Abstract
Objective
To explore the value of multiple clinical endpoints in the unique setting of ovarian cancer.
Methods
A clinical trial workgroup was established by the Society of Gynecologic Oncology to develop a consensus statement via multiple conference calls, meetings and white paper drafts.
Results
Clinical trial endpoints have profound effects on late phase clinical trial design, result interpretation, drug development, and regulatory approval of therapeutics. Selection of the optimal clinical trial endpoint is particularly provocative in ovarian cancer where long overall survival (OS) is observed. The lack of new regulatory approvals and the lack of harmony between regulatory bodies globally for ovarian cancer therapeutics are of concern. The advantages and disadvantages of the numerous endpoints available are herein discussed within the unique context of ovarian cancer where both crossover and post-progression therapies potentially uncouple surrogacy between progression-free survival (PFS) and OS, the two most widely supported and utilized endpoints. The roles of patient reported outcomes (PRO) and health related quality of life (HRQoL) are discussed, but even these widely supported parameters are affected by the unique characteristics of ovarian cancer where a significant percentage of patients may be asymptomatic. Original data regarding the endpoint preferences of ovarian cancer advocates is presented.
Conclusions
Endpoint selection in ovarian cancer clinical trials should reflect the impact on disease burden and unique characteristics of the treatment cohort while reflecting true patient benefit. Both OS and PFS have led to regulatory approvals and are clinically important. OS remains the most objective and accepted endpoint because it is least vulnerable to bias; however, the feasibility of OS in ovarian cancer is compromised by the requirement for large trial size, prolonged time-line for final analysis, and potential for unintended loss of treatment effect from active post-progression therapies. A large magnitude of effect in PFS improvement should establish benefit, and further communication with regulatory authorities to clarify acceptable endpoints should be undertaken.
Keywords: Ovarian cancer, Clinical trial endpoints, Progression free survival, Overall survival
Introduction
The proper selection of endpoints for clinical trials is paramount, and should be considered within the context and course of the disease in question. Additionally, not every clinical question can be answered by a single study. Some clinical trials are designed as hypothesis generating studies, others for regulatory purposes, and some are designed to change clinical practice. Meaningful and unambiguous trial endpoints define clinical investigation success and should reflect true patient benefit and risk from a given therapy; these landmarks justifiably influence patient care. Finally, clinical trial endpoints and the standards to which they are held significantly impact the regulatory and approval process for new drug applications. Herein we present the unique features of ovarian cancer that influence the selection of endpoints for late phase ovarian cancer clinical trials and attempt to harmonize the various viewpoints. We critically examine the validity of ovarian cancer clinical trial endpoints in the modern era, with particular attention given to surrogate endpoints. We also present novel data regarding the endpoint preferences of ovarian cancer advocates.
Improved survival in patients affected by ovarian cancer
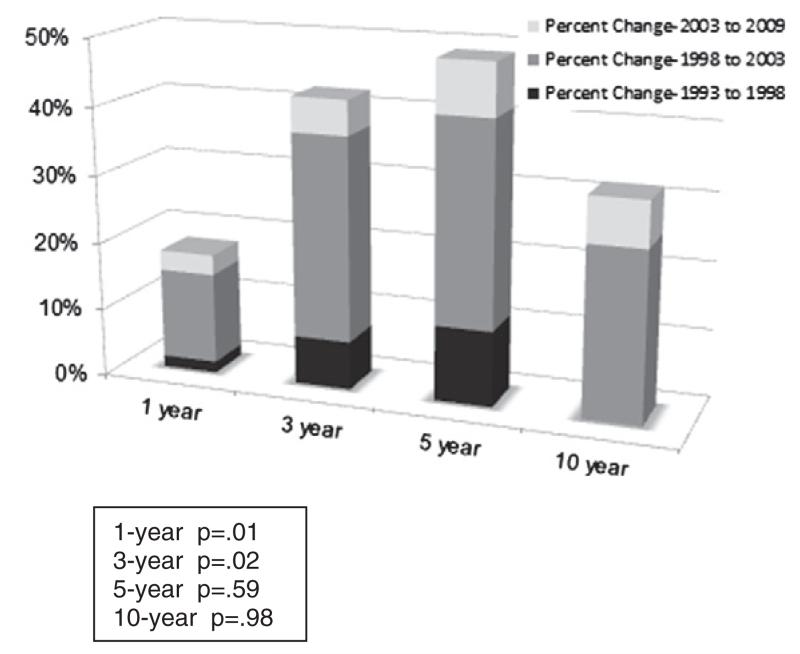
Epithelial ovarian cancer, primary peritoneal cancer and fallopian tube cancer collectively referred to as “ovarian cancer”, is a heterogeneous disease with unique molecular, pathologic and clinical features that afflicts approximately 22,400 women in the U.S. and 225,000 women worldwide annually [1,2]. Over the last 30 years, there has been a two-year improvement in the median overall survival for women in the United States with advanced stage ovarian cancer [3]. Worldwide data from FIGO covering four decades indicate that the overall 5-year survival rate for ovarian cancer has improved from 26.8% in 1958 to 49.7% in 2001 [4]. Similarly, administrative data show that the 5-year relative ovarian cancer survival rate has improved from 36.6% in 1975 to 44.0% in 2008 [5]. These improvements are not attributed to stage migration as the relative ratio of advanced to early stage disease at presentation has not changed over the years, and proportionately, similar improvements in median survival have been noted across all stages [6]. Fig. 1 depicts the percentage improvement by time interval contributed by treatment era and shows that long term survival (i.e. cure) has not improved, while short term survival improvements are likely due to improved surgical and perioperative medical management and to more effective systemic adjuvant therapy.
Fig. 1.
Relative overall survival trends: ovarian cancer. Percent changes over time adapted by Coleman RL from van Altena AM, Karim–Kos HE, de Vries E, Kruitwagen RF, Massuger LF, Kiemeney LA. Trends in therapy and survival of advanced stage epithelial ovarian cancer patients in The Netherlands. Gynecol Oncol 2012; 125(3):649-54. (Ref [6]).
Unlike other solid tumors presenting with large volume metastatic intra-peritoneal (IP) disease, newly diagnosed ovarian cancers appear to benefit from a comprehensive surgical cytoreduction. A report of advanced ovarian cancer survival showed a 29% improvement in survival (hazard ratio of 0.71) that was largely attributed to changes in treatment, including an improvement in the optimal debulking rate from 43% to 66% [7].
Refinements in systemic therapy, specifically the introduction of new agents and delivery approaches, have significantly complemented recent advances in the surgical management of ovarian cancer, particularly for patients with newly diagnosed advanced stage ovarian cancer. A review of Gynecologic Oncology Group (GOG) and other international trials over the last 20 years highlights several major contributions of systemic therapy to the improvement in median survival noted in ovarian cancer patients. Key among these developments has been the addition of taxanes (GOG 111 and OV10) [8,9] and IP chemotherapy[10-12]. Long-term follow-up of two of the three IP trials demonstrated that patients resected to no visible residual treated with IP therapy had a median OS of 9.2 years [13].
Recent trials have explored the efficacy of dose-dense and/or dose-intense IV paclitaxel administration, addition of biological agents, and the combination of the two. For example, a weekly paclitaxel regimen (dose dense and dose intense relative to the control arm) [80 mg/m2/week vs. 60 mg/m2/week] given in combinations with every 3-week carboplatin improved 3-year median survival from 65 to 72 months in patients with stages I–IV ovarian cancer (HR 0.75, p = 0.032) [14]. Ongoing phase III clinical trials, such as GOG 252 (NCT00951496), MITO-7 (NCT00660842) TRINOVA-3 (NCT01493505) and GOG 262 (NCT00687687) will clarify the impact of dose fractionated chemotherapy, with and without biological adjuvants.
Similar refinements in treatment of recurrent disease have contributed to observed incremental survival gains. Improved OS has been observed in patients with recurrent ovarian cancer who have complete resection to no visible disease [15]. Secondary surgical cytoreduction for recurrent disease is currently being studied in randomized trials (GOG 213 NCT00565851, DESKTOP III NCT01166737 and SOC-1 NCT01611766). Improved survival has been observed over time in ovarian cancer populations with similar prognostic features receiving similar treatments. As an example, recurrent, platinum sensitive ovarian cancer patients treated with the combination of gemcitabine and carboplatin from 1999 to 2002 had a median PFS of 8.6 months [16]. Similar patients treated with the same regimen from 2007 to 2010 on the control arm in the OCEANS trial had a nearly identical median PFS of 8.4 months [17]. Yet, the median OS for the group treated in the earlier study was 18 months compared to 35 months for the more contemporary cohort. One potential explanation for this difference is the potential impact of post progression therapies on outcomes for more recently diagnosed ovarian cancer patients, as well as better overall medical and supportive care.
Unfortunately fewer than 20% of patients with advanced ovarian cancer will survive long-term. The gains in median survival are largely due to an extension of survival time in patients rather than an increase in overall cure [1]. Thus, there is a clear need for novel therapeutic approaches for the thousands of patients affected by this deadly disease. The ability to encourage investigation of new therapeutic approaches for ovarian cancer given the current management and the recent gains in median survival noted with these management approaches requires a re-examination of the selection of endpoints for ovarian cancer clinical trials.
Selection of endpoints for phase III clinical trials involving women diagnosed with ovarian cancer
Traditional endpoints for epithelial ovarian cancer phase III clinical trials have included response rate (RR), time to progression (TTP), progression free survival (PFS), and overall survival (OS). Table 1 summarizes the features of each endpoint and includes novel endpoints such as time to tumor growth as well. Secondary endpoints such as toxicity and patient reported outcomes (PRO) are also being measured and reported with greater frequency and are influencing clinical decision-making by providing context through which to consider the primary endpoint observations.
Table 1.
Endpoint characteristics.
| Endpoint | Definition | Advantages | Disadvantages |
|---|---|---|---|
| Response rate (RR) | Assessed by the RECIST criteria on the basis of imaging studies. GCIG has defined changes in CA-125 as a response criterion |
Objective Quantifiable Results quickly available Tumor shrinkage appealing to patient and physician |
Difficult to measure accurately and reproducibly in ovarian cancer Not considered sufficient as a primary endpoint for phase III trials Not necessarily a clinically relevant benefit for the patient |
| Patient-recorded out- comes (PRO) |
Symptom-based parameters | Direct clinical benefit as perceived and quantified by patient |
Need randomized blinded studies Subjective and dependent on limited validation instruments |
| Progression-free surviva (PFS) |
Time from entry into trial to progression of disease, death or lost to follow up |
Provides answer sooner Avoids the impact of post-progression thera- py Preferred to TTP by regulatory agencies |
Requires blinded, placebo-controlled design Requires careful and symmetric timing of assess ments |
| Time to progression (TIP) |
Time from entry into trial to progression of disease | Similar to PFS | Does not include deaths |
| Overall survival | Time from entry into trial to death or lost to follow up | Clear cut end point of death Indisputably indicative of clinical benefit |
Longer time to answer Impacted by post-progression therapy |
| Time to tumor growth | Utilizes prescribed longitudinal tumor models | Novel metric In some models best predictor of OS Reduces time and cost |
Not validated in ovarian cancer Subjectivity in assessment |
There has been considerable controversy regarding the most appropriate endpoints for phase III clinical trials for patients with epithelial ovarian cancer. Indeed, endpoint selection remained the only unresolved issue regarding clinical trial design at the Gynecologic Cancer Intergroup’s (GCIG’s) Ovarian Cancer Consensus Conference in 2004 [18]. There was subsequent resolution with the unanimous adoption of PFS as the “most often preferred primary endpoint” for advanced ovarian cancer front-line trials in the 2010 Conference statement [19]. The clinical relevance of any endpoint can be assessed from several perspectives: the patient, the physician, or society as a whole. For patients, at least some studies have suggested that their primary health-related concerns focus on their prospects for a long and symptom-free survival[20,21]. Survival and health related quality of life (HRQoL) have therefore been endpoints upon which patients focus. Thus both PFS and OS appear to be clinically relevant from the patient’s perspective by avoiding disease progression and additional treatments.
How precisely does PFS correlate with OS? Large meta-analyses have previously indicated that PFS is a good surrogate for OS in first-line treatment trials for ovarian cancer [22,23]. One recent paper analyzed 59 phase III trials and showed an excellent correlation of differences of medians, ratio of medians, and hazard ratios for PFS and OS [24]. However in ovarian cancer, the data supporting the use of PFS as a surrogate for OS arose primarily from studies that used traditional cytotoxic chemotherapies in an era when effective second or subsequent line therapies were limited. Extrapolating this conclusion to the current era where prolonged post-progression survival (PPS) is associated with multiple lines of subsequent therapy should be examined cautiously. The results of two large recently completed trials involving bevacizumab in newly diagnosed ovarian cancer patients are emblematic of this issue [25,26]. In addition, previous studies supporting the surrogacy of PFS for OS did not permit rising CA-125 as an indication of progression. The magnitude of effect for PFS differences is influenced by how CA-125 is used in determining progression as illustrated in GOG 218 whereby CA-125 incorporation into progression determination resulted in a larger magnitude of effect difference between the treatment (arm III) and control groups (arm I) [25]. Current studies variably allow biomarker measures of progression. The extent to which this allowance strengthens or weakens the evidence for OS surrogacy is unknown. The GCIG has incorporated criteria for utilizing CA-125 as a clinical trial endpoint (Table 1—Supplemental) [27].
There are currently insufficient data to support the conclusion that PFS is a reasonable surrogate for OS in second- or third-line therapy trials [24]. Even if PFS is found to be a good surrogate for OS, it does not necessarily follow that a statistically significant PFS difference will translate into a clinically or statistically significant OS difference. Small differences in PFS, even when statistically different, have not been shown to reliably predict a non-zero treatment effect on OS [28]. Importantly, however, there is at least one randomized phase III trial in platinum-sensitive recurrent ovarian carcinoma that shows an improved HRQoL and longer time off treatment associated with improved PFS in the absence of improved OS [16].
Clinical trial endpoints should avoid known sources of bias whenever possible. Time to death can generally be objectively measured without bias. Since assessment of the cause of death can occasionally be subjective, most studies involving patients with advanced cancers will assess all-cause survival in order to remove this potential source of bias. PFS, on the other hand, is susceptible to at least two important biases. First is the subjectivity in the interpretation of progression based upon the tools of assessment. For instance, imaging studies used in recent statistically positive phase III ovarian cancer trials were plagued by a 45% variance between investigators and central review on the designation of progression. However, the overall hazard ratios for PFS and OS, as well as the interpretation of treatment effect did not differ substantially between investigator assessments and central reviews [29]. There are procedures that can be implemented to reduce or monitor this potential source of bias [30]. Clinical trials that use PFS as a primary endpoint should consider blinding the study treatments. In the event that blinding is not feasible or that the study treatments have differential toxicity profiles that undermine the blinding, a centralized audit process for randomly selected cases can be implemented.
PFS is also susceptible to assessment-time bias. Since the onset of progression can only be assessed at discrete times, the precise date for progression cannot be determined. Deviations from the pre-specified schedule for disease assessments can artificially increase or decrease the measured progression-free interval. However, there are procedures that can ameliorate the effects of assessment-time bias in non-blinded studies that use PFS as a primary endpoint [31].
To be feasible, a study endpoint must obtain a reasonable balance between patient burden and study resources. Obviously, endpoints that do not excessively burden the patient while accurately measuring safety and efficacy are preferable. In order to appreciate the feasibility for study resources, consider GOG-218. In order to attain the desired precision for estimating the hazard ratios, the study was sufficiently mature for the final analysis when at least 1045 patients had experienced either progression or death. This occurred approximately 4 years after the study opened. Had OS been selected as the primary endpoint with the same degree of precision for estimating the treatment hazard ratios imposed, the study would not have matured until at least 7 years after it opened. All of the enrolled patients in this study had gross residual disease. Had this study enrolled patients with no residual disease following their staging surgery or patients with only stage 1 or 2 disease, either the time required for study maturity would have been even longer or appreciably more patients would have had to be enrolled. Moreover, it has been argued that, as more active post-progression treatments become available, the likelihood of preserving a treatment-related effect in an OS primary analysis will become increasingly unattainable [32]. Requiring that excessively long trials be conducted conflicts with societies’ primary goal, which is to quickly identify clinically effective treatments for patients.
The impact of post-progression therapy on clinical trial endpoints
Clinical trials assessing a “proximal” treatment effect endpoint, such as objective response or progression free survival, are vulnerable to the impact of post-progression therapy for analysis of overall survival. This is because post-progression therapy may include an unbalanced crossover to the experimental agent used in the initial randomization, if available, as well as uncontrolled use, duration and assessment of subsequent standard agents. Prior to 1990, relatively few active agents for the treatment of epithelial ovarian cancers were available, thus this concern was largely mitigated. In a review of pre-2001 phase III trials, a meta-analysis shows that PFS was a reasonable surrogate for OS in 13 of 15 instances [22].
Two trials in which significant improvement in PFS was not reflected in significant improvement in OS highlight the challenges of a crossover effect. GOG 47 randomized patients to doxorubicin/cyclophosphamide ± cisplatin and showed a significantly improved PFS without a significantly improved OS. Because cisplatin was commercially available, patients assigned to doxorubicin–cyclophosphamide frequently received cisplatin upon progression with a resultant confounding of the survival endpoint. Similarly, GOG 132, a trial that randomized advanced ovarian cancer patients to paclitaxel or cisplatin or the combination of the two agents, demonstrated no survival difference. Since both cisplatin and paclitaxel were available commercially, the majority of patients assigned to the single agent regimens immediately received the other agent upon completion of the assigned protocol therapy or prior to documented progression. This crossover and post-progression therapy could have confounded the OS endpoint in these trials. In contrast, an earlier GOG trial (GOG 111), conducted in an era when paclitaxel was not commercially available, showed both PFS and OS advantages, with similar hazard ratios. GOG 172, however, was conducted in an era when effective post-progression agents were available and yet an OS advantage was preserved; thus, if a drug or intervention is sufficiently robust, a difference can be observed [12].
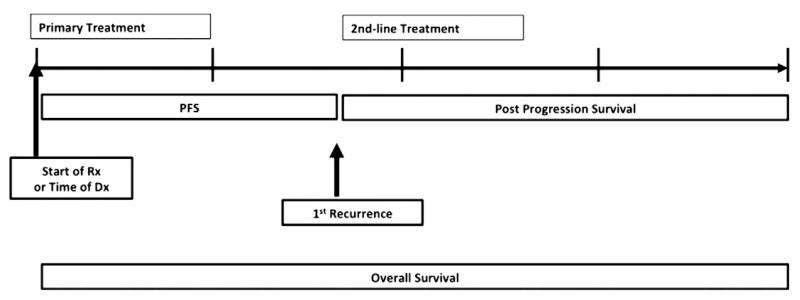
The number of active agents in ovarian cancer has escalated rapidly since 1995 leading to additional post-progression therapy options and further dilution of the correlation of PFS to OS. As such, contemporary ovarian cancer care produces a relatively long post-progression survivorship (PPS). Fig. 2 demonstrates how PPS is a major component of OS in ovarian cancer. Indeed, the ratio of the median OS to PFS times from trials involving ovarian cancer patients who appear sensitive to chemotherapy (frontline adjuvant and platinum-sensitive recurrence) range from 2.5 to 4:1 [8-12,14,16,17,33,34]. This prolonged PPS results in many ovarian cancer patients experiencing disease recurrences that feature undulating tumor burdens with multiple opportunities to intervene with subsequent therapies and makes correlation of PFS and OS less reliable (Fig. 3A).
Fig. 2.
Defining overall survival.
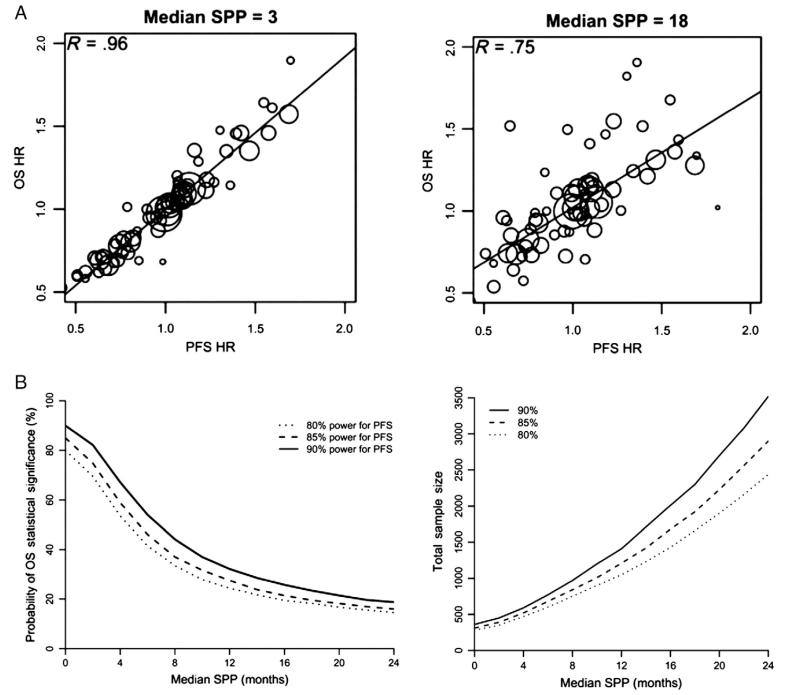
Fig. 3.
A. Loss of correlation of PFS with OS as a function of increasing PPS (post-progression survival, referred to below as SPP or survival post progression). B. Power to preserve a PFS effect. SPP (survival post progression).
Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009;101(23):1642-164927. Reprinted by permission of Oxford University Press.
The long anticipated PPS (22–40 plus months) places an enormous burden on PFS-defined treatment effect. For example, suppose that a new treatment increases the median PFS from 6 to 9 months (assuming proportional hazards). A typical study designed with 80% power to detect this treatment effect would enroll about 280 patients. If the median post-progression survival of 24 months is not affected by the study treatments then the same trial using OS as the primary endpoint would have no more than a 20% power. In order to retain 80% power for detecting this treatment effect size with an OS endpoint, the study would require 2440 patients instead (Fig. 3B). While Broglio et al. [35] emphasize the appreciable loss in statistical power in this contrived situation when OS is the primary endpoint, the low power is primarily due to the fact that this treatment effect size is so small. In fact it is much smaller than most patients reasonably expect (see survey results below) and this is the reason why this study design would be considered impractical.
When OS is a study endpoint, there are two distinct situations concerning the impact of post-progression therapies. The first case is when the study is conducted so that the standard treatments are provided to all patients once progression occurs. This is the appropriate setting for evaluating OS in phase III treatment trials when most patients in actuality receive the best available post-progression therapies. The query of whether the experimental treatment would be deemed effective theoretically where no treatments or only ineffective treatments are available is not clinically relevant to the patient. From the patients’ point of view, unless the new experimental treatment increases the duration and/or the quality of survival, “it doesn’t matter much whether the new experimental treatment failed or the subsequent standard treatments succeeded.” [36].
On the other hand, there may be some benefit in identifying agents that significantly delay disease progression as some believe that incremental OS gains have been made by cobbling the collective PFS gains of an expanding array of active agents. This hypothesis is challenged by Korn as well who argues that as long as there is not a differential benefit with subsequent treatment in the non-experimental arm then the PFS benefit initially observed should translate into an OS benefit [35]. Nonetheless, Korn also points out the clinical trial feasibility of subsequent therapies and decreasing power of the study to show an OS difference [37]. Subsequent treatment increases OS variability, thus a 60% larger sample size would be required to detect an OS improvement from 8 to 11 months versus an improvement from 6 to 9 months due to subsequent therapy that benefits both groups by 3 months. This effect is further compounded in ovarian cancer where subsequent treatment lines generally exceed the single additional line in this theoretical modeling.
In a second type of study design, the experimental study-intervention is offered to those patients, who were initially randomized to the standard treatment, after disease progression. This study design can be used to assess whether the experimental treatment should be given immediately or delayed. However, when a clinically relevant intermediate endpoint is unavailable, using this type of trial design to establish clinical efficacy is not recommended.
Optimal endpoint in maintenance treatment
The subject of optimal endpoints in the maintenance setting has been a contentious issue and most of the same arguments relevant to advanced front-line trials apply to this setting. Some have argued that maintenance therapy should confer an OS advantage and that the PFS endpoint is contaminated by extension of therapy in the maintenance cohort. Examining time off therapy and PRO/HRQoL metrics may be of value in this setting. The 2010 Consensus group opined that PFS was a viable endpoint in this setting; “unanimous consensus was that the same criteria adopted for phase III trials of advanced ovarian carcinoma should apply.” [19].
Impact of clinical trial endpoints on regulatory approval for new ovarian cancer therapeutics
Endpoints in ovarian cancer clinical trials serve different purposes. Endpoints for phase III efficacy studies generally evaluate whether a compound provides a clinical benefit such as prolongation of survival or an improvement in symptoms through tumor shrinkage (RR) or delaying progression. Phase III studies can be conducted to facilitate regulatory approval, permitting manufacturers to promote and sell their products. The history of successful FDA approvals in ovarian cancer is summarized in Table 2.
Table 2.
History of successful FDA approvals in ovarian cancer.
| Drug | Date, indication | Endpoint | Notes |
|---|---|---|---|
| Cisplatin | 1978,1st & 2nd line, refractory. | RR | Phase 2, randomized, N = 52 cisplatin vs. cisplatin/adriamycin vs. thiotepa alone or plus methotrexate RR 42% vs. 67% vs. 36% Phase 2, randomized, N = 52 cisplatin alone vs. cisplatin/ hydration/mannitol RR 42% vs. 63% |
| Carboplatin | 1989, 2nd line refractory 1991,1st line | RR survival | - |
| Altretamine | 1990, 2nd line, refractory. | RR | Received regular approval based on 2 single-armed studies: RR 20% (13/51) and 14% (3/21). Duration of response 2–36 months. |
| Paclitaxel | 1992, 2nd line, refractory. | RR | Phase 3, bifactorial design, compared 2 different doses, schedules, N = 407, RR 16.2%, 95% Cl 12.8–20.2%, Duration response, 8.3 months (3.2–21.6) |
| Topotecan | 1996, 2nd line, refractory. Treatment for metastatic ovarian cancer. |
TTP, survival, RR |
Randomized study, topotecan vs. paclitaxel. TTP(wks) 18.9 vs. 14.7 OS (wks) 63.0 vs. 53.0 RR 21% vs. 14% Duration (wks) 25.9 vs. 21.6 HR 0.97 p = 0.87 |
| Liposomal doxorubicin | June 1999, treatment for ovarian cancer. Refractory to other 1st-line therapies |
RR, OS, TTP, RR | Accelerated approval in 1999. 3 single-arm studies RR 13.8% (20/145) Duration of response 39.4 weeks. Regular approval in 2005. Randomized study liposomal doxorubicin vs. topotecan OS 14.4 months vs. 13.7 months (p = 0.55, HR 0.82) TTP4.1 months vs. 4.2 months (p = 0.617, HR 0.95) RR 19.7 vs. 17.0% Duration of response: median 6.9 months vs. 5.9 months |
| Combination gemcitabine– carboplatin |
June 2006, for treatment of patients with advanced ovarian cancer that has relapsed at least 6 months after completion of platinum-based therapy |
PFS | Single multicenter, international, open-label, randomized trial enrolling 356 patients Gemcitabine–carboplatin (GC) median PFS was 8.6 months vs. 5.8 months for C-treated patients [log rank p = 0.0038; hazard ratio 0.72 (95%, C.I. 0.57, 0.90)]. RR47% vs. 31%, p = 0.0016) OS for GC was 18.0 months vs. 17.3 months for C [p = 0.8977, hazard ratio 0.98 (95% C.1.0.78,1.24)]. |
Reprinted from Gynecol Oncol. 2007 Nov;107(2):173-6. Clinical trial endpoints in ovarian cancer: report of an FDA/ASCO/AACR Public Workshop. Bast RC, Thigpen JT, Arbuck SG, Basen-Engquist K, Burke LB, Freedman R Horning SJ, Ozols R Rustin GJ, Spriggs D, Wenzel LB, Pazdur R With permission from Elsevier.
In the United States, the Food and Drug Administration (FDA) evaluates effectiveness claims in new drug applications (NDAs), biologics license applications (BLAs), or supplemental applications. The requirement that new agents should show effectiveness is based on a 1962 amendment to the Federal Food, Drug, and Cosmetic Act. This law requires substantial evidence of effectiveness and specifies that this evidence must be derived from adequate and well-controlled clinical investigations. Similarly, the Public Health Service Act requires biological products to be safe, pure, and potent.
In spite of the increase in FDA approved drugs for ovarian cancer since the early 1990’s, there have been no new agents approved by the FDA for ovarian cancer since 2006. In contrast, the European Medicines Agency (EMA), the primary regulator serving the European Union, has approved two new agents for ovarian cancer since 2009 (Table 3).
Table 3.
Disparate regulatory approvals.
| Drug | Date, indication | Endpoint | Notes |
|---|---|---|---|
| Trabectedin Europe–Other Non-US |
September 2009, EC–approved for platinum-sensitive recurrent ovarian cancer |
Primary outcome measures: PFS assessed by independent radiology review Secondary outcome measures: OS RR, PK And safety |
April 2011, J&J voluntarily withdraws trabectedin US NDA |
| Bevacizumab Europe–Other Non-US |
2012,1st Line in combination with standard chemotherapy (carboplatin & paclitaxel) following surgery with advanced ovarian cancer. |
PFS | 2 phase III studies 19.3 months vs 16.9 months 14.7 months vs 10.6 months |
Reprinted from Gynecol Oncol. 2007 Nov;107(2):173-6. Clinical trial endpoints in ovarian cancer: report of an FDA/ASCO/AACR Public Workshop. Bast RC, Thigpen JT, ArbuckSG, Basen-Engquist K, Burke LB, Freedman R, Horning SJ, Ozols R, Rustin GJ, Spriggs D, Wenzel LB, Pazdur R. With permission from Elsevier.
Reasons for the lack of new agents being approved by the FDA for ovarian cancer since 2006 are unclear. A recent study that analyzed novel cancer drugs approved between 2003 and 2010 demonstrated that the drug-approval process was faster at the FDA than at the EMA. Thus, the drug approval process in the U.S. does not seem inordinately slow when compared to other countries.
Have the current endpoints utilized in appropriately designed ovarian cancer clinical trials to secure regulatory approval become less relevant considering the modern management of ovarian cancer? As stated at the jointly sponsored FDA, American Society of Clinical Oncology, and American Association for Cancer Research 2006 Ovarian Cancer Endpoints Workshop, survival is the gold standard in oncology treatment trials [22]. In the past, both response rate and survival have been the basis of drug approvals for first-line therapies for ovarian cancer. For second- and third-line treatments, response rate, time to progression, and survival have been the bases for approvals. If not traditional endpoints, should some new formula for regulatory approvals be considered? Clearly, the entire application including endpoints, toxicity and the magnitude of effect must be considered. Chan and colleagues suggested that the combination of a favorable PFS HR associated with minimal grade 3 and 4 toxicity predicted FDA approval of anti-neoplastic drugs between 2000 and 2011[38]. Regulatory agencies need to consider both indirect and direct PFS benefits where PFS correlates with OS in the former and additional PRO or HRQoL benefit is seen in the latter.
Should cost factor into the FDA’s decision to approve a drug, given that EMA approval does not de facto result in commercial availability of any approved agent? Also should PROs and HRQoL be worthy of consideration? Clearly, there is a need to evaluate new approaches to designing clinical trials that assess relief of the burden of ovarian cancer and that allow different pathways towards regulatory approval of new agents. Additionally, better harmonization and consistency between the EMA and FDA are needed.
Innovation in clinical trial designs and biomarkers in a world of rapid cancer discoveries
Advances in efficient study designs
A vexing problem from the perspective of novel therapeutic development has been how to make discovery more efficient in the setting of increasing regulations and prolonged survival times for diseases such as ovarian cancer. The science of clinical trials will need to keep pace with pathway and novel molecule discoveries, as the evolving dilemma is that innovation has generated more pathways and potential treatment agents than can be evaluated in available clinical trials. Efficiencies in trial design and execution such as adaptive and rolling trial constructs and the creation of newly validated surrogate markers of efficacy should be assessed and validated. Consideration of clinical trial endpoints is a pivotal part of this process as we continue to bring progress to our ovarian cancer patients.
There have been improvements in efficiencies to identify patients that would derive the most benefit from a specific treatment regimen while minimizing toxicities. The traditional phase I–IV drug development process is time-consuming, inefficient, and potentially can subject patients to ineffective therapies that can have significant toxicity [39]. Combining different phases, such as phase I/II “window” trials can decrease the amount of time that it takes to reach efficacy endpoints.
Adaptive trial designs may expose fewer patients to less effective treatments and accelerate the identification of effective treatments. Adaptive designs can incorporate an exploratory phase as well as a confirmatory phase into a single study. The exploratory phase is useful when some details concerning the study hypothesis have not been resolved [39]. These designs allow more flexibility and dynamism to the study including changes of sample sizes, or the treatment allocation ratios. For instance, in an adaptive design, assignment can be skewed to favor the treatment that is performing best thus far in a trial proportionally to the magnitude of effect.
It is a common misconception that adaptive designs provide sufficient flexibility that less pre-trial planning is required. On the contrary, experience has demonstrated that adaptive designs require more trial planning and forethought. Some of the concerns over adaptive trial designs include whether this ‘adaptation’ process can potentially introduce biases and increase false discovery (Type I error). Sometimes the adapted process can lead to positive study results that are difficult to interpret [40]. Adaptive randomization designs, by their very nature, mean that subjects enrolled later in a trial will more likely be assigned to more effective treatments. Thus, a bias might arise from patients enrolling early in adaptive design studies that might differ from those enrolling later [41]. Parmar et al. describe multi-arm, multi-stage trial designs that tend to drop ineffective treatments early based on the available data and hence accelerate evaluation of novel cancer agents [42].
Predictive biomarkers
In the era of emerging biomarker and tumor profile technologies, the ability to utilize newly identified markers that could predict improved response has a clear clinical benefit. However, the validation and reproducibility of these methods have been obstacles in making them useful for clinical trials or clinical practice. It is the onus of clinical researchers to consider overlaying new tests (companion diagnostics) and biomarkers into current clinical trials in order to determine the predictive usefulness. This would optimize enrollment of subjects who would have the greatest chance to benefit and prevent undue toxicity on a specific drug regimen.
Ovarian cancer, in general, is lagging in the development of new predictive biomarkers compared to other solid tumors. Early markers that can predict treatment efficacy, or lack thereof, are imperative. Selective biomarker approaches may also lead to cost-equipoise, or even potential cost-savings.
Most recently, ovarian cancer has been subdivided into subgroups according to morphology and molecular sub-typing [43]. For example, high-grade serous cancers are characterized by defects in homologous recombination repair and mutations in p53. Low grade serous cancers typically have molecular perturbations in the of the RAS–RAF–MAPK signaling pathways [44]. Additionally, clear cell and endometrioid carcinomas have inactivation of pathways involving ARID1A, PI3K, Wnt, and PP2A [45]. Together, this realization can be critical to future clinical trial design.
Addressing which endpoints ovarian cancer patients find relevant
Much of the dialog surrounding endpoints of therapeutic studies has come from investigators trying to bring context to observed data. As has been presented, much of this context centers around the differential impact of therapy from interventions usually focused on the median points of survival and the degree of toxicity. Data in the area of patient preferences has been sparse, but the patients are an important voice in the discussion of defining clinical benefit. Limited opinion and data exist in this arena [20].
Largely missing from the dialog is the context from patient expectations. To facilitate this aspect of the conversation, we developed a brief, online survey in collaboration with the Ovarian Cancer National Alliance (OCNA) detailing clinical scenarios and common toxicities associated with contemporary management. In addition to demographic data outlining current treatment and disease status, we provided definitions of commonly used survival and response terms, as well as “trade-off” tools balancing efficacy and toxicity for initial and recurrence therapy. We sought to identify minimally acceptable benefits from clinical studies as well as, unacceptable toxicity in the face of “positive” treatment effects.
The web-based survey tool witnessed over 2200 visits in a 3-week period with 1400 completed questionnaires. As expected, the majority of respondents were women with a current or previous history of ovarian cancer, were under active treatment, and have experienced several lines of therapy. Patients felt that both progression-free and overall survival effects were important outcome variables to clinical trials; however, they expected and wanted large differences. When asked what was “minimally acceptable”, the overwhelming majority (>70%) expected 5 or more months difference in the median variables of PFS and OS (Fig. 4A). However, patients were unwilling to assume greater toxicity from treatment even if higher efficacy was proposed. Patients acknowledged that stable disease is an important parameter, but it was less acceptable to those in remission relative to those who had experienced disease recurrence (Fig. 4B). When asked about toxicity, there was a significant shift in tolerance depending on whether the treatment was delivered in a “curative” setting; patients receiving curative therapy would accept twice the toxicity of those receiving palliative therapy.
Fig. 4.
A. Patient endpoint thresholds of minimal benefit. Ovarian cancer (N = 1204). B. How important is stable disease? Ovarian cancer (N = 1088, p < 0.001).
These data reinforce that tangible gains in endpoints from ovarian cancer trials are important for patient acceptance of new therapy. Patient expectations are high and mirror those advocated by clinicians trying to bring context of trial results to patient counseling. However, the trade-off of toxicity and survival endpoints is variably affected in the continuum of therapy and more scientific investigation into this topic is needed.
Conclusions
Effective ovarian cancer therapy remains an unmet clinical need as over 75% of patients with advanced stage disease still succumb to their disease. Moreover, since progress will be predicated on informative clinical trials, defining relevant and meaningful endpoints is a priority. Optimal clinical trial endpoints will be different for distinct populations and will be subject to debate as each candidate endpoint will have advantages and disadvantages. For instance, OS remains the most objective and accepted endpoint because it is least vulnerable to bias; however, the feasibility of OS in ovarian cancer research is compromised by several factors including the requirement for large trial size, the prolonged time-line for final analysis in some populations and the potential for unintended loss of treatment effect from active post-progression therapies. Nonetheless, any potential surrogate for OS requires validation. Recent advocacy for PFS as the endpoint of choice in solid tumors has stressed the stringency of censoring, criteria for response, and the need for real-time standardized image analysis to reduce variability in detecting progression by imaging [46].
Both OS and PFS have led to regulatory approvals for ovarian cancer and are clinically important. However, current regulatory approval guidance by the FDA indicates that surrogates for overall survival, while acceptable, must be clinically meaningful and robust. This requires that investigators provide “context” for surrogate endpoints that measurably reflect the therapeutic impact in patients. This is particularly challenging in patients who are asymptomatic at recurrence as is frequently encountered in ovarian cancer patients, thus potentially diminishing the impact of PRO/HRQoL data. Predictive biomarkers that accurately identify patients most likely to benefit from therapy and allow avoiding ineffective treatment in others are needed to magnify treatment effect. In addition, modulation of a biomarker by a therapy in the short-term could inform a post-progression treatment pathway, which could optimize subsequent treatment decisions or better place into context the impact of delayed progression or response.
Expanded and refined patient reported outcomes should be considered in the development of new metrics of clinical benefit. Since it is unlikely that additional therapy added to an existing “standard” would produce less toxicity, better metrics of treatment effect (e.g. ascite resolution, reduction in pain medication needs) are needed. Real-time reporting of adverse events and drug treatment attributions has been piloted in a recent phase II lung cancer trial demonstrating improved efficiency and accuracy of the impact of therapy [47]. In the interim, it is advisable to power ovarian cancer trials with registration intent for OS even if PFS is a primary endpoint.
Endpoint selection in ovarian cancer clinical trials should reflect the impact on disease burden and the unique characteristics of the treatment cohort (e.g. platinum-sensitive vs. platinum-resistant, primary vs. recurrent disease, high grade vs. low grade serous) relative to survival. OS as the primary endpoint may be impractical for front-line trials given that the long survival of even advanced disease patients while demanding at least an OS trend in platinum resistant disease where PPS is small, is reasonable. Table 4 proposes acceptable endpoints in different clinical scenarios. A combination of favorable PRO/HRQoL and PFS data should warrant evaluation for regulatory approval. PRO/HRQoL parameters require further validation and refinement for ovarian cancer patients. A large magnitude of effect in PFS hazard ratio improvement also should establish benefit.
Table 4.
Endpoints and study settings.
| Frontline | Platinum- sensitive |
Platinum- resistant |
|
|---|---|---|---|
| OS | Approve | Approve | Approve |
| PFS (statistically significant) + Other (QoL/PRO) | Approve | Approve | Consider |
| PFS (statistically significant) with clinically meaning MOE |
Consider | Consider | Consider |
| Response rate/CBR | No | No | Consider |
In addition to a statistically significant difference, other means of benefit would need to be demonstrated such as significant difference in time off therapy or at least an OS trend. Opportunities to develop metrics of clinical benefit that integrate response elements with context to better define treatment effect. MOE = magnitude of effect; QOL = quality of life; PRO = patient reported outcomes; CBR = Clinical Benefit Rate.
Consensus statement
In light of this discussion we consider the following metrics or benchmarks as acceptable outcomes for consideration of regulatory approval:
Statistically significant OS in any disease setting
Statistically significant PFS supported by positive PRO/HRQoL data
Statistically significant PFS alone when clinical magnitude of effect is meaningful especially for disease states with anticipated long post-progression survival times such as front-line and platinum-sensitive patients
Statistically significant response or clinical benefit rate in settings where effective options are limited such as heavily treated platinum resistant and less therapeutically responsive tumors such as clear cell and mucinous histologies
Future research should focus upon identifying more objective dynamic imaging tools or blood based tumor markers to confirm disease response and progression, which is challenging in ovarian cancer where disease volume is often small yet widely distributed. Exploration of novel endpoints such as PFS2, which is the PFS on second line therapy, as well as the PFS1:PFS2 ratio is warranted. The advantage of such an endpoint for recurrent disease is that the patient serves as her own control; however, there are significant logistical limitations, as the assessments need to be symmetric. Validation of composite endpoints while incorporating more objective means of measuring response and progression and discovery of novel biomarkers is necessary.
Supplementary Material
HIGHLIGHTS.
Ovarian cancer has unique considerations for clinical trial endpoint selection.
Optimal endpoint selection should reflect true patient benefit.
PFS as a surrogate has significant advantages and disadvantages in ovarian cancer.
Acknowledgment
Special thanks to the SGO staff especially Jessica Oldham and Ellen Sullivan for their assistance.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2013.11.008.
Conflict of interest statement
The authors declare that there are no financial disclosures, except for the following individuals:
James Thigpen, MD
Consultant: Genentech, Amgen, Jansen Biotech, Boehringer Ingelheim.
Speakers Bureau: Genentech, Amgen, Jansen Biotech, Celgene.
Thomas Herzog, MD
Consultant: Johnson & Johnson, Morphoteck, Merck (<5000).
Bradley Monk, MD
Grants/Contracts: Novartis, Amgen, Genentech, Lilly, Janssen/Johnson & Johnson, Array, Merck; Speakers Bureau/Honorarium: Roche/Genentech, Johnson & Johnson; Consultant: Qiagen, Roche/Genentech, GSK, Merck, Arno, insys, Boehringer Ingelheim, Astellas.
Robert Mannel, MD
Honoraria for Advisory Board: Genentech, Abbvie Pharmaceuticals, Amgen, Ovarian Cancer Scientific.
Ronald Alvarez, MD
Grants: Morphoteck, Pfizer, Merrimack; Honorarium GSK.
Robert L. Coleman, MD
Grants/Contracts: Novartis, Amgen, Array, Merck, Oncomed, Astra-Zeneca, Esperance.
Advisory Board (uncompensated): Roche/Genentech, Johnson & Johnson, GSK, Merck, Novartis, Boehringer Ingelheim, Astellas, Precision Therapeutics, Abbvie.
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013 Jan-Feb;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. [Internet] International Agency for Research on Cancer; Lyon, France: [accessed Aug.3, 2012]. 2010. Available from: http://globocan.iarc.fr. [Google Scholar]
- [3].Huang L, Cronin KA, Johnson KA, Mariotto AB, Feuer EJ. Improved survival time: what can survival cure models tell us about population-based survival improvements in late-stage colorectal, ovarian, and testicular cancer? Cancer. 2008;112:2289–300. doi: 10.1002/cncr.23425. [DOI] [PubMed] [Google Scholar]
- [4].Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl. 1):S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- [5].Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al., editors. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: Apr, 2012. http://seer.cancer.gov/csr/1975_2009_pops09/, based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- [6].van Altena AM, Karim-Kos HE, de Vries E, Kruitwagen RF, Massuger LF, Kiemeney LA. Trends in therapy and survival of advanced stage epithelial ovarian cancer patients in The Netherlands. Gynecol Oncol. 2012:125–3. 649–54. doi: 10.1016/j.ygyno.2012.02.033. [DOI] [PubMed] [Google Scholar]
- [7].Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival impact of maximum cytoreductive surgery for advanced ovarian carcinoma during the platinum-era: a meta-analysis of 6885 patients. J Clin Oncol. 2002;20(5):1248–59. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- [8].McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- [9].Piccart MJ, Bertelsen K, James K, Cassidy J, Mangioni C, Simonsen E, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- [10].Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–5. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- [11].Markman M, Bundy BN, Alberts DS, Fowler JM, Clarke-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–7. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- [12].Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Shashikant L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- [13].Landrum LM, Java J, Mathews CA, Lanneau GS, Jr, Copeland LJ, Armstrong DK, et al. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: a Gynecologic Oncology Group Study. Gynecol Oncol. 2013;130(1):12–8. doi: 10.1016/j.ygyno.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–8. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- [15].Zang RY, Harter P, Chi DS, Sehouli J, Jiang R, Tropé CG, et al. Predictors of surgery based on the pooled analysis of an international collaborative cohort. Br J Cancer. 2011;105(7):890–6. doi: 10.1038/bjc.2011.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG 2006. J Clin Oncol. 2006;24(29):4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- [17].Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039–45. doi: 10.1200/JCO.2012.42.0505. http://dx.doi.org/10.1200/JCO.2012.42.0505 [Epub 2012 Apr 23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].du Bois AM, Quinn M, Thigpen T, Vermorken J, Avall-Lundvist E, Bookman M, et al. consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann Oncol. 2004;2005;16(Suppl. 8):viii7–viii12. doi: 10.1093/annonc/mdi961. [DOI] [PubMed] [Google Scholar]
- [19].Stuart GC, Kitchener H, Bacon M, duBois A, Friedlander M, Ledermann J, et al. Participants of 4th Ovarian Cancer Consensus Conference (OCCC); Gynecologic Cancer Intergroup. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer May. 2011;21(4):750–5. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- [20].Erwin RL. Therapy for advanced stage cancer: what do patients want and expect? A patient advocate’s perspective. The Oncologist. 2010;15(S1):11–2. doi: 10.1634/theoncologist.2010-S1-11. http://dx.doi.org/10.1634/theoncologist.2010-S1-11. [DOI] [PubMed] [Google Scholar]; Weeks, et al. NEJM. 2010;367:43. [Google Scholar]; FDA Oncologic Drugs Advisory Committee Meeting, NDA 22334 Afinitor (everolimus) Briefing Document April 12, 2011. 2011 Retrieved Apr 22, 2013, from http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/oncologicdrugsadvisorycommittee/ucm250378.pdf.
- [21].Traeger L, Greer JA, Fernandez-Robles C, Temel JS, Pirl WF. Evidence-based treatment of anxiety in patients with cancer. J Clin Oncol. 2012;30(11):1197–205. doi: 10.1200/JCO.2011.39.5632. [DOI] [PubMed] [Google Scholar]
- [22].Brady MF. First-line therapy in ovarian cancer—surrogate endpoints for accelerated approval. FDA/ASCO/AACR Ovarian Cancer End Points Workshop. 2006 [Retrieved April 3, 2013, from http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/CancerDrugs/ucm094640.pdf]
- [23].Buyse M. Is PFS a “valid” surrogate for OS in advanced ovarian cancer? A meta-analysis Marc. FDA/ASCO/AACR Ovarian Cancer End Points Workshop. 2006 [Retrieved Aug 3, 2012, from http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/CancerDrugs/ucm094610.pdf]
- [24].Redman MW, Goldman BH, LeBlanc M, Schott A, Baker LH. Modeling the relationship between progression-free survival and overall survival: the phase II/III trial. Clin Cancer Res. 2013 May 15;19(10):2646–56. doi: 10.1158/1078-0432.CCR-12-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26):2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- [26].Perren TJ, Swart AM, Pfisterer J, Ledermann J, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365(26):2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- [27].Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, et al. New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer) J Natl Cancer Inst. 2004 Mar 17;96(6):487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- [28].Burzykowski T, Buyse M. Surrogate threshold effect: an alternative measure for meta-analytic surrogate endpoint validation. Pharm Stat. 2006;5(3):173–86. doi: 10.1002/pst.207. [DOI] [PubMed] [Google Scholar]
- [29].Herzog TJ, Vermorken JB, Pujade-Lauraine E, Provencher DM, Jagiello-Gruszfeld A, Kong B, et al. Correlation between CA-125 serum level and response by RECIST in a phase III recurrent ovarian cancer study. Gynecol Oncol. 2011 Aug;122(2):350–5. doi: 10.1016/j.ygyno.2011.04.005. [DOI] [PubMed] [Google Scholar]
- [30].Kay R, Wu J, Wittes J. On assessing the presence of evaluation-time bias in progression-free survival in randomized trials. Pharm Stat. 2011;10(3):213–7. doi: 10.1002/pst.443. [DOI] [PubMed] [Google Scholar]
- [31].Freidlin B, Korn EL, Hunsberger S, Gray R, Saxman S, Zujewski JA. Proposal for the use of progression-free survival in unblinded randomized trials. J Clin Oncol. 2007;25(15):2122–6. doi: 10.1200/JCO.2006.09.6198. [DOI] [PubMed] [Google Scholar]
- [32].Vermorken JB, Parmar MKB, Brady MF, Eisenhauer EA, Hogberg T, Ozols RF, et al. Clinical trials in ovarian carcinoma: study methodology. Ann Oncol. 2005;16(Supp. 8):viii20–9. doi: 10.1093/annonc/mdi963. [DOI] [PubMed] [Google Scholar]
- [33].Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010:28, 3107–14. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]
- [34].Bookman MA, Brady MF, McGuire WP, Harper PG, Albert DS, Friedlander M, et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: a phase III trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419–25. doi: 10.1200/JCO.2008.19.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101(23):1642–164927. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cannistra SA. Evaluating new regimens in recurrent ovarian cancer: How much evidence is good enough. J Clin Oncol. 2010;28(19):3101–3. doi: 10.1200/JCO.2010.29.7077. [DOI] [PubMed] [Google Scholar]
- [37].Korn EL, Freidlin B, Abrams JS. Overall survival as the outcome for randomized clinical trials with effective subsequent therapies. J Clin Oncol. 2011 Jun 10;29(17):2439–42. doi: 10.1200/JCO.2011.34.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chan JK, Amanam I, Kiet TK, Young-Lin N, Hoth K, Kapp DS, et al. Progress and trends for cancer drug approval — an analysis of the FDA Advisory Committee. Ann Oncol. 2012;23(Suppl. 9):460. [Google Scholar]
- [39].Wang SJ. Editorial: adaptive designs: appealing in development of therapeutics, and where do controversies lie? J Biopharm Stat. 2010;20(6):1083–7. doi: 10.1080/10543406.2010.514461. [DOI] [PubMed] [Google Scholar]
- [40].Lopez MF, Dupuy JF, Gonzalez CV. Effectiveness of adaptive designs for phase II cancer trials. Contemp Clin Trials. 2012;33(1):223–7. doi: 10.1016/j.cct.2011.09.017. [DOI] [PubMed] [Google Scholar]
- [41].Adaptive design clinical trials for drugs and biologics. FDA Guidance for Industry. Center for Drug Evaluation and Research, Food and Drug Administration; Silver Spring, MD: 2010. http://www.fda.gov/downloads/Drugs/.../Guidances/ucm201790.pdf. [Google Scholar]
- [42].Parmar MK, Barthel FM, Sydes M, Langley R, Kaplan R, Eisenhauer E, et al. Speeding up the evaluation of new agents in cancer. J Natl Cancer Inst. 2008 Sep 3;100(17):1204–14. doi: 10.1093/jnci/djn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].I P.T.S.(project leader) S.G., G.G., J.W.G. and E.R.M. (writing team) A.K. Integrated genomic analyses of ovarian carcinoma, Cancer Genome Atlas Research Network. Nature. 2011 Jun 29;474(7353):609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Diaz-Padilla I, Malpica AL, Minig L, Chiva LM, Gershenson DM, Gonzalez-Martin An ovarian low-grade serous carcinoma: a comprehensive update. Gynecol Oncol. 2012 Aug;126(2):279–85. doi: 10.1016/j.ygyno.2012.04.029. [DOI] [PubMed] [Google Scholar]
- [45].Maeda D, Shih IeM. Pathogenesis and the role of ARID1A mutation in endometriosis-related ovarian neoplasms. Adv Anat Pathol. 2013 Jan;20(1):45–52. doi: 10.1097/PAP.0b013e31827bc24d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Korn RL, Crowley J. Overview: progression-free survival as an endpoint in clinical trials with solid tumors. Clin Cancer Res. 2013;19(10):2607–12. doi: 10.1158/1078-0432.CCR-12-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pietanza MC, Basch EM, Lash A, Schwartz LH, Ginsberg MS, Zhao B, et al. Harnessing technology to improve clinical trials: study of real-time informatics to collect data, toxicities, image response assessments and patient reported outcomes in a phase II clinical trial. J Clin Oncol. 2013;31(16):2004–9. doi: 10.1200/JCO.2012.45.8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.