Abstract
Context:
Late adolescence is marked by a delay in sleep timing, which is partly driven by a delay shift of the circadian timing system. This study examined whether the sensitivity of the circadian system to light—the primary entraining stimulus to the circadian system—differs between pre- to mid-pubertal and late to postpubertal adolescents.
Objective:
The study was designed to determine the influence of puberty on the sensitivity of the circadian system to light in humans.
Methods:
Melatonin suppression to low and moderate light levels was assessed in 38 pre- to mid-pubertal (9.1–14.7 years) and 29 late to postpubertal (11.5–15.9 years) adolescents. They received 1 hour of four light levels on consecutive nights: approximately 0.1 (near-dark baseline condition), 15, 150, and 500 lux. One group received evening light beginning at 11:00 pm (n = 39); a second group received morning light beginning at 3:00 am (n = 28). Salivary melatonin was sampled every 30 minutes. Melatonin suppression for 15, 150, and 500 lux was calculated relative to unsuppressed baseline levels in the 0.1 lux setting, within individuals.
Results:
The pre- to mid-pubertal group showed significantly greater melatonin suppression to 15 lux (9.2 ± 20.5%), 150 lux (26.0 ± 17.7%), and 500 lux (36.9 ± 11.4%) during evening light exposure compared to the late to postpubertal group (−5.3 ± 17.7%, 12.5 ± 17.3%, and 23.9 ± 21.7%, respectively; P < .05). No significant differences were seen between developmental groups in morning melatonin suppression.
Conclusion:
These results indicate support for a greater sensitivity to evening light in early pubertal children. The increased sensitivity to light in younger adolescents suggests that exposure to evening light could be particularly disruptive to sleep regulation for this group.
The most prominent developmental change in sleep patterns during adolescence is a shift in the timing of sleep, such that sleep is markedly delayed (1–9). The adolescent phase delay is partly driven by psychosocial factors, such as peer and media influence, increased social opportunities, assertion of autonomy, withdrawal of parental control, and personal preference (10, 11). Several findings, however, indicate that a phase delay occurs in parallel with physical signs of puberty (12, 13), and the phase delay may therefore be initiated by a biological process involving developmental changes in the circadian timing system (14–16).
Whether the maturational process of adolescence is linked to a change in the underlying circadian timing mechanism that alters sleep timing relative to the 24-hour day/night cycle is unclear. One mechanism that can produce delayed phase position is modification of the circadian timing system's sensitivity to entraining light signals. In a previous field study of adolescents followed from ninth to tenth grades (a transition that involved a 65-minute advance of school starting time) (17), we predicted that circadian phase (salivary dim light melatonin onset) would move to an earlier hour because adolescents were exposed to more light in the early morning. In fact, however, these adolescents manifested a phase delay in spite of an earlier rising time and no change in sleep onset time (17). Thus, either the phase delay of adolescence continues into late/postpubertal development (all subjects in the study were generally late pubertal adolescents), the circadian timing mechanism is unresponsive to morning light, or the added morning light was not sufficient to produce a phase advance or to resist further delay.
The current study evaluated the hypothesis that pubertal development is associated with an increased sensitivity to evening light and decreased sensitivity to morning light. If a transformation of the circadian timing system is related to a pubertal phase delay, then more mature adolescents should show greater melatonin suppression to light in the evening and less mature adolescents should suppress more to morning light. Such a change could explain why mid- to late pubertal adolescents express a marked delay in sleep timing and why we found that waking early in the morning was ineffective in producing phase advances in circadian timing during late adolescence (17).
Subjects and Methods
Participants
A total of 67 participants aged 9.1 to 15.9 years (38 males) completed the study. Participants received brief physical examinations to determine pubertal status based on the criteria of Tanner (18). Tanner stage is based on secondary sexual characteristics and ranges from stage 1 (ie, child-like prepubertal) to 5 (postpubertal). Classification presented here is the consensus of pubic hair distribution ratings by two study physicians. Participants were screened for good health, consistent sleep-wake patterns (3 hours or less variation in weekday-weekend sleep schedules), moderate circadian phase preference (19), and absence of medication known to affect sleep or circadian rhythms. Assignment to either evening (n = 39; 13 Tanner 1, 4 Tanner 2, 6 Tanner 3, 10 Tanner 4, and 6 Tanner 5) or morning (n = 28; 8 Tanner 1, 6 Tanner 2, 1 Tanner 3, 5 Tanner 4, and 8 Tanner 5) light groups was based on participant availability. Our previous study suggested an alteration of the circadian timing system's response to light in late puberty (17). Therefore, early to mid-puberty (“early/mid”; Tanner stages 1–3) and late to postpuberty (“late/post”; Tanner stages 4 and 5) groups were formed.
Protocol
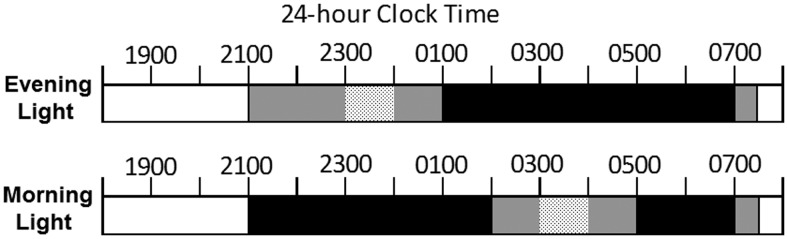
All participants were kept on a 9:00 pm to 7:00 am sleep schedule (as confirmed by nondominant wrist actigraphy; Octagonal Basic, Ambulatory Monitoring, Inc) and wore eye shades while they slept for a minimum of 10 continuous nights before the commencement of the in-laboratory study. Participants were in the laboratory for four consecutive nights of testing. On each night, they received a single, 1-hour light exposure, as illustrated in Figure 1. The order of light conditions was approximately 0.1 lux as a baseline condition followed by 15, 150, and 500 lux. Participants were kept under dim light (∼0.1 lux) conditions until light exposure at 11:00 pm for the evening group or under dim light (∼0.1 lux) or dark until light exposure at 3:00 am for the morning group (Figure 1). Light administration times were chosen to correspond to times when melatonin was expected to be elevated and during the delay (evening) and advance (morning) portions of the presumed human phase response curve to light (20), given a previous sleep schedule of 9:00 pm to 7:00 am.
Figure 1.
Participants completed either the evening (top) or morning (bottom) laboratory protocol on four consecutive nights to receive all four light levels (∼0.1, 15, 150, and 500 lux). Sleep opportunities (black horizontal bars) occurred from 1:00 to 7:00 am in the evening group and from 9:00 pm to 02:00 am and 5:00 to 7:00 am in the morning group. Room lights were off (0 lux) for sleep or dimmed (∼0.1 lux) while awake between 9:00 pm and 7:30 am during in-laboratory nights. Light exposure (dotted bars) for the evening group occurred from 11:00 pm to midnight and for the morning group from 3:00 to 4:00 am.
The evening light group slept from 1:00 to 7:00 am; the morning light group slept from 9:00 pm to 2:00 am and 5:00 to 7:00 am. Participants were in bed and continuously monitored (electroencephalography, electrooculography, electromyography, electrocardiography) from 9:00 pm to 7:00 am, and a research assistant was present in the room to ensure compliance during light exposure. Saliva was collected at 30-minute intervals while awake. Saliva samples were assayed for melatonin (Alpco, Windham). Intra-assay coefficients of variation for this radioimmunoassay kit ranges from 2.6 to 10.8%. The functional least detectable value is 0.65 pg/ml.
Melatonin analysis
Melatonin suppression was analyzed as both overall suppression using area under the curve (AUC; trapezoidal method) and melatonin suppression at 30 and 60 minutes after light exposure. For AUC, melatonin suppression was quantified as the percent change in the AUC of the salivary melatonin profile from the baseline condition (AUC baseline) to each experimental light condition (15, 150, and 500 lux; AUC light) within participants. Percent suppression was calculated using the following equation:
Therefore, higher values indicate greater suppression of melatonin production. For melatonin suppression after 30 and 60 minutes of each light exposure, suppression was calculated as the percent reduction in absolute melatonin levels (pg/ml) detected in the saliva sample taken at that time compared to levels in the sample taken at the same time during baseline (∼0.1 lux). Percent suppression was calculated separately for the 30 and 60 minutes of each light exposure using the following equation:
Dim light melatonin onset (DLMO) for each individual was defined as the clock time the salivary melatonin levels crossed the threshold value of 4 pg/ml on each test night (21). The timing of DLMO was determined by the linear interpolation of melatonin levels between the time points before and after the salivary melatonin concentration rose above the 4 pg/ml threshold. DLMO times were used to calculate the phase angle (biological time of light exposure), defined as the difference between the timing of the light exposure and DLMO.
Data analysis and statistics
Individuals were grouped according to their Tanner stage; those rated as Tanner stage 1, 2, or 3 formed the pre/midpuberty group and those rated as Tanner 4 or 5 formed the late/postpuberty group. Analyses were run separately for the evening light group and morning light group. We used independent samples t tests to compare puberty group differences on each measure of melatonin suppression. When homogeneity of variance between groups was not met, t statistics for which equal variance is not assumed are reported. Independent samples t tests were also computed to assess puberty group differences in phase angle.
Results
Melatonin suppression
Evening light.
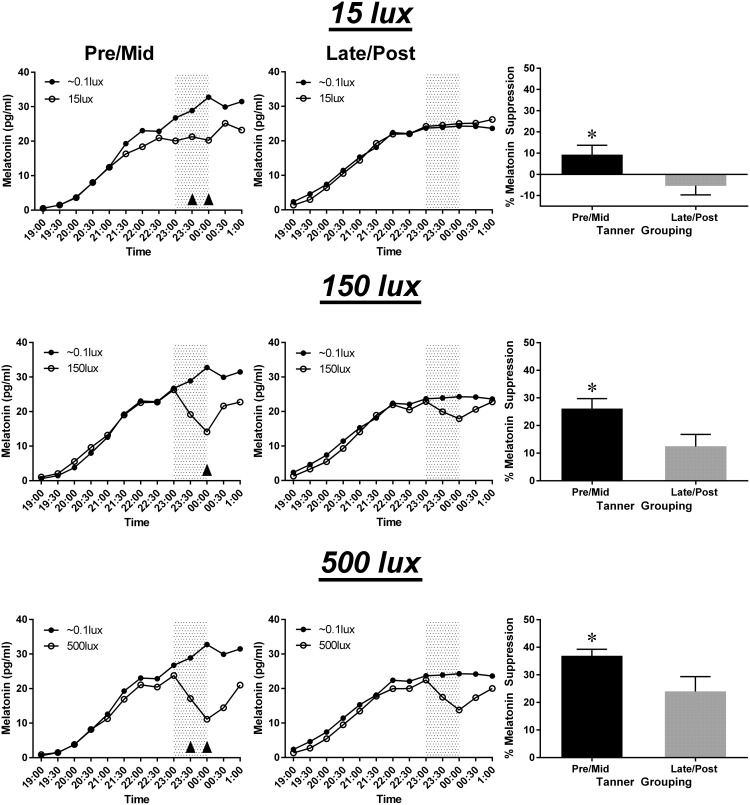
We first examined melatonin suppression across the experimental light exposures using AUC. The average percent suppression of the melatonin profile in response to the evening light conditions (15, 150, and 500 lux) was compared between groups of pre/midpubertal (n = 23) and late/postpubertal (n = 16) adolescents. Figure 2 illustrates average melatonin concentrations and the average percent AUC melatonin suppression for each puberty group and light condition. All means and standard deviations are displayed in Table 1. Exposure to all light levels (15, 150, and 500 lux) produced significantly greater melatonin suppression in the pre/midpuberty group compared to the late/postpuberty group (P < .05).
Figure 2.
Average melatonin suppression during evening light exposure (11:00 pm–midnight; gray shading) over the three experimental light conditions by puberty group. Average absolute melatonin levels at the three light levels (open circles) relative to unsuppressed baseline levels (black circles) are illustrated for the pre/midpuberty group (left column) and the late/postpuberty group (middle column). Upward arrows indicate significant pairwise comparisons (P < .05). Right column: average suppression for each light level calculated using AUC for the light exposure versus unsuppressed near-dark baseline condition. Asterisks indicate significant differences (P < .05). Error bars indicate standard error.
Table 1.
Means and sd for Melatonin Suppression (%) Within the Pre/Midpuberty and Late/Postpuberty Groups Across Morning and Evening Light Conditions (15, 150, and 500 lux)
| Light Group | Puberty Group | AUC |
30-Minute Light |
60-Minute Light |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15 lux | 150 lux | 500 lux | 15 lux | 150 lux | 500 lux | 15 lux | 150 lux | 500 lux | ||
| Evening light | Pre/mid M (sd) | 9.2a (20.5) | 26.0a (17.7) | 36.9a (11.4) | 9.48a (21.5) | 25.2c (19.3) | 43.0a (18.4) | 11.5a (23.9) | 47.3b (20.7) | 60.7a (12.4) |
| Late/post M (sd) | −5.3 (17.7) | 12.5 (17.3) | 23.9 (21.7) | −6.6 (24.5) | 12.9 (19.8) | 25.6 (21.9) | −8.9 (30.9) | 20.9 (33.9) | 39.5 (31.8) | |
| Morning light | Pre/mid M (sd) | 10.0 (18.6) | 36.3 (14.9) | 35.0 (11.8) | 12.5 (18.2) | 41.8 (15.0) | 43.3c (12.5) | 9.78 (26.6) | 61.6 (14.4) | 62.9 (14.8) |
| Late/post M (sd) | 10.9 (16.7) | 27.1 (18.1) | 27.7 (15.5) | 11.4 (21.0) | 31.4 (23.9) | 30.7 (20.8) | 16.0 (23.0) | 53.0 (17.7) | 57.6 (13.3) | |
P < .05;
P < .01;
P < .10 compared to the late/postpuberty group.
The average percent suppression of absolute melatonin levels after 30 and 60 minutes of light exposure was compared between puberty groups. At 30 minutes, the pre/midpuberty group demonstrated significantly greater average suppression in both the 15 lux and 500 lux conditions (P < .05) and tended to show greater suppression at 150 lux (P = .06) when compared to the late/postpuberty group. At 60 minutes, the pre/midpuberty group exhibited significantly greater average suppression at all light conditions (P < .05) when compared to the late/postpuberty group.
The average AUC melatonin suppression in response to each evening light condition was also compared between males (n = 22) and females (n = 17) and showed no statistically significant differences in melatonin suppression between males and females in response to any of the light conditions (P > .05).
Morning light.
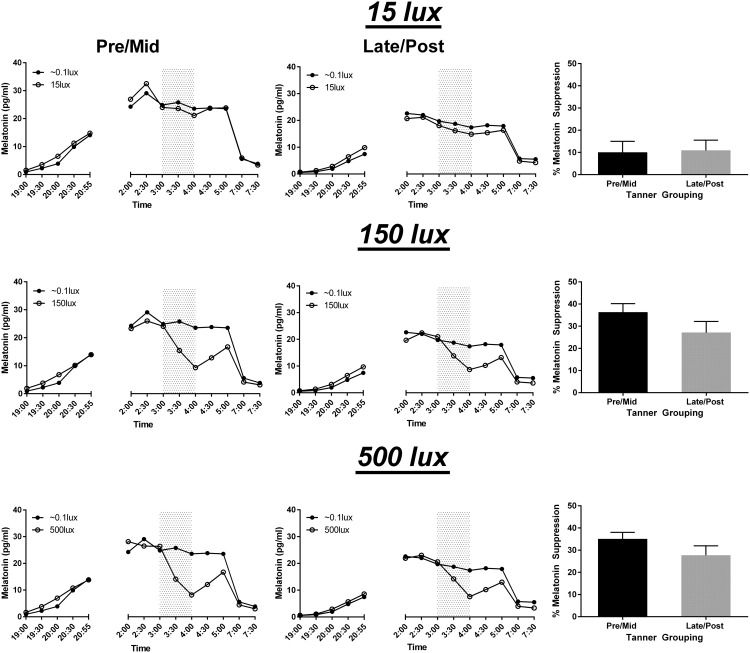
No significant differences were found between groups for melatonin suppression measured by AUC for any of the light conditions. Figure 3 depicts average melatonin values and the average percent AUC melatonin suppression for each puberty group and light condition.
Figure 3.
Average melatonin suppression during morning light exposure (3:00–4:00 am; gray shading) over the three experimental light conditions by puberty group. Average absolute melatonin levels at the three light levels (open circles) relative to unsuppressed baseline levels (black circles) are illustrated for the pre/midpuberty group (left column) and the late/postpuberty group (middle column). Right column: average suppression for each light level calculated using AUC for the light exposure versus unsuppressed near-dark baseline condition. Error bars indicate standard error.
We compared the average percent suppression of absolute melatonin levels after 30 and 60 minutes of each light condition between groups. At 30 minutes, no significant differences were observed between pubertal groups; however, a trend toward pre/midpubertal adolescents suppressing more to 500 lux compared to the late/post adolescents (P = .06) was seen. At 60 minutes, pubertal groups did not differ in melatonin suppression for any light level.
The average AUC melatonin suppression in response to each morning light condition was also compared between males (n = 16) and females (n = 12) and showed no statistically significant differences between males and females in response to any of the light conditions (P > .05).
Circadian time of light exposure
The average phase angle on each light exposure night was compared between groups. For evening light exposures, there were no statistically significant differences between puberty groups in the circadian timing of any of the evening light exposures (15, 150, or 500 lux) (P > .05). For morning light exposures, there were also no statistically significant differences between pubertal groups in the circadian timing of any of the morning light exposures (P > .05). Thus, the early/mid pubertal and late/postpubertal groups received light exposures at the same circadian times. The differences seen in melatonin suppression, therefore, were not due to systematic differences in the biological time of the light exposures.
Discussion
Our results indicate that the circadian system of pre- to mid-pubertal adolescents is more responsive to evening light than that of late to postpubertal adolescents. The less mature adolescents (pre/midpuberty group) showed greater melatonin suppression at all tested light levels in the evening, a finding contrary to our hypothesis that more mature adolescents would demonstrate greater sensitivity to evening light. Our results indicate that delayed circadian timing in older adolescents is not due to increased sensitivity to evening light. It is more likely, therefore, that older adolescents have more delayed timing due to an increase in light exposure, rather than an increase in light sensitivity. As homeostatic sleep pressure accumulation slows in late adolescence (22), mature adolescents are able to stay awake later into the evening compared to less mature adolescents. We suggest that staying awake later into the evening with the potential for phase delaying light exposure may facilitate the phase delay shift in older adolescents compared to their younger peers who fall asleep early and are less likely to be exposed to phase delaying light. Alternatively, greater sensitivity to light in late childhood and early/mid puberty may trigger the adolescent phase delay process, engendering a cascade of later sleep timing and later light exposure with adolescent maturation. We note that heightened melatonin suppression in the evening in the younger group was not due to a systematic difference in the circadian timing of light because the timing of light relative to circadian phase did not differ between the groups.
The morning melatonin suppression response was not statistically different between groups, though a trend for more suppression after 30 minutes of light in the pre/midpuberty group compared to the late/postpuberty group emerged for the 500 lux condition; the pattern appears similar in the 150 lux condition. Thus, the lack of support for our hypothesis that older adolescents would show decreased responsiveness to light in the morning may have resulted from a lack of statistical power. If mature adolescents are less responsive to morning light, this might help to maintain behaviorally induced delays in circadian rhythms (eg, due to increased evening light exposure opportunity), despite early morning light exposure on school days.
Our results support general findings in animal and human literature for a decrease in the response of the circadian system to delaying light with age. Several studies in laboratory mice have demonstrated an increased sensitivity to phase delaying light in pubertal animals relative to adult animals (16, 23, 24). Another recent study supports this finding in humans as well, with greater melatonin suppression to evening light (∼580 lux) in children (∼7 years) compared to their adult parents (∼41 years) and a trend for a greater suppression in response to dimmer room light at home in the children (∼120–140 lux) (25). A decrease in the circadian response to light may continue throughout the lifespan because young adults (∼18–30 years) show greater phase delay shifts in response to moderate light levels compared to older adults (>65 years) (26); physical changes in the lens may account for the reduction of light response in older humans.
The finding that the pre/midpuberty group showed significantly greater suppression at 15 lux in the evening is particularly striking because this light level is ineffective in producing melatonin suppression in adults (27). The circadian system of pre/midpubertal adolescents, therefore, appears to be very sensitive to light. This issue is of growing public health importance because the use of light-emitting devices is reaching a point of relative ubiquity for adolescents. For example, approximately 96% of American adolescents report using at least one form of technology in the hour before bedtime (11). Adolescents aged 13–18 years exhibit the greatest pre-bedtime use of light-emitting devices held close to the face, with 77% reporting using a computer or laptop in the hour before bed and 72% reporting using a mobile phone (11). Reported adverse effects of presleep light-emitting device use by adolescents include reduced total sleep time and delayed sleep onset (28, 29), an association that is stronger and more persistent for devices held close to the face such as mobile phones and computers (30). Consistent with our findings, younger adolescents (<13.3 years) were more likely to be affected by the negative consequences of device use on total sleep time when compared to older adolescents (13.3–17 years) (31). Pre-bedtime use of light-emitting devices acutely suppresses melatonin production, shifts rhythms later, and causes heightened arousal in adults (32, 33). Given the increased responsiveness of pre/midpubertal adolescents to the delaying effects of light, they may be exposed to an increased risk for the adverse effects of these bedtime behaviors on sleep-wake patterns and circadian timing. Children who are more sensitive to evening light may manifest a greater phase delay, leading to heightened evening arousal and short mistimed sleep. These effects may translate into acute or long-term consequences for daytime function (eg, school engagement) and health (eg, weight regulation), though future studies are needed to address these hypotheses.
Limitations.
The current study examined melatonin suppression as a measure of the responsiveness of the circadian system to light. Because we measured melatonin suppression rather than phase shifting, we cannot confirm that pubertal differences in phase delays occur. Further studies are needed to examine the effect of pubertal development on phase shifts. Thus, although melatonin suppression is an important marker of light's effects on the circadian timing system, the more relevant factor for mechanisms of entrainment during puberty may be whether maturation affects phase advances and phase delays in responses to light.
Acknowledgments
We thank Drs. Judith Owens, Victoria Dalzell, and Susan Labyak for Tanner staging and physical examinations and Dr. Linda Bausserman and the Miriam Hospital Lipid Laboratory for performing the melatonin assays. We are indebted to our research staff, including Bethany Quinn, Suni Sun, Jennifer Maxwell-Willis, Theresa Lagman, and Donna Taraborelli, and the Bradley Hospital Sleep Laboratory's research apprentices. We also like to thank the participants for their efforts.
This work was supported by Grants MH52415 and MH01358 from the National Institute of Mental Health (to M.A.C.) and HL105395 from the National Heart Lung and Blood Institute (to S.J.C.).
Disclosure Summary: C.A. is a shareholder of Jazz Pharmaceuticals plc and employee of Jazz Pharmaceuticals, Inc. who, in the course of this employment, has received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals plc; her work on this project preceded this industry involvement and the study was not supported in any way by Jazz Pharmaceuticals. S.W.C., A.B., M.A.C., and S.J.C. have indicated no financial conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH), National Institute of Mental Health (NIMH), or National Heart Lung and Blood Institute (NHLBI). The NIH, NIMH, and NHLBI had no involvement in designing the study, data collection, data analysis and interpretation, writing of the manuscript, or in the decision to submit the manuscript for publication. The authors alone are responsible for the content and writing of the paper.
Footnotes
- AUC
- area under the curve
- DLMO
- dim light melatonin onset.
References
- 1. Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. [PubMed] [Google Scholar]
- 2. Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. J Sleep Res. 2001;10:59–67. [DOI] [PubMed] [Google Scholar]
- 3. Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. J Sleep Res. 2002;11:191–199. [DOI] [PubMed] [Google Scholar]
- 4. Wolfson AR, Carskadon MA, Acebo C, et al. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26:213–216. [DOI] [PubMed] [Google Scholar]
- 5. Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. [DOI] [PubMed] [Google Scholar]
- 6. O'Brien EM, Mindell JA. Sleep and risk-taking behavior in adolescents. Behav Sleep Med. 2005;3:113–133. [DOI] [PubMed] [Google Scholar]
- 7. Yang CK, Kim JK, Patel SR, Lee JH. Age-related changes in sleep/wake patterns among Korean teenagers. Pediatrics. 2005;115:250–256. [DOI] [PubMed] [Google Scholar]
- 8. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8:602–612. [DOI] [PubMed] [Google Scholar]
- 9. Randler C. Differences in sleep and circadian preference between Eastern and Western German adolescents. Chronobiol Int. 2008;25:565–575. [DOI] [PubMed] [Google Scholar]
- 10. Van den Bulck J. Television viewing, computer game playing, and Internet use and self-reported time to bed and time out of bed in secondary-school children. Sleep. 2004;27:101–104. [DOI] [PubMed] [Google Scholar]
- 11. Gradisar M, Wolfson AR, Harvey AG, Hale L, Rosenberg R, Czeisler CA. The sleep and technology use of Americans: findings from the National Sleep Foundation's 2011 Sleep in America poll. J Clin Sleep Med. 2013;9:1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. [DOI] [PubMed] [Google Scholar]
- 13. Frey S, Balu S, Greusing S, Rothen N, Cajochen C. Consequences of the timing of menarche on female adolescent sleep phase preference. PLoS One. 2009;4:e5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carskadon MA, Acebo C, Richardson GS, Tate BA, Seifer R. An approach to studying circadian rhythms of adolescent humans. J Biol Rhythms. 1997;12:278–289. [DOI] [PubMed] [Google Scholar]
- 15. Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004;1021:276–291. [DOI] [PubMed] [Google Scholar]
- 16. Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carskadon MA, Wolfson AR, Acebo C, Tzischinsky O, Seifer R. Adolescent sleep patterns, circadian timing, and sleepiness at a transition to early school days. Sleep. 1998;21:871–881. [DOI] [PubMed] [Google Scholar]
- 18. Tanner JM. Growth at Adolescence. Oxford, UK: Blackwell; 1978. [Google Scholar]
- 19. Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74:728. [DOI] [PubMed] [Google Scholar]
- 20. Khalsa SB, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagtegaal E, Peeters T, Swart W, Smits M, Kerkhof G, van der Meer G. Correlation between concentrations of melatonin in saliva and serum in patients with delayed sleep phase syndrome. Ther Drug Monit. 1998;20:181–183. [DOI] [PubMed] [Google Scholar]
- 22. Taylor DJ, Jenni OG, Acebo C, Carskadon MA. Sleep tendency during extended wakefulness: insights into adolescent sleep regulation and behavior. J Sleep Res. 2005;14:239–244. [DOI] [PubMed] [Google Scholar]
- 23. Weinert D, Eimert H, Erkert HG, Schneyer U. Resynchronization of the circadian corticosterone rhythm after a light/dark shift in juvenile and adult mice. Chronobiol Int. 1994;11:222–231. [DOI] [PubMed] [Google Scholar]
- 24. Weinert D, Kompauerova V. Light-induced phase and period responses of circadian activity rhythms in laboratory mice of different age. Zool-Anal Complex Sy. 1998;101:45–52. [Google Scholar]
- 25. Higuchi S, Nagafuchi Y, Lee SI, Harada T. Influence of light at night on melatonin suppression in children. J Clin Endocrinol Metab. 2014;99:3298–3303. [DOI] [PubMed] [Google Scholar]
- 26. Duffy JF, Zeitzer JM, Czeisler CA. Decreased sensitivity to phase-delaying effects of moderate intensity light in older subjects. Neurobiol Aging. 2007;28:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hale L, Guan S. Screen time and sleep among school-aged children and adolescents: a systematic literature review. Sleep Med Rev. 2015;21:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bartel KA, Gradisar M, Williamson P. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep Med Rev. 2015;21:72–85. [DOI] [PubMed] [Google Scholar]
- 30. Arora T, Broglia E, Thomas GN, Taheri S. Associations between specific technologies and adolescent sleep quantity, sleep quality, and parasomnias. Sleep Med. 2014;15:240–247. [DOI] [PubMed] [Google Scholar]
- 31. Drescher AA, Goodwin JL, Silva GE, Quan SF. Caffeine and screen time in adolescence: associations with short sleep and obesity. J Clin Sleep Med. 2011;7:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang A-M, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci USA. 2015:112:1232–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cajochen C, Frey S, Anders D, et al. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J Appl Physiol (1985). 2011;110:1432–1438. [DOI] [PubMed] [Google Scholar]