Abstract
Anti-platelet autoantibodies are frequently found in systemic lupus erythematosus (SLE) patients and contribute to the development of SLE-associated immunologic thrombocytopenia (SLE-ITP). Although the correlation of anti-dsDNA autoantibody with platelet-associated antibody has been reported, the potential mechanism underlying such a correlation is incompletely understood. We have reported that anti-platelet integrin GPIIIa49-66 (CAPESIEFPVSEARVLED) autoantibodies play a major role in the development of HIV-1-related thrombocytopenia (HIV-1-ITP). The strong negative charge of GPIIIa49-66 prompts us to investigate whether GPIIIa49-66 can be an epitope mimicking dsDNA. We report here that anti-GPIIIa49-66 antibodies are found in three out of nine SLE-ITP patients. Double-stranded (ds) DNA competitively inhibited the binding of purified patient anti-dsDNA antibodies to GPIIIa49-66 peptide. Both polyclonal and monoclonal anti-GPIIIa49-66 antibodies are able to cross-react with dsDNA. Consistent with previous reports, the DNA binding activities of anti-GPIIIa49-66 antibodies are mainly dependent on the positively charged amino acid in the heavy-chain complementarity-determining region 3 (HCDR3). The HCDR3 of human SLE anti-dsDNA monoclonal antibody (mAb) 412.67 demonstrates a similar positively charged amino acid chain orientation compared with that of anti-GPIIIa49-66 mAb A11, and it cross-reacts with GPIIIa49-66 peptide. Purified anti-GPIIIa49-66 antibodies from SLE-ITP patients are able to induce platelet fragmentation in vitro and to induce thrombocytopenia in vivo. Thus, our data suggest that specific epitope cross-reaction between GPIIIa49-66 and dsDNA could be a mechanism involved in the development of SLE-associated thrombocytopenia.
Keywords: Autoantibody, anti-dsDNA autoantibody, platelet-associated antibody (PAIgG), systemic lupus erythematosus (SLE)
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune rheumatic disease characterized by the deposition of autoantibodies and immune complex, which eventually leads to tissue damage [1–4]. Immune thrombocytopenia (ITP) is a disease caused by autoantibody-mediated platelet destruction and it is frequently associated with SLE [5,6]. In fact, more than 20% of SLE patients have platelet counts less than 100 × 109/l and in severe cases the platelet counts can be as low as 30 × 109/l [7,8]. Several mechanisms may contribute to SLE-associated ITP. The potential mechanisms include, but not limit to, (1) platelet destruction induced by anti-platelet glycoprotein (GP) GPIIb/IIIa and GPIb/IX autoantibodies [9]; (2) autoantibodies against thrombopoietin receptor, c-Mpl, may result in low platelet production [10–12]; (3) platelet destruction induced by autoantibodies cross-reacting with negatively charged epitopes of proteins on platelet membrane [13,14]. In fact, it was shown that antiphospholipid monoclonal antibody (mAb) was able to specifically cross-react with negatively charged epitopes in platelet integrin GPIIIa [14]. Thus, cross-reaction of autoantibodies with platelet antigen is likely involved in the development of SLE-associated immunologic thrombocytopenia (SLE-ITP).
Anti-dsDNA autoantibodies are considered as not only a diagnostic marker but also a pathogenic factor for SLE [15]. Previous reports have shown that those anti-dsDNA antibodies may cross-react with a variety of epitopes in different proteins [16–27]. It was also showed that a synthesized peptide is able to induce anti-dsDNA antibody in mice [28,29]. Although it was suggested that the production of specific anti-platelet autoantibodies (mainly directed against GpIIb/IIIa) plays an important role in the development of SLE-ITP [14], it is not clear whether an epitope mimicking dsDNA does exist in platelet integrin GPIIIa.
We have previously described a unique anti-platelet integrin GPIIIa49-66 antibody derived from the patients with HIV-related immunologic thrombocytopenia (HIV-1-ITP), which induces complement-independent platelet oxidative fragmentation and death by generation of platelet peroxide following NADPH oxidase activation [30–34]. The presence of anti-GPIIIa49-66 Ab correlates inversely with platelet count (r = −0.71) and induces severe thrombocytopenia when injected into mice [31]. Platelet integrin GPIIIa49-66 (CAPESIEFPVSEARVLED) contains five negatively charged amino acids (underline), and monoclonal antibodies (mAbs) binding to GPIIIa49-66 mainly depend on positively charged amino acid in the heavy-chain complementarity-determining region 3 (HCDR3) [35], similar as anti-dsDNA autoantibodies binding to DNA. Thus, we investigate whether anti-dsDNA antibody could cross-react with platelet GPIIIa49-66 as it may contribute to the development of SLE-ITP.
Here, we demonstrate that (1) three out of nine SLE-ITP patients IgG cross-react with both dsDNA and platelet GPIIIa49-66, and dsDNA inhibited the binding of purified patient anti-dsDNA antibodies to GPIIIa49-66 peptide; (2) both polyclonal and monoclonal anti-GPIIIa49-66 antibodies are able to cross-react with dsDNA, and the DNA binding activities of anti-GPIIIa49-66 antibodies are mainly dependent on the positively charged amino acid in the HCDR3 region; (3) anti-GPIIIa49-66 mAbs and anti-dsDNA mAbs have similar side-chain orientation of positively charged amino acids in their HCDR3; (4) human anti-dsDNA mAb 412.67 from a SLE patient cross-reacts with GPIIIa49-66 peptide; and (5) affinity-purified anti-GPIIIa49-66 antibodies from SLE-ITP patients induced platelet fragmentation in vitro and thrombocytopenia in vivo. Therefore, our data suggest an epitope mimicry between dsDNA and GPIIIa49-66, and anti-dsDNA in SLE patients may contribute to the development of SLE-ITP.
Materials and methods
Reagents, mice, and clinical samples
All reagents were obtained from Sigma-Aldrich (St Louis, MO, USA) unless otherwise designated. Peptide GPIIIa49-66 (CAPESIEFPVSEARVLED) and irrelevant 10-mer peptide (GIGALFLGFL) were synthesized by Bio-Synthesis (Lewisville, TX, USA). Human anti-GPIIIa49-66 Abs, affinity-purified rabbit anti-GPIIIa49-66 polyclonal antibodies (pAbs) and anti-GPIIIa49-66 scFv mAbs, and its mutants were produced in our laboratory [30,31,34]. Human anti-dsDNA mAb 412.67 from a SLE patient was described previously [36–38]. Female BALB/c mice were obtained from Taconic Farms (Germantown, NY, USA). Animal work was approved by the New York University School of Medicine Animal Review Board. Human sera used in this study were collected from SLE-ITP patients and healthy subjects at Bellevue Hospital, New York. All of the SLE patients and non-SLE controls satisfied the America College of Rheumatology revised criteria for the classification of SLE [39]. SLE-ITP patients had platelet counts of 82 ± 27 × 109/l. These studies were approved by the New York University Medical Center, Institutional Review Board.
Affinity purification of IgG from sera of SLE-ITP patients
Serum IgG was isolated from SLE-ITP patients by protein A/G affinity chromatography. The monospecific anti-dsDNA fraction was purified using human placenta dsDNA-coupled CNBr-activated Sepharose 4B gel column as previously described [40]. The affinity-purified IgG was dialyzed against 0.01 M sodium phosphate buffer saline (pH 7.4; PBS).
Immunoblotting
Twenty-five micrograms of platelet lysis were separated by 12% SDS/PAGE gels; transferred to a PVDF membrane; and immunoblotted with anti-human IgG, anti-platelet integrin GPIIIa (sc-46655, Santa Cruz Biotechnology, Inc.), and a normal control IgG for 2h, followed by horse radish peroxide (HRP)-conjugated secondary IgG, and detected by chemiluminescence.
ELISA
For GPIIIa49-66 peptide, 20μg/ml of peptide in 0.1 M sodium bicarbonate buffer (pH 9.6) was adsorbed to a 96-well ELISA plate at 4°C overnight and blocked with blocking buffer (3% BSA in PBS-Tween 0.1%) at room temperature for 2h. To coat dsDNA, calf thymus dsDNA was adsorbed to a 96-well ELISA plate precoated with poly-l-lysine (50 μg/ml) at 4°C overnight [41]. After charge neutralizing the plates with poly-l-glutamate (100 μg/ml in distilled water), the plates were blocked with 2% casein (37°C, 1 h for each step). Patient samples (diluted 1:50 in PBS-Tween), anti-GPIIIa49-66 antibodies or anti-DNA mAb 412.67, were added and incubated at room temperature for 1h, followed by HRP-conjugated secondary IgG. ELISA results were read by an automated microtiter plate reader at 405 nm with 490 nm as reference. For dsDNA inhibition assay, different concentrations of calf thymus dsDNA were incubated with purified patient anti-dsDNA antibodies for 30min at 37°C for 1h. The final concentrations of dsDNAs were from 0.1 to 100 μg/ml.
Assay of platelet particle formation
Gel-filtered human platelets were isolated and labeled with anti-human CD61-fluorescein isothiocyanate as previously described [31]. Fluorescent-labeled platelets/particles were measured by flow cytometry using a FACScan (Becton Dickinson Immunocytometry Systems, Mountain View, CA, USA). Gates were adjusted for platelets by exclusion of other blood cells. Fluorescent-labeled intact platelets were monitored in the right upper quadrant with the y-axis measuring forward scatter and the x-axis measuring fluorescence. A shift in fluorescence from right upper quadrant to left upper quadrant and to left lower quadrant reflected the percentage of platelet particle induction in 10,000 enumerated events.
Induction of passive thrombocytopenia in mice
Six-week-old female BALB/c mice were randomly divided into two groups (n = 4 per group). Fifty micrograms of purified control antibody or patient anti-GPIIIa49-66 antibody were injected intraperitoneally into Balb/c mice and followed by platelet counts for 24 h. Platelet counts were determined from 20 μl of blood drawn into Unopettes (No. 365855, Becton Dickinson), containing optimal anti-coagulant concentration and diluent for quantitating platelet counts by phase contrast microscopy.
Molecular modeling
The heavy chain and light chain amino acid sequences of anti-GPIIIa49-66 antibody mAb A11 and anti-dsDNA antibody mAb 412.67 were used to generate the molecular model through WAM-Web Antibody Modeling (http://antibody.bath.ac.uk). The side chains of amino acids in the heavy chains at position 100, 102, 104, and 105 have been labeled.
Results
dsDNA inhibits IgG purified from three SLE-ITP patients binding to GPIIIa49-66
Since GPIIIa49-66 (CAPESIEFPVSEARVLED) contains five strong negatively charged amino acids (underline), we have examined potential cross-reactivity between dsDNA and GPIIIa49-66 using sera from SLE-ITP patients. We found that three out of nine patient sera cross-react with both dsDNA and GPIIIa49-66 peptide, whereas the other six patients sera only react with dsDNA, and six healthy subjects sera were not reactive (Figure 1A). Immune complexes with platelet integrin GPIIIa were also found in three SLE-ITP patients sera with anti-GPIIIa49-66 activity P5, P6, and P7 (Figure 1B). The inhibition of dsDNA to the binding of three patients purified anti-dsDNA antibodies to GPIIIa49-66 peptide suggests a potential epitope mimicry between GPIIIa49-66 and dsDNA in SLE-ITP patients (Figure 1C).
Figure 1.
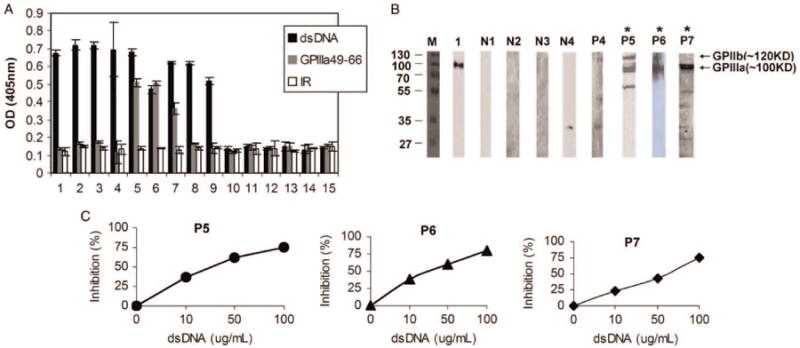
dsDNA inhibits IgG purified from three SLE-ITP patients binding to GPIIIa49-66.(A) Binding activity of serum IgG from nine SLE-ITP patients and six healthy subjects to dsDNA and platelet GPIIIa49-66. IR refers to irrelevant peptide. SEM is given. (B) Presence of anti-GPIIIa antibodies in SLE-ITP patients. Twenty-five micrograms of platelet lysis were separated by 12% SDS-PAGE gels; transferred to a PVDF membrane; and immunoblotted with patient sera(lane P4-P7), normal sera(lane N1-N4), and mouse monoclonal Ab against platelet integrin GPIIIa (lane 1), respectively. (C) Blocking of IgG purified from SLE-ITP patients binding to GPIIIa49-66 peptide by dsDNA. Affinity-purified patient anti-dsDNA antibodies incubated with varying concentrations of dsDNA as indicated before ELISA.
Cross-reactivity of anti-GPIIIa49-66 antibody with dsDNA
Since sera from three SLE-ITP patients react with platelet integrin GPIIIa49-66, we next examined whether anti-GPIIIa49-66 antibodies can also react with dsDNA. Affinity-purified rabbit anti-GPIIIa49-66 polyclonal Abs react with both GPIIIa49-66 and dsDNA compared to BSA control (Figure 2A). In addition, anti-GPIIIa49-66 mAbs can cross-react with dsDNA, whereas control 13CG2 was not reactive (Figure 2B). We have previously reported that the binding activity of anti-GPIIIa49-66 mAbs to GPIIIa49-66 is mainly dependent on the positively charged amino acid in their HCDR3 region [35].
Figure 2.
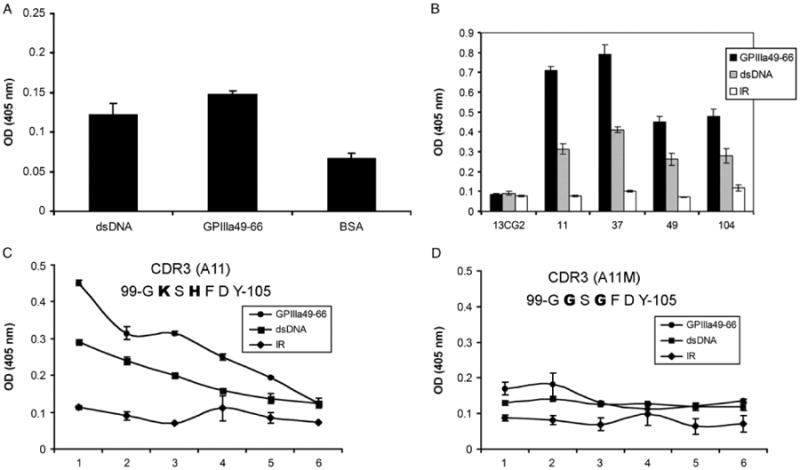
Cross-reactivity of anti-GPIIIa49-66 antibody with dsDNA. Binding activity of affinity-purified rabbit anti-GPIIIa49-66 pAbs (A) or anti-GPIIIa49-66 mAbs (B) with dsDNA measured by ELISA assay. Binding of A11 (C) and its mutant (D) to GPIIIa49-66 and dsDNA measured by ELISA assay. X-axis numbers 1–6 refer to serial doubling dilutions of 1:2 to 1:64, respectively (n = 3), SEM is given. IR refers to irrelevant peptide.
To check whether this is also the case with dsDNA, anti-GPIIIa49-66 mAb (A11) and its mutant were tested by ELISA. A11 reacts with both dsDNA and GPIIIa49-66 peptide (Figure 2C). In contrast, the binding activity of A11 mutant to dsDNA and GPIIIa49-66 peptide was significantly decreased when positively charged amino acid lysine (K104) and histidine (H102) in the HCDR3 were replaced by glycine (G) (Figure 2D). Thus, anti-GPIIIa49-66 antibodies cross-react with dsDNA and positively charged amino acids in HCDR3 are required.
Sequence comparison of CDR regions of anti-dsDNA and anti-GPIIIa49-66 antibodies
Since DNA binding activities of anti-GPIIIa49-66 antibodies are dependent on the positively charged amino acid in HCDR3, we compared the amino acid sequences of complementarity-determining region (CDR)2 and CDR3 in both anti-GPIIIa49-66 antibodies and anti-dsDNA antibodies reported by others and us [35,42]. Both anti-GPIIIa49-66 mAbs and anti-dsDNA mAbs have similar profile of positively charged amino acids in their CDR2 and CDR3 regions, suggesting the important role of positively charged amino acids in binding to both dsDNA and GPIIIa49-66 (Figure 3).
Figure 3.
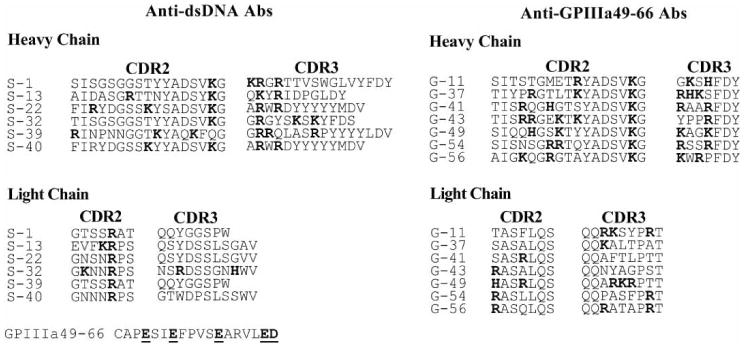
Sequence comparison of CDRs of anti-dsDNA and anti-GPIII49-66 antibody clones. Amino acid sequences of CDR2 and CDR3 of anti-dsDNA antibodies from SLE patients (S) and amino acid sequence of anti-GPIIIa49-66 antibodies generated in lab (G) were compared. The CDR2 and CDR3 alignments of both heavy and light chains are shown. Many positively charged amino acids (Arg, Lys, and His) were found in both the HCDRs and the LCDRs of two types of antibody clones.
Molecular modeling of anti-dsDNA mAb and anti-GPIIIa49-66 mAb
To examine the similarity of molecular structure between anti-dsDNA mAbs and anti-GPIIIa49-66 mAbs, we compared the molecular model of an anti-dsDNA mAb 412.67 and an anti-GPIIIa49-66 mAb A11. Indeed, anti-dsDNA mAb 412.67 shows a similar positively charged amino acid side chain orientation (K104 and R105) with anti-GPIIIa49-66 mAb A11 (K100 and H102) (Figure 4).
Figure 4.
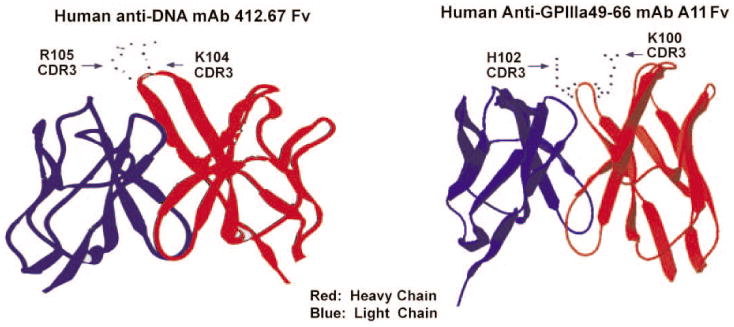
Molecular modeling of anti-dsDNA mAb 412.67 and anti-GPIIIa49-66 mAb A11.The heavy chain is in red and light chain in blue. Side chains of R105 and K104 in HCDR3 region of mAb 412.67 and side chains of H102 and K100 in HCDR3 region of mAb A11 are shown.
Binding assay of anti-dsDNA mAb with GPIIIa49-66 peptide
Since purified IgG from SLE-ITP patients is able to bind to GPIIIa49-66 and the binding activity can be inhibited by dsDNA, we next examined whether an anti-dsDNA mAb could cross-react with GPIIIa49-66. Figure 5 demonstrated that an anti-dsDNA mAb 412.67 from an SLE patient could react with GPIIIa49-66 peptide with a 50% decrease in binding comparing with dsDNA, and it was not reactive to irrelevant control peptide. This result further supports that GPIIIa49-66 could be a mimicry epitope of dsDNA on platelet.
Figure 5.
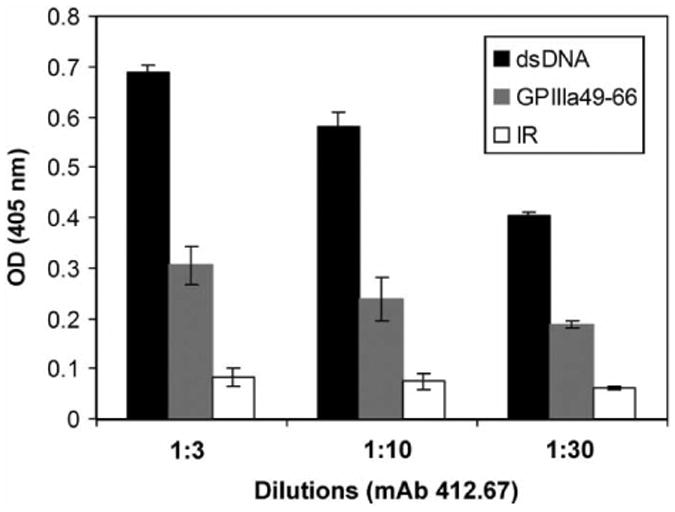
Binding assay of anti-dsDNA mAb 412.67 with GPIIIa49-66 peptide. Binding assay of anti-dsDNA mAb 412.67 with GPIIIa49-66 measured by ELISA as described in material and methods. 1:3, 1:10, 1:30 refer to serial dilutions of hybridoma supernatant, respectively (n = 3), SEM is given. IR refers to irrelevant peptide.
Effect of anti-GPIIIa49-66 antibodies from SLE-ITP patients on platelet fragmentation in vitro and in vivo
We have shown that sera from three out of nine SLE-ITP patients which contain antibodies could cross-react with both GPIIIa49-66 peptide and dsDNA. To further confirm that the anti-GPIIIa49-66 activity pathologically contributes to thrombocytopenia in SLE-ITP patient, we test the effect of anti-GPIIIa49-66 antibodies purified from these three patients on platelet fragmentation in vitro as well as inducing thrombocytopenia in vivo. We first examined whether platelet fragment and antibodies are present in immune complexes of patients with positive anti-GPIIIa49-66 activity to confirm that the platelet destruction is due to anti-GPIIIa autoantibodies. Indeed, platelet fragments were found in immune complex from patients P5, P6, and P7, but not from the three control subjects (Figure 6A). Further, affinity-purified anti-GPIIIa49-66 antibodies from patients P5, P6, and P7 induced platelet fragmentation in vitro (Figure 6B). Finally, although control IgG had no effect, affinity-purified anti-GPIIIa49-66 antibodies from patient P7 induced thrombocytopenia in Balb/c mice (Figure 6C). Thus, anti-GPIIIa49-66 antibodies in three SLE-ITP patients are likely involved in inducing thrombocytopenia.
Figure 6.
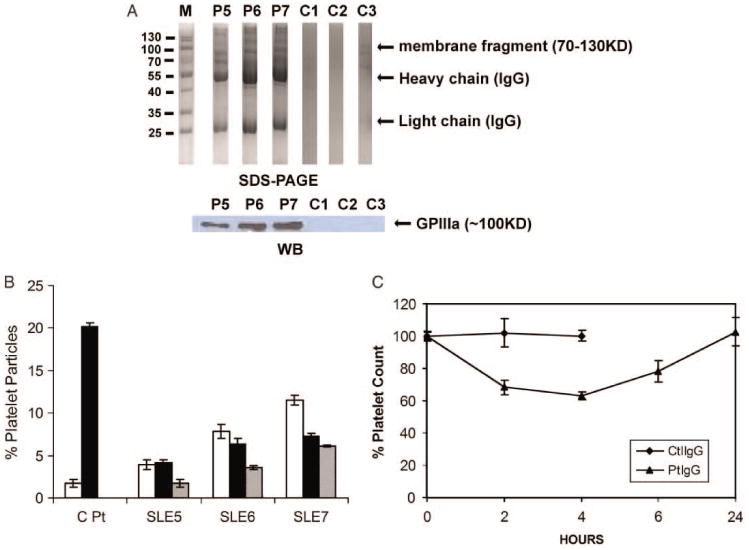
Effect of SLE-ITP patients Abs on platelet fragmentation in vitro and in vivo. (A) Presence of platelet fragments in SLE-ITP patients. Serum immune complexes from GPIIIa49-66 positive patients or healthy subjects run on 12% SDS-PAGE (upper) and immunoblotted with anti-GPIIIa49-66 antibody (lower). (B) Effect of affinity-purified anti-GPIIIa49-66 IgG from three SLE-ITP patients on platelet fragmentation in vitro. Pt refers to anti-GPIIIa49-66 Ab from HIV-ITP patients. White, dark, and gray bars refer to serial 1:1 dilutions of IgG, starting at 50 μg/ml, SEM is given. (C) Effect of affinity-purified anti-GPIIIa49-66 antibodies from Patient P7 on induction of thrombocytopenia in Balb/C mice. Purified control antibody (Ctl), patient anti-GPIIIa49-66 antibodies (50 μg) were injected intraperitoneally into Balb/C mice, and platelet counts followed for 24 h, n = 4, SEM is given.
Discussion
Anti-dsDNA autoantibodies have been reported to react with several proteins and non-protein substances [15,43]. The extensive cross-reactivity of anti-DNA autoantibodies could have implications for their pathogenic mechanism, although current findings suggested that not all anti-dsDNA antibodies are pathogenic [43]. It has been shown that high levels of anti-platelet glycoprotein antibodies, such as anti-GPIIb/IIIa and anti-GPIV, are common in SLE-ITP patients [9]. In addition, Jacob et al. reported that anti-dsDNA mAb (PME77) could cross-react with the membrane surface protein of platelets [18,20]. Consistent with these findings, we identified that a specific epitope GPIIIa49-66 with strong negative charges (CAPESIEFPVSEARVLED) reacts with anti-dsDNA antibody and may be involved in the development of SLE-ITP.
We showed here that purified anti-GPIIIa49-66 antibodies from SLE-ITP patients induce thrombocytopenia in vivo, and platelet fragments were found in the immune complexes in sera from these SLE-ITP patients. Thus, platelet destruction induced by anti-GPIIIa49-66 antibodies is not unique for HIV-1-ITP. Rather, this mechanism is likely to be involved in other immune complex-associated thrombocytopenia involved with serum immune complexes and anti-GPIIIa49-66 antibodies [44].
Basic amino acids in HCDR3 are crucial for dsDNA binding [43]. Since all four monoclonal anti-GPIIIa49-66 antibodies contain basic amino acids in their HCDR3 region, it is not a surprise finding that they all cross-react with dsDNA. We also found that sera from six out of nine SLE-ITP patients react with dsDNA but not with GPIIIa49-66, suggesting that some of the anti-dsDNA antibody may not be able to cross-react with GPIIIa49-66. Although both GPIIIa49-66 and dsDNA have a negatively charged surface, the structural difference between GPIIIa49-66 and dsDNA should be appreciated. In fact, we reported before that not only the positively charged amino acids are required for binding to GPIIIa49-66 but also the positions of these positively charged amino acids are important for GPIIIa49-66 binding [35]. In addition, light chain of anti-GPIIIa49-66 antibody also plays an important role in GPIIIa49-66 binding. Thus, while anti-GPIIIa 49-66 antibody can bind to DNA through positively charged amino acids in their HCDR3, some anti-dsDNA antibodies may not be able to bind to GPIIIa49-66 due to steric inhibition.
In these six SLE-ITP patients without anti-GPIIIa49-66 binding activity, it is most likely that thrombocytopenia can be caused by other mechanisms. There is a possibility that some anti-dsDNA antibodies may interact with other antigens on the surface of the platelet [15,43]. In addition, higher frequency of anti-GPIIb-IIIa antibodies in patients with antiphospholipid antibodies associated with thrombocytopenia was reported before [9]. Given the fact that antiphospholipid antibody is able to interact with GPIIIa [14], it is likely that antipho-spholipid antibodies frequently found in SLE can also contribute to the development of SLE. Nevertheless, in this study, we have identified a specific epitope GPIIIa49-66 that mimics dsDNA, and some anti-dsDNA antibodies can contribute to the development of SLE-ITP by cross-reacting with GPIIIa49-66.
Acknowledgments
We thank Dr Simon Karpatkin for his support and advice to initiate this work. This work is supported by NIH grants DA020816 and DA004315.
Footnotes
Declaration of interest: The authors declare no competing financial interests. The authors alone are responsible for the content and writing of the paper.
References
- 1.Atassi MZ, Casali P. Molecular mechanisms of autoimmunity. Autoimmunity. 2008;41:123–132. doi: 10.1080/08916930801929021. [DOI] [PubMed] [Google Scholar]
- 2.Zan H, Zhang J, Ardeshna S, Xu Z, Park SR, Casali P. Lupusprone MRL/faslpr/lpr mice display increased AID expression and extensive DNA lesions, comprising deletions and insertions, in the immunoglobulin locus: Concurrent upregulation of somatic hypermutation and class switch DNA recombination. Autoimmunity. 2009;42:89–103. doi: 10.1080/08916930802629554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crispin JC, Liossis SN, Kis-Toth K, Lieberman LA, Kyttaris VC, Juang YT, Tsokos GC. Pathogenesis of human systemic lupus erythematosus: Recent advances. Trends Mol Med. 2010;16:47–57. doi: 10.1016/j.molmed.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perl A. Pathogenic mechanisms in systemic lupus erythematosus. Autoimmunity. 2010;43:1–6. doi: 10.3109/08916930903374741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiraiwa A, Nugent DJ, Milner ECB. Sequence analysis of monoclonal antibodies derived from a patient with idiopathic thrombocytopenic purpura. Autoimmunity. 1990;8:107–113. doi: 10.3109/08916939008995728. [DOI] [PubMed] [Google Scholar]
- 6.Boumpas DT, Austin HA, III, Fessler BJ, Balow JE, Klippel JH, Lockshin MD. Systemic lupus erythematosus: Emerging concepts. Part 1: Renal, neuropsychiatric, cardiovascular, pulmonary, and hematologic disease. Ann Intern Med. 1995;122:940–950. doi: 10.7326/0003-4819-122-12-199506150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Ziakas PD, Routsias JG, Giannouli S, Tasidou A, Tzioufas AG, Voulgarelis M. Suspects in the tale of lupus-associated thrombocytopenia. Clin Exp Immunol. 2006;145:71–80. doi: 10.1111/j.1365-2249.2006.03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziakas PD, Poulou LS, Giannouli S, Tzioufas AG, Voulgarelis M. Thrombocytopenia in lupus: Baseline C3 as an independent risk factor for relapse. Ann Rheum Dis. 2007;66:130–131. doi: 10.1136/ard.2006.059758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macchi L, Rispal P, Clofent-Sanchez G, Pellegrin JL, Nurden P, Leng B, Nurden AT. Anti-platelet antibodies in patients with systemic lupus erythematosus and the primary antiphospholipid antibody syndrome: Their relationship with the observed thrombocytopenia. Br J Haematol. 1997;98:336–341. doi: 10.1046/j.1365-2141.1997.2243038.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuwana M, Okazaki Y, Kajihara M, Kaburaki J, Miyazaki H, Kawakami Y, Ikeda Y. Autoantibody to c-Mpl (thrombopoietin receptor) in systemic lupus erythematosus: Relationship to thrombocytopenia with megakaryocytic hypoplasia. Arthritis Rheum. 2002;46:2148–2159. doi: 10.1002/art.10420. [DOI] [PubMed] [Google Scholar]
- 11.McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoantibodies from adult patients with chronic ITP. Blood. 2004;103:1364–1369. doi: 10.1182/blood-2003-08-2672. [DOI] [PubMed] [Google Scholar]
- 12.Gernsheimer T. Chronic idiopathic thrombocytopenic purpura: Mechanisms of pathogenesis. Oncologist. 2009;14:12–21. doi: 10.1634/theoncologist.2008-0132. [DOI] [PubMed] [Google Scholar]
- 13.Galli M, Daldossi M, Barbui T. Anti-glycoprotein Ib/IX and IIb/IIIa antibodies in patients with antiphospholipid antibodies. Thromb Haemost. 1994;71:571–575. [PubMed] [Google Scholar]
- 14.Tokita S, Arai M, Yamamoto N, Katagiri Y, Tanoue K, Igarashi K, Umeda M, Inoue K. Specific cross-reaction of IgG anti-phospholipid antibody with platelet glycoprotein IIIa. Thromb Haemost. 1996;75:168–174. [PubMed] [Google Scholar]
- 15.Mageed RA, Zack DJ. Cross-reactivity and pathogenicity of anti-DNA autoantibodies in systemic lupus erythematosus. Lupus. 2002;11:783–786. doi: 10.1191/0961203302lu317oa. [DOI] [PubMed] [Google Scholar]
- 16.Dighiero G, Guilbert B, Fermand JP, Lymberi P, Danon F, Avrameas S. Thirty-six human monoclonal immunoglobulins with antibody activity against cytoskeleton proteins, thyroglobulin, and native DNA: Immunologic studies and clinical correlations. Blood. 1983;62:264–270. [PubMed] [Google Scholar]
- 17.Andre-Schwartz J, Datta SK, Shoenfeld Y, Isenberg DA, Stollar BD, Schwartz RS. Binding of cytoskeletal proteins by monoclonal anti-DNA lupus autoantibodies. Clin Immunol Immunopathol. 1984;31:261–271. doi: 10.1016/0090-1229(84)90246-0. [DOI] [PubMed] [Google Scholar]
- 18.Jacob L, Lety MA, Louvard D, Bach JF. Binding of a monoclonal anti-DNA autoantibody to identical protein(s) present at the surface of several human cell types involved in lupus pathogenesis. J Clin Invest. 1985;75:315–317. doi: 10.1172/JCI111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pisetsky DS, Hoch SO, Klatt CL, O'Donnell MA, Keene JD. Specificity and idiotypic analysis of a monoclonal anti-Sm antibody with anti-DNA activity. J Immunol. 1985;135:4080–4085. [PubMed] [Google Scholar]
- 20.Jacob L, Lety MA, Bach JF, Louvard D. Human systemic lupus erythematosus sera contain antibodies against cell-surface protein(s) that share(s) epitope(s) with DNA. Proc Natl Acad Sci USA. 1986;83:6970–6974. doi: 10.1073/pnas.83.18.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ternynck T, Avrameas S. Murine natural monoclonal autoantibodies: A study of their polyspecificities and their affinities. Immunol Rev. 1986;94:99–112. doi: 10.1111/j.1600-065x.1986.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 22.Raz E, Brezis M, Rosenmann E, Eilat D. Anti-DNA antibodies bind directly to renal antigens and induce kidney dysfunction in the isolated perfused rat kidney. J Immunol. 1989;142:3076–3082. [PubMed] [Google Scholar]
- 23.Berneman A, Ternynck T, Avrameas S. Natural mouse IgG reacts with self antigens including molecules involved in the immune response. Eur J Immunol. 1992;22:625–633. doi: 10.1002/eji.1830220303. [DOI] [PubMed] [Google Scholar]
- 24.Banks TA, Babakhani F, Poulos BT, Duffy JJ, Kibler R. Characterization of cross-reactive anti-DNA autoantibodies in murine lupus. Autoimmunity. 1993;22:229–248. doi: 10.3109/08820139309063405. [DOI] [PubMed] [Google Scholar]
- 25.Reichlin M, Martin A, Taylor-Albert E, Tsuzaka K, Zhang W, Reichlin MW, Koren E, Ebling FM, Tsao B, Hahn BH. Lupus autoantibodies to native DNA cross-react with the A and D SnRNP polypeptides. J Clin Invest. 1994;93:443–449. doi: 10.1172/JCI116980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du H, Chen M, Zhang Y, Zhao MH, Wang HY. Cross-reaction of anti-DNA autoantibodies with membrane proteins of human glomerular mesangial cells in sera from patients with lupus nephritis. Clin Exp Immunol. 2006;145:21–27. doi: 10.1111/j.1365-2249.2006.03102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elkon K, Casali P. Nature and functions of autoantibodies. Nat Rev Rheumatol. 2008;4:491–498. doi: 10.1038/ncprheum0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putterman C, Diamond B. Immunization with a peptide surrogate for double-stranded DNA (dsDNA) induces autoantibody production and renal immunoglobulin deposition. J Exp Med. 1998;188:29–38. doi: 10.1084/jem.188.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice JS, Kowal C, Volpe BT, DeGiorgio LA, Diamond B. Molecular mimicry: Anti-DNA antibodies bind microbial and nonnucleic acid self-antigens. Curr Top Microbiol Immunol. 2005;296:137–151. doi: 10.1007/3-540-30791-5_8. [DOI] [PubMed] [Google Scholar]
- 30.Karpatkin S, Nardi MA, Hymes KB. Sequestration of anti-platelet GPIIIa antibody in rheumatoid factor immune complexes of human immunodeficiency virus 1 thrombocytopenic patients. Proc Natl Acad Sci USA. 1995;92:2263–2267. doi: 10.1073/pnas.92.6.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nardi MA, Liu LX, Karpatkin S. GPIIIa-(49-66) is a major pathophysiologically relevant antigenic determinant for anti-platelet GPIIIa of HIV-1-related immunologic thrombocytopenia. Proc Natl Acad Sci USA. 1997;94:7589–7594. doi: 10.1073/pnas.94.14.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nardi M, Tomlinson S, Greco MA, Karpatkin S. Complement-independent, peroxide-induced antibody lysis of platelets in HIV-1-related immune thrombocytopenia. Cell. 2001;106:551–561. doi: 10.1016/s0092-8674(01)00477-9. [DOI] [PubMed] [Google Scholar]
- 33.Nardi M, Feinmark SJ, Hu L, Li Z, Karpatkin S. Complement-independent Ab-induced peroxide lysis of platelets requires 12-lipoxygenase and a platelet NADPH oxidase pathway. J Clin Invest. 2004;113:973–980. doi: 10.1172/JCI20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Nardi MA, Karpatkin S. Role of molecular mimicry to HIV-1 peptides in HIV-1-related immunologic thrombocytopenia. Blood. 2005;106:572–576. doi: 10.1182/blood-2005-01-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Nardi MA, Wu J, Pan R, Zhang W, Karpatkin S. Platelet fragmentation requires a specific structural conformation of human monoclonal antibody against beta3 integrin. J Biol Chem. 2008;283:3224–3230. doi: 10.1074/jbc.M705902200. [DOI] [PubMed] [Google Scholar]
- 36.Kasaian MT, Ikematsu H, Balow JE, Casali P. Structure of the VH and VL segments of monoreactive and polyreactive IgA autoantibodies to DNA in patients with systemic lupus erythematosus. J Immunol. 1994;152:3137–3151. [PMC free article] [PubMed] [Google Scholar]
- 37.Kasaian MT, Casali P. B-1 cellular origin and VH segment structure of IgG, IgA, and IgM anti-DNA autoantibodies in patients with systemic lupus erythematosus. Ann N Y Acad Sci. 1995;764:410–423. doi: 10.1111/j.1749-6632.1995.tb55856.x. [DOI] [PubMed] [Google Scholar]
- 38.Li Z, Schettino EW, Padlan EA, Ikematsu H, Casali P. Structure-function analysis of a lupus anti-DNA autoantibody: Central role of the heavy chain complementarity-determining region 3 Arg in binding of double- and single-stranded DNA. Eur J Immunol. 2000;30:2015–2026. doi: 10.1002/1521-4141(200007)30:7<2015::AID-IMMU2015>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levin RE, Weinstein A, Peterson M, Testa MA, Rothfield NF. A comparison of the sensitivity of the 1971 and 1982 American Rheumatism Association criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1984;27:530–538. doi: 10.1002/art.1780270508. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Fong KY, Chung MC, Yao ZJ. Peptide mimicking antigenic and immunogenic epitope of double-stranded DNA in systemic lupus erythematosus. Int Immunol. 2001;13:223–232. doi: 10.1093/intimm/13.2.223. [DOI] [PubMed] [Google Scholar]
- 41.Ehrenstein MR, Swana M, Keeling D, Asherson R, Hughes GR, Isenberg DA. Anti-DNA antibodies in the primary antiphospholipid syndrome (PAPS) Br J Rheumatol. 1993;32:362–365. doi: 10.1093/rheumatology/32.5.362. [DOI] [PubMed] [Google Scholar]
- 42.Barbas SM, Ditzel HJ, Salonen EM, Yang WP, Silverman GJ, Burton DR. Human autoantibody recognition of DNA. Proc Natl Acad Sci USA. 1995;92:2529–2533. doi: 10.1073/pnas.92.7.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jang YJ, Stollar BD. Anti-DNA antibodies: Aspects of structure and pathogenicity. Cell Mol Life Sci. 2003;60:309–320. doi: 10.1007/s000180300026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel H, Nardi M, Karpatkin M, Hart D, Belmont M, Karpatkin S. Differentiation of autoimmune thrombocytopenia from thrombocytopenia associated with immune complex disease: Systemic lupus erythematosus, hepatitis-cirrhosis, and HIV-1 infection by platelet and serum immunological measurements. Br J Haematol. 1999;105:1086–1091. doi: 10.1046/j.1365-2141.1999.01469.x. [DOI] [PubMed] [Google Scholar]


