Abstract
Context:
Zoledronic acid (ZA) is increasingly used in young patients with bone disorders. However, data related to the safety of ZA administration in this population are limited.
Objective:
The study aimed to characterize the short-term safety profile of ZA and identify risk factors for ZA-related adverse events (AEs) in young patients.
Design, Setting, and Participants:
This was a retrospective chart review of inpatients and outpatients less than 21 years old who received at least one ZA infusion between July 2010 and January 2014 at The Children's Hospital of Philadelphia.
Results:
Eighty-one patients (56% male; median age, 12 y; age at first infusion, 0.5 to 20 y) with diverse skeletal disorders received a total of 204 infusions. The most common indications were osteoporosis (33% of cohort) and osteogenesis imperfecta (27.2%). The median ZA dose was 0.025 mg/kg (interquartile range, 0.025–0.05); the median dosing interval was 6 months (range, 1 to 25.6 mo). AEs were mild and more common after the first ZA infusion in patients with no previous bisphosphonate exposure: hypophosphatemia (25.2% of infusions), acute phase reactions (19.1%), and hypocalcemia (16.4%). Symptomatic hypocalcemia requiring iv calcium occurred after two infusions. ZA dose was significantly associated with hypophosphatemia, but not other AEs. Hypocalcemia was more common in patients with high bone turnover as assessed by preinfusion alkaline phosphatase levels. AEs were not associated with diagnosis, baseline serum calcium, or calcium/calcitriol supplementation.
Conclusion:
Acute AEs related to ZA infusion in youths are common, occur principally after the first ZA infusion in bisphosphonate-naive patients, and are typically mild and easily managed. Future prospective studies are needed to determine the potential long-term risks, as well as benefits, of ZA therapy in the pediatric population.
Young patients with various bone disorders face an increased risk of fragility fractures, bone deformity, decreased mobility, impaired growth, and chronic pain. The conventional management of many of these bone disorders, such as osteogenesis imperfecta (OI) and idiopathic osteoporosis, includes optimization of calcium and vitamin D intake, physical therapy, and in many cases, antiresorptive therapy. Antiresorptive therapy is based on the observation that these skeletal disorders are characterized by an imbalance in bone homeostasis that favors osteoclastic bone resorption over osteoblastic bone formation. In adults, bisphosphonates are the most commonly used antiresorptive agents, and members of this class are widely used for indications such as osteoporosis in postmenopausal women and elderly men, glucocorticoid-induced osteoporosis, and Paget's disease (1–4). Differences between the various bisphosphonates arise in part due to specific modifications to R1 and R2 side chain groups, which influence the affinity for hydroxyapatite and determine the molecular action and antiosteoclastic potency of the bisphosphonate, respectively (5). Ultimately, bone resorption is inhibited, bone loss is reduced, and bone mineralization and strength are improved (6).
The current second-generation (pamidronate, ibandronate, and alendronate) and third-generation bisphosphonates (risedronate and zoledronate or zoledronic acid [ZA]) have nitrogen-containing R2 side chains that increase their antiresorptive potency by 10- to 10 000-fold relative to early non-nitrogen-containing first-generation predecessors (etidronate, clodronate, and tiludronate) (7, 8). The poor (<1%) enteral absorption of bisphosphonates, in general, and concerns regarding gastrointestinal intolerance of nitrogen-containing bisphosphonates, in particular, have increased enthusiasm for iv bisphosphonates such as ZA. Not only is ZA the most potent bisphosphonate; it also has further advantages over other iv bisphosphonates such as pamidronate due to its very short infusion time (15 to 30 min vs 2 to 4 h for pamidronate) that results in less phlebitis (9) and a duration of action that allows for less frequent dosing (10). However, adverse events (AEs) are common in the days after administration of ZA and include acute phase reactions (APRs) (flu-like symptoms including low-grade fever, myalgia and bone pain, or headache), hypocalcemia, hypophosphatemia, and less commonly, renal damage (6, 11). In addition, chronic use of ZA (and other highly potent bisphosphonates) in adults has been associated with osteonecrosis of the jaw and atypical hip fractures (12–15). A trial of ZA compared to pamidronate in children with OI reported a similar frequency of side effects between the two agents, but the authors did not collect data on postinfusion hypocalcemia or hypophosphatemia (16).
The dual conveniences of iv administration and reduced treatment frequency have led to increased usage of ZA in children and adolescents with bone disorders. Nevertheless, there are very limited published data that address the safety and efficacy of ZA in the pediatric population. The goal of our study was to characterize the short-term safety of ZA when infused in young patients with a variety of bone disorders. In this report, we describe the frequency and potential risk factors for acute AEs in young patients treated with ZA at The Children's Hospital of Philadelphia (CHOP).
Patients and Methods
Study sample
We conducted a retrospective chart review on all patients who were less than 21 years old at the time of their first ZA infusion at CHOP between July 2010 (when ZA infusions first began at CHOP) and January 2014. Patients received infusions either as inpatients or as outpatients in the day medicine infusion center under the supervision of physicians in the endocrinology, rheumatology, oncology, critical care, genetics, or metabolism divisions. This study was approved by the Institutional Review Board at CHOP, and all subjects had given assent/consent to receive ZA.
Treatment protocol
ZA treatment was recommended at the discretion of the prescribing physician. Prior to June 2012, ZA dosing and peri-infusion laboratory testing were based on the clinical judgment of the attending clinician in consultation with the CHOP clinical pharmacy. In June 2012, the authors developed a dosing and monitoring protocol for administration of ZA with the goal of standardizing ZA treatment (Tables 1 and 2). The protocol recommends ZA doses and dosing intervals that are based on a patient's underlying diagnosis. It provides guidelines for assessment of mineral metabolism both before treatment and 72 hours after infusion, with laboratory tests that include comprehensive metabolic panel, phosphorus, intact PTH, 25-hydroxyvitamin D [25(OH)D], urinary amino-terminal collagen crosslinks (NTx), and/or serum carboxy-terminal collagen crosslinks. The protocol directs that patients with suboptimal vitamin D status [serum 25(OH)D concentration of < 30 ng/mL] be supplemented with cholecalciferol before ZA treatment to achieve a serum 25(OH)D level of at least 30 ng/mL. The protocol outlines the doses of calcium and calcitriol supplements that should be administered to patients before their first (and possibly subsequent) ZA infusion(s).
Table 1.
Dosing Recommendations for ZA in Pediatric Patients
| Diagnosis | Initial Dose, mg/kg | Subsequent Doses, mg/kg | Maximum Single Dose | Dosing Interval |
|---|---|---|---|---|
| Osteoporosis, low BMD for age | 0.0125 | 0.025–0.05 | <18 y, 4 mg; ≥18 y, 5 mg | 6–12 mo |
| AVN, CRMO, fibrous dysplasia | 0.0125 | 0.025–0.05 | 4 mg | 3–12 mo |
| OI and other genetic bone diseases | 0.025 | 0.025–0.05 | <18 y, 4 mg; ≥18 y, 5 mg | 6–12 mo |
| Hypercalcemia of malignancy | 0.025 | 0.025–0.05 | 4 mg | 1–6 wk |
Table 2.
Additional Protocols for ZA Use in Pediatric Patients
| Treatment | |
|---|---|
| Laboratory monitoring parameters | |
| Preinfusion labs (7 d before infusion) | Calcium, creatinine, magnesium, phosphorus, PTH, 25(OH)D, NTx |
| Postinfusion labs (48–72 h after infusion) | Calcium (or ionized calcium), phosphorus, NTx |
| Adjuvant treatment recommendations to mitigate ZA-related AEs | |
| First infusion | |
| Scheduled starting the night before infusion | Calcium (elemental), 50 mg/kg/d up to 1500 mg, divided TID |
| Calcitriol, 0.05 μg/kg/d up to 1 μg, divided BID | |
| As needed for vitamin D insufficiency | Cholecalciferol, provide as needed for 25(OH)D <30 ng/mL |
| As needed for signs/symptoms of APR | Acetaminophen, 10–15 mg/kg every 4–6 h up to 4 g/d |
| Ibuprofen, 5–10 mg/kg every 6–8 h up to 40 mg/kg/d | |
| Subsequent infusions | Consider above recommendations on case-by-case basis |
Abbreviations: BID, twice daily; TID, three times daily.
Study measures
The following were extracted from the electronic medical record: demographics (age, sex); medical history including fracture and anthropometric data (height, weight, body mass index, and body surface area) recorded closest to the date of ZA infusion; biochemical measures within 1 month before ZA infusion and 1 week after infusion, including serum levels of calcium, ionized calcium, alkaline phosphatase, phosphorus, magnesium, creatinine, intact PTH, and 25(OH)D; urinary NTx and/or serum carboxy-terminal collagen crosslinks; pharmacy data including ZA dosing, dosing intervals, location of infusion (inpatient vs outpatient), prior bisphosphonate use, calcium supplements, vitamin D supplements, and calcitriol use; and reports of AEs within 1 week after infusion. The skeletal diagnosis was also obtained from the electronic medical record. Osteoporosis was defined, using the 2013 International Society for Clinical Densitometry (ISCD) pediatric guidelines, as vertebral compression fractures or bone mineral density (BMD)-Z score < −2 and the presence of at least two long-bone fractures before age 10 years or at least three long-bone fractures before age 19 years (16). Areal BMD (lumbar spine L1–L4 and total body less head) was determined by dual-energy x-ray absorptiometry (DXA), converted into Z-scores and adjusted for height Z-score. Low BMD was defined as height Z-adjusted DXA BMD Z-score of < −2 but not meeting other ISCD criteria for osteoporosis. Fragility fracture not otherwise specified was defined as nonvertebral fracture history but BMD Z-score > −2. Biochemical tests were performed in the CHOP clinical chemistry laboratory or other commercial laboratories using standard reagents and methods.
Adverse events
We included reports of AEs that occurred within 1 week of ZA infusions, including hypocalcemia (serum ionized calcium < 1 mmol/L or total calcium level < 8 mg/dL), hypophosphatemia (serum phosphorus level < 3 mg/dL), APRs (defined as fever and/or bone pain or myalgia), seizures, anaphylaxis, medical evaluation at an emergency department, and/or unplanned hospital admissions.
Statistical analyses
Standard descriptive statistics were used to summarize demographics, variables related to bone, and mineral metabolism and to determine the proportion of AEs. Wilcoxon rank-sum and Wilcoxon-signed rank tests were used to compare unpaired and paired medians, respectively; and χ2 analysis or Fisher's exact tests were used to compare proportions. Tests for trend were performed using an extension of the Wilcoxon rank-sum test (17). Statistical significance was defined using a two-sided P value <.05 for all analyses. All statistical analyses were conducted with Stata 12 (StataCorp LP).
Results
Descriptive characteristics
Eighty-one subjects (56% male) received a total of 204 ZA infusions during the study period (Table 3). Sixty-two of the 81 (76.5%) subjects had a history of fracture before first infusion (OI, 22 of 22 [100%]; chronic recurrent multifocal osteomyelitis [CRMO], 5 of 12 [41.7%]; avascular necrosis [AVN], 3 of 6 [50%]; low BMD, 0 of 4 [0%]; osteoporosis, 27 of 27 [100%]; fibrous dysplasia, 2 of 2 [100%]; vitamin D toxicity, 0 of 1 [0%]; cutis laxa, 0 of 1 [0%]; as part of an oncological protocol, 0 of 3 [0%]; osteolytic lesion, 2 of 2 [100%]; and fragility fracture not otherwise specified, 1 of 1 [100%]). Twelve of 81 subjects also had documentation of “bone pain” in the medical record before the first infusion, which may have influenced the decision to treat. The median age at the time of the first ZA infusion was 12 years, with a range of 6 months to 20 years. The most common underlying skeletal diagnoses were osteoporosis (33.3% of unique individuals), OI (27.2%), CRMO (14.8%), and AVN (7.4%). The 27 participants classified as having osteoporosis were clinically heterogeneous. The primary diagnoses were as follows: cerebral palsy (n = 7), chronic inflammatory disease (n = 5), muscular dystrophy (n = 4), suspected genetic bone disease (n = 4), other neuromuscular disease (n = 3), cystic fibrosis status after lung transplant (n = 1), acute lymphoblastic leukemia survivor (n = 1), small bowel resection (n = 1), and other multiple congenital anomalies (n = 1). Thirty-three percent (9 of 27) had been exposed to chronic oral or iv corticosteroids, and 56% (15 of 27) were nonambulatory at the time of initial infusion. Subjects with OI accounted for 43.1% of all ZA infusions. Most of the infusions were prescribed by an endocrinologist (61.8%); however, infusions were also prescribed by physicians in metabolism (26.5%), rheumatology (9.3%), oncology (2%), and gastroenterology (0.5%).
Table 3.
Descriptive Characteristics of Study Sample
| Unique Individuals | Unique Infusions | |
|---|---|---|
| n | 81 | 204 |
| Males, % | 55.6 | 58.8 |
| Age, y | 12 (0.5–20)a,b | 11.3 (0.5–21.9)a |
| ZA dose, mg/kg | 0.025 (0.0125–0.025)b,c | 0.025 (0.025–0.05)c |
| Dosing interval, mo | 6 (1–25.6)a | |
| No. of infusions | 2 (1–13)a | |
| Infusion location, % | ||
| Outpatient day medicine | 77.8 | 86.3 |
| Admitted for infusion | 19.8 | 12.8 |
| Already admitted for other reasons | 6.2 | 2.5 |
| Fracture history, % | 76.5 | 77 |
| Diagnosis, % | ||
| Osteoporosisd | 33.3 | 24 |
| OI | 27.2 | 43.1 |
| CRMO | 14.8 | 8.3 |
| AVN | 7.4 | 11.3 |
| Low BMDe | 4.9 | 2.9 |
| Bone metastasesf | 3.7 | 2 |
| Osteolytic lesionsg | 2.5 | 1.5 |
| Fibrous dysplasia | 2.5 | 2.9 |
| Vitamin D toxicity | 1.2 | 0.5 |
| Cutis laxa | 1.2 | 2.9 |
| Fragility fracture not otherwise specifiedh | 1.2 | 0.5 |
| Previous bisphosphonate, % | 21 | 68.6 |
| Vitamin D status, %i | ||
| Deficient | 3.9 | 5.5 |
| Insufficient | 19.2 | 25 |
| Pre-ZA medications, %j | ||
| Calcium | 40.7 | 27.5 |
| Calcitriol | 39.5 | 28.9 |
| Vitamin D | 7.4 | 8.3 |
| Pre-ZA biochemical parameters | ||
| Creatinine, mg/dLk | 0.4 (0.3–0.5)b,c | 0.4 (0.3–0.5)c |
| 25(OH)D, ng/mL | 38.7 (31.2–46.4)b,c | 37.4 (28.6–45.7)c |
| Magnesium, mg/dLl | 1.9 (1.9–2)b,c | 2 (1.9–2.1)c |
| PTH, pg/mLm | 19 (13.6–32.6)b,c | 21.3 (13.9–31.1)c |
Median (range).
First ZA infusion.
Median (IQR).
As defined by current ISCD guidelines (vertebral compression fracture or BMD-Z score <−2 and presence of at least 2 long-bone fractures before age 10 or at least 3 before age 19.
DXA BMD-Z score <−2 but not meeting other ISCD criteria for definition of osteoporosis.
All associated with neuroblastoma.
Gorham Stout and Hajdu-Cheney.
Nonvertebral fracture history but BMD Z-score >−2.
Deficient indicates 25(OH)D <20 ng/mL; insufficient indicates 25(OH)D <30 ng/mL; 25(OH)D levels in 52 individuals before 128 infusions.
Adjuvant medications added in addition to any baseline calcium or vitamin D supplements.
Creatinine levels were obtained in 72 individuals before 156 infusions.
Magnesium levels were obtained in 56 individuals before 108 infusions.
PTH levels were obtained in 38 individuals before 100 infusions.
Most (86.3%) infusions were administered in the outpatient setting. The median ZA dose for all infusions was 0.025 mg/kg/dose (interquartile range [IQR], 0.025–0.05), with 10.8% of subjects receiving a dose ≤ 0.0125 mg/kg, 52.9% receiving a dose of 0.025 mg/kg, and 36.3% receiving a dose > 0.025 mg/kg. The median dosing interval was 6 months (range, 1–25.6 mo) among the 43 subjects who received more than one infusion. Most subjects (79%) had not been treated with a bisphosphonate before their first ZA infusion. Median 25(OH)D level was 37.4 ng/mL (38.7 ng/mL for first infusion), and vitamin D deficiency (<20 ng/dL) and insufficiency (<30 ng/mL) were present before 5.5 and 25% of all infusions, respectively. Serum levels of creatinine, magnesium, and intact PTH were normal before all infusions. Postinfusion calcium levels were obtained in 67 of 81 (83%) first infusions and 134 of 204 (65.7%) total infusions; postinfusion phosphorus levels were obtained in 53 of 81 (63.4%) first infusions and 107 of 204 (52.5%) total infusions.
Adverse events
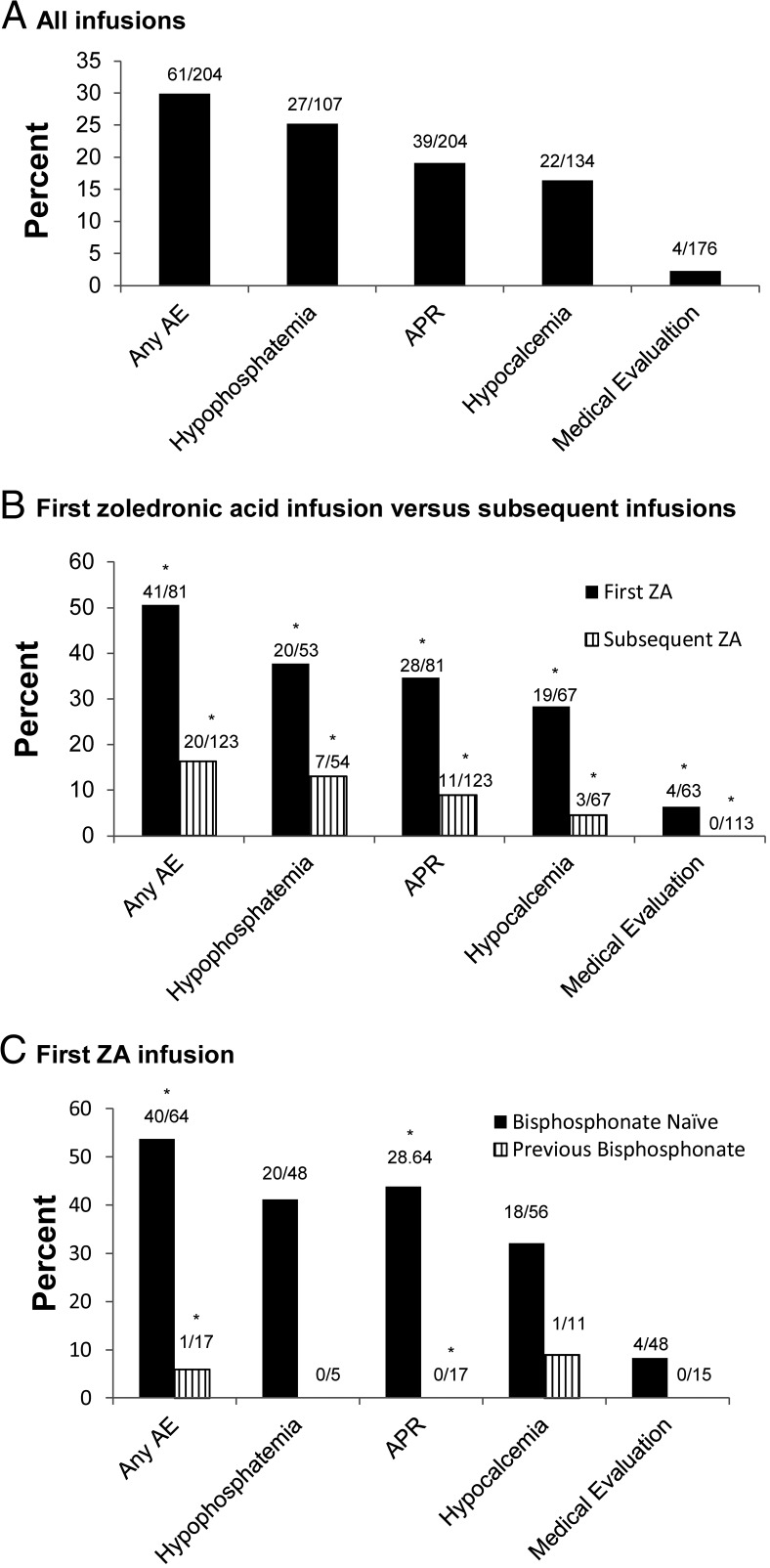
AEs occurred after 29.9% of all ZA infusions (Figure 1A). Hypophosphatemia was most common (25.2%), followed by APR (19.1%) and hypocalcemia (16.4%). Musculoskeletal complaints including pain, soreness, and weakness were more common in the setting of hypophosphatemia (11 of 27; 40.7%) compared to no hypophosphatemia (14 of 66; 17.5%; P = .01). Two subjects with hypophosphatemia received phosphate repletion. There were no instances of anaphylaxis associated with any of the 204 ZA infusions in this study. One subject was evaluated for shortness of breath during a ZA infusion; however, his symptoms were attributed to anxiety. Nausea and/or vomiting were documented in the chart after nine infusions in eight subjects; three of nine (33.3%) infusions were associated with hypocalcemia. One subject with osteopenia and an underlying seizure disorder experienced seizures within 48 hours of her first and second ZA inpatient infusions, despite calcium supplementation. Serum levels of calcium, phosphorus, and creatinine were normal at the time of the seizures. There were no reports of osteonecrosis of the jaw in study participants.
Figure 1.
Prevalence of AEs after all ZA infusions (A), in first ZA vs subsequent ZA infusions (B), and in first ZA infusion in bisphosphonate-naive vs previously bisphosphonate-exposed individuals (C). *, P < .01.
Out of all 176 outpatient ZA infusions, four infusions (2.3%) resulted in symptomatic hypocalcemia that led to evaluation in an emergency department. Two subjects were admitted and received iv calcium: 1) a 12-year-old male with type 2 Stickler syndrome who had not received any supplemental calcium or calcitriol with his first ZA infusion; and 2) a 10-year-old male with CRMO who developed emesis and diarrhea within 48 hours of his infusion. He developed hypocalcemia and hypophosphatemia despite receiving supplemental calcium (but not calcitriol) during and after the infusion. His electrocardiogram disclosed a prolonged QTc, which normalized after correction of his electrolyte abnormalities. No other cases were associated with documentation of hypocalcemia-related symptoms. Paresthesia was documented for two subjects; however, neither had hypocalcemia at the time. Asymptomatic hypocalcemia was routinely treated when detected; 13 of 18 (72.2%) cases were treated with initiation or increases in calcium and/or calcitriol supplementation.
Risk factors for development of AEs
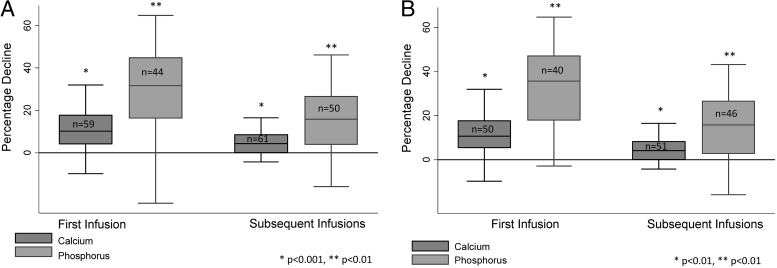
The prevalence of all AEs was greater after the first ZA infusion than after subsequent ZA infusions (Figure 1B). Subjects who were bisphosphonate naive had a greater prevalence of APRs (but not hypocalcemia or hypophosphatemia) after the initial ZA infusion than those who had received another bisphosphonate before the first ZA infusion (Figure 1C). Subjects had greater median decreases in calcium (10.2% [IQR, 4.3–15.6] vs 4.3% [IQR, 0–8.6]; P < .001) and phosphorus (31.6% [IQR,16.3–44.8] vs 15.8% [IQR, 4–26.5]; P < .001) after the first ZA infusion compared to subsequent infusions (Figure 2A). A similar effect was observed when considering only those subjects who were bisphosphonate naive at the time of the first ZA infusion (Figure 2B). There was no association between preinfusion vitamin D level and the magnitude of calcium decrease after infusion.
Figure 2.
Percentage decline in serum calcium and phosphorus levels after the first and subsequent ZA infusion among all participants (A) and participants who were bisphosphonate-naive before the first ZA infusion (B).
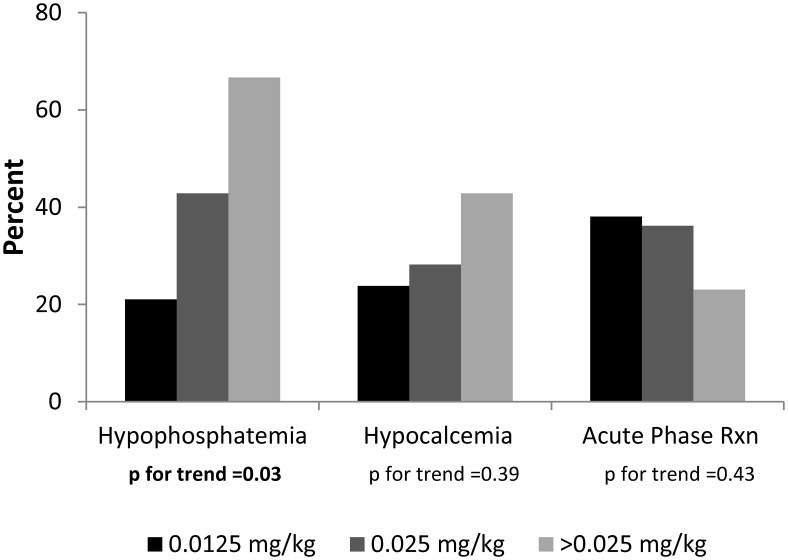
The prevalence of hypophosphatemia after the first ZA infusion increased from 21.1% in subjects who received a ZA dose of 0.0125 mg/kg to 42.9% in those who received a dose of 0.025 mg/kg and 66.7% in those who received a dose > 0.025 mg/kg (P for trend = .03) (Figure 3). No significant increases in the prevalence of hypocalcemia or APR were seen across the dose range.
Figure 3.
Prevalence of AEs after first ZA infusion across the dose range.
Subjects with alkaline phosphatase levels below 125 U/L (the upper end of the reference range of alkaline phosphatase in adults) were less likely to develop hypocalcemia after the first ZA infusion compared to those with alkaline phosphatase levels > 125 U/L (0 vs 37.8%; P = .01). The prevalence of hypophosphatemia did not vary by alkaline phosphatase status. There were no differences in the prevalence of hypocalcemia or hypophosphatemia in subjects with urine NTx levels above and below 83 nm bone collagen equivalents (BCE)/mm creatinine (the upper end of the reference range in adults) (Table 4).
Table 4.
Characteristics and Prevalence of ZA-Related AEs According to Bone Turnover Marker Status
| Alkaline Phosphatase |
Urine NTx |
|||||
|---|---|---|---|---|---|---|
| >125 U/L | ≤125 U/L | P | >83 nm BCE/mm Cr | ≤83 nm BCE/mm Cr | P | |
| n | 49 | 13 | 26 | 5 | ||
| Age, y | 11 (8–13) | 17.4 (15–18.3) | <.001 | 13.3 (10–15.4) | 19.3 (16–19.7) | .01 |
| ZA dose, mg/kg | 0.025 (0.025–0.025) | 0.025 (0.025–0.025) | .98 | 0.025 (0.0125–0.025) | 0.025 (0.025–0.025) | .15 |
| Hypocalcemia | 14/37 (37.8) | 0/12 (0) | .01 | 7/24 (29.2) | 0/3 (0) | .28 |
| Calcium decline, % | 11 (6.8–18.8) | 5.8 (1.6–12.2) | .06 | 7.4 (4.1–15.6) | 3.2 (0–13.8) | .29 |
| Hypophosphatemia | 11/30 (36.7) | 5/9 (55.6) | .31 | 5/17 (29.4) | 0/2 (0) | .37 |
| Phosphorus decline, % | 36.6 (23.3–48.9) | 36.6 (13.5–42.6) | .39 | 33.9 (22.5–40) | −5 (−24 to 13.5) | .05 |
| APR | 14/49 (25.6) | 7/13 (53.9) | .09 | 8/26 (30.8) | 1/5 (20) | .63 |
Abbreviation: Cr, creatinine. Data are expressed as median (IQR) or number (percentage).
The prevalence of AEs after all 204 ZA infusions varied by subject diagnosis, as described in Supplemental Table 1. Because OI is the most common indication for the use of ZA in the pediatric population, secondary analyses were performed comparing the prevalence of AEs in OI vs other conditions. The overall prevalence of ZA-related AEs was lower for participants with OI (13.6%) compared with other indications (42.2%); however, among subjects who had not previously received a bisphosphonate, there was no difference in the prevalence of AEs between OI and those with other conditions (58.3% for OI vs 63.5% for other indications). Additionally, the prevalence of AEs in bisphosphonate-naive subjects after the first ZA dose did not differ among the four most common diagnoses: OI (58.3%), CRMO (75%), AVN (25%), and osteoporosis (73.9%) (P = .22).
A comparison of the clinical characteristics of subjects who did or did not experience AEs after the first ZA infusion is shown in Supplemental Table 2. Subjects with at least one AE were more likely to be receiving their first bisphosphonate treatment (97.6%). Age, sex, the use of adjuvant supplemental calcium/vitamin D therapy, and the median pre-ZA infusion biochemical parameters [including 25(OH)D, calcium, phosphorus, and PTH] did not differ in individuals who developed hypocalcemia, hypophosphatemia, or APR compared to those who did not. The prevalence of vitamin D deficiency and vitamin D insufficiency in participants who developed hypocalcemia was 0% and 8%, respectfully, and it did not differ from participants without hypocalcemia.
Discussion
Despite limited published data on the safety and efficacy of ZA in the pediatric population, ZA has become an increasingly common treatment option for children with a variety of bone disorders. To date, the largest study to assess the safety profile of ZA in the pediatric population was published by Munns et al (11). In that study, there were 63 subjects whose underlying bone diseases were mainly secondary to AVN, osteoporosis, and distraction osteogenesis. In the present analysis, we reviewed the records of 81 subjects with a broader range of skeletal disorders including OI, CRMO, and bone metastases from solid tumors. Similar to previous studies, we found that AEs were common and generally mild in pediatric subjects receiving ZA therapy, and the frequency of AEs was greater after the initial ZA infusion than after subsequent infusions (9–11, 18–22).
Administration of ZA and other iv bisphosphonates is commonly associated with APRs characterized by fever, flu-like symptoms, myalgia, and/or bone pain (6, 9–11, 18–22). In our study, to simplify documentation interpretation, any subject with fever and/or bone or muscle pain within 1 week of ZA infusion was considered to have an APR. We confirmed that APRs were more frequent after initial ZA infusions, whether considering all infusions or just those administered to bisphosphonate-naive subjects. The prevalence of musculoskeletal complaints was more common in subjects with hypophosphatemia. It should be noted, however, that because of the multiple factors possibly contributing to musculoskeletal discomfort, a causal link cannot be concluded from these data alone. Consistent with previous studies, there were no life-threatening (eg, anaphylactic) reactions. On two occasions, within 48 hours of the initial and second ZA infusions, one subject experienced seizures. However, this subject had a known underlying seizure disorder, and it was thought that her seizure activity after ZA infusions was similar to her normal seizure frequency. She was not hypocalcemic at the time of the seizures. It is therefore difficult to attribute these events to ZA.
In previous studies, the frequency of hypocalcemia (serum calcium < 8–8.4 mg/dL) after initial infusion ranged from 0 to 71% (9–11, 18–22), and the frequency of hypophosphatemia (serum phosphorus < 3.1 mg/dL) was as high as 81% (22). In our retrospective chart review, utilizing similar definitions, the frequencies of hypocalcemia and hypophosphatemia after the initial ZA infusions were 28.4 and 37.7%, respectively. These disparities may relate to differences in the dose of ZA administered (eg, the highest frequencies for hypocalcemia and hypophosphatemia were in subjects who received 0.05 mg/kg of ZA) and the timing of post-ZA laboratory testing. Most previous studies monitored electrolyte abnormalities for 48 to 72 hours after ZA infusions because this time frame has been associated with a high frequency of AEs in the past (6, 10, 11, 19, 21, 22). Notably, one-third of subjects who developed hypocalcemia in our study were first noted to have hypocalcemia 72 hours or longer after ZA infusion. Because the subjects in our clinical sample did not have serial lab results checked, it is impossible to know the exact onset or nadir of hypocalcemia. However, our results indicate that hypocalcemia can occur and/or extend beyond the 48- to 72-hour postinfusion period that has been reported previously (6, 10, 11, 19, 21, 22).
Our retrospective study also indicates that in the great majority of subjects, hypocalcemia after infusion was asymptomatic, benign, and transient. Symptomatic hypocalcemia after ZA administration in children is much less common. In a study by Sbrocchi et al (19), one of five boys (20%) who were treated with ZA (0.05 mg/kg/dose) for osteoporosis associated with Duchenne muscular dystrophy developed symptomatic hypocalcemia with paresthesia and required iv calcium. In another study, two children (16.7%) with OI required iv calcium after their first dose of ZA (0.05 mg/kg) because of hypocalcemia associated with fatigue, dizziness, nausea, and muscle tremors (21). These AEs occurred despite appropriate calcium and vitamin D supplementation before the ZA infusion. In our study, there were only two ZA infusions (1.1% of outpatient infusions) in which hypocalcemia was sufficiently symptomatic to make it necessary to administer iv calcium. Of note, one of these two subjects may have been at unusually high risk for hypocalcemia after ZA because he was unable to tolerate oral calcium supplements in the context of vomiting and diarrhea that were due to a coincidental viral gastroenteritis.
Our study has several weaknesses. The retrospective design of this safety study limited the acquisition of all data from all subjects, and by intent, we did not assess therapeutic efficacy. ZA was prescribed by physicians from a number of different specialties, so there may have been variation in surveillance practices for ZA-related AEs. Some subjects were admitted to the hospital for ZA infusions or were already inpatients for other reasons (15.7 and 6% of subjects, respectively). Although there were no absolute criteria that guided decisions for planned admissions for ZA infusions, individuals with underlying medical conditions placing them at high risk for hypocalcemia-related complications were routinely admitted. Subjects who received their ZA infusions in the inpatient setting consistently received supplemental calcium and/or calcitriol. Therefore, it is possible that these subjects had a lower rate of AEs than if they had received their infusions in the outpatient setting. Due to the retrospective design of this study, our analyses of subjective complaints such as nausea/vomiting and symptoms of hypocalcemia were limited by what was documented in the chart. Because most infusions were conducted in an outpatient setting, we consider it likely that the true prevalence of subjective complaints was greater than what we were able to capture. Pubertal status was not routinely documented in the medical record; therefore, we were unable to determine the effects of pubertal maturation on ZA-related AEs. Finally, very few of our subjects received doses of ZA that were in excess of 0.025 mg/kg; thus, we cannot directly compare our results with prior studies that utilized higher doses of ZA.
The strengths of our study include the large number of subjects evaluated, the large number of repeat ZA infusions, the variability of doses used, and the diversity of diagnoses. The size of the dataset allowed for stratified analyses by diagnoses and led to the finding that subjects with OI were less likely to have an AE after the initial ZA infusion compared to subjects with other diagnoses. However, this is likely to be due to prior bisphosphonate exposure because, when considering only previously bisphosphonate-naive subjects, there was no difference in frequency of AE between subjects with and without OI. We did not find any other association between the subjects' underlying diagnosis and the development of AEs.
To date, there is no consensus regarding the most appropriate ZA dosage in children with OI and other metabolic bone disorders. In our protocol, most subjects received 0.0125 mg/kg of ZA for an initial infusion, followed by 0.025 mg/kg in subsequent infusions, with repeat dosing every 3 to 6 months. Many other centers have published observations of smaller numbers of subjects who received ZA at dosages ranging from 0.0125 to 0.05 mg/kg (6, 9–11, 18–22). Our results indicate the existence of a dose-related effect on the development of hypophosphatemia; the lack of a dose-related effect on the development of hypocalcemia is likely due to the low numbers of subjects treated with ZA doses of 0.05 mg/kg at initial infusion. Our lower frequency of hypocalcemia likely is related to differences in ZA dosing and in supplementation with calcium and calcitriol.
Hypocalcemia was more common in participants with higher alkaline phosphatase levels, consistent with previous findings that hypocalcemia is common after bisphosphonate treatment in adults with Paget's disease (23, 24), which is characterized by high bone formation rates. These findings suggest that pretreatment alkaline phosphatase may be a useful marker to predict who is at risk for the development of hypocalcemia and therefore might benefit from aggressive prophylaxis with calcium and calcitriol.
The hypophosphatemia observed in our study can be classified as mild to moderate, which is typical of bisphosphonate use (25). Although symptoms of hypophosphatemia (fatigue, myalgia, muscle weakness) have previously been associated with severely low serum phosphorus levels (<1 mg/dL) (25), it is difficult to say whether or not the hypophosphatemia seen in our study contributed to the myalgia experienced by some of our subjects after ZA infusion. Remarkably, other risk factors that we hypothesized to be associated with AEs (such as age, sex, body mass index, body surface area, ZA dosing intervals, baseline biochemical measures, and calcium/vitamin D supplementation at baseline) showed no statistically significant effect.
In conclusion, our results confirm and extend previous reports that have shown that AEs are common after ZA infusion, particularly in previously bisphosphonate-naive subjects and after the initial ZA infusion. Over the ZA dose range evaluated in this study, we found no association between the dose of ZA administered and the frequency of hypocalcemia and APRs. However, we did find that postinfusion hypophosphatemia was related to weight-adjusted ZA dose even over the dosage range that we used, raising the possibility that mild to moderate hypophosphatemia may contribute to the development of myalgia after ZA infusion. Finally, despite relatively high frequencies of electrolyte disorders, hypocalcemia and hypophosphatemia were rarely symptomatic in pediatric subjects who received 0.0125 to 0.025 mg/kg ZA. APRs were brief and, for the most part, could easily be managed at home. These results can provide some measure of reassurance to clinicians and patients that the use of ZA in the pediatric population is relatively safe in the short term. Additional prospective studies are needed to evaluate the underlying risk factors that are associated with AEs in youth to determine the optimal therapeutic dosing regimen for each specific bone disorder and to assess for possible long-term complications of ZA therapy including osteonecrosis of the jaw and atypical fracture.
Acknowledgments
The authors thank Maryjane Benton for clinical research coordination.
D.W. was supported by National Institutes of Health Grant K12HD068373.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AE
- adverse event
- APR
- acute phase reaction
- AVN
- avascular necrosis
- BCE
- bone collagen equivalents
- BMD
- bone mineral density
- CRMO
- chronic recurrent multifocal osteomyelitis
- IQR
- interquartile range
- NTx
- amino-terminal collagen crosslinks
- 25(OH)D
- 25-hydroxyvitamin D
- OI
- osteogenesis imperfecta
- ZA
- zoledronic acid.
References
- 1. Ibrahim A, Scher N, Williams G, et al. Approval summary for zoledronic acid for treatment of multiple myeloma and cancer bone metastases. Clin Cancer Res. 2003;9:2394–2399. [PubMed] [Google Scholar]
- 2. Adler RA. Glucocorticoid-induced osteoporosis: management update. Curr Osteoporos Rep. 2010;8:10–14. [DOI] [PubMed] [Google Scholar]
- 3. Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. [DOI] [PubMed] [Google Scholar]
- 4. Reid IR, Lyles K, Su G, et al. A single infusion of zoledronic acid produces sustained remissions in Paget disease: data to 6.5 years. J Bone Miner Res. 2011;26:2261–2270. [DOI] [PubMed] [Google Scholar]
- 5. Liu WC, Yen JF, Lang CL, Yan MT, Lu KC. Bisphosphonates in CKD patients with low bone mineral density. ScientificWorldJournal. 2013;2013:837573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. August KJ, Dalton A, Katzenstein HM, et al. The use of zoledronic acid in pediatric cancer patients. Pediatr Blood Cancer. 2011;56:610–614. [DOI] [PubMed] [Google Scholar]
- 7. Russell RG. Bisphosphonates: mode of action and pharmacology. Pediatrics. 2007;119(suppl 2):S150–S162. [DOI] [PubMed] [Google Scholar]
- 8. Hampson G, Fogelman I. Clinical role of bisphosphonate therapy. Int J Womens Health. 2012;4:455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panigrahi I, Das RR, Sharda S, Marwaha RK, Khandelwal N. Response to zolendronic acid in children with type III osteogenesis imperfecta. J Bone Miner Metab. 2010;28:451–455. [DOI] [PubMed] [Google Scholar]
- 10. Ooi HL, Briody J, Biggin A, Cowell CT, Munns CF. Intravenous zoledronic acid given every 6 months in childhood osteoporosis. Horm Res Paediatr. 2013;80:179–184. [DOI] [PubMed] [Google Scholar]
- 11. Munns CF, Rajab MH, Hong J, et al. Acute phase response and mineral status following low dose intravenous zoledronic acid in children. Bone. 2007;41:366–370. [DOI] [PubMed] [Google Scholar]
- 12. Assaf AT, Smeets R, Riecke B, et al. Incidence of bisphosphonate-related osteonecrosis of the jaw in consideration of primary diseases and concomitant therapies. Anticancer Res. 2013;33:3917–3924. [PubMed] [Google Scholar]
- 13. Dodson TB. Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J Oral Maxillofac Surg. 2009;67:44–52. [DOI] [PubMed] [Google Scholar]
- 14. Chang ST, Tenforde AS, Grimsrud CD, et al. Atypical femur fractures among breast cancer and multiple myeloma patients receiving intravenous bisphosphonate therapy. Bone. 2012;51:524–527. [DOI] [PubMed] [Google Scholar]
- 15. Gedmintas L, Solomon DH, Kim SC. Bisphosphonates and risk of subtrochanteric, femoral shaft, and atypical femur fracture: a systematic review and meta-analysis. J Bone Miner Res. 2013;28:1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barros ER, Saraiva GL, de Oliveira TP, Lazaretti-Castro M. Safety and efficacy of a 1-year treatment with zoledronic acid compared with pamidronate in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2012;25:485–491. [DOI] [PubMed] [Google Scholar]
- 17. Lui KJ, Cumberland WG. A Wilcoxon-type test for trend. Stat Med. 1995;14:445–446. [DOI] [PubMed] [Google Scholar]
- 18. Padhye B, Dalla-Pozza L, Little DG, Munns CF. Use of zoledronic acid for treatment of chemotherapy related osteonecrosis in children and adolescents: a retrospective analysis. Pediatr Blood Cancer. 2013;60:1539–1545. [DOI] [PubMed] [Google Scholar]
- 19. Sbrocchi AM, Rauch F, Jacob P, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int. 2012;23:2703–2711. [DOI] [PubMed] [Google Scholar]
- 20. Simm PJ, Johannesen J, Briody J, et al. Zoledronic acid improves bone mineral density, reduces bone turnover and improves skeletal architecture over 2 years of treatment in children with secondary osteoporosis. Bone. 2011;49:939–943. [DOI] [PubMed] [Google Scholar]
- 21. Vuorimies I, Toiviainen-Salo S, Hero M, Mäkitie O. Zoledronic acid treatment in children with osteogenesis imperfecta. Horm Res Paediatr. 2011;75:346–353. [DOI] [PubMed] [Google Scholar]
- 22. Högler W, Yap F, Little D, Ambler G, McQuade M, Cowell CT. Short-term safety assessment in the use of intravenous zoledronic acid in children. J Pediatr. 2004;145:701–704. [DOI] [PubMed] [Google Scholar]
- 23. Stuckey BG, Lim EM, Kent GN, Ward LC, Gutteridge DH. Bisphosphonate therapy for Paget's disease in a patient with hypoparathyroidism: profound hypocalcemia, rapid response, and prolonged remission. J Bone Miner Res. 2001;16:1719–1723. [DOI] [PubMed] [Google Scholar]
- 24. Whitson HE, Lobaugh B, Lyles KW. Severe hypocalcemia following bisphosphonate treatment in a patient with Paget's disease of bone. Bone. 2006;39:954–958. [DOI] [PubMed] [Google Scholar]
- 25. Liamis G, Milionis HJ, Elisaf M. Medication-induced hypophosphatemia: a review. QJM. 2010;103:449–459. [DOI] [PubMed] [Google Scholar]