Abstract
Despite recent advances in blood-brain barrier (BBB) research, it remains a significant hurdle for the pharmaceutical treatment of brain diseases. Focused ultrasound (FUS) is one method to transiently increase permeability of the BBB to promote drug delivery to specific brain regions. An introduction to the BBB and a brief overview of the methods which can be used to circumvent the BBB to promote drug delivery is provided. In particular, we discuss the advantages and limitations of FUS technology and the efficacy of FUS-mediated drug delivery in models of disease. MRI for targeting and evaluating FUS treatments, combined with administration of microbubbles, allows for transient, reproducible BBB opening. The integration of a real-time acoustic feedback controller has improved treatment safety. Successful clinical translation of FUS has the potential to transform the treatment of brain disease worldwide without requiring the development of new pharmaceutical agents.
Keywords: Blood-brain barrier, drug delivery, focused ultrasound, neurodegenerative disease
2. Introduction to the Blood-Brain Barrier (BBB)
The exchange of water, ions, nutrients, waste, and other substances, between the plasma, interstitial fluid and intercellular components of the body's organs occurs by a combination of diffusion, filtration, osmosis and active transport [1,2]. However, exchange from the blood to the interstitial fluid of the brain is remarkably different due to the inherent differences between the peripheral and central blood vessels. In peripheral organs, the blood vessels are ‘leaky’ and allow passage of nutrients into the organs [3]. In the central nervous system, the blood vessels are separated from the brain by a specialized, structure known as the blood brain barrier (BBB). The BBB is formed by brain-specific endothelial cells and supported by the cells of the neurovascular unit to limit the passage of polar molecules or large molecules such as proteins and peptides into or out of the brain interstitium [4,5]. The endothelial cells of the BBB have developed specific transporter systems to still allow the controlled transport of necessary peptides and proteins, even under pathological conditions [6,7,8]. The BBB functions to maintain the delicate environment required for proper neuronal function however it also serves as a serious impediment to the pharmaceutical treatment of brain diseases. This review will provide an overview of the structure and function of the BBB as well as the current approaches to overcoming the BBB for treatment of neurodegenerative disease, psychiatric illnesses and brain cancers.
a. History of the BBB
In the late 1800's, scientist Paul Ehrlich observed that intravenous injection of dyes such as Trypan blue, stained all of the organs except for the brain and spinal cord [9,10,11]. These experiments were followed up with studies by Max Lewandowsky and colleagues who showed that neurotoxic agents only affected brain function when they were injected directly into the brain. The same neurotoxic agents did not impact the brain when delivered through the vasculature [11,13]. Decades later, one of Erlich's associates demonstrated that when the same dyes were injected directly into the cerebrospinal fluid, only the brain and spinal cord became stained, thereby leading to the adoption of the term the blood-brain barrier [11,14].
The confirmation that the BBB was more than a mechanical barrier was first realized in 1967 when it was reported that the BBB is comprised of the capillary endothelial cells [15]. Using horseradish peroxidase as a tracer, the authors demonstrated that the peroxidase was unable to bypass the endothelial cells due to the presence of continuous tight junctions between adjacent endothelial cells. They also indicated that there were considerably fewer transport vesicles in the brain endothelial cells compared to other tissues. These findings now make up two of the main features of the BBB:
-
1)
presence of tight junctions which limit paracellular transport
-
2)
reduced fenestrations and transport vesicles which limit transcellular transport.
The experiments by Reese and Karnovsky were the first to define the unique anatomical features of the brain endothelial cells, which support their function as the blood-brain barrier. These histological findings combined with early physiological studies [16,17] changed the concept of the BBB from an impenetrable mechanical barrier to a dynamic structure that actively transported ions and nutrients to maintain homeostasis throughout the brain [17].
b. Structure and Function of the BBB
Endothelial cells line the blood vessels throughout the body and they support the free exchange of solutes. However, as the vessels enter the CNS, the endothelial cells form a continuous barrier connected by tight junction proteins, which limits the exchange of solutes to lipophilic molecules which are less than 400Da [18]. The tight junctions contain transmembrane proteins including occludin, claudins and junctional adhesion molecule (JAM), which form structural homodimers with adjacent cells. The importance of these proteins to the function of the BBB is varied. Occludin is a protein found exclusively in tight junctions. However, occludin knockout mice develop a normal BBB, suggesting that the brain can compensate for the loss of occludin and that the protein is not necessary for proper barrier formation [19]. On the contrary, claudin 5 deficient mice have a barrier that appears structurally normal however there are significant functional deficits likely contributing to death of the animals as neonates [20]. The role of JAM in development and maintenance of the BBB is not yet understood.
The tight junctions are strengthened by the anchoring of the transmembrane proteins to intracellular protein networks such as zonula occludens-1 (ZO-1), ensuring that strong intercellular connections inhibit paracellular transport [11].
In addition to intercellular connections, the endothelial cells have reduced fenestrations and many fewer vesicles, which leads to less transcytosis. Instead, molecules that are necessary for brain function are transported using specific transport systems.
The brain demands approximately 20% of the body's total supply of glucose daily [21]. Therefore, though the BBB excludes blood cells and plasma from the brain, other necessary nutrients, such as glucose and essential amino acids must be transported in using specific carrier proteins. The endothelial cells are rich in the glucose transport protein (GLUT-1) to ensure adequate glucose is transported across the barrier. Similarly, the essential amino acids are transported by the common receptors L1 and y+ [11]. Pumps on the luminal side of the BBB actively transport amino acids back into the circulation in order to maintain a steady concentration. Furthermore, a group of ABC efflux transporters embedded in the endothelial cells, efficiently removes drugs from the brain even if they are able to pass the BBB. Among these ABC transporters are p-glycoprotein, multidrug resistance-associated protein and breast cancer resistance protein [22].
In addition to carrier proteins, the BBB also has ion transporters to control the relative concentrations of Na+, K+ and Cl− ions across the barrier, receptors for transport via caveloae dependent endocytosis and active efflux mechanisms to ensure that any toxic molecules that do pass are shuttled back.
c. BBB Development
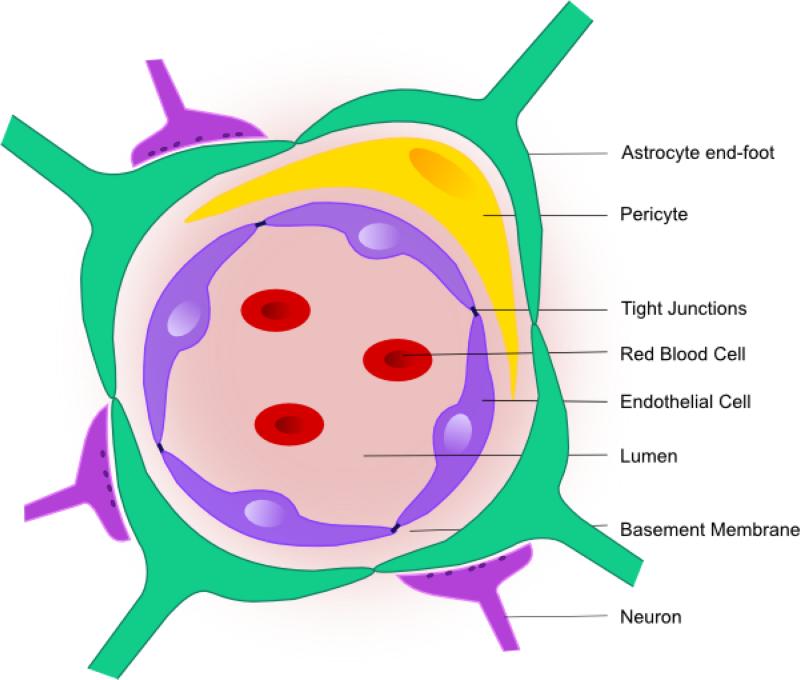
Development of a functional BBB is evident even in the early stages of embryonic brain development. Recent studies have suggested that the barrier is functional in a mouse by E15.5 [23]. The signaling pathways implicated in the development of the BBB were recently reviewed by Obermeier and colleagues [24] so instead, here we will focus on the cellular support required for proper development of the BBB. Although the endothelial cells are the main component of the BBB, the development of the BBB is governed partly by the interaction of endothelial cells with the neurovascular unit including astrocytes, pericytes and the basal lamina (Figure 1).
Figure 1. The blood-brain barrier (BBB).
The BBB is composed of endothelial cells which are connected via tight junctions to limit transport across the BBB. The endothelial cells are supported by components of the neurovascular unit which help to regulate permeability, nutrient exchange and homeostasis. These components include pericytes, astrocytes, neurons as well as the basement membrane.
Astrocytes are important for regulating the neuronal environmental milieu and they contribute to BBB regulation of water via the abundant aquaporin 4 (AQP4) channels which are present in their perivascular endfeet [25]. In addition, astrocytes have long been suggested to be crucial for proper development of a functional BBB. Original co-culture experiments demonstrated that brain endothelial cells exhibited barrier-like properties when cultured with astrocytes or astrocyte conditioned media [26]. It was suggested that astrocytes secreted soluble factors important for barrier-forming properties of the endothelial cells [27]. More recently, it has been shown that astrocytes are important to the maintenance of the BBB rather than the development. Astrocyte signaling can upregulate the expression of tight junction proteins as well as participate in the post-translational modification of these proteins [28,29]. As well, production of laminin by astrocytes is required for maintaining BBB integrity and knockout of the astrocytic laminin specifically leads to BBB breakdown [30]. Finally, astrocyte-derived proteins, such as ApoE4, can activate a pro-inflammatory pathway leading to chronic BBB opening [31].
It is known that pericytes envelop the cerebral microvessels providing structural stability. However, the role of pericytes in BBB function has been elusive due to lack of a specific marker until recent publications identified a pericyte deficiency in the platelet derived growth factor receptor beta (PDGFR-β ) knockout mice [32]. The mutation was found to be lethal, but study of the embryo suggested that the endothelial cells had an abnormal distribution of tight junction proteins and that the barrier had increased permeability. It has since been shown that pericytes are involved in the maintenance of barrier properties of the endothelial cells and reduced pericyte coverage of the BBB led to chronic BBB opening and infiltration of multiple neurotoxic products (eg. fibrin, thrombin) [33]. In mice with reduced PDGFR-β signaling, a strong negative correlation was reported between the vascular permeability and the extent of pericyte coverage of the vessel [34] and the increased permeability was suggested to be due to defective regulation of transcytosis.
The basement membrane, composed of structural proteins such as collage IV, laminin and fibronectin, also contributes to the development of barrier properties of the endothelial cells. The basement membrane positions the cells of the neurovascular unit in proximity to each other and controls their interaction. Binding of extracellular matrix molecules to transmembrane receptors on the pericytes, endothelial cells and astrocytes can lead to the activation of growth factor release and initiation of downstream signaling cascades for cell growth and differentiation [35].
d. State of the BBB in Disease
Breakdown of the BBB has been observed in several neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, Amytrophic Lateral Sclerosis (ALS) and Multiple Sclerosis [35]. At this stage, it is unclear whether BBB breakdown is a result of the pathology or if it is an underlying cause of disease. Due to the complexity of the pathology of these diseases, there are often several deficits observed. For example, reduction in p-glycoprotein expression in Parkinson's disease may lead to the influx of neurotoxins leading to neuronal death [36]. In Alzheimer's disease, pericyte deficiency and therefore, chronic BBB opening, elevates Aβ40 and Aβ42 levels and accelerates cerebral amyloid angiopathy [37]. Brain endothelial receptors, including lipoprotein receptor related protein 1 (LRP) and receptor for advanced end glycation products (RAGE), have both been implicated in amyloid transport across the BBB and therefore are potential therapeutic targets for new Alzheimer's drug development [38,39]. For ALS, the current dogma suggests that disease onset is due to toxins produced by microglia and astrocytes in the spinal cord. Leakage of plasma proteins into the cerebrospinal fluid around the spinal cord suggest BBB breakdown and provide a mechanism of activating microglia and astrocytes. Pericyte degeneration has been shown to lead to motor neuron injury in the spinal cord which may be related to ALS onset and pathology [40,41].
3. Limitation of the BBB for Pharmaceutical Treatment of Disease
In order to effectively cross the BBB, small molecule drugs must be less than 400Da and have high lipid solubility. These stringent characteristics mean that ~98% of all small molecules and 100% of the large molecule therapeutics (antibodies, proteins, gene therapeutics, cells, etc) are unable to cross the BBB in therapeutic quantities [18]. Without using a drug delivery strategy, the BBB effectively limits pharmaceutical treatment of brain diseases to depression, schizophrenia, chronic pain and epilepsy [18]. Neurodegenerative diseases, psychiatric illness and brain cancers are increasing at alarming rates and the current lack of therapeutic options for treatment of these diseases is due primarily to the presence of the BBB.
a. Current methods for overcoming BBB problem
Several techniques have been used to circumvent the BBB to promote drug delivery into the brain (Table 1). Direct injection into the brain is the most efficient for drug delivery into the brain region of interest. Stereotactic coordinates, MRI or neuronavigation systems can be used to position needles and pumps in the areas of interest. Clinical trials for Parkinson's and Alzheimer's disease have seen positive results using direct injection, however there are many risks to these types of surgical procedures including damage to the healthy brain tissue, potential for hemorrhage, and infection.. Furthermore, there are generalized risks associated with anesthesia and surgery which would eliminate some patients from receiving treatment [42]. Less invasive options would include intrathecal injection into the cerebrospinal fluid, however the rapid transport of drug from cerebrospinal fluid to the bloodstream does not eliminate the need to bypass the BBB and makes the drug concentrations at the target unpredictable [43,44].
Table 1.
Methods to circumvent the BBB for drug delivery to the brain. This table briefly summarizes the methods that have been developed to deliver drugs through the BBB.
| Category | Therapeutic Agent | Key Findings | References |
|---|---|---|---|
| Antibodies | Dopamine receptor D4 antibodies | First study to show successful antibody delivery to the brain. | [86] |
| Human epidermal growth factor receptor 2 (HER2/erbB2) | Local and non-invasive delivery of the antibody to the brain suggesting application for brain tumour | [87,88] | |
| Amyloid-β antibodies | Aβ antibodies delivered to brain, bound to plaques in mouse model of Alzheimer's disease | [78] | |
| Amyloid-β antibodies | First study to show reduction in plaque pathology upon delivery of antibodies with FUS | [57] | |
| Endogeneous IgG and IgM | Reduction in plaque pathology corresponding to significant increases in delivery of endogeneous IgG and IgM | [80] | |
| Gene Therapy Agents | cc-siRNA-Htt | FUS-delivered siRNA-Htt to the rat striatum led to significant reduction of Htt expression in a dose dependent manner. | [89] |
| Vascular endothelial growth factor | Delivery of microbubbles coated with plasmids encoding VEGF DNA were delivered in a model of stroke and resulted in attenuation of neurologic function | [90] | |
| AAV9-GFP | AAV9-GFP was delivered to the striatum and hippocampus and neurons and astrocytes were found to express GFP | [91] | |
| AAV2-GFP | AAV-GFP succesfully penetrated into the brain by MRIgFUS treatment and was able to transfect neural cells to express GFP | [92] | |
| Chemotherapy | Doxorubicin | Doxorubicin delivered by FUS and microbubbles localizes in the tumor and leads to tumour reduction in several brain tumor models | [42, 93-95] |
| Methotrexate | Methotrexate was successfully delivered to tumors and led to tumor reduction | [96] | |
| BCNU | BCNU delievery was enhanced in the tumor leading to prolonged survival periods | [97,98] | |
| Nanoparticles | 1,3-bis(2-chloroethyl_-1-nitrosourea (a chemotherapy agent) immobilized on nanoparticles | FUS improved delivery of BCNU coated nanoparticles to rodent gliomasand led to tumor suppression without damage to normal tissue. | [99,100] |
| Therapeutic magnetic nanoparticles (MNPs) | Significant increase in deposition of MNPs in brains of wildtype rats with FUS and microbubbles suggesting potential treatment for brain tumors. | [101] | |
| Specialized brain-penetrating nanoparticles (SBPNP) | Ability of MRIgFUS to deliver SBPNP across BBB showed larger and more abundant NP found throughout the brain parynchama, with increasing US pressure resulting in increased NP delivery to brain. | [102] | |
| Gold nanoparticles and nanoparticles with scattering (SERS) capability | Gold nanoparticles and nanoparticles with enhanced tracking ability were delivered to the tumor and the tumor margin respectively. | [103, 104] | |
| Cells | Iron-labeled GFP-expressing neural stem cells | Neural stem cells survived successful transplantation by MRIgFUS to the targeted brain region and expressed markers of differentiation | [105] |
| Natural Killer (NK) cells expressing chimeric Her2 antigen receptor | NK cells expressing Her2 receptor antigen were delivered to Her2 expressing tumor cells in the brain | [106] |
Methods to induce generalized BBB permeability using sugar alcohols, solvents and vasodilators have also been effective for drug delivery [45,46]. These agents induce rapid, widespread BBB permeabilization and expose the healthy brain tissue to the potentially toxic compounds in the circulatory system.
i. Carrier proteins
There are some drugs which have an expected affinity for a BBB carrier mediated transport system due to the conformational structure of the drug. One such example is the drug L-DOPA, a dopamine precursor which is an effective treatment for Parkinson's disease. L-DOPA is a neutral amino acid and it is transported into the brain using the LAT-1 carrier protein which functions to normally transport neutral amino acids [47]. However, more commonly, drugs are modified with a moiety which increases the affinity of the drug for one on the carrier proteins. Addition of cysteine, a small neutral amino acid to a non-transportable drug, can make the drug appear as a large neutral amino acid thereby increasing uptake [48]. Another method for exploiting a protein to deliver drugs across the BBB includes conjugating a drug to an antibody against a BBB receptor such as the human insulin receptor or the transferrin receptor. The antibody binds to its receptor in the BBB and initiates transport across the BBB thereby acting as a molecular Trojan Horse. These Trojan Horse proteins have been engineered to express wide classes of therapeutics including enzymes and neurotrophins [49].
ii. Pharmacological changes
To improve the transport across the BBB, small molecule drug candidates are being modified to improve their transport across the BBB. These modifications may include the ‘lipidization’ of a water-soluble drug by blocking the functional groups which form hydrogen bonds [44]. Since this can affect drug activity, it hasn't been widely used. Instead, most often, chemical modifications increase the affinity of a drug for a known carrier mediated transporter such as the ones mentioned above. Overall this strategy to promote drug delivery increases intracellular uptake by brain endothelial cells but often the drug does not reach therapeutic concentrations in the target region of the brain.
iii. Intranasal delivery
The intranasal route of administration into the brain has gained attention in recent years due to the highly vascularized nasal mucosa supporting rapid drug absorption and elimination of the first pass metabolism which enhances bioavailability of the drug in vivo [50]. As well, the nasal epithelium is adjacent to the cerebrospinal fluid flow which promotes direct delivery into the cerebral spinal fluid for direct delivery to the brain without needing to cross the BBB [51]. Recently, it was demonstrated using fluorescent tracer molecules that therapeutic agents delivered intranasally may travel through the perivascular spaces to reach the brain [52]. While these experiments have been promising in rodents, the method requires that the drug penetrate large brain regions which may be difficult in humans [44].
4. Focused Ultrasound
Focused ultrasound (FUS) is a non-invasive method where ultrasound is used to transiently open the BBB in highly targeted brain regions. This promotes movement of drugs delivered in the circulation into the brain [53]. FUS has been used to deliver a vast array of therapeutic agents in preclinical models of disease. The optimization of the method, mechanisms of induced BBB permeability and translation to clinical application will be discussed.
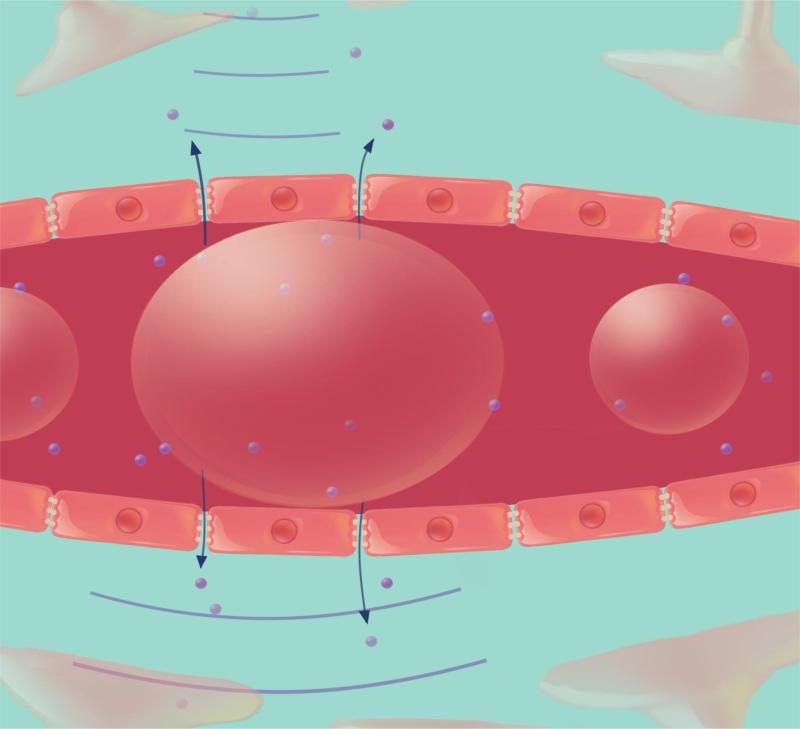
High power ultrasound had been used to open the BBB but because of the potential for thermal coagulation and the formation and collapse of gas bubbles (cavitation), the achieved bioeffects were unpredictable, varying from BBB opening to gross hemorrhage [54,55]. In 2001, Hynynen and colleagues modified the method to produce safe, reproducible BBB opening by combining low power ultrasound with the delivery of intravenous preformed microbubble contrast agent. The microbubbles act to concentrate the acoustic energy inside the blood vessel [53]. When the preformed circulating microbubbles pass through the ultrasound field, they oscillate at the frequency of the ultrasound, a process known as stable cavitation. The stable expansion and contraction of the microbubbles causes mechanical stimulation of the blood vessels leading to transient, reproducible, BBB opening (Figure 2). Since the microbubbles concentrate the ultrasound energy, the amount of ultrasound pressure required to open the BBB is significantly reduced thereby limiting the risk of skull heating and making transcranial ultrasound treatments feasible [56]. At lower pressures, in the presence of microbubbles, damage to the brain tissue was avoided except for the extravasation of a few red blood cells.
Figure 2. BBB opening with FUS.
A) Preformed microbubble contrast agent is injected intravenously and moves through the blood vessel. The microbubbles undergo stable cavitation and expand (B) and contract (C) when they travel through the low power ultrasound field. This causes the blood vessels to be mechanically stimulated and the BBB to be opened, allowing therapeutic agents temporarily to move into the brain.
The use of magnetic resonance imaging (MRI) as an imaging modality to guide and evaluate BBB opening allows precise targeting. MRI provides excellent soft tissue contrast thereby visualizing specific brain structures to be targeted for drug delivery. Furthermore, using contrast enhanced T1-weighted images the treatment can be evaluated. It has been shown that the percentage of signal enhancement on contrast-enhanced T1-weighted images is correlated to the relative amount of BBB opening and can also be correlated to the amount of drug delivery [57].
Several groups have investigated the ultrasound parameters that lead to optimal BBB opening for drug delivery. The range of frequency which is suitable for clinical transcranial ultrasound application is likely between 0.2 and 1.5 MHz but in rodents a much larger range has been tested. The threshold of ultrasound pressure required for BBB opening is related to the mechanical index which is defined as the peak negative pressure in vivo by the square root of the frequency [58]. The mechanical index indicates that higher ultrasound pressures are required for effective opening when higher frequencies are used. While ultrasound up to 8MHz has been used to open the BBB in mice, the high pressures required for BBB opening make it unlikely that these frequencies will be applicable in humans [59]. In addition to frequency, duration of the ultrasound pulse and pulse repetition frequency have been investigated for BBB opening. Pulse durations ranging from a few μs to 100 ms have been tested in rodents [53,60-62]. Short pulse lengths (2.3 - 3μs), tested for their ability to eliminate standing waves in the brain, were able to open the BBB effectively [61,62]. When shorter pulses were used, the mean enhancement observed on T1-weighted MRI images, is greater with a higher pulse repetition frequency and less with a lower pulse repetition frequency [62]. Increasing the pulse length was correlated to increased enhancement on a contrast enhanced T1-weighted image [63] with no real benefit of using pulse lengths over 10 ms [60]. For longer pulses, it has been suggested that there may be insufficient time for microbubbles to reperfuse if the pulse repetition frequency is too high [64] however, changes in repetition frequency did not significantly impact BBB permeability when tested in vivo [60]. Finally, the microbubble size and dose can also have a significant impact on BBB permeability [65-69]. Overall, these studies have suggested that at set pressures, more BBB opening (and the potential for damage) is correlated with both larger microbubble size and higher microbubble dose. Recently, it was shown that at lower ultrasound pressures, differences between microbubble preparation could have significant effects on BBB opening but that size distribution and type of microbubbles was less important at higher pressures potentially due to the induction of inertial cavitation [70].
a. Overview of advantages and challenges of FUS
There are 3 main advantages to using FUS as a method for temporary BBB opening in the brain. First, FUS is non-invasive. The application of transcranial FUS eliminates the need for invasive surgery to deliver therapeutics to the brain. This not only reduces the risk associated with surgery and anesthetic but also improves the ability for repeated treatments for chronic disease applications. Also, the microbubbles are delivered intravenously, eliminating the need for risky arterial catheterization. Second, the introduction of MRI as an imaging method for targeting the brain allows the FUS to be directed to the brain region of interest. The area of BBB opening is correlated to the size of the focal spot with higher frequency transducers producing a tighter focal spot. As an example, this would be advantageous for chemotherapeutic treatment of brain tumours where the access of the drug would be limited to the tumour and it's periphery and prevented from accessing the remaining healthy brain tissue. However, using multiple sonications, FUS treatments could also be tailored to treat diseases with more widespread pathology such as Alzheimer's disease. The concept of targeting FUS depending on the extent of the pathology has been demonstrated effectively in preclinical models of disease. For example, single sonications were successful for delivering boronophyenylalanine to the tumour in a rat glioblastoma model for boron neutron capture therapy [71] whereas, in a mouse model of Alzheimer's disease, anti-amyloid antibodies were effectively delivered to an entire hemisphere to reduce widespread plaque pathology in the cerebral cortex [72]. We believe a similar sonication strategy could be used to deliver therapeutics to an infiltrating tumour with poorly defined margins. Finally, BBB opening with FUS is transient and is closed as early as 6 hours after treatment when ultrasound parameters that don't result in damage are used. Once closed, the BBB appears to function normally and remains closed [73,74]. These data indicate that FUS does not produce long-term deficits in barrier function.
Many of the studies aimed at optimizing FUS parameters have been performed on healthy animals and it is possible that BBB opening will not be effective or that the BBB may not be able to fully repair itself when FUS is applied in models of disease. Recently, it was demonstrated using two-photon microscopy that significant differences in the kinetics of FUS-mediated BBB opening exist between a model of Alzheimer's disease and their non-transgenic littermate controls [75]. These data showed that less drug is delivered in transgenic mice compared to the non-transgenic using the same set of ultrasound parameters indicating that FUS parameters may have to be optimized for application in specific diseases. Further study is required to ensure BBB opening is effective and reliable in models of disease. As well, recent evidence has highlighted that BBB opening can lead to stimulation of new neurons in the hippocampus of the mouse brain [75,76]. These data are exciting and suggest that FUS may have beneficial effects on the brain beyond a method for drug delivery.
b. Physical and cellular mechanisms
Despite the success of FUS as a method for drug delivery in preclinical models, the exact mechanisms by which the barrier is opened are unknown. At low ultrasound pressures, microbubble oscillation in stable cavitation is likely to be the cause of opening. Microbubble oscillation creates microstreaming and generates shear stress in the vessel depending on the ultrasound frequency, vessel diameter, and microbubble size [77]. The vascular endothelial cells can respond dynamically to sheer stress [78]. In vitro experiments have shown that the stresses on the vessel wall occur during the expansion and contraction phase of the bubble oscillation however the stress is much greater in the contraction phase of the bubble [79]. Using high-speed microscopy, it was observed that the vessel wall is pulled in to the lumen which may be a cause for BBB opening [79]. In addition to shear stress, circumferential stress is generated by microstreaming of the blood around the oscillating microbubble which may also contribute to the pressure on the microvessel leading to BBB opening [80]. These mechanical stresses generated by the microbubbles may stimulate the mechanosensitive ion channels in the endothelial cells, leading to BBB opening [81]. Inertial cavitation has been suggested as a potential mechanism for BBB opening. The associated high-velocity microjetting and extreme local temperature rise associated with inertial cavitation would certainly cause BBB opening [80] however, monitoring of the acoustic emissions during FUS treatments have shown that BBB opening is possible in the absence of inertial cavitation [60,82-84]. Analysis of the spectral information indicated that increases in harmonics and ultraharmonics, indicative of microbubble activity are correlated with BBB opening Spectral analysis has demonstrated BBB opening is correlated with increases in harmonic and ultraharmonic emission [60,82]. The presence of broadband emission, indicative of inertial cavitation, was correlated with extravasation of red blood cells and edema suggesting that mechanisms of BBB opening in the absence of microhemorrhage do not involve inertial cavitation.
In vitro studies using the high-speed Brandaris camera are likely to be the key to understanding the mechanisms of BBB opening in the near future. The camera has been used to identify shear stress, microjetting and other effects of microbubbles under the effects of ultrasound in a vessel [85].
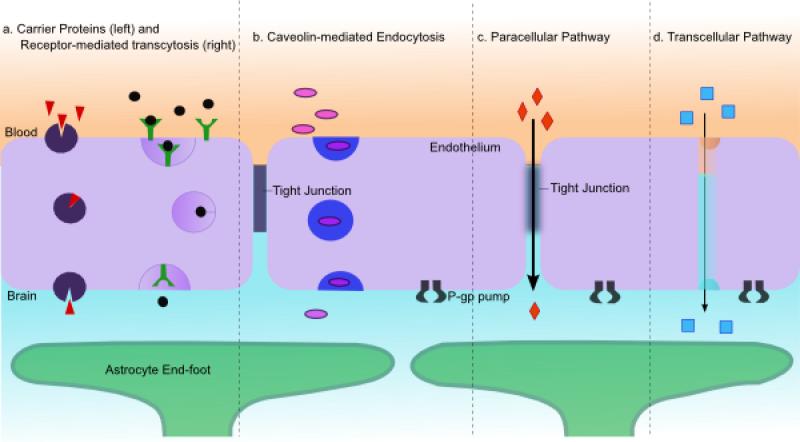
The physical stresses generated by interaction between the ultrasound and microbubbles leads to cellular changes at the BBB. Electron microscopy has been used to identify that drugs and tracer molecules pass through the BBB after FUS via transcellular and paracellular mechanisms [86,87]. In total, 4 possible routes of molecular passage across the BBB have been identified when appropriate FUS parameters are employed: transcytosis using cellular vesicles (carrier mediated transport and receptor mediated transport), endocytosis, paracellular passage through widened tight junctions, and through the cytoplasmic channels in the endothelium (Figure 3).
Figure 3. Mechanisms of transport across the BBB following FUS.
4 main methods of transport across the BBB have been identified following FUS-mediated BBB opening (redrawn after Abbott et al., 2006). A) Increased vesicles observed by electron microscopy indicated that transcytosis with either carrier proteins or receptor mediated is upregulated. B) Caveolin proteins are significantly increased at 1 hour after FUS treatment suggesting a role for caveolin-mediated endocytosis. C) Downregulation of tight junction proteins has shown that paracellular transport can occur following FUS treamtent. D) Cytoplasmic channels created potentially by the fusion of vesicles or damage to the capillary endothelium have been observed using electron microscopy.
Electron microscopy has been used to identify an increased number of vesicles in endothelial cells following FUS-mediated BBB opening [86,87]. Vesicles containing tracers and labeled drugs have been identified within the endothelial cells confirming transcellular transport [86]. The presence of cytoplasmic channels has also been identified and has suggested to be due to the fusion of several vesicles [88]. Upregulation of caveolin proteins using immunohistochemistry and Western blot procedures confirms that FUS induces active transport of molecules across the BBB [89,90].
Reduction of tight junction proteins has supported the notion that molecules move paracellularly through the BBB following FUS [86,87]. Tight junction proteins have been downregulated following FUS but returned to pre-treatment levels by 4 hours confirming that the effects of FUS on the BBB are transient [87]. The tight junction changes observed after FUS were consistent with what has been reported using mannitol. It is unknown whether FUS downregulates the expression of the proteins or if it induces reorganization causing masking of the antigen. There is evidence that the FUS downregulates a gap junction protein which connects to ZO-1 suggesting that it likely downregulates tight junction expression as well [91].
Recent experiments using two-photon microscopy have continued to explore the mechanisms of BBB opening after FUS [92,93]. Using fluorescent dyes as models for drug delivery, it was observed that leakage of the dye from intact vessels following BBB opening with FUS could be characterized as either ‘fast’ or ‘slow’. The characterization was based on the temporal dynamics of leakage with ‘fast’ describing leakage which reached extracellular peak intensity during the FUS treatment. ‘Slow’ leakage began 5-15 min after FUS treatment and occurred along the length of the vessel [93]. The authors suggested that the two leakage types corresponded to paracellular transport (fast leakage) and transcellular transport (slow leakage). Current studies aimed at histological examination of vessel leakage under two-photon microscopy are ongoing and will soon provide further information regarding the leakage type.
c. Drug delivery
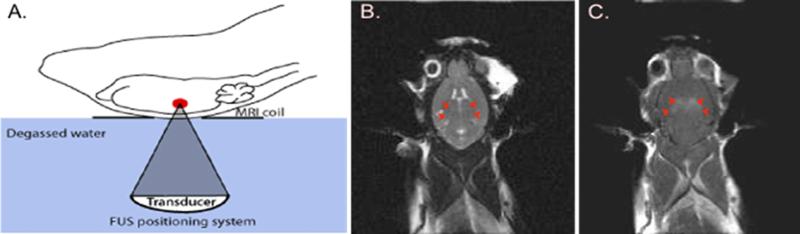
As a method for evaluating BBB opening, MRI contrast agents (500-900Da) are delivered during FUS treatment and their extravasation into the brain parenchyma is assessed in follow up imaging [53] (Figure 4). For experimental procedures using MRI, animals are placed in a secure apparatus and their head is coupled to the ultrasound transducer using water or ultrasound gel. T2-weighted MR images are used to identify and target locations of interest into the brain. Following treatment, T1-weighted images are used to visualize leakage of MR contrast agent in regions where the BBB is opened (Figure 3). Tracer molecules including Trypan and Evan's blue (~70kDa when bound to albumin in vivo) are used to identify the regions of BBB opening using gross histology [53,68,70,82,86]. In addition to tracer molecules, FUS has been used to effectively deliver a wide range of therapeutics to specific brain regions. These experiments have been summarized in Table 2.
Figure 4. Experimental set up for FUS-mediated BBB opening.
A) The animals are positioned on a 3 axis FUS positioning system with the head coupled to a tank of degassed water (adapted from Burgess et al., 2011). B) A T2-weighted MR image is taken and used to target the region of interest for drug delivery. The bilateral hippocampus is indicated by 4 red arrows. C) Following FUS treatment, contrast-enhanced T1-weighted images are used to identify leakage of contrast agent into the brain parenchyma. 4 spots of contrast enhancement correspond to the targeted spots in the hippocampus.
Table 2.
Examples of therapeutic agents delivered to the brain using FUS.
| Method | Advantages | Disadvantages |
|---|---|---|
| Surgical Injection | Effective drug delivery, targeted to region of interest using guidance system | Invasive, high risks associated with surgery and anesthesia, damage to healthy brain regions |
| Chemical BBB disruption | Rapid BBB permeabilization, transient effects | Not targeted to a specific brain region, permits widespread entry of toxins, invasive carotid artery injections are required |
| Carrier Proteins | Non-invasive, able to cross the BBB on their own, oral route of administration possible, Provide | Drug has to be safe for systemic injection, expensive to develop new drugs, widespread access to the brain, drug may not reach therapeutic concentrations in brain |
| Intranasal Delivery | Non-invasive, simple route of delivery for patients, highly nasal mucosa for efficient drug uptake | Non-targeted, drug may need to travel large areas to get to region where needed |
| Focused Ultrasound | Non-invasive (i.v injection), targeted to a specific brain region, transient BBB opening, success in pre-clinical models | Drug needs to be safe for systemic injection, method has not been tested clinically |
Large therapeutic agents including, antibodies, gene therapy agents, chemotherapeutics, nanoparticles, and cells do not cross the BBB and therefore have not been used to effectively treat brain diseases. FUS has been used to successfully deliver these classes of large therapeutic agents to the brain, showing reduction in pathology in several preclinical models of disease.
d. Effects of Ultrasound Alone
In recent studies, FUS has proven to be more than just a method for drug delivery. In a model of Alzheimer's disease, FUS-mediated BBB opening reduced plaque pathology even in the absence of drug delivery [95]. The authors found two potential mechanisms. First, they demonstrated that endogenous immunoglobulins (IgG and IgM) were increased in the brain following FUS indicating that perhaps immunoglobulins assist in the clearance of amyloid plaques. Second, FUS-mediated BBB opening led to activation of astrocytes and microglia which were found to have increased amyloid internalization [95]. In another study, FUS-mediated BBB opening was reported to increase neurogenesis in the hippocampus [76]. In this study, mice received bromodeoxyuridine (BrdU), a thymidine analog which incorporates into the DNA of dividing cells after FUS treatment. Analysis of the BrdU expressing cells post mortem indicated that a significant number of new neurons were born in the neurogenic region of the hippocampus suggesting that FUS-mediated changes in BBB permeability can stimulate new neuron growth. These data were supported in a recent study, which demonstrated that FUS treatment significantly increased the number of immature neurons in the hippocampus in a mouse model of Alzheimer's disease [75]. Although the mechanisms underlying FUS induced neurogenesis are unknown, the data correlates to a previous report that FUS can induce signaling of the Akt, pro-survival and differentiation pathway [96]. Moreover, FUS application (in the absence of microbubbles) has been shown to increase expression of brain-derived neurotrophic factor (BDNF) in the brain [97]. BDNF is known to be a potent modulator of neural plasticity and thus a combination of BDNF activation and initiation of Akt signaling may be one of the methods by which FUS-mediated BBB opening affects the brain.
e. Safety
The safety of FUS-mediated BBB opening for applications in humans was best demonstrated in recent studies using non-human primates. BBB opening was achieved in non-human primates using both a commercially available, clinical ultrasound array [74] and a single element transducer [98,99]. The design of the clinical transducer reduces the aberrations in the transmitted ultrasound created by variations in skull bone thickness and density [74]. Non-human primates were treated multiple times in multiple locations and were found to perform as well in visual and learning tests after the FUS-mediated BBB opening as they performed prior to the treatment. This evidence strongly supports the safety of FUS for BBB opening in the brain [74].
MRI has been the main tool for evaluating BBB opening after FUS treatment [53] however MRI can only be performed post-treatment and thereby is not capable of monitoring the treatments for safety purposes. As a method for providing real-time feedback, several different groups have developed systems to detect and monitor the acoustic emissions from the microbubbles [82,83,100]. One example is the development of a real-time acoustic feedback controller which increases the power in a step wise fashion with each ultrasound burst. The acoustic emissions from the microbubbles are monitored with a hydrophone at the focus. The emission data is analyzed and once ultraharmonic emissions are detected, the applied ultrasound power is reduced to a defined percentage. In rats, a reduction to 50% of the transmitted power has resulted in effective BBB opening with no red blood cell extravasation or other detrimental effects [82]. The implementation and testing of a controller system into a clinical array would allow the operator to monitor and control the FUS treatment thereby improving the safety of clinical FUS treatments.
5. Expert Commentary
The BBB has been recognized as the single most important factor limiting drug delivery to the brain for treatment of brain disorders. Current research into the development and function of the BBB has identified cells of the neurovascular unit, including pericytes, as key functional regulators of BBB permeability. Despite the recent advancement in understanding BBB development, the barrier remains a significant hurdle for therapeutic agents targeted to the brain. There have been several methods used to circumvent the BBB, each having their own advantages and disadvantages. Drug modification has been successful for getting large pharmaceutical agents into the brain, but the treatments are not targeted and it can be challenging to get therapeutic concentrations where they are required. Drug delivery methods such as intranasal delivery, has been successful in preclinical models however the ability to translate the technology to the clinic is currently unclear. We believe that FUS-mediated BBB opening is the best method to non-invasively deliver drugs of varying sizes to targeted regions of the brain. FUS has shown great success in preclinical models and recent application of FUS in the non-human primate brain using a clinical transducer has suggested that clinical translation is feasible.
The addition of microbubbles to the FUS procedure significantly improved the consistency and reproducibility of BBB opening. Using microbubbles, several groups have investigated the optimal parameters for ultrasound application which result in transient opening with limited bioeffects to the surrounding tissue. Perhaps the most significant contribution to the optimization of FUS was the development of a real-time acoustic feedback control system. The feedback controller increases the applied pressure of each burst in a step-wise fashion and analyzes the received spectral information. When the presence of ultraharmonics are detected, the controller reduces the applied pressure to a user defined percentage [82]. The controller eliminates the variability in the extent of BBB opening reported by other studies using fluctuating microbubble sizes or concentrations.
Despite the many technological advances in FUS methodology, the adoption of the technique for clinical application has been slow. We suggest that this is partially due to the general knowledge that a functioning BBB is necessary for maintaining a healthy brain. While we agree with the many studies that support the need for a properly functioning BBB, our recent work has suggested that temporary opening of the BBB is not accompanied by any adverse side effects in the healthy brain. In general, preclinical studies in both rodents and non-human primates have failed to show any adverse effects such as neuronal death or degeneration, inflammation, or infection. Furthermore, using FUS to open the BBB in rodent models of disease has actually led to improvements in pathology and behavior even in the absence of exogenous drug delivery. Since FUS can be targeted to small brain volumes, only regions which exhibit pathology are subject to BBB opening thereby limiting the proposed toxic effects of exposure to the circulation. However, there are other limitations of the FUS technology that may be contributing to the lack of widespread clinical adoption. For example, significant questions about the repeatability of the FUS procedure remain, which makes FUS currently unsuitable as a long-term treatment solution for brain disease. As well, the current dependence of the technology on MRI and access to a specially trained operator make the cost of FUS prohibitive outside of major hospital centers. As the technology continues to improve, the number of brain regions that can be treated may be increased thereby making FUS relevant for treatment of a wider range of brain diseases.
There is a growing body of literature which supports the transient, localized opening of the BBB with FUS as a method for drug delivery. Continued research to answer the questions and concerns which remain is necessary to lead to a paradigm shift whereby the BBB is known only as a temporary obstacle to overcome for effective treatment of brain disease by FUS.
While there is much work to be done to understand the full effects of FUS-mediated BBB opening on the brain and to demonstrate the safety of the technique, the research completed thus far clearly suggests that FUS represents a promising strategy that will be essential for the future treatment of brain disease.
6. Five Year View
There is much work to do to better understand the molecular and cellular mechanisms of MRIgFUS mediated BBB opening in order to improve safety measures for clinical translation. However, the next five years will be crucial for moving FUS forward as a clinical method for BBB opening for treatment of brain disease. The first clinical application of BBB opening will be for treatment of brain tumors due to their localized treatment area and vast body of evidence demonstrating that improved chemotherapeutic access through the BBB will reduce tumor size. Further, the vasculature at the tumor core is defective and thus there are fewer concerns with FUS mediated opening of the blood-tumor barrier than with the BBB. The worlds first clinical trial to assess MRIgFUS for targeted delivery of chemotherapeutics to brain tumors is set to begin in 2015 at Sunnybrook hospital in Toronto [122]. In this Phase I clinical trial, MRIgFUS will be targeted to a specific region around the brain tumor with a cancer cell density of less than 1 in 10 cells. If successful, MRIgFUS will deliver chemotherapeutic to the fringe region of the tumor, eradicate the remaining cancer cells and reduce the recurrence of brain cancer. It will be necessary to perform long-term survival studies to determine the effectiveness of this treatment.
In addition to brain tumor applications, there are other potential directions for clinical use of MRIgFUS over the next five years. Significant progress has been made in preclinical models of Alzheimer's disease indicating that BBB opening may be beneficial for treatment of the disease. However, there are many gaps in our understanding of Alzheimer's disease including the link between vascular disease and Alzheimer's. Without sufficient mouse models that exhibit all of the pathology of the disease including plaques, tangles, neuronal death, cognitive deficits and vascular pathology of the disease, it is difficult to predict the effects of FUS in a patient trial. Substantial research efforts focused on the effects of FUS on the compromised vasculature in a brain affected by Alzheimer's disease are essential to the progress of MRIgFUS as a treatment for Alzheimer's disease.
Overall, MRIgFUS has the potential to truly revolutionize the treatment of brain disease in the next five years. Should the clinical application of BBB opening for the treatment of brain tumors prove successful, MRIgFUS will transform the way that we approach cancer and provide a safe and precisely targeted method to destroy tumor tissue and prevent its recurrence. In addition, with the aid of further knowledge concerning neurodegenerative diseases such as Alzheimer's disease, and studies in complete preclinical models, MRIgFUS could provide an effective treatment for diseases that are becoming an increasingly serious concern in our society.
7. Key Issues.
The BBB in healthy individuals functions to protect the brain from harmful agents and bacteria but has also posed as a major obstacle to drug delivery into the brain, preventing the treatment of neurological damage and neurodegenerative diseases.
Almost all pharmaceuticals developed to treat neurological conditions are too large to pass through the BBB in therapeutic concentrations and it has been difficult for methods developed to circumvent the BBB (such as carrier proteins and intranasal delivery) to deliver therapeutic amounts of drug.
FUS is a non-invasive method where ultrasound is used to transiently open the BBB in highly targeted brain regions and has been shown to deliver therapeutic amounts of drugs of varying sizes to these regions in preclinical models.
Combining low power ultrasound with the delivery of intravenous preformed microbubble contrast agent causes mechanical stimulation of the blood vessel through stable cavitation when the microbubbles encounter the ultrasound field, leading to transient, reproducible BBB opening.
The degree of FUS-mediated BBB opening can be affected by the frequency (and therefore the pressure) of transmitted ultrasound, duration of the ultrasound pulse, pulse repetition frequency and microbubble size and dose.
Advantages of the use of MRIgFUS for BBB opening include the elimination of the need for invasive surgery for drug delivery to the brain, precise targeting of brain regions for treatment using MRI, and transient opening absent of long-term deficits in barrier function.
Obstacles in the way of widespread clinical adoption of FUS include questions surrounding the repeatability of the FUS procedure, unknowns about the full impact of the treatment on the target and off target brain tissue and the current dependence of the technology on MRI and a specially trained operator.
FUS has been shown to be safe in preclinical models, with safety translating to human patient trials if the further development of the clinical transducers integrate safety measures such as the real-time acoustic feedback controller which allows the operator to monitor and control the FUS treatment.
Clinical translation of MRIgFUS for treatment of brain tumors within the next five years is essential for moving MRIgFUS towards widespread clinical adoption.
Acknowledgments
K Hynynen has served as an inventor on patents owned by BWH and patent applications owned by SRI. K Hynynen also has stock ownership in and has received funding from FUS instruments for pre-clinical work.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
- 1.Ussing HH. The distinction by means of tracers between active transport and diffusion. Acta Physiol. 1949;19:43–56. [Google Scholar]
- 2.Michel C. Transport of Fluids and Solutes in Tissues. In: Bader D, Bouten C, Colin D, et al., editors. Pressure Ulcer Research. Springer Berlin Heidelberg; New York, USA: 2005. pp. 233–62. [Google Scholar]
- 3.Mann GE, Zlokovic BV, Yudilevich DL. Evidence for a lactate transport system in the sarcolemmal membrane of the perfused rabbit heart: kinetics of unidirectional influx, carrier specificity and effects of glucagon. Biochim Biophys Acta. 1985;819:241–8. doi: 10.1016/0005-2736(85)90179-8. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV, Segal MB, Begley DJ, et al. Permeability of the blood cerebrospinal fluid and blood brain barriers to thyrotropin releasing hormone. Brain Res. 1985;358:191–9. doi: 10.1016/0006-8993(85)90963-1. [DOI] [PubMed] [Google Scholar]
- 5.Zlokovic BV, Begley DJ, Chain Eliash DG. Blood brain barrier permeability to leucine-enkephalin, D-alanine2-D-leucine5-enkephalin and their N-terminal amino acid (tyrosine) Brain Res. 1985;336:125–132. doi: 10.1016/0006-8993(85)90423-8. [DOI] [PubMed] [Google Scholar]
- 6.Zlokovic BV, Hyman S, McComb JG, et al. Kinetics of arginine-vasopressin uptake at the blood-brain barrier. Biochim Biophys Acta. 1990;1025:191–8. doi: 10.1016/0005-2736(90)90097-8. [DOI] [PubMed] [Google Scholar]
- 7.Zlokovic BV, Lipovac MN, Begley DJ, et al. Transport of Leucine-Enkephalin Across the Blood-Brain Barrier in the Perfused Guinea Pig Brain. J Neurochem. 1987;49:310–5. doi: 10.1111/j.1471-4159.1987.tb03431.x. [DOI] [PubMed] [Google Scholar]
- 8.Zlokovic BV. Cerebrovascular permeability to peptides: manipulations of transport systems at the blood-brain barrier. Pharm Res. 1995;12(10):1395–1406. doi: 10.1023/a:1016254514167. [DOI] [PubMed] [Google Scholar]
- 9.Ehrlich P. Das sauerstoff-bedürfnis des organismus. In: Ehrlich P, editor. Eine Farbenanalytische Studie. Berlin, Germany: 1885. [Google Scholar]
- 10.Ehrlich P. Ueber die beziehungen von chemischer constitution, verteilung und pharmakologischer wirkung. In: Ehrlich P, editor. Gesammelte Arbeiten zur Immunitaetsforschung. Berlin, Germany: 1904. p. 574. [Google Scholar]
- 11.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2006;57:173–85. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 12.Clark DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegel GJ, Agranoff BW, Albers RW, et al., editors. Molecular, cellular and medical aspects. 6th edition Lippincott-Raven; Philadelphia, USA: 1999. pp. 637–70. [Google Scholar]
- 13.Lewandowsky M. Zur lehre von der cerebrospinalflussigkeit. Z Klin Med. 1900;40:480–94. [Google Scholar]
- 14.Goldmann EE. Vitalfarbung am zentralnervensystem. Abhandl Konigl preuss Akad Wiss. 1913;1:1–60. [Google Scholar]
- 15.Reese TS, Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1900;34:207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yudilevich DL, De Rose N. Blood-brain transfer of glucose and other molecules measured by rapid indicator dilution. Am J Physiol. 1971;220:841–46. doi: 10.1152/ajplegacy.1971.220.3.841. [DOI] [PubMed] [Google Scholar]
- 17.Davson H. The blood-brain barrier. J Physiol. 1976;255:1–28. doi: 10.1113/jphysiol.1976.sp011267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [This review underlines the problem of the BBB for the development of neurotherapeutics and covers the strategies used to overcome the barrier for drug delivery to the brain.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saitou M, Furuse M, Sasaki H, et al. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell. 2000;11:4131–42. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161(3):653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clark DE. Rapid calculation of polar molecular surface area and its application to the prediction of transport phenomena. 2. Prediction of blood-brain barrier penetration. J Pharm Sci. 1999;87:815–21. doi: 10.1021/js980402t. [DOI] [PubMed] [Google Scholar]
- 22.Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10:1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Zvi A, Lacoste B, Kur E, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–11. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat. 2002;200(6):629–38. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbott NU, Rönbäck L, Hansson E. Astrocyte-endothelial interaction at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 27.Tonra JR. Cerebellar susceptibility to experimental autoimmune encephalomyelitis in SJL/J mice: Potential interaction of immunology with vascular anatomy. Cerebellum. 2002;1:57–68. doi: 10.1080/147342202753203096. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–31. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 29.Wosik K, Cayrol R, Dodelet-Devillers A, et al. Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci. 2007;27(34):9032–42. doi: 10.1523/JNEUROSCI.2088-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao Y, Chen Z, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5(3413):1–12. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–6. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daneman R, Zhou L, Kebede AA, et al. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–66. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68(3):409–27. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Armulik A, Guillem Genové, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57(2):178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Kortekaas R, Leenders KL, van Oostrom JC, et al. Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol. 2005;57:176–79. doi: 10.1002/ana.20369. [DOI] [PubMed] [Google Scholar]
- 37.Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. doi: 10.1038/ncomms3932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Deane R, Wu Z, Sagare A, et al. LRP/Amyloid β-Peptide Interaction Mediates Differential Brain Efflux of Aβ Isoforms. Neuron. 2004;43:333–44. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 39.Deane R, Singh I, Sagare AP, et al. A multimodal RAGE-specific inhibitor reduces amyloid β–mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–92. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winkler EA, Sengillo JD, Bell RD, et al. Blood-spinal cord barrier pericyte reductions contribute to increased capillary permeability. J Cerebr Blood Flow Metab. 2012;32(10):1841–52. doi: 10.1038/jcbfm.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winkler EA, Sengillo JD, Sagare AP, et al. Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. PNAS USA. 2014;111(11):1035. doi: 10.1073/pnas.1401595111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gross ME, Nelson ET, Mone MC, et al. A comparison of postoperative outcomes utilizing a continuous preperitoneal infusion versus epidural for midline laparotomy. Am J Surg. 2011;202(6):765–70. doi: 10.1016/j.amjsurg.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Fishman RA, Christy NP. Fate of adrenal cortical steroids following intrathecal injection. Neurology. 1965;15:1–6. doi: 10.1212/wnl.15.1.1. [DOI] [PubMed] [Google Scholar]
- 44.Pardridge WM. Drug transport across the blood-brain barrier. Journal of Cerebral Blood Flow and Metab. 2012;32:1959–72. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rapoport S. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin. Investig. Drugs. 2001;10:1809–18. doi: 10.1517/13543784.10.10.1809. [DOI] [PubMed] [Google Scholar]
- 46.Matsukado K, Sugita M. Intracarotid low dose bradykinin infusion selectively increases tumor permeability through activation of bradykinin B2 receptors in malignant gliomas. Brain Res. 1998;792:10–15. doi: 10.1016/s0006-8993(97)01502-3. [DOI] [PubMed] [Google Scholar]
- 47.Pardridge WM, Oldendorg WH. Kinetics of blood-brain barrier transport of hexoses. BBA Biomem. 1975;282:377–92. doi: 10.1016/0005-2736(75)90279-5. [DOI] [PubMed] [Google Scholar]
- 48.Killian DM, Hermeling S, Chikhale PJ. Targeting the cerebrovascular large neutral amino acid transporter (LAT1) isoform using a novel disulfide-based brain drug delivery system. Drug Deliv. 2007;14(1):25–31. doi: 10.1080/10717540600559510. [DOI] [PubMed] [Google Scholar]
- 49.Pardridge WM, Boado RJ. Reengineering biopharmaceuticals for targeted delivery across the blood-brain barrier. Methods Enzymol. 2012:274–78. doi: 10.1016/B978-0-12-396962-0.00011-2. [DOI] [PubMed] [Google Scholar]
- 50.Jadhav AS, Pathare DB, Shigare MS. Development and validation of enantioselective high performance liquid chromatographic method for Valacyclovir, an antiviral drug in drug substance. J. Pharm. Biomed. Anal. 2007;43(4):1568–72. doi: 10.1016/j.jpba.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 51.Kristensson K, Olsson Y. Uptake and retrograde axonal transport of peroxidase in hypoglossal neurons. Acta Neuropathol. 1971;19:1–9. doi: 10.1007/BF00690948. [DOI] [PubMed] [Google Scholar]
- 52.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv. Drug. Delivery Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 53•.Hynynen K, McDannold N, Vykhodtseva N, Jolesz FA. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220:640–46. doi: 10.1148/radiol.2202001804. [This landmark study introduced the use of microbubbles to the FUS procedure, resulting in reduced ultrasound powers and reproducible, transient BBB opening. eproducible blood-brain barrier opening.] [DOI] [PubMed] [Google Scholar]
- 54.Vykhodtseva NI, Hynynen K, Damianou C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med. Biol. 1995;21:969–79. doi: 10.1016/0301-5629(95)00038-s. [DOI] [PubMed] [Google Scholar]
- 55.Vykhodtseva N, MacDannold N, Hynynen K. Progress and problems in the application of focused ultrasound for blood-brain barrier disruption. Ultrasonics. 2008;48:279–96. doi: 10.1016/j.ultras.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hynynen K. MRI-guided focused ultrasound treatments. Ultrasonics. 2010;50(2):221–9. doi: 10.1016/j.ultras.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Treat LH, McDannold N, Vykhodtseva N, et al. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer. 2007;121:901–7. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 58.McDannold N, Vykhodtseva N, Hynynen K. Blood-brain disruption induced by focused ultrasound and circulating preformed microbubbles appears to be characterized by the mechanical index. Ultrasound Med. Biol. 2008;34:834–40. doi: 10.1016/j.ultrasmedbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bing KF. Dissertation. Duke University; Durham: 2008. The potential for ultrasonic image-guided therapy using a diagnostic system. [Google Scholar]
- 60.McDannold N, Vykhodtseva N, Hynynen K. Effects of Acoustic Parameters and Ultrasound Contrast Agent Dose on Focused-Ultrasound Induced Blood-Brain Barrier Disruption. Ultrasound Med. Biol. 2008;34:930–37. doi: 10.1016/j.ultrasmedbio.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi JJ, Selert K, Gao Z, et al. Noninvasive and localized blood-brain barrier disruption using focused ultrasound can be achieved at short pulse lengths and low pulse repetition frequencies. J Cerebr Blood F Met. 2011;31:725–37. doi: 10.1038/jcbfm.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O'Reilly MA, Waspe AC, Ganguly M, Hynynen K. Closely-Timed Short Pulses: Influence of Sonication Parameters and Injection Rate. Ultrasound Med Biol. 2011;37:587–94. doi: 10.1016/j.ultrasmedbio.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samiotaki G, Konofagou EE. Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE T Ultrason Ferr. 2013;60:2257–65. doi: 10.1109/TUFFC.2013.6644731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goertz DE, Wright C, Hynynen K. Contrast Agent Kinetics in the Rabbit Brain During Exposure to Therapeutic Ultrasound. Ultrasound Med Biol. 2010;36:916–24. doi: 10.1016/j.ultrasmedbio.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McDannold N, Vykhodtseva N, Hynynen K. Use of ultrasound pulses combined with Definity for targeted blood-brain barrier disruption: a feasibility study. Ultrasound Med Biol. 2007;33(4):584–90. doi: 10.1016/j.ultrasmedbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang F, Fu W, Chen W, et al. Quantitative evaluation of the use of microbubbles with transcranial focused ultrasound on blood–brain-barrier disruption. Ultrason Sonochem. 2008;15:636–43. doi: 10.1016/j.ultsonch.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Weng J, Wu S, Yang F, Lin W, Tseng WI. Pulse sequence and timing of contrast-enhanced MRI for assessing blood-brain barrier disruption after transcranial focused ultrasound in the presence of hemorrhage. JMRI-J Magn Reson Im. 2010;31:1323–30. doi: 10.1002/jmri.22174. [DOI] [PubMed] [Google Scholar]
- 68.Choi JJ, Feshitan JA, Baseri B, et al. Microbubble-Size Dependence of Focused Ultrasound-Induced Blood-Brain Barrier Opening in Mice In Vivo. IEEE T Bio-Med Eng. 2010;54:145–54. doi: 10.1109/TBME.2009.2034533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vlachos F, Tung Y, Konofagou E. Permeability dependence study of the focused ultrasound-induced blood–brain barrier opening at distinct pressures and microbubble diameters using DCE-MRI. Magnet Reson Med. 2011;66:821–30. doi: 10.1002/mrm.22848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S, Samiotaki G, Olumolade O, Feshitan JA, Konofagou EE. Microbubble type and distribution dependence of focused ultrasound-induced blood-brain barrier opening. Ultrasound Med Biol. 2014;40:130–7. doi: 10.1016/j.ultrasmedbio.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alkins RD, Broderson PM, Sodhi RNS, Hynynen K. Enhancing drug delivery for boron neutron capture therapy of brain tumors with focused ultrasound. J Neuro-oncol. 2013;15:1225–35. doi: 10.1093/neuonc/not052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jordão JF, Ayala-Grosso CA, Markham K, et al. Antibodies targeted to the brain with image-guided focused ultrasound reduces amyloid-beta plaque load in the TgCRND8 mouse model of Alzheimer's disease. PloS One. 2010;5:e10549. doi: 10.1371/journal.pone.0010549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McDannold N, Vykhodtseva N, Raymond S, Jolesz F, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med Biol. 2005;31(11):1527–37. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 74•.McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS. Temporary disruption of the blood-brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012;72(14):3652–63. doi: 10.1158/0008-5472.CAN-12-0128. [This landmark study in non-human primates demonstrates that FUS-mediated BBB opening, performed using a clinical transducer, does not negatively impact visual or cognitive testing.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Burgess A, Hynynen K. Drug delivery across the blood-brain barrier using focused ultrasound. Expert Opin Drug Deliv. 2014;11:711–21. doi: 10.1517/17425247.2014.897693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scarcelli T, Jordão JF, O`Reilly MA, et al. Stimulation of hippocampal neurogenesis by transcranial focused ultrasound and microbubbles in adult mice. Brain Stimul. 2014;7:304–307. doi: 10.1016/j.brs.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hosseinkhah N, Hynynen K. A three-dimensional model of an ultrasound contrast agent gas bubble and its mechanical effects on microvessels. Exp Opin Drug Deliv. 2012;57(3):785–808. doi: 10.1088/0031-9155/57/3/785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krizanac-Bengez L, Mayberg MR, Janigro D. The cerebral vasculature as a therapeutic target for neurological disorders and the role of shear stress in vascular homeostatis and pathophysiology. Neurol Res. 2004;26(8):846–853. doi: 10.1179/016164104X3789. [DOI] [PubMed] [Google Scholar]
- 79.Chen H, Kreider W, Brayman AA, Bailey MR, Matula TJ. Blood vessel deformations on microsecond time scales by ultrasonic cavitation. Phys Rev Lett. 2011;106(3):034301. doi: 10.1103/PhysRevLett.106.034301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nyborg WL. Biological effects of ultrasound: development of safety guidelines. Part II: general review. Ultrasound Med Biol. 2001;27:301–33. doi: 10.1016/s0301-5629(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 81.Traub O, Ishida T, Ishida M, Tupper JC, Berk BC. Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. J Biol Chem. 1999;274(29):20144–50. doi: 10.1074/jbc.274.29.20144. [DOI] [PubMed] [Google Scholar]
- 82•.O`Reilly MA, Hynynen K. Blood-brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology. 2012;263:96–106. doi: 10.1148/radiol.11111417. [This study describes the development of a real-time acoustic feedback controller which increases the consistency of BBB opening and increases the safety of FUS treatment.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arvanitis CD, Livingstone MS, Vykhodtseva N, McDannold N. Controlled ultrasound-induced blood-brain barrier disruption using passive acoustic emissions monitoring. PloS One. 2012;7:e45783. doi: 10.1371/journal.pone.0045783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu SY, Tung YS, Marquet F, et al. Transcranial cavitation detection in primates during blood-brain barrier opening-a performance assessment study. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61(6):966–78. doi: 10.1109/TUFFC.2014.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Van Rooij T, Luan Y, Renaud G, et al. Response to ultrasound of two types of lipid-coated microbubbles observed with a high-speed optical camera. J Acoust Soc Am. 2014;135:2370. [Google Scholar]
- 86•.Sheikov N, McDannold N, Vykhodtseva N, Jolesz F, Hynynen K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med Biol. 2004;30:979–89. doi: 10.1016/j.ultrasmedbio.2004.04.010. [Electron microscopy was used to demonstrate that tracer molecules pass through the BBB using both paracellular and transcellular pathways after focused ultrasound application in the brain.] [DOI] [PubMed] [Google Scholar]
- 87.Sheikov N, McDannold N, Sharma S, Hynynen K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol. 2008;34:1093–104. doi: 10.1016/j.ultrasmedbio.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sheikov N, McDannold N, Jolesz F, Zhang Y, Tam K, Hynynen K. Brain arterioles show more active vesicular transport of blood-borne tracer molecules than capillaries and venules after focused ultrasound-evoked opening of the blood-brain barrier. Ultrasound Med Biol. 2006;32:1399–409. doi: 10.1016/j.ultrasmedbio.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 89.Lionetti V, Fittipaldi A, Agostini S, et al. Enhanced caveolae-mediated endocytosis by diagnostic ultrasound in vitro. Ultrasound Med Biol. 2009;35:136–43. doi: 10.1016/j.ultrasmedbio.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 90.Deng J, Huang Q, Wang F, et al. The Role of Caveolin-1 in Blood–Brain Barrier Disruption Induced by Focused Ultrasound Combined with Microbubbles. J Mol Neurosci. 2012;46:677–87. doi: 10.1007/s12031-011-9629-9. [DOI] [PubMed] [Google Scholar]
- 91.Choi JJ, Pernot M, Brown TR, Small SA, Konofagou EE. Spatio-temporal analysis of molecular delivery through the blood–brain barrier using focused ultrasound. Phys Med Biol. 2007;52(18):5509–30. doi: 10.1088/0031-9155/52/18/004. [DOI] [PubMed] [Google Scholar]
- 92.Alonso A, Reinz E, Fatar M, Hennerici MG, Meairs S. Clearance of albumin following ultrasound-induced blood-brain barrier opening is mediated by glial but not neuronal cells. Brain Res. 2011;1411:9–16. doi: 10.1016/j.brainres.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 93.Raymond SB, Treat LH, Dewey JD, et al. Ultrasound enhanced delivery of molecular imaging and therapeutic agents in Alzheimer's disease mouse models. 2008;3(5):e2175. doi: 10.1371/journal.pone.0002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cho EE, Drazic J, Ganguly M, Stefanovic B, Hynynen K. Two-photon fluorescence microscopy study of cerebrovascular dynamics in ultrasound-induced blood-brain barrier opening. J Cerebr Blood F Met. 2011;31(9):1852–62. doi: 10.1038/jcbfm.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jordão JF, Thévenot E, Markham-Coultes K, et al. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp Neurol. 2013;248:16–29. doi: 10.1016/j.expneurol.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jalali S, Huang Y, Dumont DJ, Hynynen K. Focused ultrasound-mediated bbb disruption is associated with an increase in activation of AKT: experimental study in rats. BMC Neurol. 2010;10:114–24. doi: 10.1186/1471-2377-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tufail Y, Matyushov A, Baldwin N, et al. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron. 2010;66:681–94. doi: 10.1016/j.neuron.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Marquet F, Wu S, Tung Y, et al. Real-time, transcranial monitoring of safe blood-brain barrier opening in non-human primates. Ultrason. 2012;2012:1–4. doi: 10.1371/journal.pone.0084310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tung Y, Wu S, Marquet F, Konofagou EE. Quantification of stable cavitation dose during FUS-induced blood-brain barrier opening in mice and in non-human primates. Ultrason. 2012;2012:244–7. [Google Scholar]
- 100.McDannold N, Vykhodtseva N, Hynynen K. Targeted disruption of the blood–brain barrier with focused ultrasound: association with cavitation activity. Phys Med Biol. 2006;51:793–807. doi: 10.1088/0031-9155/51/4/003. [DOI] [PubMed] [Google Scholar]
- 101.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem Biophys Res Commun. 2006;340:1085–90. doi: 10.1016/j.bbrc.2005.12.112. [DOI] [PubMed] [Google Scholar]
- 102.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proceedings Natl Acad Sci USA. 2006;103:11719–23. doi: 10.1073/pnas.0604318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Controlled Release. 2012;163:277–84. doi: 10.1016/j.jconrel.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burgess A, Huang Y, Querbes W, et al. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Controlled Release. 2012;163:125–9. doi: 10.1016/j.jconrel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang HB, Yang L, Wu J, et al. Reduced ischemic injury ater stroke in mice by angiogenic gene delivery via ultrasound targeted microbubble destruction. J Neuropathol Exp Neurol. 2014;73:548–558. doi: 10.1097/NEN.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 106.Thévenot E, Jordão JF, O'Reilly MA, et al. Targeted delivery of scAAV9 to the brain using MRI-guided focused ultrasound. Human Gene Ther. 2012;23:1144–55. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsu PH, Wei KC, Huang CY, et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8:e57682. doi: 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Treat LH, McDannold N, Zhang Y. Improved Anti-Tumor Effect of Liposomal Doxorubicin after Targeted Blood-Brain Barrier Disruption by MRI-Guided Focused Ultrasound in Rat Glioma. Ultrasound Med Biol. 2012;38:1716–25. doi: 10.1016/j.ultrasmedbio.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aryal M, Vykhodtseva N, Zhang YZ. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood-brain barriers improve outcomes in a rat glioma model. J Control Release. 2013;169:103–11. doi: 10.1016/j.jconrel.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kovacs Z, Werner B, Rassi A, Sass JO, Martin-Fiori E, Bernascni M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Control Release. 2014 doi: 10.1016/j.jconrel.2014.05.033. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 111.Mei J, Cheng Y, Song Y, et al. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging-guided focused ultrasound. J Ultrasound Med. 2009;28:871–80. doi: 10.7863/jum.2009.28.7.871. [DOI] [PubMed] [Google Scholar]
- 112.Liu HL, Hua MY, Yang HW, et al. Magnetic resonance monitoring of focused ultrasound / magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci USA. 2010;107:15205–10. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fan CH, Ting CY, Liu HL, et al. Antiangiogenic drug-loaded microbubbles combined with focused ultrasound for glioma treatment. Biomaterials. 2013;34:2142–2155. doi: 10.1016/j.biomaterials.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 114.Chen PY, Liu HL, Hua MY, et al. Novel magnetic / ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro Oncol. 2010;12:1050–60. doi: 10.1093/neuonc/noq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ting CY, Fan CH, Liu HL, et al. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials. 2012;33:704–12. doi: 10.1016/j.biomaterials.2011.09.096. [DOI] [PubMed] [Google Scholar]
- 116.Liu HL, Hua MY, Yang HW, et al. Magnetic resonance monitoring of focused ultrasound/magnetic nanoparticle targeting delivery of therapeutic agents to the brain. Proc Natl Acad Sci. 2010;107:15205–10. doi: 10.1073/pnas.1003388107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Timbie K, Burke C, Nance E, et al. Ultrasound-targeted delivery of systemically administered therapeutic nanoparticles. J Acoust Soc Am. 2013;134:4047. [Google Scholar]
- 118.Etame AB, Diaz RJ, O'Reilly MA, et al. Enhanced delivery of gold nanoparticles with therapeutic potential into the brain using MRI-guided focused ultrasound. Nanomed. 2012;8:1133–1142. doi: 10.1016/j.nano.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Diaz RJ, McVeigh PZ, O'Reilly MA, et al. Focused ultrasound delivery of Raman naonparticles across the blood-brain barrier: Potential for targeting experimental brain tumors. Nanomedicine. 2013 doi: 10.1016/j.nano.2013.12.006. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Burgess A, Ayala-Grosso CA, Ganguly M, Jordão JF, Aubert I, Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6:e27877. doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Alkins R, Burgess A, Ganguly M, et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013;73:1892–1899. doi: 10.1158/0008-5472.CAN-12-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hynynen K. Human brain's ultimate barrier to open for first time: It's neuroscience's final frontier. Tiny bubbles will open the blood-brain barrier to sneak drugs into tumours – and we might treat Alzheimer's the same way. New Scientist. 2014 [Google Scholar]
- 123.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nature Reviews Neuroscience. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]