Abstract
The concurrent management of type 2 diabetes mellitus (T2DM) and chronic congestive heart failure presents several therapeutic challenges. Of concern is that insulin and insulin-sensitizing medications detrimentally “flood” the heart with energy-providing substrates, including fats and glucose. In this population, treatment of T2DM should focus on the reduction of increased substrate supply. Sodium glucose cotransporter-2 (SGLT-2) inhibitors, a new class of antidiabetic medication, operate via this principle by blocking the reabsorption of glucose in the kidney and subsequently releasing glucose through the urine. In this review, we begin with an examination of the mechanisms of glucotoxicity and lipotoxicity in the heart. Then we analyze the potential role of SGLT-2 inhibitor therapy in patients with concurrent T2DM and chronic heart failure. Based on the available evidence, SGLT-2 inhibitors are safe and can be recommended to treat T2DM in patients with chronic heart failure and intact renal function. Further studies are in progress to assess long-term survival benefits.
Introduction
Of the 29.1 million people with type 2 diabetes (T2DM) in the United States,1 about 30% will develop heart failure.2 Of all patients hospitalized for heart failure with low ejection fraction, 40% have T2DM. Diabetes mellitus (DM) itself confers increased cardiovascular (CV) mortality and heart failure–related hospitalization as well as longer hospital stays when compared to patients without DM.3 Notably, besides hyperglycemia, T2DM patients are characterized by an increased amount of myocardial fat.4,5 The mechanisms of these disorders are complex and tied to insulin resistance. A salient feature of myocardial insulin resistance is an imbalance between the supply of energy-providing substrates—specifically an increased fuel supply of fat and glucose—and reduced substrate oxidation. Together, increased supply and reduced breakdown (or “demand”) are present in muscle, including the heart muscle.6–8 Insulin resistance predicts heart failure incidence independently of multiple traditional risk factors9 and the failing human heart shows histologic evidence of insulin resistance and lipid overload.10 Furthermore, there is some evidence suggesting that heart failure itself may be an insulin resistant state, which increases the risk of developing diabetes later.11
In this review we propose that in chronic heart failure, the treatment of T2DM should focus more on reducing substrate supply,8 rather than promoting substrate uptake by muscle. This principle is exemplified by the class of sodium glucose cotrasporter-2 (SGLT-2) inhibitors, which reduce blood glucose levels by blocking reabsorption of glucose by the kidney.12 Biochemically, fatty acids and glucose inhibit each other as they enter cellular pathways of oxidation.13 Epidemiological studies show an association between higher mortality rates and tight glycemic control, primarily through the use of insulin, in patients with both diabetes and heart failure.14–16 It is proposed that insulin and insulin-sensitizing medications are flooding the heart with energy-providing substrates, including fats and sugars.8 We and others have previously reported the possible effects multiple insulin and noninsulin therapies on glycemic control and heart failure.8,17,18 However, little has been reported on SGLT-2 inhibitors, which affect both supply and demand pathways in diabetics with chronic heart failure.12
SGLT-2 inhibitor therapy may be considered in T2DM patients with chronic heart failure, both with reduced ejection fraction (HFREF) and preserved ejection fraction (HFPEF). HFREF is usually defined by a left ventricular heart ejection fraction (LVEF) of ≤50%, increased LV mass, increased end diastolic volume, and increased end systolic volume. Examples include ischemic cardiomyopathy, diabetic cardiomyopathy, and many types of valvular heart disease. HFPEF is characterized by an LVEF>50%, increased LV mass, unchanged or decreased end diastolic volume, and unchanged or decreased end systolic volume. Examples include hypertensive heart disease, restrictive cardiomyopathy, and hypertrophic obstructive cardiomyopathy.19,20 Part of the SGLT-2 inhibitor mechanism includes diuresis, which leads to a preload reduction. This preload reduction is often desirable in patients with HFREF. However, diuretics should be used with caution in patients with HFPEF, as there is a concern for excessive preload reduction. Furthermore, any diuretic therapy should be used carefully in severe heart failure associated with the cardiorenal syndrome.19,20
We begin with a review of the mechanisms of lipotoxicity and glucotoxicity of the heart. Next we examine the potential role of SGLT-2 inhibitor therapy in T2DM patients with chronic heart failure. This review will not address the treatment of acute heart failure.
Lipotoxicity and Glucotoxicity in the Heart
Cardiac lipotoxicity is a complex process characterized by increased free fatty acid delivery to the heart and impaired fatty acid oxidation.10 Fatty acids are the primary lipids metabolized by the heart, and provide 70% of the heart's energy requirement.7 In diabetic and nondiabetic rodent models, myocardial triglyceride levels accumulate when rates of fat uptake exceed rates of fatty acid oxidation. Fatty acid intermediates are also converted to toxic metabolites such as diacylglycerol and ceramides, which activate signaling pathways that convey insulin resistance and/or injure cardiac myocytes via apoptosis and oxidative stress.3,21,22 It is generally thought that the accumulation of metabolites leads to contractile dysfunction, myocardial hypertrophy, and lipid accumulation.22–24 This environment also fosters impaired left ventricular filling, diastolic dysfunction, and biatrial enlargement.25 Notably, insulin is a key regulator of myocardial substrate metabolism, and defective insulin signaling might exacerbate cardiac lipotoxicity.26,27 Lipid accumulation leads to cardiomyocte insulin resistance resulting in increased utilization of fatty acids for cardiac energy, decreased contractive response to insulin, and diminished cardiac efficiency.24,26,28–31 SGLT-2 inhibitors are known to improve overall insulin sensitivity,32,33 though cardiac-specific effects in humans require further investigation. Also of special note, the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-α) is activated by long chain fatty acids, inducing the expression of proteins which increase the uptake and oxidation of fatty acids. When PPAR-α is overexpressed in the heart, myocardial triglyceride (TG) deposition increases and contractile dysfunction ensues.21,34,35 Lipoprotein lipase serves a protective role by exporting triglyceride away from the heart 36 and increasing fatty acid oxidation.37,38 PPAR-Δ and apolipoprotein B may have protective roles in reducing myocardial TG deposition.38–40
In hearts of patients with nonischemic cardiomyopathy, after removal (and prior to heart transplant), some of these hearts showed intramyocardial lipid overload, found more commonly in the hearts of obese patients and diabetic patients. Also, these individuals had a gene expression profile similar to that of the Zucker diabetic fatty model of lipotoxic and cardiac dysfunction. All of these hearts had in common increased expression levels of PPARα-regulated genes but not of PPARα itself.10
Using magnetic resonance spectroscopy techniques, Rijzewijk et al. showed that myocardial TG accumulation in men with uncomplicated T2DM was associated with LV diastolic dysfunction, independently of age, body mass index, heart rate, diastolic blood pressure, and visceral fat.4 Korosoglou et al. showed the same in both men and women with T2DM, independently of age, duration of diabetes, blood pressure, and fasting serum glucose.41 Some studies suggest that increased myocardial TG and diastolic dysfunction may begin to develop during phases of insulin resistance and/or impaired glucose tolerance,42–45 while others suggest these findings are limited to T2DM.46 In healthy men and women subjected for 6 hours to a hyperglycemic, hyperinsulinemic clamp, short-term myocardial lipid accumulation, and altered myocardial function were observed.47 While there is clearly an association between myocardial TG and diastolic dysfunction in T2DM patients, no conclusions can be made regarding causality.48
Glucotoxicity also has a deleterious effect on the heart. We have shown that glucose uptake increases more than glucose oxidation when a mammalian heart is placed under metabolic stress; the stress is then eliminated with treatment of rapamycin (an mTOR inhibitor) or metformin (via adenosine monophosphate-activated protein kinase activation). After mechanical unloading via left ventricular assistance device, levels of glucose-6-phosphate and endoplasmic reticulum stress markers are decreased.49 Other potential glucotoxic mechanisms leading to heart failure include the inactivation of sarcoplasmic endoplasmic reticulum calcium ATPase via oxidative stress,50 nonenzymatic glycation and oxidation of lipids and proteins leading to the formation of advanced glycation end products, and increased activation of protein kinase C/diacylglycerol signaling.3,51 Collectively, the literature suggests major consequences of dysregulated fuel supply on cardiac structure and function.
SGLT-2 Inhibitors
Mechanisms
Glycosuria is a hallmark of diabetes mellitus since the days of von Mering and Minkowski,52 even dating back to the Egyptian pharaohs.53 In physiological terms, increased glucose excretion by the kidney has been considered an adaptive mechanism that prevents glucose overload and hyperosmosis in the circulation.54 In pharmacological terms, inhibitors of the SGLT-2 block the glucose reabsorption via the SGLT-2 channel of the proximal tubules of the nephron, causing increased urinary glucose excretion.55 SGLT-2 inhibitors result in moderate (30%–50%) inhibition of glucose reabsorption.56 Kidney disease and decreased glomerular filtration rate decrease the efficacy of SGLT-2 inhibitors. There are currently three approved SGLT-2 inhibitors in the United States: canagliflozin, dapagliflozin, and empagliflozin. In placebo-controlled phase-3 trials in patients with T2DM, these agents improve glucose control both as monotherapy and in combination with other agents, with low risk of hypoglycemia. The overall glucose-lowering effect of SGLT-2 inhibitors has been documented across several studies12 in spite of increased gluconeogenesis32,33 and increased glucagon secretion.57 Furthermore, there is a reduction of body weight and blood pressure as well as effects on plasma lipids (increases in high- and low-density lipoprotein cholesterol). The main reported side effects are genital and urinary tract infections, especially in females. There is also a risk of adverse events related to volume depletion.12
Reduction in body mass
There is an increased association between obesity (in particular, increased visceral or abdominal adipose tissue) and increased cardiovascular risk, metabolic syndrome, and diabetes.58 Antihyperglycemic drugs that can target multiple links in this association have a better chance at reducing the multifactorial cardiovascular risk. With SGLT-2 inhibitors, the amount of glucose excreted in the urine is equivalent to 200–300 calories per day,59 and the negative energy balance is associated with weight loss.60 Although total body weight reduction is a desirable effect in these patients, it is imperative to know that the reduction is based on decreasing fat and not fluid. After 24 weeks of dapagliflozin therapy, a placebo-corrected total body weight reduction of 2.1 kg was seen, with two-thirds of this weight loss attributable to fat mass reduction. This shows that the predominant weight loss is from caloric loss and not from osmotic fluid loss. Dapagliflozin reduces both visceral adipose tissue and abdominal subcutaneous adipose tissue when compared with placebo.58 The weight loss and fat reduction continues with prolonged dapagliflozin use, with 68.3% weight loss attributable to loss of body fat after 102 weeks.61 Similar results have been seen with canagliflozin62 and empagliflozin.63 Visceral adiposity is known to cause increased cardiovascular risk and insulin resistant diabetes. It is tempting to speculate that the decrease of visceral fat with SGLT-2 inhibitors has a direct effect on overall cardiovascular risk. It seems that these effects also have positive outcomes on reducing fuel supply to the heart.
Fuel supply and demand
At its normal resting state the heart is predominantly fueled by fatty acids. In the state of hyperlipidemia, upregulation of fatty acid metabolizing proteins ensues, thus increasing cardiac myocyte uptake of fatty acids.7 When fatty acid availability exceeds its rate of oxidation, the imbalance leads to triglyceride accumulation in the cardiac myocyte. The metabolic flexibility of the heart is exemplified by the hypertrophied or atrophied heart, which switches its major substrate usage from fatty acids to glucose as the main fuel.64 In diabetics, the body is in a hyperglycemia state, which encourages glucose uptake by cardiac cells. The uncoupling of glycolysis and pyruvate oxidation leads to an accumulation of glycolytic intermediates in the heart.7 The accumulation of lipid deposition and glucose metabolites is detrimental to the function of the heart. We propose that antidiabetic drugs which enhance substrate uptake (such as thiazolidinediones, sulfonylureas, and insulin) help to fuel an already fuel-filled heart, thereby potentially contributing to worsening heart failure.8 SGLT-2 inhibitors decrease the substrate glucose by inhibiting absorption of glucose in the kidney, causing glycosuria. Although this mechanism is different from metformin, which inhibits hepatic gluconeogenesis, both metformin and SGLT-2 inhibitors allow the body to decrease the excess fuel to the heart, possibly allowing it to be safe in chronic heart failure.17,65–67
Reduction of blood pressure and arterial stiffness
Another cardiovascular benefit of SGLT-2 inhibitors is sodium excretion and the reduction of blood pressure, in part due to the osmotic diuresis.68 A 3–5 mm Hg reduction in systolic and 2 mm Hg reduction in diastolic blood pressure has been seen without any compensatory increase in heart rate.69 A meta-analysis of 27 randomized controlled trials shows that SGLT-2 inhibitors reduce systolic blood pressure by 4.0 mm Hg and diastolic blood pressure by 1.6 mm Hg.68 Dapagliflozin has been directly compared to hydrochlorothiazide, showing that after 12 weeks of treatment dapagliflozin reduced systolic blood pressure by 3.3 mm Hg as compared with hydrochlorothiazide which reduced by 6.6 mm Hg.70 Thus, blood pressure reduction due to osmotic diuresis is independent of the blood pressure reduction due to weight loss. Some studies have shown that osmotic diuresis can only account for the initial short-term blood pressure decrease. After 12 weeks of canagliflozin, urine volumes return to pretreatment levels, without blood pressure levels returning to baseline.71 It could thus be postulated that other mechanisms are at work to provide long-term blood pressure reduction, which include renal remodeling, reduced arterial stiffness and weight loss.
Furthermore, SGLT-2 inhibitors likely reduce arterial stiffness via a multifactorial mechanism which incorporates weight loss,72 decreased fasting insulin levels,73 vascular smooth muscle relaxation, and multiple beneficial anti-inflammatory effects.72,74 Under clamped euglycemic and hyperglycemic conditions, SGLT-2 inhibitors reduce measures of arterial stiffness, specifically decreasing radial augmentation index, carotid augmentation index, and aortic augmentation index.75
Conclusion
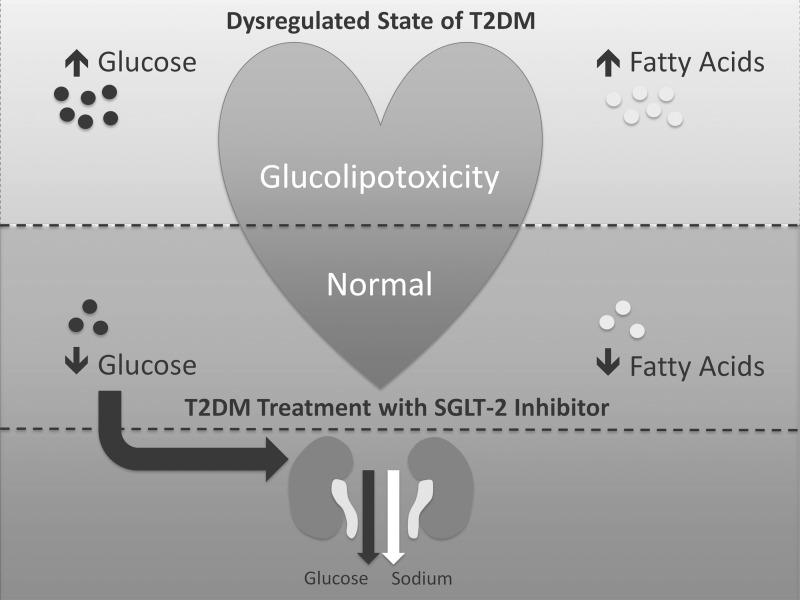
SGLT-2 inhibitors are safe and can be recommended in diabetic patients with chronic heart failure and intact renal function. While insulin and some oral antihyperglycemic medications (thiazolidinediones, sulfonylureas) increase the flow of toxic substrates to the heart,8,17 SGLT-2 inhibitors decrease this substrate supply, thereby inhibiting myocardial glucolipotoxicity67,76 and preserving heart function (Fig. 1). Their clinical use has been too short to show any survival benefits, and large cardiovascular outcome trials are currently in progress.12 However, in this brief review we have presented a compelling line of reasoning for use of SGLT-2 inhibitors in this population.
FIG. 1.
The role of sodium glucose cotrasporter-2 (SGLT-2) inhibitors in supply and demand of the heart in type 2 diabetes (T2DM). The top portion of the schematic shows the heart in the dysregulated state of T2DM, with resulting glucolipotoxicity.76 Glucotoxicity occurs when glucose uptake exceeds glucose oxidation.49 Lipotoxicity is characterized by both increased fatty acid delivery and impaired fatty acid oxidation.10 The middle portion represents T2DM treatment with an SGLT-2 inhibitor. Resolution of hyperglycemia leads to decreased myocardial glucose uptake7 and possibly decreased myocardial fatty acid uptake.67 The bottom portion represents the impact of the SGLT-2 inhibitor on the kidneys. SGLT-2 inhibitors enhance lipolysis and fatty acid oxidation in the body.77 Furthermore, a rodent model showing improved left ventricular function after SGLT-2 inhibitor therapy introduces the possibility of these effects in the heart.67 Reabsorption of glucose and sodium is inhibited; their excretion leads to lower serum glucose levels and decreased fluid volume.
Acknowledgments
This work was supported by the Center for Clinical and Translational Sciences, which is funded by National Institutes of Health Clinical and Translational Award UL1 TR000371 and KL2 TR000370 from the National Center for Advancing Translational Research (Dr. Gutierrez), and by NHLBI 2R01-HL-61483 (Dr. Taegtmeyer).
Author Disclosure Statement
Dr. Gutierrez is on the Speakers Bureau for AstraZeneca Pharmaceuticals. The other authors have no conflicts to disclose.
References
- 1.Centers for Disease Control and Prevention, Division of Diabetes Translation. National diabetes statistics report, 2014. Accessed at www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf March 23, 2015
- 2.Cowie CC, Rust KF, Byrd-Holt DD, et al. . Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988–2006. Diabetes Care 2010;33:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dei Cas A, Khan SS, Butler J, et al. . Impact of Diabetes on Epidemiology, Treatment, and Outcomes of Patients With Heart Failure. JACC Heart Fail 2015;3:136–145 [DOI] [PubMed] [Google Scholar]
- 4.Rijzewijk LJ, van der Meer RW, Smit JW, et al. . Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol 2008;52:1793–1799 [DOI] [PubMed] [Google Scholar]
- 5.Young ME, McNulty P, Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation 2002;105:1861–1870 [DOI] [PubMed] [Google Scholar]
- 6.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000;106:171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation 2002;105:1727–1733 [DOI] [PubMed] [Google Scholar]
- 8.Khalaf KI, Taegtmeyer H. After avandia: the use of antidiabetic drugs in patients with heart failure. Tex Heart Inst J 2012;39:174–178 [PMC free article] [PubMed] [Google Scholar]
- 9.Ingelsson E, Sundstrom J, Arnlov J, et al. . Insulin resistance and risk of congestive heart failure. JAMA 2005;294:334–341 [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Adrogue JV, Golfman L, et al. . Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 2004;18:1692–1700 [DOI] [PubMed] [Google Scholar]
- 11.Preiss D, Zetterstrand S, McMurray JJ, et al. . Predictors of development of diabetes in patients with chronic heart failure in the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) program. Diabetes Care 2009;32:915–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inzucchi SE, Zinman B, Wanner C, et al. . SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res 2015;12:90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taegtmeyer H, Hems R, Krebs HA. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J 1980;186:701–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshaghian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J 2006;151:191. [DOI] [PubMed] [Google Scholar]
- 15.Aguilar D, Bozkurt B, Ramasubbu K, et al. . Relationship of hemoglobin A1C and mortality in heart failure patients with diabetes. J Am Coll Cardiol 2009;54:422–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pocock SJ, Wang D, Pfeffer MA, et al. . Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J 2006;27:65–75 [DOI] [PubMed] [Google Scholar]
- 17.Ekeruo IA, Solhpour A, Taegtmeyer H. Metformin in diabetic patients with heart failure: safe and effective? Curr Cardiovasc Risk Rep 2013;7:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasir S, Aguilar D. Congestive heart failure and diabetes mellitus: balancing glycemic control with heart failure improvement. Am J Cardiol 2012;110:50B–57B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braunwald E. Heart failure. JACC Heart Fail 2013;1:1–20 [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee K. Pathophysiology of systolic and diastolic heart failure. Med Clin North Am 2012;96:891–899 [DOI] [PubMed] [Google Scholar]
- 21.Zhou YT, Grayburn P, Karim A, et al. . Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A 2000;97:1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu HC, Kovacs A, Ford DA, et al. . A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 2001;107:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finck BN, Han X, Courtois M, et al. . A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A 2003;100:1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young ME, Guthrie PH, Razeghi P, et al. . Impaired long-chain fatty acid oxidation and contractile dysfunction in the obese Zucker rat heart. Diabetes 2002;51:2587–2595 [DOI] [PubMed] [Google Scholar]
- 25.Chiu HC, Kovacs A, Blanton RM, et al. . Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 2005;96:225–233 [DOI] [PubMed] [Google Scholar]
- 26.Drosatos K, Schulze PC. Cardiac lipotoxicity: molecular pathways and therapeutic implications. Curr Heart Fail Rep 2013;10:109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SY, Cho YR, Kim HJ, et al. . Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes 2005;54:3530–3540 [DOI] [PubMed] [Google Scholar]
- 28.Iozzo P, Chareonthaitawee P, Dutka D, et al. . Independent association of type 2 diabetes and coronary artery disease with myocardial insulin resistance. Diabetes 2002;51:3020–3024 [DOI] [PubMed] [Google Scholar]
- 29.Mazumder PK, O'Neill BT, Roberts MW, et al. . Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes 2004;53:2366–2374 [DOI] [PubMed] [Google Scholar]
- 30.How OJ, Aasum E, Severson DL, et al. . Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes 2006;55:466–473 [DOI] [PubMed] [Google Scholar]
- 31.Belke DD, Larsen TS, Gibbs EM, et al. . Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab 2000;279:E1104–1113 [DOI] [PubMed] [Google Scholar]
- 32.Merovci A, Solis-Herrera C, Daniele G, et al. . Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrannini E, Muscelli E, Frascerra S, et al. . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dewald O, Sharma S, Adrogue J, et al. . Downregulation of peroxisome proliferator-activated receptor-alpha gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation 2005;112:407–415 [DOI] [PubMed] [Google Scholar]
- 35.Park SY, Cho YR, Finck BN, et al. . Cardiac-specific overexpression of peroxisome proliferator-activated receptor-alpha causes insulin resistance in heart and liver. Diabetes 2005;54:2514–2524 [DOI] [PubMed] [Google Scholar]
- 36.Bjorkegren J, Veniant M, Kim SK, et al. . Lipoprotein secretion and triglyceride stores in the heart. J Biol Chem 2001;276:38511–38517 [DOI] [PubMed] [Google Scholar]
- 37.Yagyu H, Chen G, Yokoyama M, et al. . Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest 2003;111:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng L, Ding G, Qin Q, et al. . Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 2004;10:1245–1250 [DOI] [PubMed] [Google Scholar]
- 39.Nielsen LB, Bartels ED, Bollano E. Overexpression of apolipoprotein B in the heart impedes cardiac triglyceride accumulation and development of cardiac dysfunction in diabetic mice. J Biol Chem 2002;277:27014–27020 [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama M, Yagyu H, Hu Y, et al. . Apolipoprotein B production reduces lipotoxic cardiomyopathy: studies in heart-specific lipoprotein lipase transgenic mouse. J Biol Chem 2004;279:4204–4211 [DOI] [PubMed] [Google Scholar]
- 41.Korosoglou G, Humpert PM, Ahrens J, et al. . Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging 2012;35:804–811 [DOI] [PubMed] [Google Scholar]
- 42.McGavock JM, Lingvay I, Zib I, et al. . Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007;116:1170–1175 [DOI] [PubMed] [Google Scholar]
- 43.Utz W, Engeli S, Haufe S, et al. . Myocardial steatosis, cardiac remodelling and fitness in insulin-sensitive and insulin-resistant obese women. Heart 2011;97:1585–1589 [DOI] [PubMed] [Google Scholar]
- 44.Kankaanpaa M, Lehto HR, Parkka JP, et al. . Myocardial triglyceride content and epicardial fat mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J Clin Endocrinol Metab 2006;91:4689–4695 [DOI] [PubMed] [Google Scholar]
- 45.Stahrenberg R, Edelmann F, Mende M, et al. . Association of glucose metabolism with diastolic function along the diabetic continuum. Diabetologia 2010;53:1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krssak M, Winhofer Y, Gobl C, et al. . Insulin resistance is not associated with myocardial steatosis in women. Diabetologia 2011;54:1871–1878 [DOI] [PubMed] [Google Scholar]
- 47.Winhofer Y, Krssak M, Jankovic D, et al. . Short-term hyperinsulinemia and hyperglycemia increase myocardial lipid content in normal subjects. Diabetes 2012;61:1210–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindsey JB, Marso SP. Steatosis and diastolic dysfunction: the skinny on myocardial fat. J Am Coll Cardiol 2008;52:1800–1802 [DOI] [PubMed] [Google Scholar]
- 49.Sen S, Kundu BK, Wu HC, et al. . Glucose regulation of load-induced mTOR signaling and ER stress in mammalian heart. J Am Heart Assoc 2013;2:e004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teshima Y, Takahashi N, Saikawa T, et al. . Diminished expression of sarcoplasmic reticulum Ca(2+)-ATPase and ryanodine sensitive Ca(2+)Channel mRNA in streptozotocin-induced diabetic rat heart. J Mol Cell Cardiol 2000;32:655–664 [DOI] [PubMed] [Google Scholar]
- 51.Gawlowski T, Stratmann B, Stork I, et al. . Heat shock protein 27 modification is increased in the human diabetic failing heart. Horm Metab Res 2009;41:594–599 [DOI] [PubMed] [Google Scholar]
- 52.Brogard JM, Vetter T, Blickle JF. Discovery of pancreatic diabetes in Strasbourg. Diabete Metab 1992;18:104–114 [PubMed] [Google Scholar]
- 53.Doram K, Bouland DL. Diabetes mellitus–new hope for an old disease. West J Med 1994;160:250–251 [PMC free article] [PubMed] [Google Scholar]
- 54.Pasquel FJ, Umpierrez GE. Hyperosmolar hyperglycemic state: a historic review of the clinical presentation, diagnosis, and treatment. Diabetes Care 2014;37:3124–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jabbour SA, Goldstein BJ. Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int J Clin Pract 2008;62:1279–1284 [DOI] [PubMed] [Google Scholar]
- 56.Lu Y, Griffen SC, Boulton DW, et al. . Use of systems pharmacology modeling to elucidate the operating characteristics of SGLT1 and SGLT2 in renal glucose reabsorption in humans. Front Pharmacol 2014;5:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonner C, Kerr-Conte J, Gmyr V, et al. . Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512–517 [DOI] [PubMed] [Google Scholar]
- 58.Bolinder J, Ljunggren O, Kullberg J, et al. . Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab 2012;97:1020–1031 [DOI] [PubMed] [Google Scholar]
- 59.List JF, Woo V, Morales E, et al. . Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009;32:650–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 2009;53:875–883 [DOI] [PubMed] [Google Scholar]
- 61.Bolinder J, Ljunggren O, Johansson L, et al. . Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 2014;16:159–169 [DOI] [PubMed] [Google Scholar]
- 62.Cefalu WT, Leiter LA, Yoon KH, et al. . Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013;382:941–950 [DOI] [PubMed] [Google Scholar]
- 63.Ridderstrale M, Andersen KR, Zeller C, et al. . Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol 2014;2:691–700 [DOI] [PubMed] [Google Scholar]
- 64.Doenst T, Goodwin GW, Cedars AM, et al. . Load-induced changes in vivo alter substrate fluxes and insulin responsiveness of rat heart in vitro. Metabolism 2001;50:1083–1090 [DOI] [PubMed] [Google Scholar]
- 65.Sasaki H, Asanuma H, Fujita M, et al. . Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation 2009;119:2568–2577 [DOI] [PubMed] [Google Scholar]
- 66.Xie Z, Lau K, Eby B, et al. . Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 2011;60:1770–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Younis FM, Hollander K, Mayoux EW, et al. . Effect of prophylactic treatment of empagliflozin on cardiac function and diabetes in CRDH rats. Diabetes 2014;63:A273 (1056-P). [Google Scholar]
- 68.Baker WL, Smyth LR, Riche DM, et al. . Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 2014;8:262–275 [DOI] [PubMed] [Google Scholar]
- 69.Vasilakou D, Karagiannis T, Athanasiadou E, et al. . Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013;159:262–274 [DOI] [PubMed] [Google Scholar]
- 70.Lambers Heerspink HJ, de Zeeuw D, Wie L, et al. . Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013;15:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sha S, Polidori D, Heise T, et al. . Effect of the sodium glucose co-transporter 2 inhibitor canagliflozin on plasma volume in patients with type 2 diabetes mellitus. Diabetes Obes Metab 2014;16:1087–1095 [DOI] [PubMed] [Google Scholar]
- 72.Cooper JN, Buchanich JM, Youk A, et al. . Reductions in arterial stiffness with weight loss in overweight and obese young adults: potential mechanisms. Atherosclerosis 2012;223:485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hughes TM, Althouse AD, Niemczyk NA, et al. . Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol 2012;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oelze M, Kroller-Schon S, Welschof P, et al. . The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One 2014;9:e112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cherney DZ, Perkins BA, Soleymanlou N, et al. . The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El-Assaad W, Joly E, Barbeau A, et al. . Glucolipotoxicity alters lipid partitioning and causes mitochondrial dysfunction, cholesterol, and ceramide deposition and reactive oxygen species production in INS832/13 ss-cells. Endocrinology 2010;151:3061–3073 [DOI] [PubMed] [Google Scholar]
- 77.Yokono M, Takasu T, Hayashizaki Y, et al. . SGLT2 selective inhibitor ipragliflozin reduces body fat mass by increasing fatty acid oxidation in high-fat diet-induced obese rats. Eur J Pharmacol 2014;727:66–74 [DOI] [PubMed] [Google Scholar]