Abstract
Nucleophilic substitution reactions of acetals with benzyloxy groups four carbon atoms away can be highly diastereoselective. The selectivity in several cases increased as the reactivity of the nucleophile increased, in violation of the reactivity-selectivity principle. The increase in selectivity with reactivity suggests that multiple conformational isomers of reactive intermediates can give rise to the products.
Keywords: nucleophilic substitution, carbocations, synthetic methods, through-space interactions, allylation
Neighboring-group participation[1] is a powerful strategy for controlling the stereochemical outcomes of acetal substitution reactions. This approach is used extensively in carbohydrate chemistry, where a substituent at the 2-position, usually an acyloxy group, controls the stereochemical configuration of substitution.[2-4] An acyloxy group can form a fused ring system resembling 1 (Figure 1), and opening of the five-membered ring by a nucleophile installs the nucleophile trans to the participating group.[2,5] Acyloxy groups at remote positions can also exert influences on stereoselectivity.[6-10] By contrast, participation by an alkoxy group via an oxonium ion is not generally considered to occur.[10-15]
Figure 1.
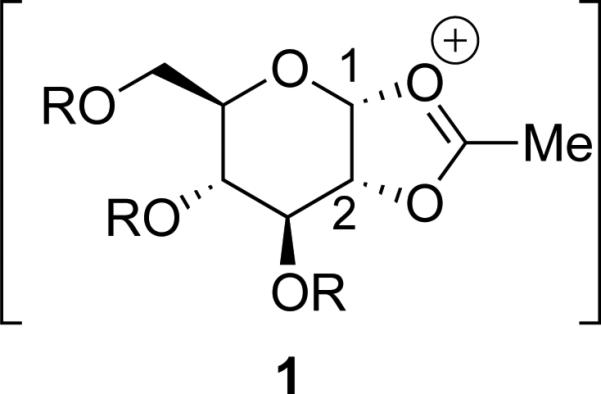
Participation by an acetoxy group at C-2 of a carbohydrate.
In this Communication, we demonstrate that an alkoxy group, positioned to form a five-membered ring as in oxonium ion 1, can control the stereochemical course of nucleophilic substitution reactions of an acetal.[16-18] These reactions exhibit an atypical trend for acetal substitution reactions:[19-20] as the reactivity of the nucleophile increased, selectivity increased, in direct opposition to the “reactivity-selectivity principle,” a frequently misapplied concept.[21] We provide evidence that selectivity can increase as reactivity increases if the product can be formed by reactions of multiple reactive intermediates.
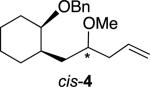
Consistent with their ability to accelerate ionization of acetals,[22-23] alkoxy groups exert considerable control on the stereoselectivity of nucleophilic substitution reactions of acetals. Treatment of alkoxy-substituted acetal trans-2 with allyltrimethylsilane (3) and a Lewis acid gave the product trans-4 with high selectivity [Eq. (1)]. The yield was highest using BF3·OEt2 as the Lewis acid in CH2Cl2, although the use of other Lewis acids (TiCl4, SnCl4, Me3SiOTf) and solvents (toluene, MeCN) gave similar results.
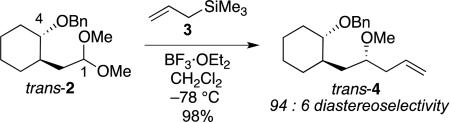 |
(1) |
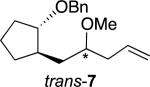
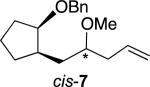
The allylations of related acetals proceeded with a range of selectivities. Table 1 reports those stereoselectivities and the relative rates of hydrolysis of the acetals, which indicate the extent of interaction of the benzyloxy group with the acetal during ionization. The stereochemical configuration of alkenes trans-4 and 6 were established by X-ray crystallographic analysis of derivatives,[24] but all efforts to obtain crystalline products from alkene 5 were unsuccessful. The assignment of stereochemistry for alkene 5 was made by analogy to alkenes trans-4 and 6, so it is tentative. The stereochemical configurations of the other products were not assigned.
Table 1.
Diastereoselectivities for acetal substitution reactions similar to Eq. (1).
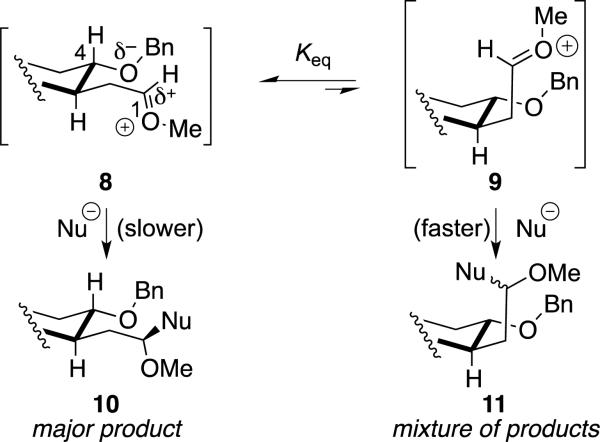
The results presented in Table 1 can be understood by considering the structures of the relevant oxocarbenium ion intermediates (8, Scheme 1). The alkoxy group at C-4, which bears partial negative charge, would approach the partially positively charged carbon atom (C-1) in this cation.[25] The substrates that showed close interactions between the benzyloxy group and the acetal carbon atom based upon their rates of ionization[22-23] would position those two groups relatively close to each other. Nucleophilic attack on 8 from the more accessible front face would lead to the major product (10).[26]
Scheme 1.
Origin of stereoselectivity with weaker nucleophiles.
Selectivity of substitution would depend upon how much the benzyloxy group stabilized the oxocarbenium ion. If there were little interaction between the benzyloxy group and the acetal carbon atom, the oxocarbenium ion would be far from the benzyloxy group, so little facial discrimination would be expected.[27] The low selectivities for the formation of alkenes cis-4, trans-7, and cis-7 result from that relatively small interaction.[22-23]
The observation of lower selectivity when the interaction between the two groups was large, as observed for the acetals leading to products 6 and 7, can be explained by considering that the product is formed from more than one reactive intermediate. The stabilized form of the oxocarbenium ion (8) would react more slowly with nucleophiles than a less stabilized form,[29] so reaction could occur through higher energy conformational isomers of the oxocarbenium ion,[30] in accord with the Curtin–Hammett principle.[31] Those conformational isomers in which electrostatic stabilization is weaker, such as 9, would react more rapidly, and because the benzyloxy groups and oxocarbenium ion are further apart in these conformers, the faces of the cation are not as well differentiated.
Table 2 and Eq. (2) indicate that substitution reactions can be highly diastereoselective for alkoxy-substituted acetals.[32] The major stereoisomers in all cases possess the same stereochemical configurations as the products shown in Table 1.[24] The general increase in selectivity as the reactivity of the nucleophile increases is opposite to what has been observed in systematic studies of other acetal substitution reactions,[19-20] although some substitutions exhibit similar patterns.[27,33-35]
 |
(2) |
Table 2.
| Nu–M | N b | 14 | 15 | 16 |
|---|---|---|---|---|

|
1.8 | 94 : 6 | 87 : 13 | 82 : 18 |
|
3.8 | – | 96 : 4 | 96 : 4 |

|
4.4 | 97 : 3 | ≥97 : 3 | ≥97 : 3 |
|
5.5 | ≥ 97 : 3 | ≥97 : 3 | 94 : 6 |

|
6.2 | ≥97 : 3 | ≥97 : 3 | ≥97 : 3 |
Determined by 1H NMR spectroscopy. Isolated yields are 60-90%.
Higher nucleophilicity parameters (N) correspond to more reactive nucleophiles.[29]
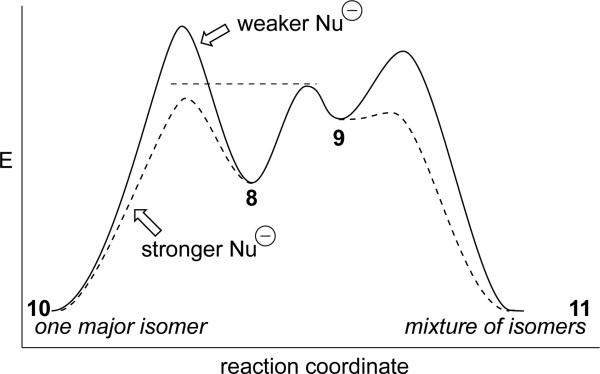
The trend of higher selectivity with increased reactivity of the nucleophile is consistent with a kinetic scenario where two forms of a reactive intermediate can lead to product (Scheme 2).[31] With weak nucleophiles, reaction with the more reactive conformer (cation 9 in Scheme 1) would be faster than reactions with the more stabilized conformer 8 (solid line in Scheme 2). When strong nucleophiles are used (dashed line), however, the more stabilized intermediate, oxocarbenium ion 8, could react with the nucleophile directly. The major conformer 8, as noted above, exhibits better discrimination between the two diastereotopic faces of the electrophile than the higher energy conformer 9, so selectivity would increase with increased reactivity.
Scheme 2.
Reaction coordinate diagram illustrating how selectivity could increase with reactivity. Compound numbers refer to Scheme 1.
Additional studies using a highly reactive nucleophile also suggest that the products would be formed from multiple reactive intermediates. Substitution reactions with methallyltributylstannane (21)[29] resulted in diverging stereoselectivities. Whereas the stereoselectivity with the cyclohexane-derived acetal trans-2 decreased, the selectivity for the eight-membered ring acetal 13 remained high [Eq. (3)].
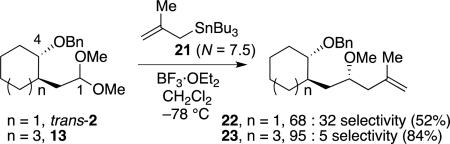 |
(3) |
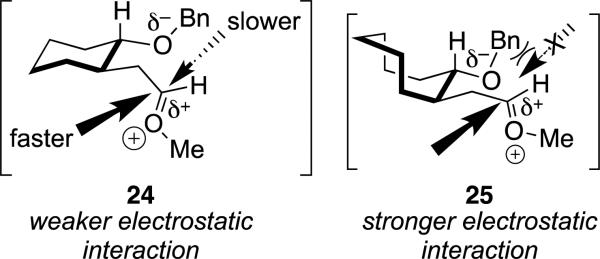
The difference in selectivity shown in Eq. (3) can be understood by considering how an oxocarbenium ion's stability influences its reactivity. With a highly reactive nucleophile, the products would be formed exclusively from the lower-energy oxocarbenium ion (Scheme 2). The cyclohexane-derived oxocarbenium ion 24 involves weaker interaction between the benzyloxy group and the cationic carbon atom compared to the cyclooctane-derived ion 25 (Scheme 3). Computational studies on these oxocarbenium ions reveal that the distance between the oxygen atom of the benzyloxy group and the cationic carbon atom are longer (1.99 Å) for the cyclohexane-derived oxocarbenium ion 24 than for the cyclooctane-derived cation 25 (1.61 Å).[23] As a result, the back face of the oxocarbenium ion 24 is accessible, although attack from this face should be slower because it is more sterically hindered. As the rates of nucleophilic attack on the oxocarbenium ion 24 increase with strong nucleophiles,[19] selectivity should decrease because the rate of addition from both faces would increase asymptotically to the diffusion rate limit.[21] By contrast, the back face of eight-membered ring oxocarbenium ion 25 is much more sterically hindered because of the close interaction between the benzyloxy group and the oxocarbenium ion carbon atom, so attack from this face would be prohibitively slow. The increased stabilization of oxocarbenium ion 25 relative to cation 24 also suggest that the rate of addition would remain below the diffusion rate limit, so reactions would continue to be selective even with highly reactive nucleophiles.
Scheme 3.
Stabilization of the oxocarbenium ion and consequences for stereoselectivity.
In conclusion, an alkoxy group can control the stereochemical course of substitution reactions of acetals, in many cases with ≥97% diastereoselectivity. Electrostatic stabilization is strong enough to form a structure that can be trapped by nucleophiles to give products with high diastereoselectivity. The observation of increased selectivity with increased reactivity suggests that the products were formed from multiple conformational isomers of the reactive intermediates, leading to inverse correlations of reactivity and selectivity.
Supplementary Material
Acknowledgements
This research was supported by the National Institutes of Health, National Institute of General Medical Sciences (GM-61066). We thank Olga Lavinda for assistance with computations studies, Dr. Chin Lin for help with NMR spectroscopy and mass spectrometric data, and Dr. Chunhua Hu for his assistance with crystallographic structure determination.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- 1.Capon B. Q. Rev. Chem. Soc. 1964;18:45–111. [Google Scholar]
- 2.Guo J, Ye X-S. Molecules. 2010;15:7235–7265. doi: 10.3390/molecules15107235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D'Souza FW, Cheshev PE, Ayers JD, Lowary TL. J. Org. Chem. 1998;63:9037–9044. [Google Scholar]
- 4.Castro S, Fyvie WS, Hatcher SA, Peczuh MW. Org. Lett. 2005;7:4709–4712. doi: 10.1021/ol051912e. [DOI] [PubMed] [Google Scholar]
- 5.Whitfield DM, Nukada T. Carbohydr. Res. 2007;342:1291–1304. doi: 10.1016/j.carres.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Demchenko AV, Rousson E, Boons G-J. Tetrahedron Lett. 1999;40:6523–6526. [Google Scholar]
- 7.Fan E, Shi W, Lowary TL. J. Org. Chem. 2007;72:2917–2928. doi: 10.1021/jo062542q. [DOI] [PubMed] [Google Scholar]
- 8.Yang F, Zhu Y, Yu B. Chem. Commun. 2012;48:7097–7099. doi: 10.1039/c2cc33155a. [DOI] [PubMed] [Google Scholar]
- 9.Ngoje G, Li Z. Org. Biomol. Chem. 2013;11:1879–1886. doi: 10.1039/c3ob26994a. [DOI] [PubMed] [Google Scholar]
- 10.Zeng Y, Ning J, Kong F. Carbohydr. Res. 2003;338:307–311. doi: 10.1016/s0008-6215(02)00455-x. [DOI] [PubMed] [Google Scholar]
- 11.Fascione MA, Adshead SJ, Stalford SA, Kilner CA, Leach AG, Turnbull WB. Chem. Commun. 2009:5841–5843. doi: 10.1039/b913308a. [DOI] [PubMed] [Google Scholar]
- 12.Tosin M, Murphy PV. Org. Lett. 2002;4:3675–3678. doi: 10.1021/ol026629j. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta F, Garegg PJ. Carbohydr. Res. 1990;202:225–238. doi: 10.1016/0008-6215(90)84082-6. [DOI] [PubMed] [Google Scholar]
- 14.Demchenko AV. Synlett. 2003:1225–1240. [Google Scholar]
- 15.4-Alkoxy-substituted acetals undergo stereoselective reactions in some cases using Lewis acids such as Me3SiOTf and nucleophiles such as Me3SiCN. These reactions are proposed to proceed via oxonium ions: Molander GA, Haar JP. J. Am. Chem. Soc. 1993;115:40–49.
- 16.Bartlett PA, Johnson WS, Elliott JD. J. Am. Chem. Soc. 1983;105:2088–2089. [Google Scholar]
- 17.Denmark SE, Almstead NG. J. Am. Chem. Soc. 1991;113:8089–8110. [Google Scholar]
- 18.Sammakia T, Smith RS. J. Am. Chem. Soc. 1994;116:7915–7916. [Google Scholar]
- 19.Krumper JR, Salamant WA, Woerpel KA. Org. Lett. 2008;10:4907–4910. doi: 10.1021/ol8019956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaver MG, Woerpel KA. J. Org. Chem. 2010;75:1107–1118. doi: 10.1021/jo902222a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayr H, Ofial AR. Angew. Chem. Int. Ed. 2006;45:1844–1854. doi: 10.1002/anie.200503273. [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006;118:1876–1886. [Google Scholar]
- 22.Garcia A, Otte DAL, Salamant WA, Sanzone JR, Woerpel KA. Angew. Chem. Int. Ed. 2015;54:3061–3064. doi: 10.1002/anie.201410043. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2015;127:3104–3107. [Google Scholar]
- 23.Garcia A, Otte DAL, Salamant WA, Sanzone JR, Woerpel KA. J. Org. Chem. 2015;80:4470–4480. doi: 10.1021/acs.joc.5b00338. [DOI] [PubMed] [Google Scholar]
- 24.Details are provided as supporting information [Google Scholar]
- 25.Woods RJ, Andrews CW, Bowen JP. J. Am. Chem. Soc. 1992;114:859–864. [Google Scholar]
- 26.The six-membered version of oxocarbenium ion 8 (i.e., 24 in Scheme 3) was calculated (B3LYP/6-31G*, reference 23) to be lower in energy (0.4 kcal/mol) than a conformer that exposes the other face of the oxocarbenium ion to nucleophilic attack, likely because it minimizes steric interactions between the OMe group and the OBn group.
- 27.Denmark SE, Almstead NG. J. Org. Chem. 1991;56:6485–6487. [Google Scholar]
- 28.Otte DAL, Borchmann DE, Lin C, Weck M, Woerpel KA. Org. Lett. 2014;16:1656–1659. doi: 10.1021/ol403776k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayr H, Kempf B, Ofial AR. Acc. Chem. Res. 2003;36:66–77. doi: 10.1021/ar020094c. [DOI] [PubMed] [Google Scholar]
- 30.Beaver MG, Billings SB, Woerpel KA. J. Am. Chem. Soc. 2008;130:2082–2086. doi: 10.1021/ja0767783. [DOI] [PubMed] [Google Scholar]
- 31.Seeman JI. Chem. Rev. 1983;83:83–134. [Google Scholar]
- 32.As with acetal hydrolysis reactions (references 22-23), no debenzylated products were observed in any reaction mixtures, indicating that oxonium ions were not likely to be reactive intermediates: Chao C-S, Lin C-Y, Mulani S, Hung W-C, Mong K.-k. T. Chem. Eur. J. 2011;17:12193–12202. doi: 10.1002/chem.201100732.
- 33.Minehan TG, Kishi Y. Tetrahedron Lett. 1997;38:6815–6818. [Google Scholar]
- 34.Štimac A, Kobe J. Carbohydr. Res. 2000;324:149–160. doi: 10.1016/s0008-6215(99)00293-1. [DOI] [PubMed] [Google Scholar]
- 35.Friestad GK, Lee HJ. Org. Lett. 2009;11:3958–3961. doi: 10.1021/ol901613k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.