Abstract
Background and objectives
The population incidence of dialysis-requiring AKI has risen substantially in the last decade in the United States, and factors associated with this temporal trend are not well known.
Design, setting, participants, & measurements
We conducted a retrospective cohort study using data from the Nationwide Inpatient Sample, a United States nationally representative database of hospitalizations from 2007 to 2009. We used validated International Classification of Diseases, Ninth Revision codes to identify hospitalizations with dialysis-requiring AKI and then, selected the diagnostic and procedure codes most highly associated with dialysis-requiring AKI in 2009. We applied multivariable logistic regression adjusting for demographics and used a backward selection technique to identify a set of diagnoses or a set of procedures that may be a driver for this changing risk in dialysis-requiring AKI.
Results
From 2007 to 2009, the population incidence of dialysis-requiring AKI increased by 11% per year (95% confidence interval, 1.07 to 1.16; P<0.001). Using backward selection, we found that the temporal trend in the six diagnoses, septicemia, hypertension, respiratory failure, coagulation/hemorrhagic disorders, shock, and liver disease, sufficiently and fully accounted for the temporal trend in dialysis-requiring AKI. In contrast, temporal trends in 15 procedures most commonly associated with dialysis-requiring AKI did not account for the increasing dialysis–requiring AKI trend.
Conclusions
The increasing risk of dialysis-requiring AKI among hospitalized patients in the United States was highly associated with the changing burden of six acute and chronic conditions but not with surgeries and procedures.
Keywords: acute kidney injury, temporal trend, dialysis, epidemiology, cohort studies, humans, incidence, renal dialysis, retrospective studies, sepsis
Introduction
AKI is one of the most common and serious complications among hospitalized patients (1,2). Those patients who suffer from the most severe form of AKI (requiring dialysis) have particularly high rates of adverse outcomes during and after hospitalization, including the development of CKD (3–5). (For simplicity, we use the term dialysis here and throughout this paper to encompass both acute intermittent dialysis and continuous RRT.) We recently reported that the United States population incidence of dialysis-requiring AKI (AKI-D) has increased in the past decade (6), and we concluded that this trend could not be accounted for fully by secular changes in demographics or in four prespecified risk factors (sepsis, acute heart failure, cardiac catheterization, and mechanical ventilation) (6).
We undertook this study to uncover whether temporal changes in other risk factors could be potential reasons for this observed increase in AKI-D incidence. Identifying potentially modifiable risk factors is important, because this may lead to new insights regarding how to decrease the population burden of AKI-D and its associated complications.
Another goal of the study is to evaluate potential outcome misclassification. Our previous incidence estimates relied on the best-available algorithm of administrative codes to identify patients with AKI-D at the time of the study (6,7). More recent data suggest that some patients with ESRD on chronic dialysis may be misclassified as patients with AKI-D (8). Thus, we examined the effect on disease estimates if we eliminated all labeled patients with AKI-D that also had concurrent diagnoses of ESRD.
Materials and Methods
Data Source
We extracted 2007–2009 data from the Nationwide Inpatient Sample (NIS), a United States nationally representative administrative database of hospitalizations (9). We chose to start this analysis in 2007 to allow at least 1 year to fully implement the International Classification of Diseases, Ninth Revision (ICD-9-CM) code for ESRD requiring dialysis (585.6), which was introduced in October of 2005 (10). We chose to end the study in 2009, because this was the last year of our previous study, and we wanted this overlap (6). In a sensitivity analysis, we extended the study period to 2011, the latest year possible for this analysis because of changes in the NIS sampling scheme starting in 2012 (9).
The NIS is the largest all–payer, publicly available hospital database; it comprises a 20% stratified sample of all short–term, nonfederal, nonrehabilitation hospitals. Hospitals are sampled according to geographic region, location, teaching status, ownership, and bed size; all discharges from sampled hospitals are included. Each observation represents an individual hospitalization and includes demographic variables, discharge diagnoses, and procedures. The NIS was developed as part of the Healthcare Cost and Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality. Using the supplied sampling weights, NIS data represent hospitalizations at a national level.
To identify potential explanatory variables for changes in AKI-D incidence, we used Clinical Classifications Software (CCS), a classification system that collapses the >14,000 standardized diagnosis codes in the ICD-9-CM into a smaller number of clinically significant categories. The CCS for ICD-9-CM is developed as part of the HCUP, and the NIS supplies corresponding CCS codes for each of the listed ICD-9-CM diagnostic and procedure codes. We looked at both CCS diagnoses and CCS procedures as potential variables associated with the temporal trend in AKI-D. The NIS contains ≤15 CCS diagnostic codes for each hospital discharge from years 2007–2008 and ≤25 CCS diagnostic codes for 2009. For consistency and to avoid ascertainment bias caused by the increase in the number of supplied codes since 2009, we only used the first 15 ICD-9-CM CCS diagnostic codes listed in the study period. For CCS procedure codes, ≤15 were listed in all 3 years of the study period.
Definition of Primary Outcome
We used the same algorithm to define AKI-D as before (6): requiring both a diagnostic code for acute renal failure in any position (584.5, 584.6, 584.7, 584.8, or 584.9) and a procedure code for dialysis in any position (39.95, V45.1, V56.0, or V56.1) along with the absence of procedure codes for arteriovenous fistula creation/revision (39.27, 39.42, 39.43, or 39.93) (6,7).
Selection of Predictor Variables, Model Selection, and Temporal Trend Analyses
To explore potential reasons for temporal changes in AKI-D, we devised a strategy to first reduce the candidate diagnoses to those most highly associated with AKI-D (versus non–AKI-D) hospitalizations. Then, we sought to see if temporal trends in these variables could attenuate the increase in AKI-D incidence, because we reasoned that our chances of better understanding the temporal trend would be highest if we focused on factors more strongly associated with the development of AKI-D. Thus, we divided data from the latest NIS year from our last analysis, 2009, into two subgroups: discharges with AKI-D and discharges without AKI-D. We calculated the event rate of each CCS code within each subgroup and subsequently, calculated the event rate ratios for each CCS code (AKI-D versus no AKI-D). The 15 CCS diagnoses and the 15 CCS procedures with the highest event rate ratios were selected for analyses, excluding certain predetermined codes that are deemed to represent the outcome of interest: CCS diagnostic codes 157 (acute and unspecified renal failure) and 156 (nephritis, nephrosis, or renal sclerosis) and CCS procedure codes 58 (hemodialysis), 110 (diagnostic procedures of urinary tract), and 195 (diagnostic ultrasound of urinary tract). In addition, we also excluded CCS diagnostic code 158 (CKD) from analysis, owing to the fact that it encompasses not only ICD-9-CM codes for CKD (585.1–585.5 and 585.9) but also, ESRD (585.6). Any CCS diagnostic or procedure code that did not exist in all 3 years of the dataset was also excluded from analysis.
Similar to our prior approach (6), we fit a univariate logistic regression model with calendar year as the primary predictor and AKI-D as the primary outcome to quantify the change in risk of AKI-D among hospitalized patients per year. Appropriate discharge–level sampling weights were applied. Owing to the large number of patient-level observations in the NIS (5–8 million per year), we took a random 25% subsample of each year’s data to facilitate the computations. This also allowed us to have separate derivation and validation datasets.
Multivariate logistic regression was used to examine whether and to what degree the previously selected CCS diagnoses and procedures along with age, sex, and race/ethnicity accounted for the temporal trend in risk of AKI-D. We decided a priori to analyze one series of models with the 15 identified CCS diagnostic codes and a separate series of models with the 15 identified CCS procedure codes. We analyzed diagnoses and procedures separately to avoid diagnoses and procedures that may be collinear with one another, such as respiratory failure and mechanical intubation or myocardial infarction and cardiac catheterization. The goal of multivariate modeling is to see if inclusion of specific sets of diagnoses (or procedures) eliminated the positive direction of the year term in risk of AKI-D seen in the crude model. For example, using AKI-D as the outcome and year as the primary predictor, we adjusted for demographics and the previously identified 15 diagnoses in the model to see if the coefficient of the year term is reversed from >1 (rising temporal trend) to <1. If so and to find the minimal set of diagnoses that could reverse the trend, we then used a backward selection technique. We sequentially removed the diagnoses with the largest P value and refit the model, checking to see at what point the temporal trend became positive, at which point we reached our final model.
After development of the final model as described above, we validated its performance with a second independent random 25% subsample. We derived the correlation matrix of the selected codes to test for collinearity. All data were analyzed using STATA/SE 13.0 (StataCorp., College Station, TX).
Analyses Excluding Patients with ESRD Discharge Diagnosis
A secondary aim of this study was to define the temporal trend in AKI-D hospitalizations that did not concurrently have discharge diagnostic codes for ESRD (585.6). A similar modeling approach was taken to determine whether using this conservative case definition and codes identified above could still attenuate the temporal trend in AKI-D.
Results
Conditions Most Strongly Associated with AKI-D Versus Non–AKI-D
Characteristics of patients with AKI-D versus without AKI-D are listed in Table 1. Patients who suffered AKI-D were more likely to be older, men, and black compared with patients without AKI-D. The patient fatality rate for AKI-D during this period was 22%.
Table 1.
Characteristics of hospitalized patients with dialysis-requiring AKI versus nondialysis-requiring AKI from 2007 to 2009
| Characteristics | AKI-D (n=448,755) | No AKI-D (n=118,413,269) | P Value |
|---|---|---|---|
| Age, yr (mean) | 63.2 | 48.0 | <0.001 |
| Age >75 yr old, % | 28.3 | 21.3 | <0.001 |
| Men, % | 57.1 | 41.6 | <0.001 |
| Race/ethnicity, % | <0.001 | ||
| White, non-Hispanic | 51.0 | 52.6 | |
| Black, non-Hispanic | 16.5 | 11.3 | |
| Hispanic | 9.9 | 10.3 | |
| Asian | 2.6 | 2.1 | |
| Other or missing | 20.0 | 23.7 | |
| Died during hospitalization, % | 22.0 | 1.9 | <0.001 |
AKI-D, dialysis-requiring AKI.
The 15 diagnoses and the 15 procedures most strongly associated with AKI-D by having the highest event rate ratios (patients with AKI-D versus no AKI-D) in 2009 are listed in Table 2. These diagnoses include both acute conditions, such as shock, cardiac arrest, and septicemia, and chronic conditions, like hypertension and multiple myeloma.
Table 2.
Diagnoses and procedures most strongly associated with dialysis–requiring AKI (versus nondialysis–requiring AKI) hospitalizations in 2009 (ranked by order of event rate ratios)
| Diagnoses and Procedures | Event Rate Ratio (AKI-D Versus Non–AKI-D Hospitalizations) | 95% CI |
|---|---|---|
| Diagnoses | ||
| Shock | 20.5 | 20.0 to 20.9 |
| Cardiac arrest and ventricular fibrillation | 10.4 | 9.9 to 11.0 |
| Septicemia | 9.5 | 9.3 to 9.6 |
| Multiple myeloma | 8.5 | 7.8 to 9.2 |
| Respiratory failure | 7.5 | 7.4 to 7.6 |
| Coagulation and hemorrhagic disorders | 6.6 | 6.5 to 6.7 |
| Hypertension | 6.5 | 6.5 to 6.6 |
| Coma, stupor, and brain damage | 5.9 | 5.6 to 6.2 |
| Liver diseases | 5.7 | 5.5 to 5.8 |
| Mycoses | 5.6 | 5.5 to 5.8 |
| Peritonitis and intestinal abscess | 5.6 | 5.2 to 6.0 |
| Gangrene | 5.5 | 5.1 to 6.0 |
| Injuries or conditions caused by external injuries | 5.3 | 5.2 to 5.4 |
| Aspiration pneumonitis | 5.2 | 5.0 to 5.4 |
| Complication of device, implant, or graft | 5.2 | 5.0 to 5.3 |
| Procedures | ||
| Coronary and vascular stents | 33.7 | 33.4 to 34.0 |
| Nonkidney solid organ transplantation | 25.2 | 22.0 to 28.9 |
| Tracheostomy | 18.2 | 17.3 to 19.1 |
| Swan–Ganz catheterizations | 15.3 | 14.2 to 16.6 |
| Skin and breast procedures | 11.5 | 11.1 to 12.0 |
| Respiratory intubation/mechanical ventilation | 10.0 | 9.9 to 10.1 |
| Nephrotomy/nephrostomy | 9.6 | 8.7 to 10.6 |
| Endobronchial procedures | 8.7 | 8.1 to 9.4 |
| Cardioversion | 8.7 | 8.3 to 9.1 |
| Skin diagnostic procedures | 8.4 | 7.3 to 9.6 |
| Abdominal paracentesis | 7.4 | 7.0 to 7.8 |
| Bronchoscopy | 7.2 | 6.9 to 7.5 |
| Noncardiac vascular catheterizations | 6.8 | 6.7 to 6.9 |
| Bone marrow transplantation | 6.5 | 5.3 to 8.0 |
| Enteral and parenteral nutrition | 6.4 | 6.2 to 6.6 |
AKI-D, dialysis-requiring AKI; 95% CI, 95% confidence interval.
Temporal Trend Analyses
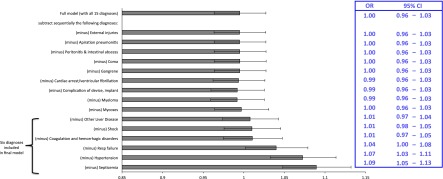
From 2007 to 2009, the United States population incidence of AKI-D increased from 445 to 533 patients per million person-years. The odds of developing AKI-D among hospitalized patients increased annually by 11% per year (odds ratio [OR], 1.11; 95% confidence interval [95% CI], 1.07 to 1.16; P<0.001), similar to what we had reported previously (6). Adjustment for age, sex, and race reduced the annual increase to 9% (OR, 1.09; 95% CI, 1.05 to 1.13; P<0.001). Additional adjustment for the 15 identified diagnoses most associated with AKI-D in 2009 achieved our a priori goal of eliminating the positive direction of the year term (OR, 0.99; 95% CI, 0.96 to 1.03) (Table 3). None of the identified diagnoses were found to be strongly collinear with one another. Using backward selection, we were able to determine the minimum subset of diagnoses that led to an OR≥1 for the year term in the model (Figure 1). For example, successive eliminations of external injuries, aspiration pneumonitis, and peritonitis from the model did little to change the year parameter (OR, 0.99). However, after successive eliminations of additional diagnoses as predictors from the multivariable model, we were able to derive a final model adjusting for age, sex, and race, as well as six final diagnoses of septicemia, hypertension, respiratory failure, coagulation/hemorrhagic disorders, liver disease, and shock, that reached an OR≥1.0 for the year term (Figure 1, Table 3). This model was validated in a second random 25% subsample, in which the same six conditions (along with demographic variables) succeeded in eliminating the temporal trend in AKI-D (Table 3).
Table 3.
Diagnoses associated with the temporal trend in risk of dialysis-requiring AKI among hospitalized patients from 2007 to 2009
| Model | Primary 25% Subsample | Validation 25% Subsample | ||
|---|---|---|---|---|
| OR per yr (95% CI) | P Value | OR per yr (95% CI) | P Value | |
| Crude | 1.11 (1.07 to 1.16) | <0.001 | 1.11 (1.07 to 1.15) | <0.001 |
| Adjusted for age, sex, and race | 1.09 (1.05 to 1.13) | <0.001 | 1.09 (1.05 to 1.13) | <0.001 |
| Adjusted for age, sex, race, and the top 15 diagnosesa | 0.99 (0.96 to 1.03) | 0.75 | 0.99 (0.97 to 1.03) | 0.93 |
| Adjusted for age, sex, race, septicemia, hypertension, respiratory failure, coagulation/hemorrhagic disorders, shock, and liver diseases | 1.00 (0.96 to 1.03) | 0.86 | 1.00 (0.97 to 1.04) | 0.97 |
OR, odds ratio; 95% CI, 95% confidence interval.
The top 15 diagnoses are the ones shown in Table 2: shock, cardiac arrest/ventricular fibrillation, septicemia, multiple myeloma, respiratory failure, coagulation and hemorrhagic disorders, hypertension, coma/stupor/brain damage, liver diseases, mycoses, peritonitis/intestinal abscess, gangrene, injuries or conditions caused by external injuries, aspiration pneumonitis, and complication of device/implant/graft.
Figure 1.
Multivariable logistic regression and a backward selection technique were applied to identify a final set of diagnoses to sufficiently account for the temporal trend in dialysis-requiring AKI. Listed diagnoses are covariates in the multivariable model (with calendar year as the primary predictor and dialysis-requiring AKI as the outcome), with each horizontal bar representing the odds ratio (OR; with 95% confidence interval [95% CI]) for the calendar year term after the corresponding diagnosis is dropped from the model. For example, after successively dropping external injuries and aspiration pneumonitis from the model, the OR for the year term remained <1, but after dropping mycoses, the model with the remaining six diagnoses resulted in the OR of the year term ≥1. The bracket indicates the six diagnoses selected for the final model. Error bars indicate 95% CIs. The embedded table displays the corresponding ORs with 95% CIs.
In contrast, adjusting for the 15 selected procedures most associated with AKI-D in 2009 along with demographic variables failed to achieve our a priori goal of eliminating the positive direction of the year term (OR, 1.08; 95% CI, 1.04 to 1.12).
In sensitivity analysis, where we extended follow-up to 2011, we noted that the incidence of AKI-D continued to rise in 2010 and 2011. Taking into account the entire 2007–2011 period, the odds of developing AKI-D among hospitalized patients now increased by 6% per year (OR, 1.06; 95% CI, 1.04 to 1.08; P<0.001). Controlling for the same six diagnoses (along with demographic variables) also abolished the temporal trend (OR, 0.96; 95% CI, 0.95 to 0.98; P<0.001).
Analyses Excluding Patients with Concurrent ESRD Discharge Diagnosis
We found that, among the AKI-D hospitalizations, over one third (36%) had concurrent discharge diagnostic codes for ESRD. After excluding hospitalizations with both AKI-D and ESRD codes, there remained a strong temporal trend in increasing incidence of AKI-D from 297 patients per million person-years in 2007 to 325 patients per million person-years in 2009. The odds of AKI-D among all hospitalized patients without concurrent ESRD diagnoses increased annually by 6% per year (OR, 1.06; 95% CI, 1.02 to 1.11; P<0.01).
Using the same six diagnoses from the final model listed in Table 3 (derived from the full sample, including patients with AKI-D and concurrent discharge diagnosis of ESRD), we were also able to abolish the yearly increase in AKI-D (excluding patients with concurrent discharge diagnosis of ESRD; OR, 0.95; 95% CI, 0.92 to 0.99; P=0.01).
Discussion
We analyzed a nationally representative sample of United States hospitalizations from 2007 to 2009 and showed that the risk of AKI-D among hospitalized patients increased during this period. This increase is only partially related to changing demographics but could be statistically accounted for after taking into consideration concurrent temporal trends in six acute and chronic conditions (septicemia, hypertension, respiratory failure, coagulation and hemorrhagic disorders, shock, and liver diseases) among hospitalized patients. In contrast, temporal trends in procedures most commonly associated with AKI-D did not explain the increasing risk of AKI-D over this period.
Our results are consistent with prior studies showing a rising secular trend in incidence of AKI-D in the United States (6,11–13). In contrast to our prior attempt (6), we may be more successful in gaining some insight into potential reasons behind the rise in AKI-D here. This may be because we formulated a strategy to select diagnoses most associated with the outcome of interest, AKI-D. In addition, we took advantage of the ICD-9-CM CCS system to expand the capture of our set of comorbidities rather than relying on the more restricted ICD-9-CM codes.
Our results provide novel insight as to the relative significance of both acute conditions and chronic comorbidities in exploring why AKI-D has increased over time. The major effect of acute conditions, such septicemia and shock, in the model may not be surprising given studies showing increasing rates of sepsis and that shock is a well known cause of AKI (14–16). Similarly, respiratory failure rates have risen over time (17,18), and patients with respiratory failure and on mechanical ventilation have a high risk of AKI (19). In addition to these acute conditions, we showed hypertension as a major risk factor and a potent component in the trend of hospitalized patients with AKI-D. Although hypertension is a well recognized risk factor for CKD, the relationship between hypertension and AKI is less well understood. Some studies (20,21) (although not all [22,23]) have shown that preexisting hypertension increases risk of AKI. More pervasive use of antihypertensives, specifically blockers of the renin-angiotensin axis (24), may play a role in susceptibility to severe AKI among those undergoing high-risk procedures (25–27). Although the adult prevalence of hypertension in the United States has been stable in the past decade (28), the annual number of hospital discharges with hypertension as a listed diagnosis has increased from 2006 to 2010 (29,30). Vigilant outpatient follow-up, management, and renal function assessment in those with prevalent hypertension may help reduce admissions and hence, severe AKI in this high-risk group.
Interestingly, cardiovascular procedures well known to cause AKI, such as coronary artery bypass graft surgery (31) and percutaneous coronary intervention, were not as strongly associated with the temporal trend in AKI-D. Reasons for this may be that the number of coronary artery bypass graft surgeries performed in the United States has been declining and that percutaneous coronary intervention rates have plateaued over time (32). Additionally, one well conducted study showed that the risk of AKI after cardiac catheterizations among patients with acute myocardial infarction has lessened over time (33,34). Thus, a combination of decreasing exposures and improving processes of care (such as success at preventing contrast-induced AKI) may be why high–risk cardiovascular procedures do not seem to be a major contributor to the AKI-D trend over time. We note that diabetes mellitus did not come up as a leading diagnosis associated with AKI-D using our selection methods; this may be because of known undercoding of diabetes with complications as a comorbidity in administrative data (35).
The second set of findings in this study is that at least one third of all hospital discharges with diagnosis of AKI-D also had a concurrent diagnosis of ESRD requiring dialysis. The precise reason for this observation is not known. Although some patients with ESRD on maintenance dialysis may be misclassified as patients with AKI-D, it is also possible that, in numerous patients, the ESRD diagnosis was applied at the end of hospitalization to patients with severe AKI-D who did not recover enough renal function to come off dialysis, and these patients were discharged from the hospital to continue outpatient dialysis. In our prior work using data from a large integrated health care system in northern California, in which we had quantification of pre–AKI baseline GFR estimated from serum creatinine, we documented that among 1764 patients with AKI-D observed between 1996 and 2003, 585 (33%) failed to recover renal function and were considered to have developed ESRD (3,4). Thus, it is possible that much of the coexistence of diagnostic and procedure codes for AKI-D and diagnostic codes for ESRD observed is caused by true progression of AKI-D toward ESRD. The cross-sectional nature of each year’s data in the NIS and the inability to identify repeated hospitalizations in the same individual limit our ability to come to more definitive conclusions. Even if we take a conservative approach and exclude all AKI-D hospitalizations with concurrent discharge diagnosis of ESRD, AKI-D disease incidence was still increasing during our study period. Also, we found that the same six explanatory variables were able to eliminate the temporal trend in increasing risk of AKI-D.
Although our study is strengthened by the use of a very large, nationally representative hospital discharge sample, allowing for excellent power and generalizability, we recognize a number of limitations. We lacked clinical data, such as serum creatinine, to ascertain the outcome of interest but relied on the best-validated set of ICD-9-CM diagnostic and procedure codes to capture AKI-D (7,36). Although the use of ICD-9-CM CCS codes allowed us to more broadly capture diagnoses and procedures to be selected as covariates, the validity of those codes has not been definitively established. Nevertheless, we believe that our analysis sheds some light on the potential reasons why AKI-D may have become more common over time, although we clearly must acknowledge that association is not causation.
In conclusion, United States representative hospital discharge data suggest that the risk of AKI-D among hospitalized patients increased 11% per year from 2007 to 2009, and this temporal trend seems to be strongly associated with the increased burden of the following six conditions: septicemia, hypertension, respiratory failure, coagulation/hemorrhagic disorders, shock, and liver diseases. Temporal changes in surgeries and procedures do not seem to underlie the rising risk of AKI-D among hospitalized patients. Misclassification of ESRD as AKI-D may be present but does not account for the rise in AKI-D over time.
Disclosures
None.
Acknowledgments
This publication was supported by Cooperative Agreement Number 1U58DP003839 from the Centers for Disease Control and Prevention (CDC). This research was additionally supported by National Institutes of Health Grants KL2TR000143 (to R.K.H.), K23DK100468 (to R.K.H.), and K24DK092291 (to C.-y.H.).
Part of the data were presented at the American Society of Nephrology Kidney Week 2013 (TH-OR002) on November 7, 2013 in Atlanta, GA.
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
The CDC CKD Surveillance Team consists of members from groups led by the University of California, San Francisco (N.R.P. [principal investigator], Tanushree Banerjee, Yunnuo Zhu, Delphine Tuot, C.-y.H., C.E.M., Deidra Crews, R.K.H., Vanessa Grubbs, Kirsten Bibbins-Domingo), the University of Michigan (R.S. [principal investigator], Brenda Gillespie, William Herman, V.B.S., Hal Morgenstern, M.H., Yi Li, Friedrich Port, Bruce Robinson, William McClellan, Jennifer Bragg-Gresham, Diane Steffick, Anca Tilea, Sai Dharmarajan, Patrick Albertus, Jerry Yee, Rajesh Balkrishnan, Kara Zivin, April Wyncott), and the CDC (Desmond Williams, N.R.B. [technical advisor], Mark Eberhardt, Paul Eggers, Linda Geiss, Juanita Mondesire, Bernice Moore, Priti Patel, M.E.P., Deborah Rolka, Sharon Saydah, Sundar Shrestha, Larry Waller).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Temporal Trends in AKI: Insights from Big Data,” on pages 1–3.
References
- 1.Bellomo R, Kellum JA, Ronco C: Acute kidney injury. Lancet 380: 756–766, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Lameire NH, Bagga A, Cruz D, De Maeseneer J, Endre Z, Kellum JA, Liu KD, Mehta RL, Pannu N, Van Biesen W, Vanholder R: Acute kidney injury: An increasing global concern. Lancet 382: 170–179, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS: Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol 4: 891–898, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY: Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liangos O, Wald R, O’Bell JW, Price L, Pereira BJ, Jaber BL: Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clin J Am Soc Nephrol 1: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY: Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 24: 37–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL: Validity of International Classification of Diseases, Ninth Revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Patel UD, Hardy NC, Smith DH, Gurwitz JH, Hsu CY, Parikh CR, Brunelli SM, Baker M, Forrow S, Comins C, Bourdreau DM, Liu C, Pawloski PA, Selvam N, Selvan MS, Stratton S, VanWormer JJ, Aggrey G, Blank M, Archdeacon P: Mini-Sentinel Validation of Acute Kidney Injury Cases, 2013. Available at: http://www.minisentinel.org/methods/outcome_validation. Accessed May 1, 2015
- 9.HCUP Nationwide Inpatient Sample (NIS): Healthcare Cost and Utilization Project (HCUP), 2007–2011, Rockville, MD, Agency for Healthcare Research and Quality, 2013
- 10.National Kidney Foundation : K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 11.Hsu CY, McCulloch CE, Fan D, Ordoñez JD, Chertow GM, Go AS: Community-based incidence of acute renal failure. Kidney Int 72: 208–212, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Siew ED, Davenport A: The growth of acute kidney injury: A rising tide or just closer attention to detail? Kidney Int 87: 46–61, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL: Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med 35: 1244–1250, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Martin GS, Mannino DM, Eaton S, Moss M: The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Carson SS, Cox CE, Holmes GM, Howard A, Carey TS: The changing epidemiology of mechanical ventilation: A population-based study. J Intensive Care Med 21: 173–182, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM: The epidemiology of mechanical ventilation use in the United States. Crit Care Med 38: 1947–1953, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Murray PT: The kidney in respiratory failure and mechanical ventilation. Contrib Nephrol 165: 159–165, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Kane-Gill SL, Sileanu FE, Murugan R, Trietley GS, Handler SM, Kellum JA: Risk factors for acute kidney injury in older adults with critical illness: A retrospective cohort study. Am J Kidney Dis 65: 860–869, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naik BI, Colquhoun DA, McKinney WE, Smith AB, Titus B, McMurry TL, Raphael J, Durieux ME: Incidence and risk factors for acute kidney injury after spine surgery using the RIFLE classification. J Neurosurg Spine 20: 505–511, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Bahar I, Akgul A, Ozatik MA, Vural KM, Demirbag AE, Boran M, Tasdemir O: Acute renal failure following open heart surgery: Risk factors and prognosis. Perfusion 20: 317–322, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Cartin-Ceba R, Kashiouris M, Plataki M, Kor DJ, Gajic O, Casey ET: Risk factors for development of acute kidney injury in critically ill patients: A systematic review and meta-analysis of observational studies. Crit Care Res Pract 2012: 691013, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu Q, Burt VL, Dillon CF, Yoon S: Trends in antihypertensive medication use and blood pressure control among United States adults with hypertension: The National Health And Nutrition Examination Survey, 2001 to 2010. Circulation 126: 2105–2114, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Rim MY, Ro H, Kang WC, Kim AJ, Park H, Chang JH, Lee HH, Chung W, Jung JY: The effect of renin-angiotensin-aldosterone system blockade on contrast-induced acute kidney injury: A propensity-matched study. Am J Kidney Dis 60: 576–582, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Arora P, Rajagopalam S, Ranjan R, Kolli H, Singh M, Venuto R, Lohr J: Preoperative use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers is associated with increased risk for acute kidney injury after cardiovascular surgery. Clin J Am Soc Nephrol 3: 1266–1273, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yacoub R, Patel N, Lohr JW, Rajagopalan S, Nader N, Arora P: Acute kidney injury and death associated with renin angiotensin system blockade in cardiothoracic surgery: A meta-analysis of observational studies. Am J Kidney Dis 62: 1077–1086, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Yoon SS, Ostchega Y, Louis T: Recent trends in the prevalence of high blood pressure and its treatment and control, 1999-2008. NCHS Data Brief 48: 1–8, 2010 [PubMed] [Google Scholar]
- 29.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, WRITING GROUP MEMBERS. American Heart Association Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics--2010 update: A report from the American Heart Association. Circulation 121: e46–e215, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics Committee and Stroke Statistics Subcommittee : Heart disease and stroke statistics--2014 update: A report from the American Heart Association. Circulation 129: e28–e292, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP: A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol 16: 162–168, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Epstein AJ, Polsky D, Yang F, Yang L, Groeneveld PW: Coronary revascularization trends in the United States, 2001-2008. JAMA 305: 1769–1776, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amin AP, Salisbury AC, McCullough PA, Gosch K, Spertus JA, Venkitachalam L, Stolker JM, Parikh CR, Masoudi FA, Jones PG, Kosiborod M: Trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction. Arch Intern Med 172: 246–253, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Hsu RK, Hsu CY: Acute kidney injury: Comment on “trends in the incidence of acute kidney injury in patients hospitalized with acute myocardial infarction.” Arch Intern Med 172: 253–254, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quan H, Parsons GA, Ghali WA: Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care 40: 675–685, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Grams ME, Plantinga LC, Hedgeman E, Saran R, Myers GL, Williams DE, Powe NR, CDC CKD Surveillance Team : Validation of CKD and related conditions in existing data sets: A systematic review. Am J Kidney Dis 57: 44–54, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]