipients of ECD allografts age <60 years old (eGFR of SCD versus ECD, 1 year: 55.0±17.4 versus 39.9±15.3 ml/min per 1.73 m2; P<0.001; 5 year: 53.5±38.7 versus 38.7±16.0 ml/min per 1.73 m2; P<0.001) and ≥60 years old (1 year: 55.0±17.4 versus 41.1±13.7 ml/min per 1.73 m2; P<0.001; 5 year: 55.0±36.2 versus 36.2±12.4 ml/min per 1.73 m2; P<0.001) compared with recipients of SCD kidneys.
Table 1.
Characteristics of recipients of kidney transplants stratified by recipient age and donor types
| Variables | SCD<60 yr old (n=2411) | ECD<60 yr old (n=650) | SCD Versus ECD P Value | SCD≥60 yr old (n=539) | ECD≥60 yr old (n=222) | SCD Versus ECD P Value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age at transplant (yr), mean±SD | 44.2±10.2 | 45.1±10.1 | 0.04 | 64.2±3.5 | 64.4±3.4 | 0.35 |
| Men, n (%) | 1518 (63.0) | 437 (67.2) | 0.04 | 341 (63.3) | 136 (61.3) | 0.62 |
| Race, n (%) | <0.01 | 0.73 | ||||
| White | 1881 (78.0) | 474 (72.9) | 469 (87.0) | 191 (86.0) | ||
| Not white | 530 (22.0) | 176 (27.1) | 70 (13.0) | 31 (14.0) | ||
| Patient survival (yr), median (IQR) | 5.8 (2.8–9.0) | 4.6 (1.8–7.8) | <0.001 | 4.2 (1.6–75) | 3.4 (1.5–6.4) | <0.001 |
| Graft survival (yr), median (IQR) | 5.2 (2.1–8.3) | 3.0 (3.0–3.0) | <0.001 | 3.8 (1.4–6.9) | 3.0 (3.0–4.0) | 0.02 |
| Comorbidities at time of first transplant, n (%) | ||||||
| Smoking status | 0.79 | 0.38 | ||||
| Current | 391 (16.2) | 104 (16.0) | 32 (5.9) | 16 (7.2) | ||
| Former | 734 (30.4) | 207 (31.8) | 232 (43.0) | 84 (37.8) | ||
| Never | 1286 (53.3) | 339 (52.2) | 275 (51.0) | 122 (55.0) | ||
| Diabetes mellitus | 322 (13.4) | 90 (13.8) | 0.75 | 86 (16.0) | 27 (12.2) | 0.27 |
| Coronary artery disease | 182 (7.5) | 62 (9.5) | 0.10 | 109 (20.2) | 35 (15.8) | 0.19 |
| Peripheral vascular disease | 111 (4.6) | 43 (6.6) | 0.04 | 49 (9.1) | 16 (7.2) | 0.48 |
| Primary kidney disease, n (%) | 0.75 | 0.45 | ||||
| Diabetes mellitus | 252 (10.7) | 71 (11.0) | 51 (9.6) | 15 (6.9) | ||
| GN | 1199 (50.9) | 326 (50.7) | 231 (43.3) | 110 (50.7) | ||
| Analgesic nephropathy | 13 (0.6) | 2 (0.3) | 17 (3.2) | 9 (4.1) | ||
| Renovascular disease | 88 (3.7) | 31 (4.8) | 54 (10.1) | 19 (8.8) | ||
| Polycystic kidney disease | 355 (15.1) | 89 (13.8) | 114 (21.4) | 39 (18.0) | ||
| Others | 448 (19.0) | 124 (19.3) | 66 (12.4) | 25 (11.5) | ||
| Immunologic status | ||||||
| HLA mismatches, median (IQR) | 3 (2–5) | 3 (3–3) | 0.99 | 3 (2–5) | 3 (3–4) | 0.76 |
| PRA≥85%, n (%) | 130 (5.4) | 34 (5.2) | 0.37 | 16 (3.0) | 11 (5.0) | 0.17 |
| Induction with T cell depletion agent, n (%) | 124 (5.1) | 51 (7.8) | <0.01 | 24 (4.5) | 14 (6.3) | 0.29 |
| No. of rejection episodes, n (%) | <0.01 | 0.17 | ||||
| 0 | 1688 (70.0) | 420 (64.6) | 439 (81.4) | 171 (77.0) | ||
| ≥1 | 723 (30.0) | 230 (35.4) | 100 (18.6) | 51 (23.0) | ||
| Time on dialysis (yr), n (%) | <0.01 | 0.35 | ||||
| 0–1 | 836 (34.7) | 200 (30.8) | 165 (30.6) | 62 (27.9) | ||
| >1–3 | 769 (31.9) | 188 (28.9) | 187 (34.7) | 68 (30.6) | ||
| >3–5 | 388 (16.1) | 127 (19.5) | 82 (15.2) | 38 (17.1) | ||
| >5 | 418 (17.3) | 135 (20.8) | 105 (19.5) | 54 (24.3) | ||
| Transplant era, n (%) | <0.001 | 0.05 | ||||
| 1997–1999 | 534 (22.1) | 125 (19.2) | 100 (18.6) | 37 (16.7) | ||
| 2000–2002 | 643 (26.7) | 121 (18.6) | 118 (21.9) | 33 (14.9) | ||
| 2003–2005 | 541 (22.4) | 145 (22.3) | 128 (23.7) | 52 (23.4) | ||
| 2006–2009 | 693 (28.7) | 259 (39.8) | 193 (35.8) | 100 (45.0) | ||
| Donor information | ||||||
| Donor age, mean±SD | 35.7±14.7 | 62.7±6.4 | <0.001 | 36.6±14.5 | 63.7±7.1 | <0.001 |
| Donor man, n (%) | 1443 (59.9) | 349 (53.7) | <0.01 | 314 (58.3) | 125 (56.3) | 0.62 |
| Preretrieval creatinine (mg/dl), mean±SD | 0.99±0.58 | 1.08±0.85 | <0.01 | 1.06±0.78 | 1.04±0.58 | 0.68 |
| Preretrieval creatinine >1.5 mg/dl, n (%) | 209 (8.9) | 75 (11.7) | 0.03 | 62 (11.8) | 29 (13.4) | 0.55 |
| Donor hypertension, n (%) | 163 (6.9) | 354 (56.1) | <0.001 | 52 (9.9) | 124 (57.4) | <0.001 |
| Cerebrovascular causes of brain death, n (%) | 1042 (43.2) | 543 (83.5) | <0.001 | 251 (46.6) | 185 (83.3) | <0.001 |
| Allograft function | ||||||
| eGFR at 1 yr (ml/min per 1.73 m2), mean±SDa | 55.0±17.4 | 39.9±15.3 | <0.001 | 55.0±17.4 | 41.1±13.7 | <0.001 |
| eGFR at 5 yr (ml/min per 1.73 m2), mean±SDa | 53.5±38.7 | 38.7±16.0 | 55.0±36.2 | 36.2±12.4 | <0.001 | |
| Graft failure, n (%) | 283 (11.7) | 115 (17.7) | <0.001 | 42 (7.8) | 24 (10.8) | 0.18 |
| Death with functional graft, n (%) | 182 (7.5) | 67 (10.3) | 0.02 | 105 (19.5) | 43 (19.4) | 0.29 |
| Cause of mortality, n (%) | 0.15 | 0.28 | ||||
| Cardiovascular | 115 (40.9) | 51 (50.5) | 42 (32.3) | 22 (42.3) | ||
| Malignancy | 60 (21.1) | 24 (23.8) | 40 (30.8) | 9 (17.3) | ||
| Infection | 51 (18.0) | 13 (12.9) | 34 (26.2) | 14 (26.9) | ||
| Others | 57 (20.1) | 13 (12.9) | 14 (10.8) | 7 (13.5) |
SCD, standard criteria deceased donor; ECD, expanded criteria deceased donor; IQR, interquartile range; PRA, panel reactive antibody.
eGFR was estimated using the Modification of Diet in Renal Disease equation (9).
Incidence of All-Cause and Cause-Specific Mortality
In total, 567 (14.8%) recipients of transplants died during the follow-up period. Cardiovascular disease was the most common cause of death (n=230; 40.6%) followed by infection (n=133; 23.5%) and cancer (n=112; 19.8%).
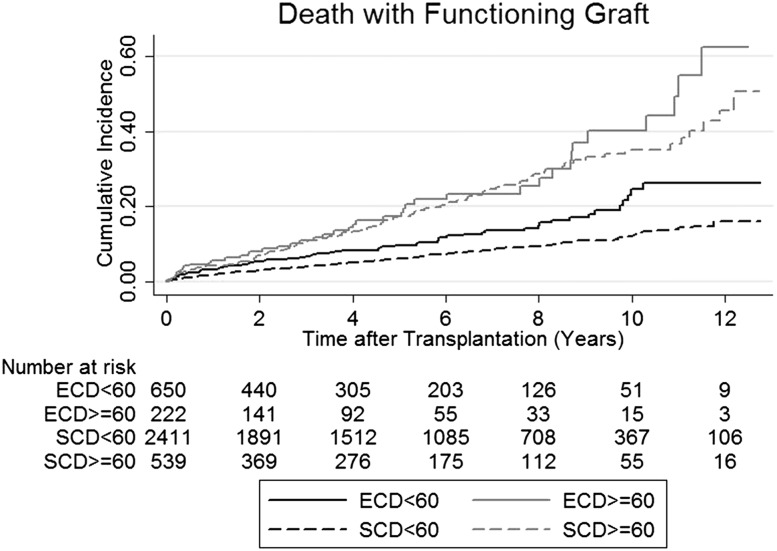
Figures 1 and 2 show the Kaplan–Meier estimates of all-cause mortality and death with a functioning graft among all recipients of transplants stratified by recipient age and donor types. The 10-year all–cause mortality rates for younger recipients with SCD kidneys, younger recipients with ECD kidneys, older recipients with SCD kidneys, and older recipients with ECD kidneys were 18.0%, 29.3%, 40.5%, and 46.2% respectively (P value for pairwise comparison between young SCD versus young ECD; P<0.001). Similar patterns were observed for death with functioning graft. The 10-year death rates (with functioning graft) of younger recipients of SCD and ECD kidneys and older recipients with SCD and ECD kidneys were 12.1%, 24.6%, 35.1%, and 40.2%, respectively (Table 2).
Figure 1.
Kaplan–Meier estimate of all-cause mortality of recipients of transplants stratified by recipient age and donor types. Ten-year mortality rates for standard criteria deceased donor (SCD)<60, expanded criteria deceased donor (ECD)<60, SCD≥60, ECD≥60: 18.0%, 29.3%, 40.5%, and 46.2%, respectively (P value for pairwise comparison between SCD<60 and ECD<60=0.001).
Figure 2.
Kaplan–Meier estimate of death with functioning graft of recipients of transplants stratified by recipient age and donor types. Ten-year death with functioning graft rate for standard criteria deceased donor (SCD)<60, expanded criteria deceased donor (ECD)<60, SCD≥60, ECD≥60: 12.1%, 4.6%, 35.1%, and 40.2%, respectively.
Table 2.
The incidence of all-cause mortality and death with functioning graft by recipient age and donor types
| Patient's Group | 3 yr, % | 5 yr, % | 10 yr, % | SCD Versus ECD Log-Rank Test P Value |
|---|---|---|---|---|
| All-cause mortality | ||||
| SCD<60 yr old | 5.2 | 8.4 | 18.0 | <0.001 |
| ECD<60 yr old | 8.3 | 12.6 | 29.3 | |
| SCD≥60 yr old | 13.0 | 20.9 | 40.5 | 0.58 |
| ECD≥60 yr old | 10.9 | 19.1 | 46.2 | |
| Death with functioning graft | ||||
| SCD<60 yr old | 4.0 | 6.3 | 12.1 | <0.001 |
| ECD<60 yr old | 6.8 | 9.7 | 24.6 | |
| SCD≥60 yr old | 10.7 | 17.5 | 35.1 | 0.32 |
| ECD≥60 yr old | 10.9 | 17.3 | 40.2 |
SCD, standard criteria deceased donor; ECD, expanded criteria deceased donor.
Association between Recipients’ Age, Donor Type, and All-Cause Mortality
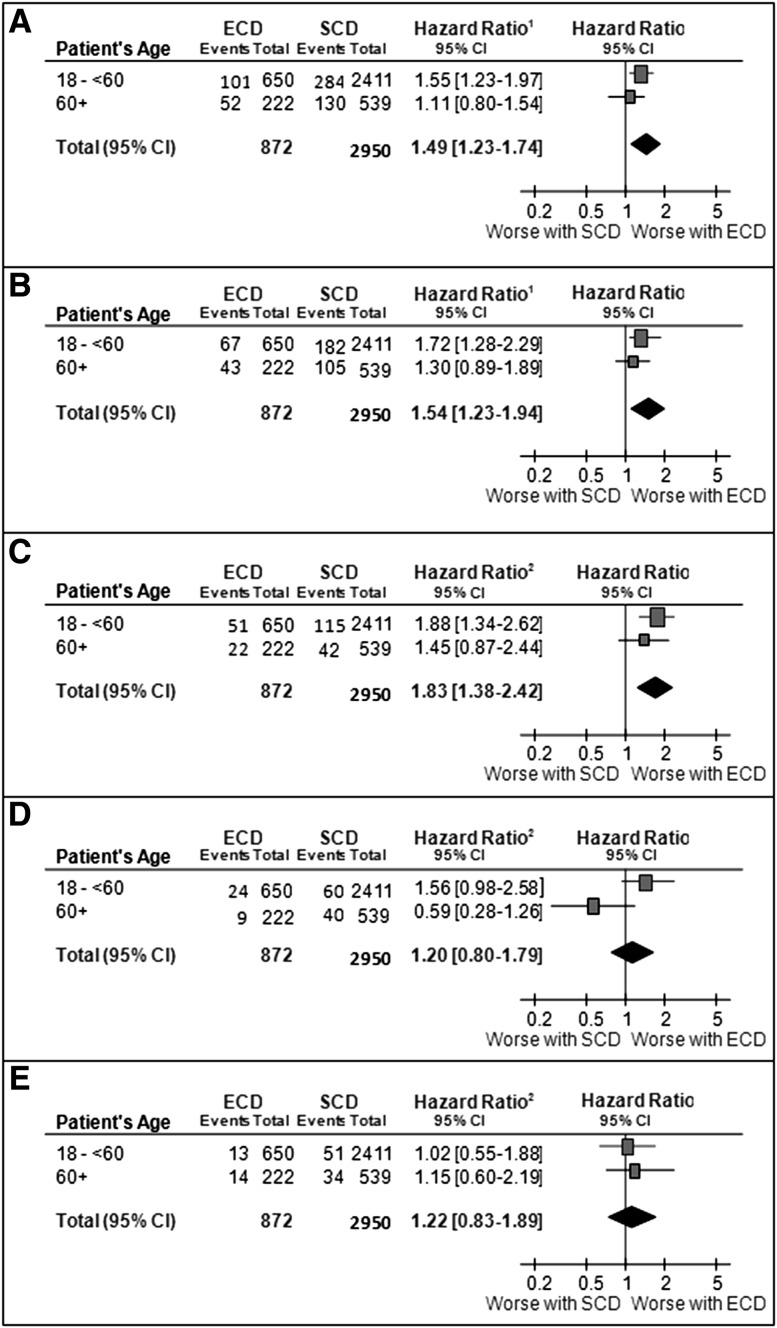
Overall, the association between donor types and all-cause mortality was modified by donor age (P value of interaction =0.05). Compared with younger recipients with SCD kidneys, the adjusted HR estimate for all-cause mortality among younger recipients of ECD kidneys was 1.55 (95% CI, 1.23 to 1.97; P<0.001). There were no statistically significant differences between the risks of all-cause mortality among older recipients who received an SCD or an ECD kidney (adjusted HR, 1.11; 95% CI, 0.80 to 1.54; P=0.54) (Figure 3A, Table 3).
Figure 3.
Younger expanded criteria deceased donor (ECD) kidney recipients had excess risk of all-cause mortality, death with functioning graft and cardiovascular death compared with young standard criteria deceased donor (SCD) recipients but no statistically significant association among older recipients. Relevance of donor types and recipient’s age to (A) all-cause mortality, (B) death with functioning graft, (C) cardiovascular mortality, (D) infected mortality, and (E) cancer-related mortality. 95% CI, 95% confidence interval; ECD, expanded criteria deceased donor; SCD, standard criteria deceased donor.
Table 3.
Adjusted hazard ratios of all-cause mortality after first kidney transplantation in recipients ages <60 and ≥60 years old
| Variables | Recipients <60 yr old | Recipients ≥60 yr old | ||||
|---|---|---|---|---|---|---|
| Adjusted HR | 95% CI | P Value | Adjusted HR | 95% CI | P Value | |
| Patient demographics | ||||||
| Men | 0.96 | 077 to 1.19 | 0.68 | 1.07 | 0.77 to 1.49 | 0.68 |
| Comorbidities at time of transplant | ||||||
| Coronary artery disease | 1.68 | 1.24 to 2.27 | 0.001 | 1.27 | 0.87 to 1.84 | 0.22 |
| Peripheral artery disease | 1.66 | 1.15 to 2.38 | <0.01 | 1.24 | 0.76 to 2.01 | 0.40 |
| Diabetes | 2.06 | 1.57 to 2.68 | <0.001 | 1.53 | 1.03 to 2.26 | 0.04 |
| Cerebrovascular disease | 1.86 | 1.21 to 2.86 | <0.01 | 1.16 | 0.62 to 2.17 | 0.64 |
| Smoking status | ||||||
| Never | Reference | Reference | ||||
| Chronic | 1.55 | 1.19 to 2.03 | 0.001 | 1.48 | 0.85 to 2.56 | 0.16 |
| Former smoker | 1.21 | 0.96 to 1.53 | 0.11 | 0.98 | 0.71 to 1.36 | 0.92 |
| Time on dialysis, yr | ||||||
| 0–1 | Reference | Reference | ||||
| >1–3 | 1.30 | 1.01 to 1.67 | 0.04 | 1.41 | 0.98 to 2.02 | 0.06 |
| >3–5 | 1.34 | 0.99 to 0.83 | 0.06 | 1.02 | 0.63 to 1.66 | 0.93 |
| >5 | 1.89 | 1.41 to 2.55 | <0.001 | 1.58 | 1.00 to 2.49 | 0.05 |
| Donor types | ||||||
| SCD | Reference | Reference | ||||
| ECD | 1.55 | 1.23 to 1.97 | <0.001 | 1.11 | 0.80 to 1.54 | 0.54 |
HR, hazard ratio; 95% CI, 95% confidence interval; SCD, standard criteria deceased donor; ECD, expanded criteria deceased donor.
Association between Recipients’ Age, Donor Type, and Death with Functioning Graft
Similar findings were observed when we considered death with functioning graft as an outcome of interest. There was a significant interaction between donor age, donor type, and death with functioning graft (P value for interaction =0.04). Compared with younger recipients with SCD kidneys, the adjusted HR estimate for death with functioning graft among younger recipients of ECD kidneys was 1.72 (95% CI, 1.28 to 2.29; P<0.001). There were no statistically significant differences between the risks of death with functioning graft among older recipients who received an SCD or an ECD kidney (adjusted HR, 1.30; 95% CI, 0.89 to 1.89; P=0.17) (Figure 3B).
Association between Recipients’ Age, Donor Type, and Death with Cause-Specific Mortality
There was an association between donor types and cardiovascular-related mortality among younger but not older recipients of transplants (P value for interaction =0.05). Compared with younger recipients with SCD kidneys, the adjusted HR estimate for cardiovascular disease–related mortality was 1.88 (95% CI, 1.34 to 2.62; P<0.001). There was no statistically significant association between donor types and cardiovascular-related mortality in older recipients of kidney transplants (adjusted HR, 1.45; 95% CI, 0.87 to 2.44; P=0.16) (Figure 3C).
Compared with younger recipients who received an SCD kidney, the adjusted HR for infection-related mortality among younger recipients of transplants with ECD kidneys was 1.56 (95% CI, 0.98 to 2.58; P=0.06). There was no statistically significant association between donor types and infection-related death in older recipients (adjusted HR, 0.59; 95% CI, 0.28 to 1.26; P=0.18; P value for interaction =0.05) (Figure 3D).
There was no statistically significant association between donor types and cancer-related mortality, irrespective of the age of the recipients (younger recipients: adjusted HR, 1.02; 95% CI, 0.55 to 1.88; P=0.96; older recipients: adjusted HR, 1.15; 95% CI, 0.60 to 2.19; P=0.67) (Figure 3E).
Association between Donor Types and Death–Censored Graft Loss
Four hundred sixty-four (12.1%) patients experienced graft loss during the median follow-up period of 4.9 years (n=283 [11.7%] for younger SCD, n=115 [17.7%] for younger ECD, n=42 [7.8%] for older SCD, and n=24 [10.8%] for older ECD). Recipient’s age was not an effect modifier between donor types and death–censored graft loss (P value for interaction =0.61). The incidences of death–censored graft loss 10 years after transplantation for those who received SCD and ECD allografts were 10.4 and 15.6 per 1000 patients-year, respectively. The adjusted HR for death–censored graft loss among those who received an ECD compared with an SCD allograft was 1.85 (95% CI, 1.51 to 2.26).
Discussion
In our retrospective cohort study involving 3822 recipients of kidney transplants with a median follow-up time of 5.3 years, we showed that recipient age was an effect modifier of donor type on the risk of death. Younger recipients of kidney transplants with ECD kidneys had an excess risk of all-cause mortality and death with functioning graft compared with recipients who received SCD kidneys, but there was no statistically significant association among older recipients (>60 years of age). This association seemed to be mediated through an excess risk from cardiovascular disease.
Previous analyses from the US Renal Data System Registry have reported that the overall adjusted patient survival (and not death with functioning graft) was significantly reduced in recipients who had received ECD kidneys compared with recipients who had received SCD kidneys (8). The adjusted patient survival was 5% lower at 1 year and 8%–12% lower at 3–5 years for all recipients of kidney transplants with ECD kidneys compared with recipients who have received SCD kidneys (12,13). Our study findings suggested that the increased risk of death was only observed in younger recipients with ECD kidneys, and the cause of death was attributed predominantly to an excess risk of cardiovascular disease. In part, this may be explained by an increased risk of cardiovascular-related disease associated with poorer graft function (and reduced eGFR) in ECD kidneys (8,13). Alternately, it may be attributed to the chronic inflammatory and immunosuppressive effects induced by uremia (14), leading to an accelerated risk of cardiovascular disease. The differential effect of age on the incidence of all-cause mortality and death with functioning graft by donor types was an interesting finding. This may suggest that donor function plays a less important role in influencing long–term patient outcomes in older recipients. This finding further strengthens the recommendations that older recipients may benefit from accepting and receiving ECD kidneys early after the development of ESRD rather than remaining on the waiting list (15).
With increasing availability of ECD kidneys for transplantation, the optimal use of ECD kidneys remains unclear and has been the subject of intense debate over the last decade. Matching the expected longevity of donor kidneys to the expected survival of transplant candidates would confer maximal use of the available donor kidneys but at the expense of equity. Matching donor and recipient age by preferential allocation of ECD kidneys to older transplant candidates and SCD kidneys to younger candidates is one such approach design to improve the use of donor kidneys (16,17). The disadvantage of receiving ECD kidneys, which may result in earlier graft loss compared with SCD kidneys, may be traded against the expected survival advantage of accepting ECD kidneys at an earlier time point, negating the risk of death associated with increasing waiting time (6,18,19). The Eurotransplant Senior Program is an age–matching allocation system, whereby transplant candidates age ≥65 years old are only allocated kidneys from donors age >65 years old (20). Since the inception of the program, the waiting time for transplantation among elderly patients has significantly reduced from 4.64 to 3.55 years (21). Although a similar age–matching program has not been implemented in the United States, identifying better–quality donor kidneys using a derived kidney donor profile index (KDPI) allows preferential allocation of better-quality kidneys from donors with a KDPI≤20% to transplant candidates with the longest expected survivals (i.e., those in the top 20th percentile of the longest estimated post–transplant survival) (22). The new national allocation ECD program in the United States explicitly considers each transplant candidate’s preferences and willingness in receiving marginal or ECD kidneys defined as donor kidneys with calculated KDPI>0.85 (22,23). In Australia, donor/recipient age matching is not an explicit criteria for deceased donor allocation, but it is important for physicians and younger transplant candidates to be cognizant of the survival effect of accepting ECD kidneys relative to SCD kidneys, balancing between the acceptance of poorer–quality donor kidneys and potential shorter waiting time on dialysis.
Our study has both strengths and potential limitations. It is a contemporary analysis that has explicitly explored the interaction between donor types, recipient age, and all-cause and cause-specific mortality in a large national cohort of recipients of kidney transplants. The prospective nature and the completeness of the dataset suggest that ascertainment biases in the exposure and study factors are minimized. Unlike other studies, we had chosen all-cause mortality and death with graft function as the two main outcome measures. All-cause mortality takes into account the risk of death after graft failure, because patients who commenced on dialysis after failing their allografts have high mortality and may benefit from retransplantation (24). Death with a functioning graft may provide an accurate estimation of the gain/loss of potential functioning graft years without confounding by the adverse effect on survival associated with graft loss and return to dialysis. Our findings are robust and consistent for both outcomes. Age of the recipient is an effect modifier of donor types, such that the effect on death is greater in the young and largely driven by the excess in cardiovascular events.
Although multiple confounding factors were adjusted for, there may be unmeasured residual confounders, such as the changing nature of donor characteristics (such as donor comorbidities [e.g., diabetes], BP, and vasopressor used before donation); other donor-, recipient-, and transplant-related factors (such as the severity of recipient comorbidities); patient compliance, and center-specific effects, that could have modified the association between donor type and mortality. Although there were no statistically significant differences in the baseline comorbidities of recipients who received the different donor types, selection bias may exist, because there may be systematic differences in how kidneys were allocated at the local and national levels and also, inherent differences in the acceptance of particular donor kidneys by the physicians and/or patients. Although chronic conditions and comorbidities may be strong predictors of mortality in the younger population, this may not necessarily be the case in the elderly. Functional limitation may be a better predictor for adverse outcomes, such as death, in the very old (25), but functional status of recipients of transplants is not routinely collected by the ANZDATA Registry. Finally, our study did not account for the dynamic variations in the proportions of ECD and SCD donors within the donor pool and the changing characteristics of the transplant candidates over time. Our study was also conducted using data from the Australian and New Zealand transplant cohort. As such, the findings may not be generalizable to other ethnic groups.
Compared with SCD kidneys, acceptance of ECD kidneys was associated with poorer survival in younger recipients of kidney transplants but not in older recipients. Economic evaluation to assess the benefits and costs of the current allocation model in Australia compared with an allocation system whereby ECD kidneys are preferentially allocated to older transplant candidates would establish the cost-effectiveness of implementing such a program. In addition, the potential tradeoff between acceptance of ECD kidneys at an earlier time point and the expected survival benefits associated with a reduction in the overall waiting time on dialysis should be explored.
Younger recipients who received ECD kidneys seemed to have a greater risk of all-cause mortality and death with functioning graft compared with young recipients with SCD kidneys. The observed excess risk of death is cause specific for cardiovascular disease and infections among younger recipients of kidney transplants who have received ECD kidneys compared with those with SCD kidneys. The decision to accept ECD kidneys for younger transplant candidates should, therefore, be undertaken with caution. Future studies exploring the potential tradeoff between the acceptances of ECD kidneys versus longer waiting time for SCD kidney would be warranted.
Disclosures
None.
Acknowledgments
The authors acknowledge the substantial contributions of the entire Australian and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients) to provide information to and maintain the Australian and New Zealand Dialysis and Transplant (ANZDATA) Registry database.
The data reported here have been supplied by the ANZDATA Registry. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the ANZDATA Registry.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Waiting for a Deceased Kidney Donor Transplant: Better a Small Fish Than an Empty Dish?,” on pages 12–13.
References
- 1.Excell L, Hee K, Russ G: ANZOD Registry Report, Adelaide, South Australia, Australia and New Zealand Organ Donation Registry, pp 1–54, 2012
- 2.OPTN/SRTR 2012 Annual Data Report, Rockville, MD, US Department of Health and Human Services, 2014 [Google Scholar]
- 3.Mohan S, Tanriover B, Ali N, Crew RJ, Dube GK, Radhakrishnan J, Hardy MA, Ratner LE, McClellan W, Cohen D: Availability, utilization and outcomes of deceased diabetic donor kidneys: Analysis based on the UNOS registry. Am J Transplant 12: 2098–2105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA, Held PJ: Donor characteristics associated with reduced graft survival: An approach to expanding the pool of kidney donors. Transplantation 74: 1281–1286, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Savoye E, Tamarelle D, Chalem Y, Rebibou JM, Tuppin P: Survival benefits of kidney transplantation with expanded criteria deceased donors in patients aged 60 years and over. Transplantation 84: 1618–1624, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 294: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK: Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol 12: 589–597, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM: Expanded criteria donors for kidney transplantation. Am J Transplant 3[Suppl 4]: 114–125, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G, National Kidney Foundation : National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann Intern Med 139: 137–147, 2003 [DOI] [PubMed] [Google Scholar]
- 10.International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Geneva, Switzerland, World Health Organization, 2010
- 11.Fine J, Gray R: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 12.Lim WH, Chadban S, Campbell S, Cohney S, Russ G, McDonald S: A review of utility-based allocation strategies to maximize graft years of deceased donor kidneys. Nephrology (Carlton) 16: 368–376, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Pascual J, Zamora J, Pirsch JD: A systematic review of kidney transplantation from expanded criteria donors. Am J Kidney Dis 52: 553–586, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Betjes MG: Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol 9: 255–265, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Schold JD, Meier-Kriesche HU: Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 1: 532–538, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Meier-Kriesche HU, Schold JD, Gaston RS, Wadstrom J, Kaplan B: Kidneys from deceased donors: Maximizing the value of a scarce resource. Am J Transplant 5: 1725–1730, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Lim WH, Chang S, Chadban S, Campbell S, Dent H, Russ GR, McDonald SP: Donor-recipient age matching improves years of graft function in deceased-donor kidney transplantation. Nephrol Dial Transplant 25: 3082–3089, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Al-Shraideh Y, Farooq U, Farney AC, Palanisamy A, Rogers J, Orlando G, Buckley MR, Reeves-Daniel A, Doares W, Kaczmorski S, Gautreaux MD, Iskandar SS, Hairston G, Brim E, Mangus M, Stratta RJ: Influence of recipient age on deceased donor kidney transplant outcomes in the expanded criteria donor era. Clin Transplant 28: 1372–1382, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL: Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant 14: 2310–2316, 2014 [DOI] [PubMed]
- 20.Smits JM, Persijn GG, van Houwelingen HC, Claas FH, Frei U: Evaluation of the Eurotransplant Senior Program. The results of the first year. Am J Transplant 2: 664–670, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Frei U, Noeldeke J, Machold-Fabrizii V, Arbogast H, Margreiter R, Fricke L, Voiculescu A, Kliem V, Ebel H, Albert U, Lopau K, Schnuelle P, Nonnast-Daniel B, Pietruck F, Offermann R, Persijn G, Bernasconi C: Prospective age-matching in elderly kidney transplant recipients—A 5-year analysis of the Eurotransplant Senior Program. Am J Transplant 8: 50–57, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, Wang X, Shteyn E, Cherikh W, Stewart D, Samana CJ, Chung A, Hart A, Kasiske BL: New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol 25: 1842–1848, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen DJ, St Martin L, Christensen LL, Bloom RD, Sung RS: Kidney and pancreas transplantation in the United States, 1995–2004. Am J Transplant 6: 1153–1169, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Gill JS, Abichandani R, Kausz AT, Pereira BJ: Mortality after kidney transplant failure: The impact of non-immunologic factors. Kidney Int 62: 1875–1883, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Guralnik JM, Ferrucci L: Assessing the building blocks of function: Utilizing measures of functional limitation. Am J Prev Med 25[Suppl 2]: 112–121, 2003 [DOI] [PubMed] [Google Scholar]