Abstract
Background
Randomized controlled trials and retrospective studies in ANCA-associated vasculitis (AAV) concurred that rituximab (RTX) is effective to induce and maintain remission. Infections and hypogammaglobulinemia during RTX were usually infrequent and uncomplicated. But in the Tromsø study cohort, 45 % of patients with granulomatosis with polyangiitis (GPA) developed hypogammaglobulinemia during RTX maintenance leading to its discontinuation in 62 %.
Methods
To explain these differences in outcome when using RTX in AAV to maintain remission, we used statistical structural methods to compare the Tromsø study cohort with other published cohorts.
Results
GPA patients’ characteristics of the Tromsø study cohort were not so different compared with other cohorts. Rates of hypogammaglobulinemia and discontinuation of RTX seemed closely related to the cut-off used and to the levels of immunoglobulin (Ig) at baseline. Combination of low IgG serum levels at baseline (7.7 g/L) and low cut-off to define hypogammaglobulinemia in the Tromsø study cohort explained the high rate of hypogammaglobulinemia and discontinuation of RTX.
Conclusions
Patients’ characteristics in the Tromsø study cohort were not skewed, apart from IgG levels. Low IgG level at baseline seemed to contribute the most to hypogammaglobulinemia and its complications.
Keywords: ANCA-associated vasculitis, Rituximab, Induction, Maintenance, Immunoglobulin, Hypogammaglobulinemia, Discontinuation, Adverse event, Principal component analysis, Correspondence analysis
Background
Granulomatosis with polyangiitis (GPA) is an ANCA-associated vasculitis (AAV) affecting usually the small and medium vessels and is closely related to proteinase3-ANCA (PR3-ANCA). GPA often involves the upper and lower airways tracts and kidneys and was a fatal disease prior to the use of cyclophosphamide (CYC) [1].
Rituximab (RTX) is a chimeric monoclonal antibody against CD20 that depletes B cells [2] and is effective in inducing and maintaining remission in AAV patients in randomized controlled studies [3–5]. In two retrospective studies of remission maintenance with RTX, severe infections and hypogammaglobulinemia were frequent adverse events: 26–29 % had severe infections and 41–45 % had hypogammaglobulinemia [6–8]. However interpretation of the risk of hypogammaglobulinemia and its practical implications differed between the two studies. In the Tromsø study cohort, 17 % received intravenous immunoglobulin (IVIG) replacement and 62 % who developed hypogammaglobulinemia discontinued maintenance remission with RTX [8].
To explain differences in outcome, we compared using statistical structural methods the patients’ characteristics of the Tromsø study cohort with other published studies of induction and maintenance with RTX in AAV.
Methods
Identification of induction and maintenance studies
Two studies from the Northern Norwegian vasculitis register that was approved in 2001 by the Regional Ethical Committee for Medical and Health Research Ethics (REK-V 41/2001) were included [6, 8]. Patients (or the patients’ parents or guardians in case of children) gave written informed consent in accordance to the declaration of Helsinki at registry inclusion.
All other induction and maintenance studies with RTX in AAV were retrieved either from searching PubMed (www.ncbi.nlm.nih.gov/pubmed) in January 2015 using indexing terms ANCA vasculitis, rituximab maintenance and induction, or from reviewing the reference lists.
All studies of nine or more AAV patients were included in the analysis. Eighteen induction [3, 4, 6, 9–24] and 11 maintenance studies [5–8, 24–32] were identified. Definition of severe infections was similar in all studies, while definition of hypogammaglobulinemia differed.
Statistical analysis
Principal component analysis (PCA) finds the best linear combinations of important variables at baseline. Number of patients, age, proportion of men, PR3-ANCA status, kidney and lung involvement, Birmingham vasculitis score (BVAS) at baseline, proportion of patients exposed to CYC and type of RTX induction regimen were included in the PCA of induction studies. Number of patients, age, PR3-ANCA status, kidney and lung involvement, exposure to CYC, disease duration prior RTX and follow-up in months were included in the PCA of maintenance studies. Correspondence analysis (CA) of adverse events during RTX maintenance included studies that reported the risks of both severe infections and hypogammaglobulinemia.
Statistical analysis was done with R (R project for statistical computing www.r-project.org).
Results
Induction
Ten studies out of 18 were included in the PCA. Missing data from studies not included in the PCA were PR3-ANCA status [20, 21, 23], lung involvement [3, 22], CYC exposure [13, 17] and BVAS [12, 20] (Table 1 and Fig. 1). All studies concluded that RTX was effective in inducing remission in AAV patients, even though patients’ characteristics and induction regimen were different. The two first PCA dimensions explained 59 % of the variance of the cohorts’ characteristics included in the analysis.
Table 1.
Characteristics of ANCA-associated vasculitis patients who received rituximab for remission induction
| Study place | N | Men | Age | PR3-ANCA | Kidney | Lung | Orbital Subglottic | CYC exposed | Time to RTX | FU | RTX 1gx2 | BVAS | IgG prior | IgG after | HypoG | SI | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | y | % | % | % | % | % | mo | mo | % | g/L | g/L | % | % | ||||
| Linköping, Sweden | 9 | 56 | 59 | 78 | 78 | 44 | NA | 100 | 36 | 12 | 0 | 6 | 8.4 | 6.4 | NR | 0 | 10 |
| Rochester, US | 10 | 70 | 57 | 100 | 70 | 40 | 10 | 100 | 59 | 9 | 0 | 6 | NR | NR | NR | 20 | 11 |
| MC, UK | 65 | 52 | 47 | 57 | 10 | 40 | 20 | 97 | 72 | 20 | 49 | NR | 7.9 | 8.0 | 0 | 20 | 12 |
| Boston, US | 39 | 49 | 60 | 62 | NR | NR | NR | NR | 67 | 18 | 90 | 1 | NR | NR | 0 | 3 | 13 |
| Bad-Bramstedt Germany | 59 | 59 | 54 | 86 | 44 | 41 | 46 | 100 | 37 | 7 | 0 | 11 | 8.6 | 6.9 | 12 | 26 | 14 |
| Freiburg, Germany | 37 | 57 | 62 | 81 | 60 | 81 | NR | 92 | 99 | 30 | 81 | 13 | 9.9 | 9.0 | 27 | 16 | 24 |
| Goteborg, Sweden | 29 | 52 | 51 | 97 | 62 | 59 | 38 | 100 | 31 | 21 | 0 | 6 | NR | NR | NR | 10 | 15 |
| Stockholm, Sweden | 16 | 56 | 60 | 81 | 50 | 50 | NR | 100 | 68 | 20 | 38 | 10 | NR | NR | NR | 38 | 16 |
| London UK 2014 | 19 | 26 | 61 | 53 | 53 | 32 | NR | NR | 40 | 11.5 | 0 | 7 | NR | NR | 0 | 0 | 17 |
| Munich, Germany | 17 | 59 | 58 | 76 | 71 | 59 | 53 | 76 | 40 | 24 | 0 | 13 | NR | NR | NR | 24 | 18 |
| Cleveland, US | 105 | 48 | 49 | 75 | 58 | 83 | NA | 88 | 55 | 23 | 73 | 4 | NR | NR | NR | 7 | 19 |
| London, UK 2009 | 10 | 50 | 49 | NR | 40 | 0 | 70 | 90 | 78 | 12 | 100 | NR | NR | NR | NR | 0 | 20 |
| Torino, Italy | 11 | 55 | 58 | NR | 64 | 27 | NR | 82 | 22 | NR | 0 | 23 | 8.0 | 6.7 | NR | NR | 21 |
| London, UK 2011 | 23 | 52 | 59 | 70 | 100 | NR | NR | 100 | 1 | 36 | 100 | 21 | NR | NR | 4 | 9 | 22 |
| Registry Germany | 58 | 48 | 50 | NR | 45 | 59 | 19 | 60 | 54 | 18 | 47 | 13 | NR | NR | NR | 7 | 23 |
| RAVE US | 99 | 46 | 54 | 67 | 66 | 52 | NR | 42 | 30 | 6 | 0 | 9 | NR | NR | NR | 7 | 4 |
| RITUXIVAS EUVAS | 33 | 52 | 68 | 53 | 100 | NR | NR | 100 | 1 | 12 | 0 | 19 | NR | NR | 3 | 19 | 3 |
| Tromsø, Norway | 29 | 52 | 50 | 86 | 59 | 66 | 62 | 97 | 57 | 49 | 100 | 10 | 7.7 | NR | NR | NR | 8 |
BVAS Birmingham Vasculitis Activity Score, CYC cyclophosphamide, FU follow-up during maintenance remission with rituximab, HypoG rate of hypogammaglobulinemia, MC multicentric study, N number of patients, NR not reported, RTX rituximab, Ref reference, SI rate of severe infection
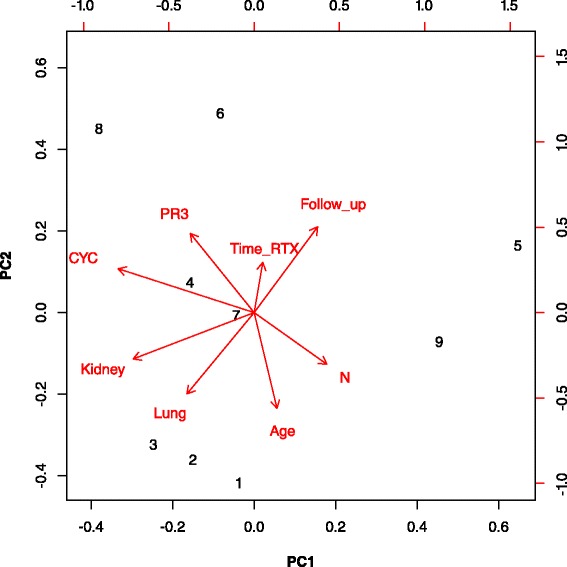
Fig. 1.

Principal component analysis of rituximab induction studies. 1: DE-Bad-Bramstedt [14]; 2: US-Cleveland [19]; 3: DE-Freiburg [24]; 4: DE-Munich [18]; 5: SE-Gothenburg; [15]; 6: SE-Stockholm [16]; 7: RAVE study [4]; 8: US-Rochester [11], 9: SE-Linköping [10]; 10: NO-Tromsø [6]. Age: age in years; BVAS: Birmingham Vasculitis Activity Score; CYC: proportion of patients exposed to cyclophosphamide; Kidney: proportion of patients with kidney involvement; Lung: proportion of patients with lung involvement; Men: proportion of men in studies; N: number of patients in studies; PR3: proportion of patients who are PR3-ANCA positive; RTX_1gx2: proportion of patients who received rituximab 1g twice given one fortnight apart (rheumatoid arthritis protocol)
The RAVE and Cleveland Clinic studies recruited the most patients [4, 19]. The two 1g- RTX infusions given one fortnight apart were used more often at induction in the studies from Freiburg and Tromsø [8, 24].
Patients in the Tromsø cohort only had GPA, were more often PR3-ANCA positive, had more lung and less kidney involvement, received more frequently CYC and had a higher BVAS at baseline (Fig. 1).
Maintenance
The PCA did not include two studies that reported neither age [31] nor exposure to CYC [29]. Studies were very different in term of patients’ characteristics at baseline, however all reported decreased disease activity and relapse rates during RTX maintenance (Table 2 and Fig. 2). The two first PCA dimensions explained 64 % of the variance of the cohorts’ characteristics included in the analysis.
Table 2.
Characteristics of ANCA-associated vasculitis patients who received rituximab for remission maintenance
| Study place | N | Age | Men | PR3-ANCA | CYC | CYC | RTX | Kidney | Lung | Orb-Subg | BVAS | Time to RTX | FU mo | IgG before | IgG after | SI | HypoG | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| y | % | % | % | g | g | % | % | % | mo | g/L | g/l | % | % | |||||
| Nottingham, UK | 11 | 41 | 64 | 100 | 100 | NR | 8 | 55 | 46 | NR | 14 | 72 | 32 | NR | NR | NR | 9 | 25 |
| Paris 2011, France | 28 | 51 | 60 | 68 | 100 | 48 | NR | 32 | 64 | 14 | 15 | 84 | 38 | 7.8 | 7.0 | 11 | 11 | 26 |
| Rochester, US | 53 | 46 | 47 | 98 | 100 | NR | NR | 42 | 49 | NR | 5 | 120 | 53 | 8.4 | 5.7 | NR | NR | 28 |
| Freiburg, Germany | 37 | 62 | 57 | 81 | 92 | 12 | NR | 60 | 81 | NR | 13 | 99 | 30 | 9.9 | NR | 16 | 27 | 24 |
| Boston, US | 172 | 60 | 45 | 43 | NR | NR | NR | 62 | 44 | NR | 2 | NR | 25 | NR | NR | 15 | 10 | 29 |
| Paris 2014, France | 66 | 50 | 49 | 80 | 89 | 29 | 4.6 | 21 | 47 | 14 | 10 | 67 | 34 | 8.3 | 7.5 | 14 | 2 | 30 |
| Uppsala, Sweden | 12 | NR | 42 | 100 | 100 | 61 | NR | 50 | 100 | 42 | 9 | 35 | 79 | 8.5 | 6.8 | 33 | NR | 31 |
| MC, France | 80 | 53 | NR | 84 | 98 | 13 | NR | 55 | 71 | 10 | 7 | 54 | 18 | NR | NR | 15 | NR | 32 |
| Mainritsan, France | 57 | 54 | 65 | 77 | 100 | 7.3 | 2.5 | 70 | 58 | NR | NR | 3 | 28 | 6.1 | 6.9 | 19 | NR | 5 |
| Cambridge, UK | 69 | 52 | 41 | 74 | 91 | 13.5 | 6 | 12 | 29 | 12 | NR | 60 | 59 | 9.3 | 8.0 | 29 | 41 | 7 |
| Tromsø, Norway | 29 | 50 | 52 | 86 | 97 | 17 | 9 | 59 | 66 | 62 | 10 | 57 | 49 | 7.7 | 4.9 | 24 | 45 | 8 |
BVAS Birmingham Vasculitis Activity Score, CYC cyclophosphamide, FU follow-up during maintenance remission with rituximab, HypoG rate of hypogammaglobulinemia, MC multicentric study, N number of patients, NR not reported, Orb-Subg frequency of orbital-subglottic involvement, RTX rituximab, Ref reference, SI rate of severe infection
Fig. 2.
Principal component analysis of rituximab maintenance studies. 1: DE-Freiburg [24]; 2: FR-MC [32]; 3: FR-MAINRITSAN study [5]; 4: NO-Tromsø [6, 8]; 5: UK-Cambridge [7]; 6: US-Rochester [28]; 7: FR-Paris 2012 [26]; 8: UK-Nottingham [25]; 9: FR-Paris 2014 [30]. Age in years; CYC: proportion of patients exposed to cyclophosphamide; Follow_up: follow-up of remission maintenance with rituximab; Kidney: proportion of patients with kidney involvement; Lung: proportion of patients with lung involvement; N: number of patients included in the studies; PR3: proportion of patients with positive PR3-ANCA; Time_RTX: disease duration prior to rituximab
Studies from Freiburg [24] and two other multicentric French studies [5, 32] were closely related, as their patients were older at RTX induction, had more kidney and lung involvement and had the shortest follow-up after RTX. Almost all patients were PR3-ANCA positive in the studies from Mayo Clinic and Nottingham [25, 28]. Cohorts from Tromsø and Paris were closest to the centroid (point 0) [8, 26].
Patients in the Tromsø cohort only had GPA, were younger, more often PR3-ANCA positive and received more frequently CYC prior to RTX (Fig. 2).
Severe infection and hypogammaglobulinemia during maintenance
A total of 18 % (range 11–33 %) of patients had severe infections during RTX maintenance [5–7, 24, 29–32]; 18 % (2 – 45 %) developed hypogammaglobulinemia [7, 8, 24, 25, 29, 30]. The CA included five cohorts [6–8, 24, 29, 30] as data of both severe infection and hypogammaglobulinemia were lacking in most studies. The Tromsø and Cambridge study cohorts had more severe infection and hypogammaglobulinemia [6–8] compared with Freiburg, Paris and Boston [24, 29, 30] (Fig. 3). Risks of severe infections and hypogammaglobulinemia were equivalent in the studies of Cambridge and Tromsø [6–8]. While severe hypogammaglobulinemia occurred in 7 % of the patients in the Cambridge cohort [7], hypogammaglobulinemia led to RTX discontinuation in 62 % and 53 % in the Tromsø and Boston cohorts [8, 29].
Fig. 3.
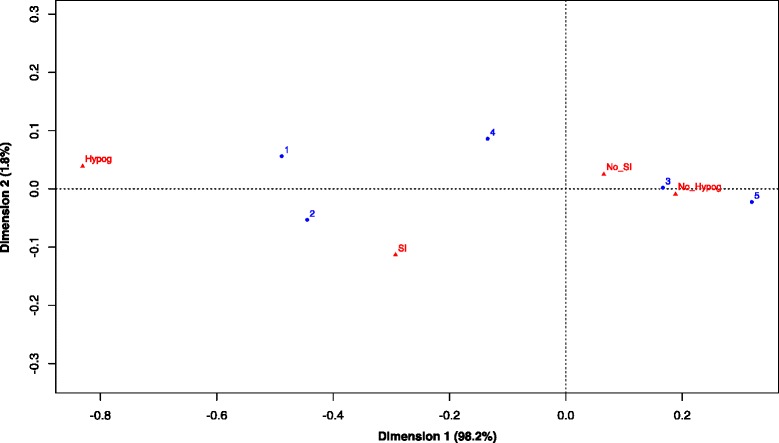
Correspondence analysis of adverse events in studies on RTX maintenance in AAV. Hypog: hypogammaglobulinemia; No_Hypog: absence of hypogammaglobulinemia; No_SI: absence of severe infection; SI: severe infection. 1: Tromsø Norway [6, 8]; 2: Cambridge UK [7]; 3: Boston USA [29]; 4: Freiburg Germany [24]; Paris France [30]
Ig levels were lower in patients from the Tromsø study cohort both at baseline and during RTX maintenance: IgG 7.7 compared with > 8.3 g/L at baseline in the other studies [7, 24, 29, 30] and IgG 4.9 during maintenance compared with > 7.0 in Boston [29], 7.5 in Paris [30], 8.0 in Cambridge [7] and 9.0 in Freiburg [24].
Discussion
The Tromsø study cohort only included GPA patients; they were more frequently PR3-ANCA positive and were often exposed to CYC compared with other cohorts. Patients’ characteristics of the Tromsø cohort were similar to Freiburg during induction [24] and to an older cohort from Paris during maintenance [26]. The Tromsø study cohort seemed representative of maintenance studies since it was close to the centroid of the PCA.
In AAV, the risks of hypogammaglobulinemia and of severe infection seemed equivalent at 18 % during remission maintenance with RTX. While severe infection was defined alike in all studies, the definition of hypogammaglobulinemia was not standardized and identified patients with different Ig serum levels conferring different risk to receive IVIG and to discontinue RTX. Hypogammaglobulinemia cutoff was lowest in the Boston study: total IgG < 4 g/L [29] vs. IgG < 6 in Cambridge [7], IgG < 7 [24] in Freiburg, total Ig < 6 (corresponding to IgG < 5 g/L) in Tromsø [8] and total Ig < 7 g/L in Paris [30]. Difference in cutoffs explained the difference in results between studies, but hypogammaglobulinemia in the Tromsø study was still more frequent and severe.
Fifty three and 62 % discontinued RTX maintenance due to hypogammaglobulinemia in the Boston and Tromsø cohorts [8, 29]. However the risk of hypogammaglobulinemia was four times higher in the Tromsø cohort since the Boston study used a lower cutoff to define hypogammaglobulinemia [29].
The Cambridge and Tromsø studies had an increased risk of hypogammaglobulinemia, respectively 41–45 % [6–8]. However the impact of hypogammaglobulinemia was more severe in the Tromsø study. In the Tromsø study, patients had lower IgG levels during RTX maintenance and 17 % required intravenous immunoglobulin (IVIG) replacement [8]. Only 7 % required IVIG in the Cambridge study [7].
PR3-ANCA status and exposure to CYC prior to RTX were similar in the Tromsø and Cambridge cohorts. Nevertheless others factors, such as organ involvement and treatment strategy during remission maintenance, could also explain the differences in severity of hypogammaglobulinemia between both studies. GPA patients in the Tromsø study had more lung and kidney involvement than in Cambridge. They also used concomitant immunosuppressive drugs for a median of two years and continued with pre-emptive RTX maintenance for a median of four years. While in the Cambridge study, patients did not use concomitant immunosuppressive drugs, received RTX remission maintenance during two years and were only re-treated with RTX in case of relapse [7].
Conclusions
In summary, GPA patients’ characteristics in the Tromsø cohort were not skewed compared with the other cohorts, apart from IgG levels. In agreement with a previous study on RTX use in autoimmune diseases [33], low IgG level at baseline seemed to contribute the most to hypogammaglobulinemia and its complications during RTX maintenance in AAV.
Ethics approval and consent to participate
Human data originated from the Northern Norwegian vasculitis register that was approved in 2001 by the Regional Ethical Committee for Medical and Health Research Ethics (REK-V 41/2001). Patients (or the patients’ parents or guardians in case of children) gave written informed consent in accordance to the declaration of Helsinki at registry inclusion.
Abbreviations
- AAV
ANCA-associated vasculitis
- ANCA
antineutrophil cytoplasmic antibodies
- BVAS
Birmingham vasculitis activity score
- CA
correspondence analysis
- CYC
cyclophosphamide
- GPA
granulomatosis with polyangiitis
- Ig
immunoglobulin
- IVIG
intravenous immunoglobulin
- PCA
principal component analysis
- PR3
proteinase 3
- RTX
rituximab
Footnotes
Competing interests
The author declares that he has no competing interests.
Author’s contribution
EB acquired, analyzed and interpreted the data. EB was involved in drafting the manuscript and revising it critically. EB has given formal approval of the version to be published. EB agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The author read and approved the final manuscript.
References
- 1.Fauci AS, Wolff SM. Wegener’s granulomatosis: studies in eighteen patients and a review of the literature. Medicine. 1973;52:535–61. doi: 10.1097/00005792-197311000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Leandro MJ, de la Torre I. Translational mini-review series on B cell directed therapies: the pathogenic role of B cells in autoantibody-associated autoimmune diseases – lessons from B cell-depletion therapy. Clin Exp Immunol 2009:doi:10.1111/j.1365-2249.2009.03977.x [DOI] [PMC free article] [PubMed]
- 3.Jones RB, Cohen Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363:211–20. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 4.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371:1771–80. doi: 10.1056/NEJMoa1404231. [DOI] [PubMed] [Google Scholar]
- 6.Besada E, Koldingsnes W, Nossent JC. Long-term efficacy and safety of pre-emptive maintenance therapy with rituximab in granulomatosis with polyangiitis: results from a single centre. Rheumatology. 2013;52:2041–7. doi: 10.1093/rheumatology/ket257. [DOI] [PubMed] [Google Scholar]
- 7.Alberici F, Smith RM, Jones RB, Roberts DM, Willcocks LC, Chaudhry A et al. Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatology 2014; doi:10.1093/rheumatology/keu452. [DOI] [PMC free article] [PubMed]
- 8.Besada E, Koldingsnes W, Nossent JC. Serum immunoglobulin levels and risk factors for hypogammaglobulinemia during long-term maintenance therapy with rituximab in patients with granulomatosis with polyangiitis. Rheumatology. 2014;53:1818–24. doi: 10.1093/rheumatology/keu194. [DOI] [PubMed] [Google Scholar]
- 9.Venhoff N, Effelsberg NM, Salzer U, Warnatz K, Peter HH, Lebrecht D et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. Plos ONE 7(5):e37626. doi:10.1371/journal.pone.0037626. [DOI] [PMC free article] [PubMed]
- 10.Eriksson P. Nine patients with anti-neutrophil cytoplasmic antibody-positive vasculitis successfully treated with rituximab. J Intern Med. 2005;257:540–8. doi: 10.1111/j.1365-2796.2005.01494.x. [DOI] [PubMed] [Google Scholar]
- 11.Keogh KA, Ytterberg SR, Fervenza FC, Carlsson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173:180–7. doi: 10.1164/rccm.200507-1144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones RB, Ferraro AJ, Chaudhry AN, Brogan P, Salama AD, Smith KG, et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009;60:2156–68. doi: 10.1002/art.24637. [DOI] [PubMed] [Google Scholar]
- 13.Rhee EP, Laliberte KA, Niles JL. Rituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody –associated vasculitis. Clin J Am Soc Nephrol. 2010;5:1394–400. doi: 10.2215/CJN.08821209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holle JU, Dubrau C, Herlyn K, Heller M, Ambrosch P, Noelle B, et al. Rituximab for refractory granulomatosis with polyangiitis (Wegener’s granulomatosis): comparison of efficacy in granulomatous versus vasculitic manifestations. Ann Rheum Dis. 2012;71:327–33. doi: 10.1136/ard.2011.153601. [DOI] [PubMed] [Google Scholar]
- 15.Pullerits R, Ljevak M, Vikgren J, Bokarewa M. Off-trial evaluation of the B-cell targeting treatment in the refractory cases of antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis: long-term follow-up from a single centre. Scand J Immunol. 2012;76:411–20. doi: 10.1111/j.1365-3083.2012.02747.x. [DOI] [PubMed] [Google Scholar]
- 16.Wendt M, Gunnarsson I, Bratt J, Bruchfeld A. Rituximab in relapsing or refractory ANCA-associated vasculitis: a case series of 16 patients. Scand J Rheumatol. 2012;41:116–9. doi: 10.3109/03009742.2011.620573. [DOI] [PubMed] [Google Scholar]
- 17.Turner-Stokes T, Sandhu E, Pepper RJ, Stolagiewicz NE, Ashley C, Dinneen D, et al. Induction treatment of ANCA-associated vasculitis with a single dose of rituximab. Rheumatology. 2014;53:1395–403. doi: 10.1093/rheumatology/ket489. [DOI] [PubMed] [Google Scholar]
- 18.Moog P, Probst M, Kuechle C, Hauser C, Heemann U, Thuermel K. Single-dose rituximab for remission induction and maintenance therapy in ANCA-associated vasculitis: a retrospective analysis of 17 patients. Scand J Rheumatol. 2014;43:519–23. doi: 10.3109/03009742.2014.918172. [DOI] [PubMed] [Google Scholar]
- 19.Azar L, Springer J, Langford CA, Hoffman GS. Rituximab with or without a conventional maintenance agent in the treatment of relapsing granulomatosis with polyangiitis (Wegener’s) Arthritis Rheum. 2014;66:2862–70. doi: 10.1002/art.38744. [DOI] [PubMed] [Google Scholar]
- 20.Taylor S, Salama AD, Joshi L, Pusey CD, Lightman SL. Rituximab is effective in the treatment of refractory ophthalmic Wegener’s granulomatosis. Arthritis Rheum. 2009;60:1540–7. doi: 10.1002/art.24454. [DOI] [PubMed] [Google Scholar]
- 21.Roccatello D, Sciascia S, Rossi D, Alpa M, Naretto C, Russo A, et al. Long-term effects of rituximab added to cyclophosphamide in refractory patients with vasculitis. Am J Nephrol. 2011;34:175–80. doi: 10.1159/000329535. [DOI] [PubMed] [Google Scholar]
- 22.Mansfield N, Hamour S, Habib AM, Tarzi R, Levy J, Griffith M, et al. Prolonged disease-free remission following rituximab and low-dose cyclophosphamide therapy for renal ANCA-associated vasculitis. Nephrol Dial Transplant. 2011;26:3280–6. doi: 10.1093/ndt/gfr127. [DOI] [PubMed] [Google Scholar]
- 23.Roll P, Ostermeier E, Haubitz M, Lovric S, Unger L, Holle J, et al. Efficacy and safety of rituximab treatment in patients with antineutrophil cytoplasmic antibody-associated vasculitides: results from a German registry (GRAID) J Rheumatol. 2012;39:2153–6. doi: 10.3899/jrheum.120482. [DOI] [PubMed] [Google Scholar]
- 24.Venhoff N, Niessen L, Kreuzaler M, Rolink AG, Hässler F, Rizzi M, et al. Reconstitution of the peripheral B lymphocyte compartment in patients with ANCA-associated vasculitides treated with rituximab for relapsing or refractory disease. Autoimmunity. 2014;47:401–8. doi: 10.3109/08916934.2014.914174. [DOI] [PubMed] [Google Scholar]
- 25.Rees F, Yazdani R, Lanyon P. Long-term follow-up of different refractory systemic vasculitides treated with rituximab. Clin Rheumatol. 2011;30:1241–5. doi: 10.1007/s10067-011-1756-8. [DOI] [PubMed] [Google Scholar]
- 26.Roubaud-Baudron C, Pagnoux C, Meaux-Ruault N, Grasland A, Zoulim A, Le Guen J, et al. Rituximab maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis. J Rheumatol. 2012;39:125–30. doi: 10.3899/jrheum.110143. [DOI] [PubMed] [Google Scholar]
- 27.Smith RM, Jones RB, Guerry MJ, Laurino S, Catapano F, Chaudhry A, et al. Rituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64:3760–9. doi: 10.1002/art.34583. [DOI] [PubMed] [Google Scholar]
- 28.Cartin-Ceba R, Golbin JM, Keogh KA, Peikert T, Sanchez-Menendez M, Ytterberg SR, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener’s) Arthritis Rheum. 2012;64:3770–8. doi: 10.1002/art.34584. [DOI] [PubMed] [Google Scholar]
- 29.Pendergraft WF, III, Cortazar FB, Wenger J, Murphy AP, Rhee EP, Laliberte KA, et al. Long-term maintenance therapy using rituximab-induced continuous B-cell depletion in patients with ANCA vasculitis. Clin J Am Soc Nephrol. 2014;9:736–44. doi: 10.2215/CJN.07340713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calich AL, Puéchal X, Pugnet G, London J, Terrier B. Charles P et al. Rituximab for induction and maintenance therapy in granulomatosis with polyangiitis (Wegener’s). Results of a single-center cohort study on 66 patients. J Autoimmun. 2014;50:135–41. [DOI] [PubMed]
- 31.Knight A, Hallenberg H, Baecklund E. Efficacy and safety of rituximab as maintenance therapy for relapsing granulomatosis with polyangiitis — a case series. Clin Rheumatol. 2014;33:841–8. doi: 10.1007/s10067-013-2351-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charles P, Néel A, Tieulié N, Hot A, Pugnet G, Decaux O, et al. Rituximab for induction and maintenance of ANCA associated vasculitides: a multicenter retrospective study on 80 patients. Rheumatology. 2014;53:532–9. doi: 10.1093/rheumatology/ket381. [DOI] [PubMed] [Google Scholar]
- 33.Roberts DM, Jones RB, Smith RM, Alberici F, Kumaratne DS, Burns S, et al. Rituximab-associated hypogammaglobulinemia: Incidence, predictors ad outcomes in patients with multi-system autoimmune disease. J Autoimmun. 2015;57:60–5. doi: 10.1016/j.jaut.2014.11.009. [DOI] [PubMed] [Google Scholar]


