Abstract
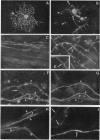
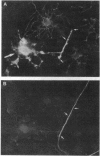
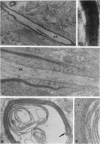
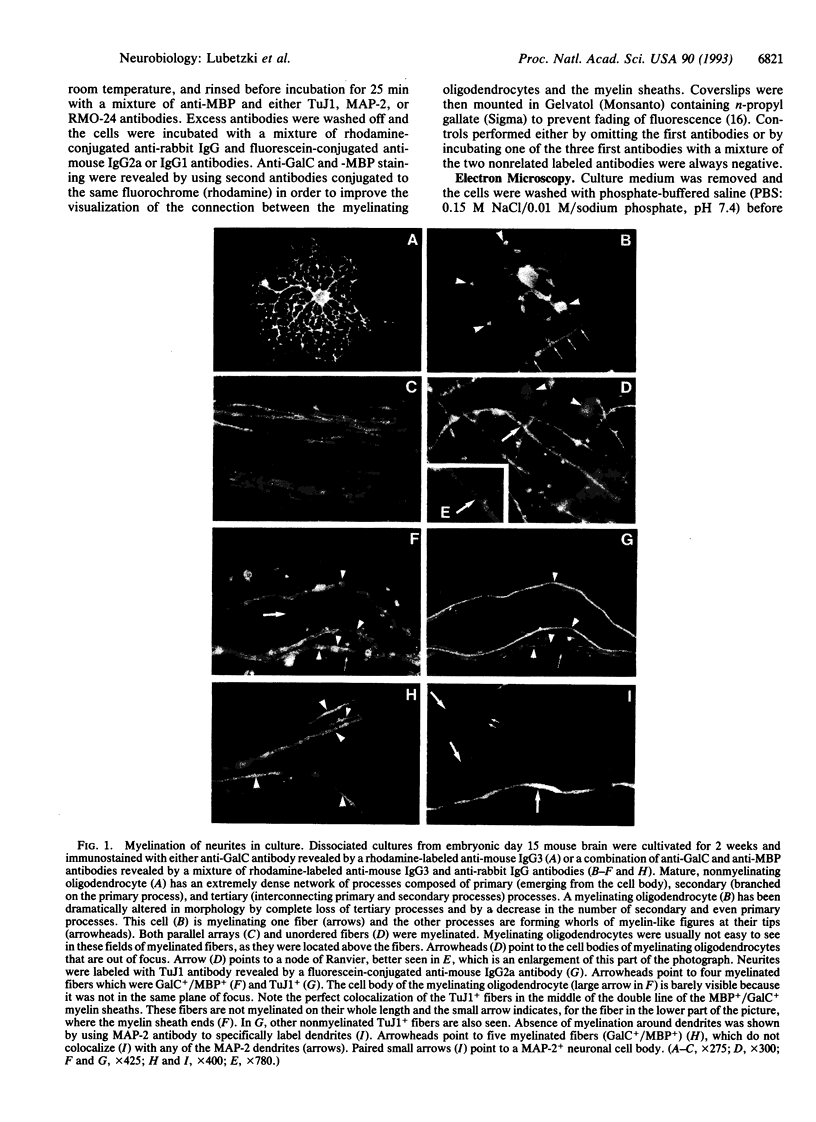
Cerebral hemispheres from mouse embryos at 15 days of gestation were dissociated and maintained in culture for several weeks in a medium which permitted homochronic and homotypic oligodendrocytes and neurons to interact in the presence of other central nervous system cells. After 13-14 days in culture a few oligodendrocytes changed from highly branched, "sun-like," nonmyelinating cells to sparcely branched myelinating cells. The number of fibers myelinated per oligodendrocyte ranged from 1 to 10, similar to that described previously in vivo in the corpus callosum. When an oligodendrocyte began to myelinate, it immediately myelinated a maximum number of fibers, suggesting that the number of axons to be myelinated by the oligodendrocyte was predetermined. When only one fiber was in the vicinity of a myelinating oligodendrocyte, whorls of myelin-like figures were seen at the tip of oligodendrocyte processes that had not reached an axon. Myelinated fibers were unambiguously identified as axons both by immunostaining and by electron microscopy. Myelin was not observed around astrocyte processes or around dendrites. The exclusive myelination of axons suggests the existence of a specific axonal recognition signal which attracts oligodendrocyte processes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Charron L., Bray G. M. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976 Oct;5(8):565–573. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- Althaus H. H., Montz H., Neuhoff V., Schwartz P. Isolation and cultivation of mature oligodendroglial cells. Naturwissenschaften. 1984 Jun;71(6):309–315. doi: 10.1007/BF00396614. [DOI] [PubMed] [Google Scholar]
- Bansal R., Pfeiffer S. E. Developmental expression of 2',3'-cyclic nucleotide 3'-phosphohydrolase in dissociated fetal rat brain cultures and rat brain. J Neurosci Res. 1985;14(1):21–34. doi: 10.1002/jnr.490140103. [DOI] [PubMed] [Google Scholar]
- Bernhardt R., Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1984 Jun 20;226(2):203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. Differential localization of MAP-2 and tau in mammalian neurons in situ. Ann N Y Acad Sci. 1986;466:145–166. doi: 10.1111/j.1749-6632.1986.tb38392.x. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cáceres A., Banker G. A., Binder L. Immunocytochemical localization of tubulin and microtubule-associated protein 2 during the development of hippocampal neurons in culture. J Neurosci. 1986 Mar;6(3):714–722. doi: 10.1523/JNEUROSCI.06-03-00714.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Behar T., Hudson L., Lazzarini R. A. Emergence of three myelin proteins in oligodendrocytes cultured without neurons. J Cell Biol. 1986 Feb;102(2):384–392. doi: 10.1083/jcb.102.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A. L., Pfeiffer S. E. Oligodendrocyte progenitors isolated directly from developing telencephalon at a specific phenotypic stage: myelinogenic potential in a defined environment. Development. 1989 May;106(1):119–132. doi: 10.1242/dev.106.1.119. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Hartman B. K., Agrawal H. C., Kalmbach S., Shearer W. T. A comparative study of the immunohistochemical localization of basic protein to myelin and oligodendrocytes in rat and chicken brain. J Comp Neurol. 1979 Nov 15;188(2):273–290. doi: 10.1002/cne.901880206. [DOI] [PubMed] [Google Scholar]
- Knapp P. E., Bartlett W. P., Skoff R. P. Cultured oligodendrocytes mimic in vivo phenotypic characteristics: cell shape, expression of myelin-specific antigens, and membrane production. Dev Biol. 1987 Apr;120(2):356–365. doi: 10.1016/0012-1606(87)90238-7. [DOI] [PubMed] [Google Scholar]
- Lee M. K., Tuttle J. B., Rebhun L. I., Cleveland D. W., Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17(2):118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Schlaepfer W. W., Trojanowski J. Q. Monoclonal antibodies distinguish several differentially phosphorylated states of the two largest rat neurofilament subunits (NF-H and NF-M) and demonstrate their existence in the normal nervous system of adult rats. J Neurosci. 1987 Nov;7(11):3474–3488. doi: 10.1523/JNEUROSCI.07-11-03474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubetzki C., Goujet-Zalc C., Gansmüller A., Monge M., Brillat A., Zalc B. Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J Neurochem. 1991 Feb;56(2):671–680. doi: 10.1111/j.1471-4159.1991.tb08202.x. [DOI] [PubMed] [Google Scholar]
- Matthews M. A., Duncan D. A quantitative study of morphological changes accompanying the initiation and progress of myelin production in the dorsal funiculus of the rat spinal cord. J Comp Neurol. 1971 May;142(1):1–22. doi: 10.1002/cne.901420102. [DOI] [PubMed] [Google Scholar]
- Monge M., Kadiiski D., Jacque C. M., Zalc B. Oligodendroglial expression and deposition of four major myelin constituents in the myelin sheath during development. An in vivo study. Dev Neurosci. 1986;8(4):222–235. doi: 10.1159/000112255. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Glial cell diversification in the rat optic nerve. Science. 1989 Mar 17;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Ranscht B., Clapshaw P. A., Price J., Noble M., Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remahl S., Hilderbrand C. Relation between axons and oligodendroglial cells during initial myelination. I. The glial unit. J Neurocytol. 1990 Jun;19(3):313–328. doi: 10.1007/BF01188401. [DOI] [PubMed] [Google Scholar]
- Rosen C. L., Bunge R. P., Ard M. D., Wood P. M. Type 1 astrocytes inhibit myelination by adult rat oligodendrocytes in vitro. J Neurosci. 1989 Oct;9(10):3371–3379. doi: 10.1523/JNEUROSCI.09-10-03371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlieve L. L., Rao G. S., Campbell G. L., Pieringer R. A. Investigations on myelination in vitro: biochemical and morphological changes in cultures of dissociated brain cells from embryonic mice. Brain Res. 1980 May 5;189(1):79–90. doi: 10.1016/0006-8993(80)90008-6. [DOI] [PubMed] [Google Scholar]
- Seil F. J. Tissue culture studies of demyelinating disease: a critical review. Ann Neurol. 1977 Oct;2(4):345–355. doi: 10.1002/ana.410020417. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Itoyama Y., Kies M. W., Webster H deF Immunocytochemical method to identify basic protein in myelin-forming oligodendrocytes of newborn rat C.N.S. J Neurocytol. 1978 Apr;7(2):251–263. doi: 10.1007/BF01217922. [DOI] [PubMed] [Google Scholar]
- Szuchet S., Polak P. E., Yim S. H. Mature oligodendrocytes cultured in the absence of neurons recapitulate the ontogenic development of myelin membranes. Dev Neurosci. 1986;8(4):208–221. doi: 10.1159/000112254. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bunge R. P. Evidence that axons are mitogenic for oligodendrocytes isolated from adult animals. Nature. 1986 Apr 24;320(6064):756–758. doi: 10.1038/320756a0. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bunge R. P. Myelination of cultured dorsal root ganglion neurons by oligodendrocytes obtained from adult rats. J Neurol Sci. 1986 Jul;74(2-3):153–169. doi: 10.1016/0022-510x(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Williams A. K. Oligodendrocyte proliferation and CNS myelination in cultures containing dissociated embryonic neuroglia and dorsal root ganglion neurons. Brain Res. 1984 Feb;314(2):225–241. doi: 10.1016/0165-3806(84)90045-2. [DOI] [PubMed] [Google Scholar]
- Wood P., Okada E., Bunge R. The use of networks of dissociated rat dorsal root ganglion neurons to induce myelination by oligodencrocytes in culture. Brain Res. 1980 Aug 25;196(1):247–252. doi: 10.1016/0006-8993(80)90732-5. [DOI] [PubMed] [Google Scholar]
- Yavin Z., Yavin E. Synaptogenesis and myelinogenesis in dissociated cerebral cells from rat embryo on polylysine coated surfaces. Exp Brain Res. 1977 Aug 8;29(1):137–147. doi: 10.1007/BF00236881. [DOI] [PubMed] [Google Scholar]