Abstract
Background and objectives
Partial nephrectomy or radical nephrectomy is the standard of care for patients with kidney neoplasms, but surgery may result in loss of renal function. We sought to identify patient characteristics associated with renal functional recovery following radical nephrectomy.
Design, setting, participants, & measurements
We performed a retrospective study among 572 patients with kidney neoplasms who underwent RN between 2006 and 2013. The primary endpoint was recovery of postoperative eGFR to the preoperative level. We plotted the trajectory of each patient’s eGFR from their first postoperative visit up to 3 years after surgery. Cumulative incidence and competing risks regression estimated associations between patient and clinical characteristics and eGFR recovery, stratified by preoperative eGFR.
Results
Median age was 61.5 years; 68% of patients were male, and 89% were white. Overall, eGFR increased over time following an initial postoperative decrease. Median postoperative follow-up among survivors was 10.8 (minimum, 0.03; maximum, 36.0) months; during follow-up, 263 patients achieved eGFR recovery. Median time to eGFR recovery was 25.3 months. Two-year cumulative incidence of eGFR recovery was 49% overall and 44% and 58% among those with preoperative eGFR≥60 and <60 ml/min per 1.73 m2, respectively (P<0.001). On multivariable analysis, younger age at surgery and female sex were significantly associated with a higher chance of eGFR recovery among patients with preoperative eGFR<60 ml/min per 1.73 m2. Among patients with preoperative eGFR≥60 ml/min per 1.73 m2, hypertension was significantly associated with a lower chance of eGFR recovery, whereas increased tumor size was significantly associated with a higher chance of eGFR recovery.
Conclusions
Overall, almost half of the patients in this study recovered to their preoperative eGFR by 2 years following surgery. Distributions of preoperative risk factors differed by preoperative eGFR, leading to distinct factors that were significantly associated with chance of eGFR recovery.
Keywords: nephrectomy, kidney cancer, chronic kidney disease, renal function, follow-up studies, glomerular filtration rate, humans, kidney, postoperative period, risk factors
Introduction
The incidence of kidney cancer is increasing in the United States (1), likely because of increased rates of incidental detection (2,3). Partial nephrectomy (PN) or radical nephrectomy (RN) is the standard surgical treatment for renal cortical tumors but may result in long-term loss of renal function given the reduction in renal mass. Although young and healthy individuals who donate a kidney appear to do well with respect to survival and long-term kidney function (4), it is less clear what factors affect postoperative outcomes among patients with renal tumors, particularly those who undergo radical procedures. Patients with renal tumors tend to be older and many have significant medical comorbidities, including diabetes, hypertension, CKD, and cardiovascular disease. These medical comorbidities, independently or in combination, may have an adverse effect on kidney function before or after RN.
Most prior studies have focused on differences in postoperative eGFR and risk of developing new-onset CKD between patients undergoing PN and those undergoing RN, finding that patients who undergo RN have significantly lower postoperative eGFR and significantly higher risk of developing new-onset CKD after their operation (5–7). In recent studies conducted among patients who underwent a radical procedure, lower preoperative eGFR, older age, and history of comorbidities were associated with lower postoperative eGFR (8,9), whereas older age, history of comorbidities, and tobacco use have been independently associated with the development of CKD following nephrectomy (10–13). Notably, not all patients develop CKD, and indeed some patients experience improved renal function after surgery (5). It remains unclear to what extent recovery of eGFR can occur and, if it does occur, during what time frame.
In this study we describe the natural history of eGFR after RN in a large patient population with kidney neoplasms treated at a tertiary referral center. Given its potential effect on renal function recovery, we report associations separately by preoperative eGFR. The goals of this analysis were to (1) evaluate long-term trends in postoperative eGFR and (2) identify patient characteristics associated with postoperative recovery to preoperative eGFR levels.
Materials and Methods
Data on 661 patients who underwent RN for kidney neoplasms from 2006 to 2013 were obtained from a kidney cancer surgical database at Memorial Sloan Kettering Cancer Center after institutional review board approval. Patients were excluded if they had benign tumors (n=57) or metastatic disease (n=12), were of unknown race (n=19), or were receiving platinum-based chemotherapy (n=1).The final sample size for analysis was 572 patients, with a total of 9887 eGFR measurements.
Serum creatinine values were collected at the preoperative visit and at all postoperative visits. Patients are seen for follow-up visits in the clinic every 3–6 months postoperatively, on average. We used serum creatinine to calculate eGFR values using the CKD-Epidemiology Collaboration formula (14) as follows:
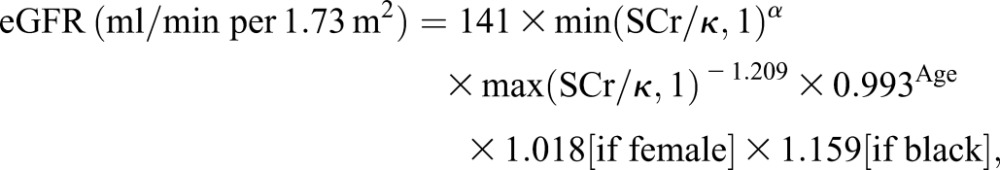 |
where SCr is serum creatinine (mg/dl), κ is 0.7 for female patients and 0.9 for male patients, α is −0.329 for female patients and −0.411 for male patients, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1.
To explore trends in the data, we plotted the trajectory of each patient’s eGFR from his or her immediate postoperative visit up to 3 years postoperatively, and we used locally weighted scatterplot smoothing to look at trends both overall and by preoperative eGFR. Preoperative eGFR was dichotomized as ≥60 versus <60 ml/min per 1.73 m2 for the purposes of this analysis. We analyzed the association between patient and disease characteristics with preoperative eGFR using a Wilcoxon rank-sum test for continuous variables and a Fisher exact test for categorical variables. The primary endpoint was postoperative recovery to preoperative eGFR, within a 5% margin of error. The data were analyzed in a survival analysis framework, with eGFR recovery as the primary event of interest. Follow-up time was calculated from the date of surgery to the date of eGFR recovery. Patients who did not achieve eGFR recovery at any time were censored at the date of their last eGFR measurement or at 36 months, whichever came first. Death from any cause was treated as a competing event. Competing risks methods estimated the cumulative incidence of eGFR recovery, and the Gray test was used for by-group comparisons. Competing risks regression allowed for univariable and multivariable regression modeling.
A P value <0.05 was considered to represent a statistically significant difference. All analyses were conducted using R software, version 3.1.0 (R Core Development Team, Vienna, Austria) including the cmprsk package.
Results
Of the 572 patients included in this analysis, 65 (11%) had eGFR≥90 ml/min per 1.73 m2, 298 (52%) had an eGFR of 60–89 ml/min per 1.73 m2, 201 (35%) had an eGFR of 30–59 ml/min per 1.73 m2, 5 (1%) had an eGFR of 15–29 ml/min per 1.73 m2, and 3 (1%) had an eGFR<15 ml/min per 1.73 m2 preoperatively. For the purposes of this analysis, we dichotomized preoperative eGFR as ≥60 (n=363; 63%) versus <60 (n=209; 37%) ml/min per 1.73 m2. Overall, median preoperative eGFR was 64.9 (minimum, 13.9; maximum, 137.4) ml/min per 1.73 m2; median preoperative eGFR was 75.1 (minimum, 60.0; maximum, 137.4) ml/min per 1.73 m2 and 51.7 (minimum, 13.9; maximum, 59.9) ml/min per 1.73 m2 in the eGFR≥60 and <60 ml/min per 1.73 m2 groups, respectively. Patient and disease characteristics both overall and by preoperative eGFR are presented in Table 1. Median patient age was 61.5 years (minimum, 25.1; maximum, 91.6 years); 67.7% of patients were male, and 88.5% were white. Patients with higher preoperative eGFR (≥60 ml/min per 1.73 m2) tended to be younger, had lower American Society of Anesthesiologists stage and less frequently had diabetes, hypertension, and coronary artery disease compared with patients with lower preoperative eGFR (<60 ml/min per 1.73 m2) (all P<0.05). Tumor size also significantly differed across preoperative eGFR, such that patients with preoperative eGFR<60 ml/min per 1.73 m2 had smaller tumors than those with preoperative eGFR≥60 ml/min per 1.73 m2.
Table 1.
Patient characteristics by preoperative eGFR
| Variable | Overall (n=572) | Preoperative eGFR | P Value | |
|---|---|---|---|---|
| ≥60 ml/min per 1.73 m2 (n=363 [63%]) | <60 ml/min per 1.73 m2 (n=209 [37%]) | |||
| Median age at surgery, yr | 61.5 (25.1, 91.6) | 57.5 (25.1, 91.6) | 68.2 (28.3, 87.9) | <0.001 |
| Median BMI, kg/m2a | 28.7 (15.5, 69.7) | 28.4 (18.9, 69.7) | 29.0 (15.5, 51.0) | 0.36 |
| Sex | 0.06 | |||
| Female | 185 (32.3) | 107 (29.5) | 78 (37.3) | |
| Male | 387 (67.7) | 256 (70.5) | 131 (62.7) | |
| Race | 0.10 | |||
| Other | 66 (11.5) | 48 (13.2) | 18 (8.6) | |
| White | 506 (88.5) | 315 (86.8) | 191 (91.4) | |
| Diabetes | 96 (16.8) | 51 (14) | 45 (21.5) | 0.03 |
| Hypertension | 357 (62.4) | 205 (56.5) | 152 (72.7) | <0.001 |
| Coronary artery disease | 62 (10.8) | 30 (8.3) | 32 (15.3) | 0.01 |
| Smokingb | 0.73 | |||
| Never | 281 (49.1) | 180 (49.6) | 101 (48.3) | |
| Ever | 289 (50.5) | 181 (49.9) | 108 (51.7) | |
| NA | 2 (0.3) | 2 (0.6) | 0 (0) | |
| ASA grade | 0.03 | |||
| 1, 2 | 236 (41.3) | 162 (44.6) | 74 (35.4) | |
| 3, 5 | 336 (58.7) | 201 (55.4) | 135 (64.6) | |
| Median tumor size, cmc | 7.7 (0.4, 32) | 8 (1.3, 32) | 7.2 (0.4, 23) | 0.01 |
| T stage | 0.01d | |||
| Tx, T0 | 10 (1.7) | 7 (1.9) | 3 (1.4) | |
| T1 | 111 (19.4) | 63 (17.4) | 48 (23) | |
| T2 | 71 (12.4) | 57 (15.7) | 14 (6.7) | |
| T3, T4 | 380 (66.4) | 236 (65) | 144 (68.9) | |
| Type of surgery | 0.43 | |||
| Laparoscopic/robotic | 104 (18.2) | 70 (19.3) | 34 (16.3) | |
| Open | 468 (81.8) | 293 (80.7) | 175 (83.7) | |
Median values are expressed with minimum, maximum values in parentheses. All other values are expressed as number (percentage). BMI, body mass index; NA, not available; ASA, American Society of Anesthesiologists.
BMI value missing for one patient.
Smoking values missing for two patients.
Tumor size value missing for one patient.
P value from chi-squared test comparing Tx, T0, T1 versus T2 versus T3, T4.
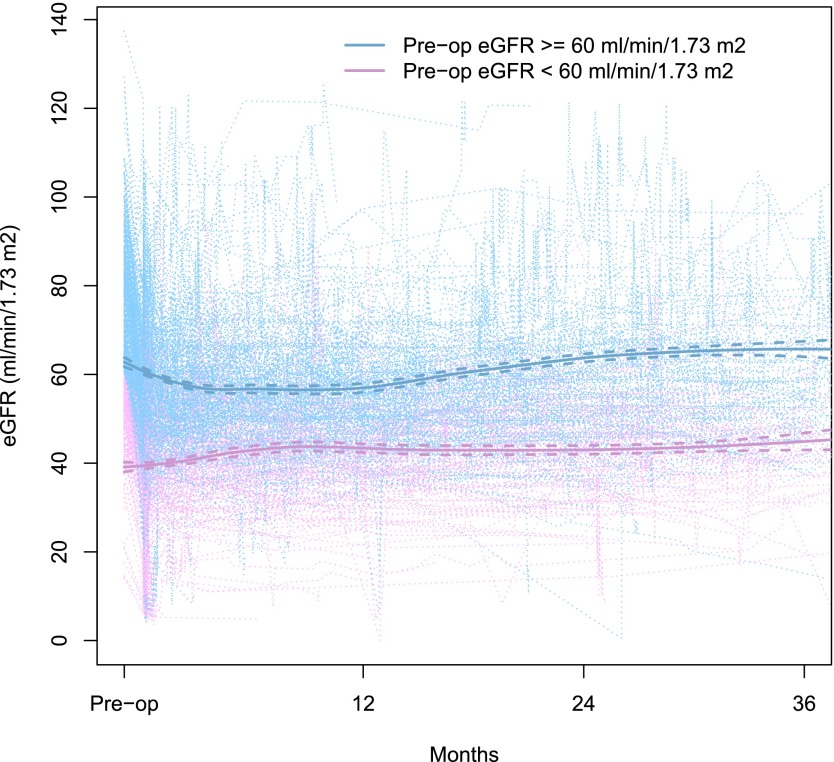
Longitudinal postoperative eGFR trajectories for each patient are plotted in Figure 1. Patients had 2–115 eGFR measurements during the 36-month follow-up period (median, 11). On average, patients with eGFR≥60 ml/min per 1.73 m2 preoperatively had a decline in eGFR after surgery until around 8 months, whereas patients with eGFR<60 ml/min per 1.73 m2 preoperatively had a general increasing trend in eGFR over time after surgery. Median postoperative follow-up among survivors was 10.8 months (minimum, 0.03; maximum, 36.0). During follow-up, 263 patients achieved eGFR recovery to preoperative values, with a median time to eGFR recovery of 25.3 months (95% confidence interval [95% CI], 20.6 to not reached). Whereas 175 patients recovered to within 5% of their preoperative eGFR level, 88 patients recovered to an eGFR >5% higher than their preoperative value. Among these 88 patients, the median increase in eGFR above their preoperative value was 7.4 (minimum, 2.2; maximum, 49.8) ml/min per 1.73 m2. Thirty-three patients died without eGFR recovery and were treated as competing events.
Figure 1.
Patients with eGFR≥60 ml/min per 1.73 m2 preoperatively had a decline in eGFR after surgery until around 8 months, whereas patients with eGFR<60 ml/min per 1.73 m2 preoperatively had a general increasing trend in eGFR over time after surgery. Individual patient trajectories of postoperative eGFR (ml/min per 1.73 m2) over time (light purple and blue dotted lines). Purple lines indicate patients with preoperative eGFR<60 ml/min per 1.73 m2, and blue lines indicate patients with preoperative eGFR≥60 ml/min per 1.73 m2. Locally weighted scatterplot smooths by preoperative eGFR are shown with bold solid lines; 95% confidence intervals are shown with bold dashed lines.
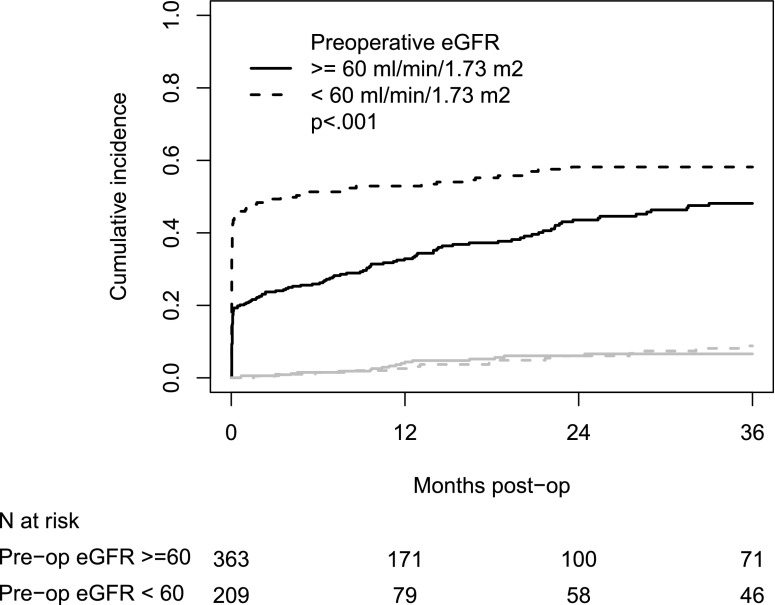
Figure 2 shows the cumulative incidence of eGFR recovery by preoperative eGFR. Overall, 40% and 49% of patients recovered to their baseline eGFR by 1 year and 2 years postoperatively, respectively. The cumulative incidence of eGFR recovery was significantly greater among those with preoperative eGFR<60 ml/min per 1.73 m2 (P<0.001). The 1-year cumulative incidences of eGFR recovery were 33% and 53% among those with preoperative eGFR≥60 and <60 ml/min per 1.73 m2, respectively; 2-year cumulative incidences of eGFR recovery were 46% and 58%, respectively. Cumulative incidence of deaths without eGFR recovery did not significantly differ between the two groups (P=0.63).
Figure 2.
Cumulative incidence of eGFR recovery was significantly greater among those with preoperative eGFR<60 ml/min per 1.73 m2 as compared to those with eGFR≥60 ml/min per 1.73 m2. Cumulative incidence of eGFR recovery by preoperative eGFR. Black lines indicate eGFR recovery and gray lines indicate death.
Table 2 presents univariable associations of patient and disease characteristics with eGFR recovery, both overall and by preoperative eGFR. The 10 patients with T stage Tx or T0 were combined with the T1 patients for analysis. Among patients with eGFR<60 ml/min per 1.73 m2 preoperatively, older age at surgery was significantly associated with a lower chance of eGFR recovery (hazard ratio [HR], 0.97 [95% CI, 0.96 to 0.99]; P<0.001). Among patients with eGFR≥60 ml/min per 1.73 m2 preoperatively, hypertension was associated with lower chance of eGFR recovery (HR, 0.64 [95% CI, 0.46 to 0.88]; P=0.01) whereas increased tumor size (HR, 1.11 [95% CI, 1.08 to 1.15]; P<0.001) and higher T stage (T2 versus Tx/T0/T1 HR, 1.96 [95% CI, 1.08 to 3.55]; T3/T4 versus Tx/T0/T1 HR, 1.93 [95% CI, 1.17 to 3.18]; P=0.03) were significantly associated with a higher chance of eGFR recovery. Because T stage also contains information about tumor size and the two are highly correlated, only tumor size was used in primary multivariable analysis, and a secondary multivariable analysis incorporated T stage in place of tumor size.
Table 2.
Univariable competing risks regression results for associations with eGFR recovery, overall and stratified by preoperative eGFR
| Variable | Overall | Preoperative eGFR | ||||
|---|---|---|---|---|---|---|
| ≥60 ml/min per 1.73 m2 | <60 ml/min per 1.73 m2 | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at surgery | 0.99 (0.98 to 1.00) | 0.29 | 0.99 (0.97 to 1.00) | 0.09 | 0.97 (0.96 to 0.99) | <0.001 |
| BMIa | 0.99 (0.97 to 1.01) | 0.40 | 0.99 (0.97 to 1.02) | 0.62 | 0.99 (0.96 to 1.02) | 0.47 |
| Sex | 0.02 | 0.16 | 0.15 | |||
| Male | 1.00 | 1.00 | 1.00 | |||
| Female | 1.34 (1.05 to 1.71) | 1.28 (0.91 to 1.81) | 1.27 (0.92 to 1.75) | |||
| Race | 0.38 | 0.33 | 0.78 | |||
| White | 1.00 | 1.00 | 1.00 | |||
| Other | 1.18 (0.82 to 1.69) | 1.27 (0.79 to 2.03) | 0.93 (0.54 to 1.58) | |||
| Diabetes | 1.31 (0.97 to 1.75) | 0.07 | 1.01 (0.63 to 1.63) | 0.96 | 1.39 (1.00 to 1.95) | 0.05 |
| Hypertension | 0.87 (0.69 to 1.09) | 0.22 | 0.64 (0.46 to 0.88) | 0.006 | 1.09 (0.76 to 1.56) | 0.65 |
| Coronary artery disease | 0.93 (0.62 to 1.41) | 0.74 | 0.6 (0.28 to 1.28) | 0.19 | 1.04 (0.66 to 1.64) | 0.85 |
| Smokingb | 0.33 | 0.26 | 0.93 | |||
| Never | 1.00 | 1.00 | 1.00 | |||
| Ever | 1.12 (0.89 to 1.42) | 1.21 (0.87 to 1.67) | 1.01 (0.74 to 1.39) | |||
| ASA grade | 0.31 | 0.85 | 0.41 | |||
| 1, 2 | 1.00 | 1.00 | 1.00 | |||
| 3, 5 | 1.13 (0.89 to 1.43) | 1.03 (0.75 to 1.42) | 1.15 (0.82 to 1.62) | |||
| Tumor sizec | 1.07 (1.04 to 1.10) | <0.001 | 1.11 (1.08 to 1.15) | <0.001 | 1.03 (0.99 to 1.08) | 0.13 |
| T stage | 0.02 | 0.03 | 0.28 | |||
| Tx, T0, T1 | 1.00 | 1.00 | 1.00 | |||
| T2 | 1.46 (0.95 to 2.25) | 1.96 (1.08 to 3.55) | 1.30 (0.64 to 2.67) | |||
| T3, T4 | 1.60 (1.15 to 2.22) | 1.93 (1.17 to 3.18) | 1.40 (0.93 to 2.12) | |||
| Type of surgery | 0.13 | 0.09 | 0.57 | |||
| Laparoscopic/robotic | 1.00 | 1.00 | 1.00 | |||
| Open | 0.81 (0.61 to 1.06) | 0.72 (0.50 to 1.05) | 0.89 (0.59 to 1.33) | |||
HR, hazard ratio; 95% CI, 95% confidence interval; BMI, body mass index; ASA, American Society of Anesthesiologists.
BMI value missing for one patient.
Smoking values missing for two patients.
Tumor size value missing for one patient.
On multivariable analysis, there were significant interactions between age and preoperative eGFR (P=0.03) and tumor size and preoperative eGFR (P=0.02), so stratified results are presented. Older age at surgery remained significantly associated with a lower chance of eGFR recovery among patients with preoperative eGFR<60 ml/min per 1.73 m2 (HR, 0.97 [95% CI, 0.96 to 0.99]; P=0.001) and female sex was significantly associated with a higher chance of eGFR recovery compared with male sex (HR, 1.41 [95% CI, 1.01 to 1.95]; P=0.04) (Table 3). Among patients with preoperative eGFR≥60 ml/min per 1.73 m2, hypertension remained significantly associated with a lower chance of eGFR recovery (HR, 0.67 [95% CI, 0.47 to 0.94]; P=0.02) and increased tumor size remained significantly associated with a higher chance of eGFR recovery (HR, 1.11 [95% CI, 1.08 to 1.15]; P<0.001) (Table 3). When T stage rather than tumor size was used in the multivariable models, patterns of association remained the same. T stage was not significantly associated with eGFR recovery among patients with preoperative eGFR<60 ml/min per 1.73 m2; however, among patients with preoperative eGFR≥60 ml/min per 1.73 m2, those with T3/T4 disease had significantly higher chance of eGFR recovery compared with those with Tx, T0, or T1 disease (HR, 1.96 [95% CI, 1.18 to 3.24]) (data not shown).
Table 3.
Multivariable competing risks regression results for associations with eGFR recovery, stratified by preoperative eGFR
| Variable | Preoperative eGFR | |||
|---|---|---|---|---|
| ≥60 ml/min per 1.73 m2 | <60 ml/min per 1.73 m2 | |||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age at surgery | 1.00 (0.98 to 1.01) | 0.64 | 0.97 (0.96 to 0.99) | 0.001 |
| Sex | 0.13 | 0.04 | ||
| Male | 1.00 | 1.00 | ||
| Female | 1.32 (0.93 to 1.88) | 1.41 (1.01 to 1.95) | ||
| Diabetes | 1.35 (0.84 to 2.17) | 0.22 | 1.29 (0.91 to 1.81) | 0.15 |
| Hypertension | 0.67 (0.47 to 0.94) | 0.02 | 1.06 (0.74 to 1.51) | 0.76 |
| Tumor size (cm)a | 1.11 (1.08 to 1.15) | <0.001 | 1.02 (0.98 to 1.07) | 0.29 |
HR, hazard ratio; 95% CI, 95% confidence interval.
Tumor size value missing for one patient, so the sample size for multivariable analysis is n=571.
Discussion
In this study we described trends in eGFR over time following RN for kidney neoplasms and found several characteristics measured at the time of surgery that are associated with a return to preoperative eGFR. Recovery to baseline eGFR was common in this patient population, perhaps even more so among those with lower preoperative eGFR. Patients with lower preoperative eGFR were older; more frequently had diabetes, hypertension, and coronary artery disease; and had smaller tumors compared with those with greater preoperative eGFR. Among patients with preoperative eGFR<60 ml/min per 1.73 m2, younger age at surgery and female sex were associated with a higher chance of eGFR recovery, whereas among patients with preoperative eGFR≥60 ml/min per 1.73 m2, absence of hypertension and larger tumor size were associated with a higher chance of eGFR recovery. The finding that patients with low baseline eGFR (<60 ml/min per 1.73 m2) are more likely to recover to their preoperative level is clinically significant because a permanent drop in eGFR among these patients, who already have a more limited renal function reserve, would have worse clinical implications. Our findings suggest that reduced eGFR at baseline should not be seen as a contraindication for radical procedures, especially among younger patients and female patients, because >50% of these patients recovered to baseline eGFR by 1 year after surgery. While diabetes is a known risk factor for CKD, we did not find an effect in this study, possibly because of the small number (n=96) of patients with diabetes in our population.
A novel and interesting finding of our study is the association between increased tumor size and increased chance of eGFR recovery among patients with preoperative eGFR≥60 ml/min per 1.73 m2. Patients with larger tumors may experience a greater reduction in kidney function in the affected kidney than those with smaller tumors, and therefore the contralateral kidney may have already undergone hypertrophic and/or functional compensation for the loss of renal function in the affected kidney. We hypothesize that the normal contralateral kidney is the major contributor to the total eGFR in patients with large tumors and therefore is less affected by the surgical removal of the affected kidney. In contrast, among patients with preoperative eGFR<60 ml/min per 1.73 m2, tumor size was not associated with renal function recovery, suggesting that other factors, such as old age, have more influence on recovery in this segment of the population. Future studies involving the systematic use of nuclear renography to assess differential function before and after surgery are needed to test this hypothesis.
Most studies evaluating kidney function following nephrectomy have focused on differences in outcome between patients undergoing RN versus PN. In a recent review, Li et al. (15) found worse postoperative renal outcomes among patients undergoing RN than those having PN. These studies have resulted in the recommendation to perform PN whenever technically feasible to best preserve renal function for patients undergoing a nephrectomy for renal cell carcinoma (15). Indeed, studies from our own institution have shown that after 25 months of follow-up, the mean postoperative serum creatinine is significantly higher in patients undergoing RN than in those undergoing PN (5,16). Moreover, in other studies we demonstrated that the 3-year postoperative probability of freedom from new-onset CKD was 80% after PN versus 35% after RN (5). However, RN remains a necessity for many patients, particularly for those with large or centrally located renal cortical tumors where PN is not feasible and for patients with advanced renal cortical tumors.
Several studies have examined factors associated with changes in eGFR following radical surgery for renal masses. Chung et al. (8) analyzed 2068 patients treated with RN for renal cortical tumors with median follow-up time of 33 months and found that renal function began to stabilize and then gradually increase after an initial postoperative decrease (8). In support of our finding that patients with lower preoperative eGFR had increased cumulative incidence of eGFR recovery, on univariable analysis they found that patients with preoperative eGFR of 15–29 ml/min per 1.73 m2 had the steepest increase in eGFR following RN (8). Although these authors did not examine tumor size, significant predictors of increases in postoperative eGFR over time included higher preoperative eGFR, younger age at surgery, and no history of diabetes or of hypertension (8). Jeon et al. (9) examined the association between tumor size and postoperative eGFR among 1371 patients who underwent RN for renal cell carcinoma and found that the decrease in eGFR from preoperatively to 7 days postoperatively was greater in patients with tumors <4 cm than in those with tumors 4–7 cm or ≥7 cm and that it remained lower up to 1 year postoperatively. This finding supports our hypothesis that patients with larger tumors are less affected by the surgical removal of the affected kidney due to prior contralateral kidney compensation or hypertrophy.
It is known that in addition to the type of surgery, factors such as increased age, diabetes, hypertension, and tobacco use are independently associated with the development of CKD after nephrectomy (9–13). To our knowledge, our study is the first to examine the endpoint of eGFR recovery following RN for a renal mass. The biologic mechanisms underlying renal functional compensation or hypertrophy in the contralateral kidney and factors that may influence this effect are not well known. Takagi et al. (17) examined the parenchymal volume and function of the contralateral kidney in 113 patients undergoing PN and 59 undergoing RN both before and after surgery. They found that the median increase in eGFR in the contralateral kidney was 2.3% and 21.1% following PN and RN, respectively. Further, they found that increased percentage of parenchymal loss and higher Charlson Comorbidity Index score were both significantly associated with a decrease in contralateral kidney eGFR overall (17). Choi et al. (18) investigated functional changes in the kidney following RN for renal cell carcinoma and found that preoperative volume of both the operative and the remnant kidney was significantly higher among patients with lower preoperative CKD stage. However, they found that the increase in GFR/functional renal volume was significantly higher in patients with stage 3 CKD than in those with no CKD or stage 2 CKD. They concluded that functional adaptive hyperfiltration is more important for the recovery of renal function than structural adaptive hypertrophy (18).
Most studies to date have focused on development of CKD following nephrectomy at early time points (6 months), but this early time point may not capture the conditions that may lead to, or time required to, achieve partial, near-complete, or complete functional recovery. A recent study found that surgically induced CKD was not a significant predictor of survival in patients without preexisting CKD and that annual functional renal decline was only 0.7% in patients with surgically induced CKD, compared with 4.7% in patients with preexisting CKD (19). The patients with surgically induced CKD did not have the additional medical comorbidities or preexisting CKD, which may be etiologic factors in the development of kidney and urothelial cancers and can be considered a healthier cohort, more akin to the kidney donor population than the average patient with kidney tumor (20,21).
We acknowledge the limitations of our study. First, as in any longitudinal analysis, there may be bias due to nonrandom drop-out. Patients not only are dying during follow-up but could also be lost to follow-up for other disease-related issues, such as recurrence or competing illnesses. We attempted to address this concern by using a time-to-event framework with death from any cause treated as a competing event. To further address this possible source of bias, we examined associations between factors of interest in this study and length of follow-up for each participant. We found that patients with a worse American Society of Anesthesiologists stage or a higher T stage tended to have shorter follow-up time, suggesting that sicker patients with worse disease are not being followed as long in this study. However, eGFR did not differ by length of follow-up. Other possible sources of bias include the retrospective nature of this study and the lack of detailed information on the severity of comorbidities, such as hypertension, diabetes, coronary artery disease, and smoking. It is also possible that other unmeasured confounders, such as changes in a patient’s nutrition or hydration practices following surgery, could affect renal functional recovery in this study, and we cannot account for their effect in this analysis. This study should be replicated in a patient population with longer follow-up because the current study found that median time to recovery to preoperative eGFR was 25.3 months but the median follow-up time among survivors in this study was only 10.8 months.
In conclusion, to further elucidate factors that influence postoperative renal function in patients undergoing RN for kidney cancer, we examined factors associated with eGFR recovery to the preoperative value. Overall, 49% of patients recovered to preoperative eGFR by 2 years following surgery. Encouragingly, patients with lower preoperative eGFR had greater chance of eGFR recovery. Factors associated with eGFR recovery differed by preoperative eGFR, and this should be considered in planning postoperative patient care and follow-up.
Disclosures
E.A.J. serves on the editorial board of the American Journal of Physiology – Renal Physiology and has consulted with Relypsa (Redwood City, CA) and Spears, Moore, Rebman, and Williams (Chattanooga, TN). No other authors have relevant disclosures.
Acknowledgments
John Musser and Roy Mano of Memorial Sloan Kettering Cancer Center made substantial contributions to data acquisition and verification.
These studies were supported by intramural research funds (to P.R.), the Core Grant (P30 CA008748 to E.C.Z.), and a Byrne Research Fund (to E.A.J.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A: Cancer statistics, 2014. CA Cancer J Clin 64: 9–29, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Patard JJ, Rodriguez A, Rioux-Leclercq N, Guillé F, Lobel B: Prognostic significance of the mode of detection in renal tumours. BJU Int 90: 358–363, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Russo P: Renal cell carcinoma: Presentation, staging, and surgical treatment. Semin Oncol 27: 160–176, 2000 [PubMed] [Google Scholar]
- 4.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ: Long-term consequences of kidney donation. N Engl J Med 360: 459–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P: Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol 7: 735–740, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong HJ, Park JS, Kim DY, Shin HS, Jung HJ: Renal function following curative surgery for renal cell carcinoma: Who is at risk for renal insufficiency? Korean J Urol 54: 830–833, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mariusdottir E, Jonsson E, Marteinsson VT, Sigurdsson MI, Gudbjartsson T: Kidney function following partial or radical nephrectomy for renal cell carcinoma: A population-based study. Scand J Urol 47: 476–482, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Chung JS, Son NH, Byun SS, Lee SE, Hong SK, Jeong CW, Lee SC, Chae DW, Choi WS, Park YH, Hong SH, Kim YJ, Kang SH: Trends in renal function after radical nephrectomy: a multicentre analysis. BJU Int 113: 408–415, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Jeon HG, Choo SH, Sung HH, Jeong BC, Seo SI, Jeon SS, Choi HY, Lee HM: Small tumour size is associated with new-onset chronic kidney disease after radical nephrectomy in patients with renal cell carcinoma. Eur J Cancer 50: 64–69, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J: Predicting renal functional outcomes after surgery for renal cortical tumours: A multifactorial analysis. BJU Int 106: 489–492, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Clark MA, Shikanov S, Raman JD, Smith B, Kaag M, Russo P, Wheat JC, Wolf JS, Jr, Matin SF, Huang WC, Shalhav AL, Eggener SE: Chronic kidney disease before and after partial nephrectomy. J Urol 185: 43–48, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Klarenbach S, Moore RB, Chapman DW, Dong J, Braam B: Adverse renal outcomes in subjects undergoing nephrectomy for renal tumors: A population-based analysis. Eur Urol 59: 333–339, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Malcolm JB, Bagrodia A, Derweesh IH, Mehrazin R, Diblasio CJ, Wake RW, Wan JY, Patterson AL: Comparison of rates and risk factors for developing chronic renal insufficiency, proteinuria and metabolic acidosis after radical or partial nephrectomy. BJU Int 104: 476–481, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Lau WL, Rhee CM, Harley K, Kovesdy CP, Sim JJ, Jacobsen S, Chang A, Landman J, Kalantar-Zadeh K: Risk of chronic kidney disease after cancer nephrectomy. Nat Rev Nephrol 10: 135–145, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P: Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4-7 cm. BJU Int 97: 939–945, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Takagi T, Mir MC, Sharma N, Remer EM, Li J, Demirjian S, Kaouk JH, Campbell SC: Compensatory hypertrophy after partial and radical nephrectomy in adults. J Urol 192: 1612–1618, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Choi DK, Jung SB, Park BH, Jeong BC, Seo SI, Jeon SS, Lee HM, Choi HY, Jeon HG: Compensatory structural and functional adaptation after radical nephrectomy for renal cell carcinoma according to preoperative stage of chronic kidney disease. J Urol 194: 910–915, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Lane BR, Campbell SC, Demirjian S, Fergany AF: Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol 189: 1649–1655, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Christensson A, Savage C, Sjoberg DD, Cronin AM, O'Brien MF, Lowrance W, Nilsson PM, Vickers AJ, Russo P, Lilja H: Association of cancer with moderately impaired renal function at baseline in a large, representative, population-based cohort followed for up to 30 years. Int J Cancer 133: 1452–1458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowrance WT, Ordoñez J, Udaltsova N, Russo P, Go AS: CKD and the risk of incident cancer. J Am Soc Nephrol 25: 2327–2334, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]