Abstract
In this study, we used synaptic vesicles purified from the electric organ of marine electric rays to search for novel molecules which have important functions in synaptic transmission. Proteins that copurified with synaptic vesicles were used to immunize rats, and the resulting antisera were then used to further characterize the vesicle proteins. One of the antisera recognizes a protein of 34 kDa, p34, that has several characteristics which suggest it is a synaptic vesicle specific protein: (1) it copurifies exclusively with the synaptic vesicle peak during permeation chromatography on a controlled pore glass beads column, (2) it can be immunoprecipitated with intact synaptic vesicles and (3) it is specifically localized to the nervous system. The results suggest that p34 is a synaptic vesicle specific protein with a widespread distribution in the nervous system.
Keywords: Synaptic vesicle, Synaptic vesicle protein, Synaptic vesicle purification, Proton ATPase, Synaptotagmin, Vamp, SV2, o-rab3
INTRODUCTION
The discovery and analysis of the proteins that make up synaptic vesicles provide a starting point for studying the molecular mechanisms of synaptic transmission. The ability to obtain purified synaptic vesicles has facilitated the characterization of the protein components of synaptic vesicles, and as a result, detailed structural information from cDNA analysis has been obtained for many of these proteins12,16.
Studies utilizing purified synaptic vesicles from the electric organ of marine rays have made several contributions to our knowledge of the specific proteins that make up a synaptic vesicle. For example, expression screening of an electric lobe cDNA library using an antiserum directed against electric organ synaptic vesicles led to the discovery of vamp, an 18 kDa synaptic vesicle protein with a membrane inserted carboxy-terminus, a highly conserved central charged region, and a proline rich amino-terminus15. Amino acid sequence analysis of a 62 kDa protein in electric organ synaptic vesicles led to the discovery and cDNA cloning of synaptotagmin B and the characterization of a family of synaptotagmin molecules which are differentially localized in the nervous system20. Another synaptic vesicle protein, SV2, was first identified with a monoclonal antibody raised against electric organ synaptic vesicles2. This monoclonal has since proven useful in the cloning of the cDNA encoding SV2 in mammals1,5.
In an effort to identify additional synaptic vesicle proteins, we immunized rats with proteins which copurify with electric organ synaptic vesicles. Three of the resulting antibodies recognized synaptic vesicle specific proteins. One of these antibodies has been shown to recognize synaptotagmin B20, and another has been shown to recognize o-rab317, each of which are established synaptic vesicle proteins. The third antibody recognizes a protein, p34, that has several characteristics which suggest that it is also specifically localized to synaptic vesicles.
MATERIALS AND METHODS
Synaptic vesicle purification
Synaptic vesicles were purified from the electric organs of Discopyge ommata (obtained from Saltwater City in San Francisco) as previously described3,9 with the following modificatons. Purifications were scaled up to 235 g of electric organ. The initial grinding of the frozen electric organ was done by powderizing the tissue chunks in a Waring blender in the presence of liquid nitrogen for 30–40 s. The resuspension of the 100,000× g membrane pellet was done with six up/down strokes of a 0.004–0.006 inch clearance teflon-glass dounce (Kontes) followed by one down stroke with a zero-clearance dounce (Kontes) cooled to 0°C in ice water. In addition, a larger CPG column was made by fusing two 2.5 cm x 120 cm columns (BioRad). The resulting column was filled with 1,000 mls of controlled pore glass beads (Electro-nucleonics) to make a bed length of 203 cm. The column was run at a flow-rate of 31 ml/h.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of synaptic vesicle proteins
Proteins from either the excluded peak fractions or synaptic vesicle fractions were precipitated in 10% trichloroacetic acid (TCA) and the resulting pellets were washed with 80% acetone followed by 100% acetone. Protein samples were subjected to SDS-PAGE as described8. In order to adequately resolve proteins in the 34 kDa region, separating gels of 20 cm (8–13% acrylamide gradient) were used for Fig. 1 and Fig. 3. Separating gels of 6 cm (15% acrylamide) were used for Fig. 2, and 9 cm (10% acrylamide) for Fig. 4 and Fig. 5. Proteins were then visualized by staining the gel with Coomassie blue.
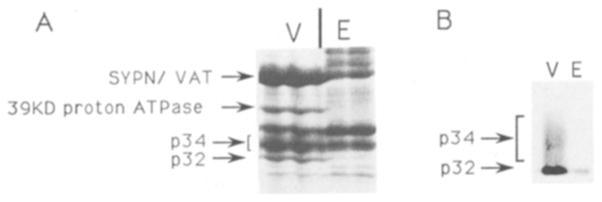
Fig. 1.
A: region of gel containing p34. SDS-PAGE fractionated proteins (90 μg) from the synaptic vesicle peak (V) and the excluded peak (E) of the CPG column. Proteins were visualized by Coomassie blue staining. Only the region of the gel between 30 kDa and 43 kDa is shown. Locations of the molecular weight markers are indicated on the right. The locations of synaptophysin (SYPN), VAT, the 39 kDa subunit of the proton ATPase, p34, and p32 are indicated with arrows on the left. The locations of synaptophysin and of VAT have previously been determined by Western blot analysis4 and amino terminal sequence analysis 9 The locations of p32 and p34 were determined by Western blot analysis. The 39 kDa proton ATPase subunit was identified by protein sequencing of two CNBr fragments (see Materials and Methods). B: p34 is found in purified vesicles, but not excluded peak protein. Western blot analysis of an equal amount of protein (3 μg) from the excluded peak and vesicle fractions of the CPG column. The blot was probed with the antiserum raised against p32. The p34 immunoreactivity found in the same serum is evident as a diffuse band above p32.
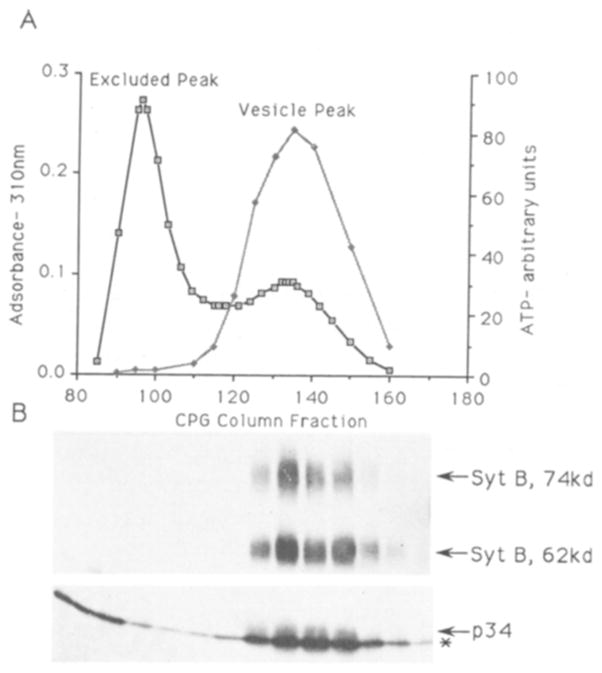
Fig. 3.
A: CPG column profile of a synaptic vesicle purification. Fractions from the final stage of the synaptic vesicle purification (the controlled pore glass beads (CPG) column were analyzed for light scattering material (membranes and protein) by adsorbance at 310 nm (open boxes) and for intact vesicles by ATP content (shaded diamonds). The fractions corresponding to membranes excluded from the glass bead pores (300 nm) and those containing synaptic vesicles are indicated. B: p34 copurifies with the synaptic vesicle containing fractions. Western blot analysis of fractions from the CPG column (equal volume). After transer, the blot was cut into strips which were probed with either Synaptotagmin B antibodies or the p32/p34 antiserum. Synaptotagmin B immunoreactivity, both the 62 kDa and 74 kDa forms, and p34 immunoreactivity are each indicated by arrows, p32 immunoreactivity is indicated by an asterisk.
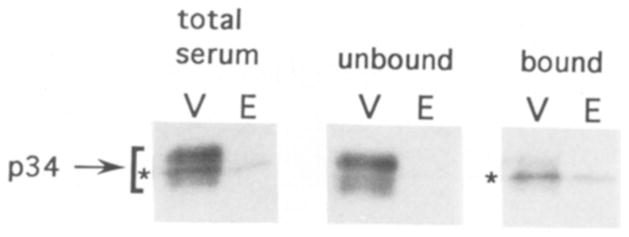
Fig. 2.
Antibodies specific to p34 can be separated from p32 antibodies. Western blot analysis of proteins from the vesicle peak (V, 1 μg) and excluded peak (E, 4 μg) of the CPG column. Blots were separately probed with the p32/p34 antiserum (total serum), antibodies which did not bind to the p32 affinity column (unbound), or antibodies which were eluted from the p32 affinity column after washing (bound). The position of p34 immunoreactivity in the blots is indicated with a bracket. The position of p32 immunoreactivity, which comigrated with p34 in this gel, is indicated with an asterisk.
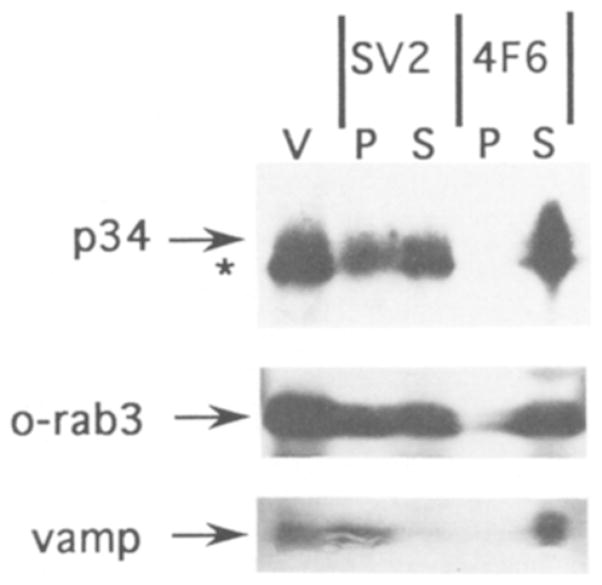
Fig. 4.
p34 can be immunoprecipitated with SV2-containing vesicles. Western blot analysis of proteins immunoprecipitated with either a monoclonal antibody to SV2 or a control monoclonal to an Aplysia antigen (4F6). Proteins from the pellet (P) and supernatant (S) of each immunoprecipitation are indicated above the lanes. A lane of purified synaptic vesicle protein (V) was also included. Blots were cut into strips and separately probed with antibodies to p32/p34, vamp, and ommata rab3 (o-rab3).
Fig. 5.
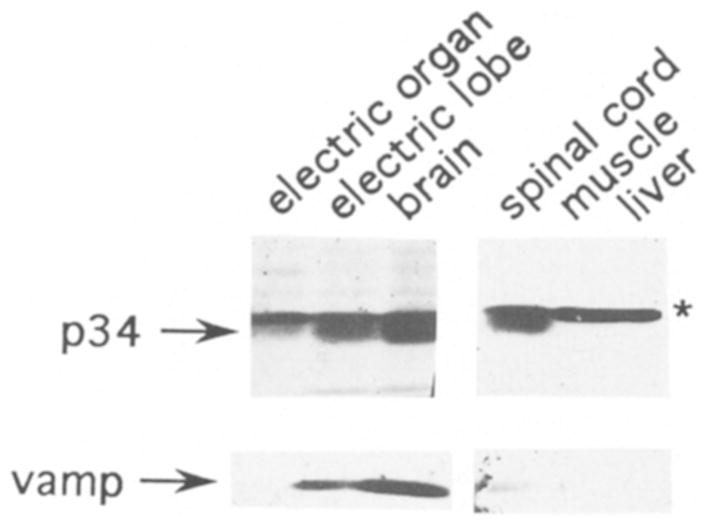
p34 is specific to the nervous system. Western blot analysis of SDS-solubilized proteins (30 μg each lane) from electric organ, electric lobe (brainstem), whole brain, spinal cord, muscle, and liver. Blots were cut into strips and probed with either the p32/p34 antiserum or a polyclonal antibody to vamp, an integral membrane protein of the synaptic vesicle. Immunoreactivity corresponding to p34 and vamp are indicated with arrows, and p32 immunoreactivity is indicated with an asterisk. In the gel system used for this blot, the p34 band migrated erratically and appears below the p32 band. This was probably because a shorter resolving gel was used in combination with a different percentage of acrylamide (see methods). A control lane of synaptic vesicles was used to confirm the location of p34 immunoreactivity relative to p32.
Cyanogen bromide cleavage and amino acid sequencing
Cyanogen bromide cleavage and amino acid sequencing of the 39 kDa subunit of the proton ATPase was performed using a previously described method20. To prepare p32 for amino-terminal sequencing, purified synaptic vesicle proteins were fractionated by SDS-PAGE, blotted to Immobilon-P, and stained with Coomassie blue. The band corresponding to p32 was then cut from the blot and sequenced.
Production of antibodies
The o-rab3 and p32/p34 antisera were produced, as previously described20, by injecting gel-purified proteins into Sprague-Dawley rats. O-rab3 antiserum was produced by immunizing rats with a 28 kDa protein from purified synaptic vesicles which has been identified as o-rab3 by protein sequencing. The p32/p34 antiserum was produced by injecting p32, which probably also contained some p34. The primary injections contained approximately 5 μg of protein followed by two boosts of 5 μg each at 3-week intervals. Controls using normal rat serum showed no immunoreactivity for o-rab3, p32, or p34 in purified vesicles. O-rab3 antiserum was further affinity purified, as previously described11,20, using o-rab3 on Immobilon-P as the solid support.
Affinity depletion of p32 antibodies
Purified synaptic vesicles were fractionated on an SDS-PAGE gel and p32 was cut out and electroeluted according to a previously described procedure7. The SDS was removed by bringing the sample to 50 mM KCl, incubating on ice 30 min and spinning at 15,000× g for 10 min. The supernatant containing approximately 15μg of p32 protein was coupled to a 1:1 mixture of Affigel 10 and Affigel 15 (Biorad) in MOPS pH 7.5 buffer overnight at 4°C. After the coupling reaction, the supernatant was assayed by immunoblotting to confirm a transfer of p32 to the solid phase. The coupling reaction was stopped by incubating with 0.2 M glycine for 90 min at 4°C. After washing out the glycine, whole antiserum diluted 1:4 with PBS was mixed with the beads overnight at 4°C. The beads (1 ml) were then washed with 10 vols. of PBS. Unbound antibodies consisted of the flow-through from the column plus the first milliliter of PBS wash. Bound antibodies were eluted with 6 × 1 ml 0.1 M glycine pH 2.8. The first three fractions were pooled and neutralized with Tris base.
Preparation of tissues for Western blotting
Three regions of the central nervous system were used for Western blot analysis: (1) spinal cord—consisting of the entire spinal cord from its junction with the brainstem to the posterior end. (2) Electric lobe—consisting of the brainstem, but dominated by the electromotor nuclei (electric lobes) which innervate the electric organ. (3) Brain—consisting of the rest of the central nervous system (cerebellum, midbrain, forebrain) which does not include the spinal cord or brainstem. Proteins from frozen tissues were prepared for electrophoresis by grinding the tissue to a powder under liquid nitrogen and resuspending the powder in 1% SDS. DNA was sheared by passing through a narrow gauge needle. Protein concentration was determined by the Lowry method.
Western blotting
Western blotting was performed essentially as described14. Rat anti-synaptotagmin B antibodies (used at 1:10) are polyclonal, affinity-purified antibodies which have been previously described20. Affinity purified rabbit anti-vamp antibodies (gift of Dr. William Trimble, used at 1:50) were produced against a 15 amino acid synthetic peptide corresponding to amino acids 55 (valine) through 69 (alanine) of rat vamp I. This region is 100% identical to the corresponding regions of rat vamp II and ommata vamp. The p32/p34 antiserum, produced as described above, was used at a dilution of 1:500. The affinity depleted p32/p34 antiserum and the affinity purified p32 antibodies were used at dilutions equivalent to 1:750 of the starting antiserum. For Fig. 1B biotin-conjugated secondary antibodies were used, followed by avidin-coupled horse radish peroxidase (Cappel) and color development using 3,3′-diaminobenzidine (DAB) (Sigma) as a substrate. Fig. 2 employed 125I-labeled secondary antibodies (Amersham), and Figs. 3–5 employed alkaline phosphatase-conjugated secondary antibodies (Bio-Rad) used in conjunction with the 5-bromo-4-chloro-3-inolyl phosphate (BCIP) and nitroblue tetrazolium (NBT) color development system (Bio-Rad), according to manufacturer’s instructions.
Immunoprecipitation of synaptic vesicles
For each immunoprecipitation, 20 μ1 of SV2 monoclonal supernatant2 (gift of Dr. Regis Kelly) or a control monoclonal supernatant (4F6) was added to 125 μ1 of freshly prepared electric organ synaptic vesicles (1.5 μg) and incubated for 4 h at 4°C. 10 μl of rabbit anti-mouse Immunobeads (BioRad) were then added and allowed to mix for 16 h at 4°C. Beads were pelleted by a brief microfuge spin and the supernatants were removed and saved. The pellet of beads was then washed 6 times under iso-osmotic conditions, alternating between 0.4 M NaCl, 10 mM HEPES [pH 7.0] and 0.45 M sucrose, 0.175 M NaCl, 10 mM HEPES [pH 7.0]. Supernatants from the immunoprecipitations were precipitated with TCA and prepared for electrophoresis as described above. Proteins from the pelleted beads were solubilized with SDS-PAGE sample buffer, and both supernatant and pellet proteins were subjected to Western blot analysis as described above, except that no DTT was included in the SDS-PAGE sample buffer to prevent antibody heavy and light chains from co-migrating with the proteins of interest.
RESULTS
The final step in our synaptic vesicle purification employs permeation chromatography on a controlled pore glass beads (CPG) column to separate synaptic vesicles from larger membranes which are excluded from the glass bead pores. It has previously been shown that proteins which are specifically localized to synaptic vesicles, such as vamp and synaptotagmin, cannot be detected in the excluded peak fractions20. To identify additional synaptic vesicle specific proteins, proteins from the excluded peak and synaptic vesicle peak of the CPG column were fractionated by SDS-PAGE and analyzed by Coomassie blue staining. Those proteins that were enriched or exclusively found in the synaptic vesicle peak were then used to raise antibodies or to obtain protein sequence. Fig. 1 shows a region of such a gel which appears to contain two synaptic vesicle enriched bands and two synaptic vesicle specific bands, as well as several bands that are equally present in the excluded and vesicle peaks. One of the vesicle enriched bands has been shown in previous studies to contain both synaptophysin and VAT, two proteins which comigrate under these conditions. Although synaptophysin is specific to the vesicle fractions of the prep, Western blot analysis has shown that synaptophysin is specific to the synaptic vesicle fractions whereas VAT is present in both excluded peak and synaptic vesicle fractions 4,9. The other synaptic vesicle enriched band (labeled p34) comigrated with an abundant protein in the excluded peak, so it was not possible to determine if the p34 band on this gel contained a synaptic vesicle specific protein (Fig. 1A).
Amino acid sequence was obtained for both of the synaptic vesicle specific proteins (39 kDa and 32 kDa). The 39 kDa protein was identified as a subunit of the vacuolar proton ATPase by sequence analysis of two cyanogen bromide fragments. The following are the amino acid sequences obtained for the 39 kDa protein (single-letter code where X represents an unidentified residue) followed by the numbers of the amino acid positions corresponding to the 39 kDa subunit of the proton ATPase from bovine chromaffin granules 19. Positions where the amino acids are identical between the 2 proteins are underlined: XXEIEXNTLHEAYXN (99–113), XPILEFNADNDASIFNI (131–147). In addition, it is likely that before cleavage each fragment lay adjacent to a methionine residue, since CNBr cleaves on the carboxy-terminal side of methionine 6. The bovine sequences also each show a methionine at this location.
Twenty-two amino acids of the 32 kDa protein, p32, were identified by amino-terminal sequencing, but no significant matches were found in the NBRF protein database. The sequence obtained was as follows: ML-GRVVAALVSFLRSLXNVFLK.
Identification of p34
To further investigate p32, rats were immunized with gel-purified p32. The resulting serum was analyzed using Western blot analysis of purified vesicles and excluded peak protein (Fig. 1B). Two regions of immunoreactivity were observed: a sharp band corresponding to p32, which was present in both vesicles and excluded proteins, and a diffuse band with an average mol. wt. of 34 kDa (p34), which was detectable only in purified vesicles. It is difficult to identify the region on the Coomassie stained gel that corresponds to the p34 immunoreactivity because this region also contains two bands which are common to both the excluded and vesicle peaks (Fig. 1A). Although p32 appears enriched in vesicles (or specific to vesicles in Fig. 1A), it is important to note that the gels in Fig. 1 were loaded with equal amounts of excluded and vesicle peak protein. Since the excluded peak fractions contain about three times as much protein as the vesicle fractions, the excluded peak proteins are under-represented in these gels.
Since p32 and p34 have different electrophoretic mobilities and differ in their distribution across the CPG column fractions, it is likely that the antiserum recognizes two different proteins. This is probably due to the presence of some p34 in the p32 band which was used for the immunizations. To further test this possibility, we have separated p32 and p34 specific antibodies by affinity depletion of p32 specific antibodies. The depleted serum was then tested on a Western blot of excluded peak and synaptic vesicle proteins (Fig. 2). While the p32 and p34 immunoreactivities were not completely resolved in this gel, p32 is observed as a sharp band against the more diffuse p34 background. The unbound antibodies recognized primarily p34, while antibodies eluted from the affinity column (bound) showed an enrichment in p32 immunoreactivity.
Copurification of p34 with synaptic vesicles
To determine if the presence of p34 in purified synaptic vesicles is due to its copurification with the vesicles, a Western blot analysis was done on fractions spanning the excluded and vesicle peaks of the CPG column (Fig. 3A,B). P34 immunoreactivity copurified with the synaptic vesicle fractions, and the pattern of p34 immunoreactivity parallels that of the synaptic vesicle proteins synaptotagmin B (Fig. 3B) and vamp 20. P34 immunoreactivity also copurified with synaptic vesicles in the sucrose gradient, which is the step immediately preceding the CPG column in the vesicle purification procedure. These results demonstrate that p34, like synaptotagmin B and vamp, copurifies with synaptic vesicles.
P32 immunoreactivity, on the other hand, was clearly present in the excluded peak fractions. It is difficult to say if there is an enrichment of p32 in the vesicle fractions due to the close migration of p34, which may be contributing to the intensity of the p32 immunoreactivity in the vesicle fractions. Although this result demonstrates that p32 does not copurify with synaptic vesicles, a possible association of p32 with synaptic vesicles (in addition to other membrane compartments) cannot be excluded.
Immunoprecipitation of p34 with SV2-containing vesicles
To determine if p34 is directly associated with synaptic vesicles, intact synaptic vesicles were immunoprecipitated with a monoclonal antibody against SV2, an integral membrane protein of synaptic vesicles2. The immunoprecipitates were then examined by Western blot analysis for the presence of p34, p32, and two synaptic vesicle proteins (o-rab3 and vamp) (Fig. 4). As expected, the majority of the immunoreactivity for vamp, which is an integral membrane protein, was found in the pellet of the SV2 immunoprecipitation. P34 immunoreactivity was also present in the SV2 immunoprecipitate, whereas no p34 immunoreactivity was present in the control immunoprecipitate. The relative amounts of p34 immunoreactivity in the supernatant and pellet of the SV2 immunoprecipitate was similar to o-rab3, a small GTP-binding protein of synaptic vesicles17.
Although the p32 immunoreactivity appears confined to the supernatant of the SV2 immunoprecipitation (since there is no distinct band in the pellet), it is difficult to determine the distribution of p32 with confidence due to the close migration of p32 and p34 in this gel.
Tissue distribution of p34
To determine the distribution of p34 among the tissues of D. ommata, a Western blot analysis was performed on several tissues (Fig. 5). Strong p34 immunoreactivity was seen in electric lobe (brainstem), brain, and spinal cord, while no p34 immunoreactivity was detected in muscle or liver (Fig. 5). Weak p34 immunoreactivity was seen in electric organ. No vamp immunoreactivity was detected in electric organ under these conditions, although vamp is easily detectable in purified electric organ synaptic vesicles (Fig. 4). Synaptotagmin immunoreactivity is also less intense in electric organ than central nervous system tissues 20, suggesting that electric organ has a lower density of synapses than brain tissue. The distribution of vamp was otherwise similar to p34, showing exclusive localization to the nervous system. P32 immunoreactivity, on the other hand, was found in all tissues tested and was especially strong in muscle and liver.
The distribution of p34 among the tissues tested suggests that it is exclusively localized to the nervous system. Furthermore, the results demonstrate that p34 is not unique to the region of the brain that innervates the electric organ, and thus does not have a function unique to the electric organ. P32, on the other hand, was found in all tissues tested and thus is likely to participate in a general cellular function.
DISCUSSION
The results described three proteins that appear enriched or specific to purified electric organ synaptic vesicles. One of these proteins was identified as the 39 kDa subunit of the vacuolar proton ATPase. This ATPase is a large multisubunit protein complex which produces a proton gradient in several types of vesicular organelles, including chromaffin granules, clathrin-coated vesicles, lysosomes, and synaptic vesicles10. Thus, it is expected that some of the ATPase subunits will be found in purified synaptic vesicles.
Another protein that appeared specific to synaptic vesicles, p32, was used to generate antibodies. The resulting antiserum showed two immunoreactivities in purified vesicles: the 32 kDa band used for the immunizations, and a diffuse band of 34 kDa (p34). The results demonstrate three significant differences between p32 and p34: (1) During electrophoresis, p32 migrates as a sharp band whereas p34 migrates as a diffuse band, (2) using affinity chromatography, antibodies specific to p34 can be separated from p32 antibodies, and (3) p32 and p34 differ in their fractionation during the synaptic vesicle purification and in their tissue distribution. Thus, the results suggest that p32 and p34 are unrelated proteins. Although we initially identified p32 as a synaptic vesicle specific protein, subsequent Western blot analysis revealed that it does not copurify with synaptic vesicles, and that it is not specific to the nervous system. Thus it is likely to be a protein involved in a general cellular function.
P34, on the other hand, has characteristics which suggest that it is specifically localized to synaptic vesicles. P34 shows a strict copurification with electric organ synaptic vesicles, and is not detectable in the excluded peak fractions. The results also show that p34 can be specifically immunoprecipitated with intact synaptic vesicles using a monoclonal antibody to SV2, an integral component of the synaptic vesicle membrane. This suggests that p34 is directly associated with synaptic vesicles. The distribution of p34 among the tissues tested suggests that it is exclusively localized to the nervous system. Furthermore, the results demonstrate that it is not unique to the region of the brain that innervates the electric organ, as evidenced by the strong immunoreactivity found in all brain regions tested. It is likely therefore, that p34 is a component of most or all regions of the ray central nervous system.
It is difficult to determine whether this is the same p34 that has been described previously in preparations of electric organ synaptic vesicles. A protein of 34 kDa was one of 14 proteins identified in a preparation of purified Torpedo marmorata synaptic vesicles 13. However, it was described as one of the major Coomassie stained bands in the vesicle prep, and the p34 we are describing generally migrates as a diffuse, poorly staining band which is difficult to resolve from other proteins in the region. Wagner and Kelly reported proteins of 33 and 35.5 kDa which co-purify with Narcine brasiliensis electric organ synaptic vesicles, as judged by Coomassie staining of fractions from the CPG column18, and it is possible that one of these proteins is identical to the 34 kDa protein we are describing. However, no antibodies studies have been reported for these proteins, and thus it has not been determined if they are present in excluded peak fractions, if they can be immunoprecipitated with synaptic vesicles, or if they are specific to the nervous system.
In summary, the results demonstrate that p34 fits several criteria for being a synaptic vesicle specific protein, including copurification with electric organ synaptic vesicles, immunoprecipitation with SV2-containing vesicles, and nervous system specific localization. Future studies, aimed at cloning the cDNA for this protein, will provide valuable probes for the further analysis of p34, and may also reveal sequence homologies relevant to synaptic vesicle function.
Acknowledgments
The authors would like to thank Dr. William Trimble for supplying vamp antibodies, Dr. Regis Kelly for supplying SV2 antibody, Dr. Wayne Sossin for supplying 4F6 monoclonal antibody, and Dr. James Schilling for generating cyanogen bromide peptides, and for sequence analysis of the 39 kDa vacuolar proton ATPase subunit and p32. The authors would also like to thank Dr. Sandra Bajjalieh and Dr. Johnny Ngsee for critical reading of the manuscript. This work is supported by a grant to R.H.S. from the National Institutes of Mental Health.
References
- 1.Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science. 1992;257:1271–1273. doi: 10.1126/science.1519064. [DOI] [PubMed] [Google Scholar]
- 2.Buckley K, Kelly RB. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985;100:1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlson SS, Wagner JA, Kelly RB. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochemistry. 1978;17:1188–1199. doi: 10.1021/bi00600a009. [DOI] [PubMed] [Google Scholar]
- 4.Cowan D, Linial M, Scheller RH. Torpedo synaptophysin: evolution of a synaptic vesicle protein. Brain Res. 1990;509:1–7. doi: 10.1016/0006-8993(90)90301-q. [DOI] [PubMed] [Google Scholar]
- 5.Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell. 1992;70:861–867. doi: 10.1016/0092-8674(92)90319-8. [DOI] [PubMed] [Google Scholar]
- 6.Gross E. The cyanogen bromide reaction. Meth Enzymol. 1967;11:238–255. [Google Scholar]
- 7.Hunkapiller MW, Lujan E, Ostrander F, Hood LE. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Meth Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Linial M, Miller K, Scheller RH. VAT-1: an abundant membrane protein from torpedo cholinergic synaptic vesicles. Neuron. 1989;2:1265–1273. doi: 10.1016/0896-6273(89)90311-5. [DOI] [PubMed] [Google Scholar]
- 10.Nelson N. Structure, molecular genetics, and evolution of vacuolar H+-ATPases. J Bioenerg Biomembr. 1989;21:553–571. doi: 10.1007/BF00808113. [DOI] [PubMed] [Google Scholar]
- 11.Snyder M, Elledge S, Sweetser D, Young RA, Davis RW. Lambda gt 11: gene isolation with antibody probes and other applications. Meth Enzymol. 1987;154:107–128. doi: 10.1016/0076-6879(87)54073-3. [DOI] [PubMed] [Google Scholar]
- 12.Sudhof TC, Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991;6:665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- 13.Tashiro T, Stadler H. Chemical composition of cholinergic synaptic vesicles from Torpedo marmorata based on improved purification. Eur J Biochem. 1978;90:479–487. doi: 10.1111/j.1432-1033.1978.tb12627.x. [DOI] [PubMed] [Google Scholar]
- 14.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trimble WS, Cowan DM, Scheller RH. VAMP-1: a synaptic vesicle-associated membrane protein. Proc Natl Acad Sci USA. 1988;85:4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trimble WS, Linial M, Scheller RH. Cellular and molecular biology of the presynaptic nerve terminal. Annu Rev Neurosci. 1991;14:93–122. doi: 10.1146/annurev.ne.14.030191.000521. [DOI] [PubMed] [Google Scholar]
- 17.Volknandt W, Pevsner J, Elferink LA, Schilling J, Scheller RH. A synaptic vesicle specific GTP-binding protein from ray electric organ. Mol Brain Res. 1991;11:283–290. doi: 10.1016/0169-328x(91)90037-x. [DOI] [PubMed] [Google Scholar]
- 18.Wagner JA, Kelly RB. Topological organization of proteins in an intracellular secretory organelle: the synaptic vesicle. Proc Natl Acad Sci USA. 1979;76:4126–4130. doi: 10.1073/pnas.76.8.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Moriyama Y, Mandel M, Hulmes JD, Pan YE, Danho W, Nelson H, Nelson N. Cloning of cDNA encoding a 32-kDa protein, an accessory polypeptide of the H+-ATPase from chromaffin granules. J Biol Chem. 1988;263:17638–17642. [PubMed] [Google Scholar]
- 20.Wendland B, Miller KG, Schilling J, Scheller RH. Differential expression of the p65 gene family. Neuron. 1991;6:993–1007. doi: 10.1016/0896-6273(91)90239-v. [DOI] [PMC free article] [PubMed] [Google Scholar]