Abstract
Context:
Adiponectin levels (ADPN) are lower in individuals with central obesity and cardiometabolic diseases. Conversely, studies have shown paradoxical hyperadiponectinemia (HA) in metabolically healthy obese (MHO) individuals of non-European descent. Moreover, individuals with higher sc to visceral adipose tissue (ie, higher peripheral adiposity) distribution have higher ADPNs. However, it is not known whether metabolically healthy individuals have predominantly peripheral adiposity along with higher ADPNs.
Objective:
This study aimed to evaluate the association of ADPN and adiposity distribution with metabolic health in white individuals.
Design and Setting:
This was a cross-sectional study of members of “Take Off Pounds Sensibly” weight loss club and their relatives.
Participants:
We recruited 2486 (72% women, 61% obese) individuals. They were defined as metabolically healthy by absence of hypertension, diabetes, and dyslipidemia; and they were further classified into metabolically healthy nonobese (MHNO), metabolically unhealthy nonobese (MUNO), metabolically healthy obese (MHO), and metabolically unhealthy obese (MUO). Waist-to-hip ratios (WHRs) were used as markers of adiposity distribution. Insulin resistance was measured using homeostasis model assessment.
Results:
Among the four groups, MHNO had the lowest WHRs (higher peripheral adiposity) and highest ADPN, and MUO had highest WHRs (higher central adiposity) and lowest ADPN (P < .001). Among both nonobese and obese, metabolically healthy individuals had higher ADPN than metabolically unhealthy individuals (P < .05) after adjustment for age, sex, and body mass index. MHNO also had lower WHRs compared with MUNO (P < .01). Although WHRs were lower among MHO compared with MUO, the difference was not significant. In addition, nonobese and obese individuals with HA (defined using sex-specific cutoffs) had lower homeostasis model assessment and dyslipidemia compared with individuals without HA.
Conclusions:
Higher ADPN and lower WHRs (higher peripheral adiposity) are associated with better metabolic health in both nonobese and obese white individuals. These results suggest that ADPN and peripheral adiposity play a key role in determining the metabolic health independent of body mass index.
Obesity is accumulation of excessive adipose tissue and is associated with multiple metabolic complications including type 2 diabetes mellitus, hypertension, dyslipidemia, and ultimately, cardiovascular disease (1). Previously seen solely as an energy storage organ, adipose tissue is now known to exert important metabolic and humoral influences through its secretion of adipokines, and the organism-wide effect of this signaling cannot be underestimated (2). Adiponectin is one such protein hormone that is exclusively synthesized in adipose tissue in adult humans and possesses insulin-sensitizing, antiapoptotic, and anti-inflammatory properties (3, 4). Intriguingly, it has long been established that adiponectin levels (ADPNs) are lower in obese individuals (particularly those with central obesity) and in those with cardiometabolic diseases and nonalcoholic fatty liver disease, and the reasons for these lower ADPNs despite high adipose tissue mass are not well understood (5–9).
Contrary to the above data, some recent studies among African American and Hispanic populations have suggested that some obese individuals have higher plasma ADPNs (termed paradoxical hyperadiponectinemia [HA]), and that these obese individuals are metabolically healthier compared with obese individuals with lower ADPNs (10–12). In addition, we have previously shown that individuals with a higher sc to visceral adipose tissue ratio have higher circulating ADPNs (13). However, it is not known whether metabolically healthy individuals have predominantly higher sc adipose tissue volumes along with higher ADPNs. In the current study, we hypothesized that metabolically healthy individuals have higher ADPNs irrespective of their body mass index (BMI) and may have predominantly peripheral distribution of adipose tissue.
Materials and Methods
Subjects
A total of 2486 individuals (men = 700; women = 1786) were recruited for the study from the membership of “Take Off Pounds Sensibly” (TOPS Club, Inc) as has been described previously (13–15). The study was initiated in 1994 with the formulation of a nine-page questionnaire that collected information on family structure, health, and behavior status, and detailed family and personal history of obesity and its health complications. Questionnaire data was received from 60 000 respondents. Of these respondents, families with at least two obese siblings (BMI ≥ 30 kg/m2) and at least one nonobese sibling and/or parent (BMI ≤ 27 kg/m2) from Wisconsin, Illinois, Kentucky, and West Virginia were invited to participate in the study and resulted in recruitment of 1436 individuals (14, 15). Further recruitment of an additional 1450 individuals was carried out in the states of Indiana, Iowa, Michigan, Minnesota, Missouri, and Ohio by sending out one-page flyers in 1996 to increase the sample size and to reach individuals who did not complete the detailed questionnaire. Participation was voluntary. Subjects with a history of type 1 diabetes mellitus, cancer, renal or hepatic disease, active coronary artery disease, substance abuse, corticosteroids, thyroid medications above the replacement dose (either for goiter or thyroid cancer), or history of weight loss of more than 10% of body weight in the preceding 12 months were excluded from the study. All procedures were approved by the Medical College of Wisconsin's Institutional Review Board and conform to the relevant ethical guidelines for human research. Informed consent was provided by each participant (both for questionnaires and the actual study).
Clinical and biochemical measurements
Weight, height, and blood pressure (BP) were measured using standardized methods. Waist circumference was measured at the level of the navel, and hip circumference was measured at the widest point of the buttock region. BMI and waist-to-hip ratio (WHR) were calculated. WHR was used as surrogate marker for adiposity distribution, with lower WHR suggesting lower central (or higher peripheral) adiposity. Subjects were fasting at the time of laboratory measurements. Plasma glucose was measured in triplicate using a Glucose Analyzer II (Beckman Instruments) with a glucose oxidase method. Plasma insulin was measured using a double antibody equilibrium RIA (Linco Research) specific to human insulin. Coefficient of variance for insulin was 7.6%. The homeostasis model assessment (HOMA) method was used for calculation of insulin resistance index (HOMA-IR) in patients without type 2 diabetes (16). Plasma triglycerides were measured using a glycerylphosphate oxidase method (Stanbio Laboratory, Inc). High-density lipoprotein cholesterol (HDL-C) was measured using phosphotungstic acid or MgC12 precipitate (Roche-Boehringer). Low-density lipoprotein cholesterol (LDL-C) was calculated using Friedewald's formula (17). Plasma adiponectin was determined using a double antibody equilibrium RIA (Millipore) (18). Coefficient of variance for adiponectin was 6.5%. Markers of inflammation were measured in a subsample of 933 individuals. TNF-α, IL-6, and IL-1β were measured at Texas Biomedical Research Institute using kits from Linco Research, Inc. on a Luminex 100 IS instrument and multiplex technology. According to the manufacturer, intra-assay precision was 5.6–9.0% depending on the analyte tested. Interassay precision ranged from 3.1–18.4%.
Definitions
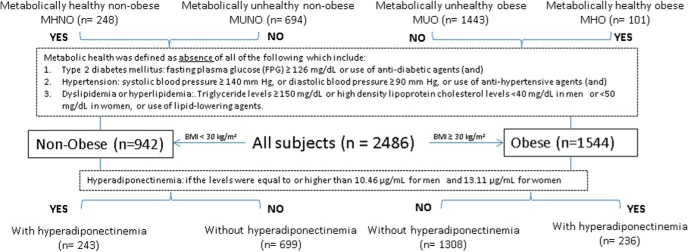
Obesity was defined by BMI greater than or equal to 30 kg/m2. Type 2 diabetes mellitus was defined as fasting plasma glucose at least 7.0 mmol/L (126 mg/dL) or use of insulin, incretin mimetics, or other oral hypoglycemic agents including metformin. Hypertension was defined as either systolic BP (SBP) at least 140 mm Hg, or diastolic BP (DBP) at least 90 mm Hg, or use of antihypertensive agents. Dyslipidemia was characterized by presence of triglyceride levels at least 1.69 mmol/L (150 mg/dL) or high-density lipoprotein cholesterol (HDL-C) levels less than 1.04 mmol/L (40 mg/dL) in men or less than 1.29 mmol/L (50 mg/dL) in women, or being on any of the lipid-lowering agents. Subjects were classified as metabolically healthy if all three diseases (hypertension, type 2 diabetes mellitus, and dyslipidemia) were absent. Plasma ADPNs were defined as high if the levels were equal to or higher than the top tertile (ie, 67th percentile) among metabolically healthy individuals with a BMI less than 25 kg/m2. In this study, HA was set at 10.46 μg/mL for men and 13.11 μg/mL for women. Using the definitions set above, we classified the individuals into four groups: metabolically healthy nonobese (MHNO), metabolically unhealthy nonobese (MUNO), metabolically healthy obese (MHO), and metabolically unhealthy obese (MUO) (Figure 1). Lastly, hormone therapy (HT) was defined by either use of oral contraceptive pills, estrogen and/or progesterone hormone replacement therapy in postmenopausal women, or T therapy in men.
Figure 1.
Subject stratification based on metabolic health and BMI status.
Statistical analysis
Estimates are presented with mean and SE and are adjusted for within-family clustering. The primary analysis tool was generalized estimating equations (GEE) modeling with an exchangeable working correlation structure. This approach provides a generalization for t test/ANOVA for continuous outcomes and logistic regression for categorical outcomes in the presence of clustering. Patient clusters were defined by families. Clustering-adjusted partial associations were computed from a GEE model using the method of Natarajan et al (19). The pairwise comparison between multiple groups was adjusted for multiple testing using Tukey's method within the GEE framework. Covariate-adjusted means, also called least-squares means, are predicted population margins: that is, they estimate the marginal means over a balanced population. Each variable was analyzed separately. Analyses were performed using SAS 9.3 (SAS Institute).
Results
A total of 2486 white individuals (72% women, 28% men) were included in the analysis and were stratified as nonobese (39%) or obese (61%) based on the BMI. Their anthropometric and metabolic characteristics are presented in Table 1. Overall, the obese individuals had a higher prevalence of hypertension, type 2 diabetes, and dyslipidemia. The obese individuals also had lower ADPNs, higher insulin resistance index (HOMA-IR), and markers of inflammation (TNF-α, IL-6, and IL-1b) compared with nonobese individuals (Table 1). There was similar use of hormonal therapy in both groups. Age and sex-adjusted correlations of WHR and plasma ADPNs in the overall sample, nonobese, and obese individuals are presented in Table 2. In all groups, WHR was positively correlated with BP, insulin resistance index (HOMA-IR), and triglycerides. WHR was also negatively correlated with HDL-C among the overall group and nonobese. Adiponectin, in contrast, showed negative correlation with BMI, WHR, BP, and triglycerides, and positive correlation with HDL-C in all groups. HOMA-IR was negatively associated with ADPN in the overall group and obese individuals but not in nonobese. In the subgroup of individuals in whom inflammatory markers were measured, there was no statistically significant correlation between the markers of inflammation (TNF-α, IL-6, and IL-1b) with adiponectin or WHR.
Table 1.
Distribution of Anthropometric and Metabolic Characteristics by BMI (Mean ± SE)
| Variable | Nonobese (n = 942) | Obese (n = 1544) |
|---|---|---|
| Age, y | 45.5 ± 0.6 | 48.7 ± 0.4b |
| Women, % | 64.2 ± 1.7 | 80.3 ± 1.4b |
| BMI, kg/m2 | 24.8 ± 0.1 | 38.9 ± 0.21b |
| WHR | 0.85 ± 0.01 | 0.90 ± 0.01b |
| Hypertension, % | 26.9 | 52.6b |
| SBP, mm Hg | 122 ± 0.7 | 132 ± 0.5b |
| DBP, mm Hg | 76 ± 0.4 | 82 ± 0.3 b |
| Type 2 diabetes mellitus, % | 4.2 ± 0.7 | 16.2 ± 1.0b |
| HOMA-IR | 8.52 ± 0.83 | 15.9 ± 0.9b |
| Dyslipidemia, % | 67.1 ± 1.7 | 86.8 ± 0.9b |
| Total cholesterol, mmol/L, mg/dL | 5.00 ± 0.05 | 5.23 ± 0.03 |
| (193 ± 2) | (202 ± 1)b | |
| Triglycerides, mmol/L, mg/dL | 1.22 ± 0.03 | 1.71 ± 1.03 |
| (108 ± 3) | (151 ± 3)b | |
| LDL-C, mmol/L, mg/dL | 3.11 ± 0.05 | 3.39 ± 0.05 |
| (Calculated) | (120 ± 2) | (131 ± 2)b |
| HDL-C, mmol/L, mg/dL | 1.11 ± 0.03 | 1.04 ± 0.01 |
| (43 ± 1) | (40 ± 0.4)b | |
| Adiponectin, μg/mLa | 10.4 ± 0.2 | 8.9 ± 0.1b |
| Nonobese (n = 379) | Obese (n = 554) | |
| TNF-α, pg/mLa | 4.33 ± 0.16 | 5.53 ± 0.16b |
| IL-6, pg/mLa | 5.17 ± 0.85 | 7.78 ± 0.95c |
| IL-1b, pg/mL | 0.38 ± 0.04 | 0.59 ± 0.7c |
SI units not available.
P ≤ .001.
P ≤ .05.
Table 2.
Age and Sex-Adjusted Correlations of Adiponectin and WHR With Metabolic Parameters Among All Subjects, Obese, and Nonobese
| Characteristic | All Subjects |
Nonobese |
Obese |
|||
|---|---|---|---|---|---|---|
| WHR | Adiponectin | WHR | Adiponectin | WHR | Adiponectin | |
| BMI | 0.34a | −0.37a | 0.32b | −0.31b | 0.08d | −0.14e |
| Waist circumference | 0.44a | −0.48a | 0.27b | −0.30b | 0.29e | −0.29e |
| SBP | 0.27a | −0.20a | 0.23b | −0.09 | 0.13e | −0.10d |
| DBP | 0.25a | −0.23a | 0.19c | −0.22b | 0.13e | −0.11f |
| HOMA-IR | 0.20a | −0.11a | 0.39b | −0.06 | 0.17e | −0.16e |
| Triglycerides | 0.11a | −0.13a | 0.17b | −0.32b | 0.07f | −0.12e |
| Total Cholesterol | 0.00 | −0.02 | −0.01 | 0.02 | −0.05 | 0.01 |
| LDL-C | 0.01 | 0.01 | −0.06 | 0.03 | −0.09 | 0.04 |
| HDL-C | −0.14a | 0.26a | −0.15c | 0.21b | −0.05 | 0.23e |
Among all subjects: a P ≤ .001.
Among nonobese subjects: b P ≤ .001; c P ≤ .01.
Among obese subjects: d P ≤ .01; e P ≤ .001; f P ≤ .05.
The nonobese and obese groups were further stratified into metabolically healthy or unhealthy and their metabolic characteristics are presented in Table 3. Approximately 26% of the nonobese individuals were classified as metabolically healthy based on the predetermined definition (and 74% were classified as unhealthy). Seven percent of the obese individuals were metabolically healthy (93% were unhealthy) based on the criteria we used. When all four groups were compared, MHNO had the highest ADPNs, whereas MUO individuals had the lowest ADPNs (P < .001). There was no statistically significant difference in the ADPN among MHNO and obese individuals. The results did not change when these levels were adjusted for age, BMI, sex, and HT. In addition, when all four groups were compared, MHNO individuals had the lowest WHR and MUO individuals had the highest WHR (P < .001). We subsequently analyzed the subsample in whom inflammatory markers were available (Table 3), and found that MHNO had the lowest TNF-α and MUO had the highest TNF-α levels (P < .001). IL-6 levels were higher among MUO individuals compared with MHO individuals. No significant differences was found in the levels of IL-1b levels between metabolically healthy vs unhealthy.
Table 3.
Distribution of Anthropometric and Metabolic Characteristics by BMI and Metabolic Health (Unadjusted Means ± se)
| Variable | MHNO (n = 248) | MUNO (n = 694) | MHO (n = 101) | MUO (n = 1443) |
|---|---|---|---|---|
| Age, y | 41.8 ± 0.9 | 46.9 ± 0.7c | 45.1 ± 1.2 | 48.97 ± 0.4e |
| Women, % | 60.6 | 61.4 | 73.3 | 78.7 |
| BMI, kg/m2 | 23.5 ± 0.2 | 25.3 ± 0.1c | 36.3 ± 0.6 | 39.1 ± 0.2f |
| WHR | 0.84 ± 0.01 | 0.86 ± 0.00d | 0.88 ± 0.01 | 0.90 ± 0.00 |
| Hypertension, %a | 0 | 36.5 | 0 | 56.2 |
| SBP, mm Hga | 114 ± 1 | 125 ± 1 | 121 ± 1 | 132 ± 1 |
| DBP, mm Hga | 73 ± 1 | 78 ± 1 | 78.0 ± 0.5 | 82 ± 0 |
| Diabetes mellitus, %a | 0 | 5.2 | 0 | 17.1 |
| HOMA-IR | 6.1 ± 0.5 | 9.4 ± 1c | 10.1 ± 0.9 | 16.3 ± 1.0f |
| Dyslipidemia, %a | 0 | 91.4 | 0 | 93.3 |
| Total cholesterol, mmol/L, mg/dL | 4.92 ± 0.08 | 5.02 ± 0.05 | 5.13 ± 0.10 | 5.23 ± 0.03 |
| (190 ± 3) | (194 ± 2) | (198 ± 4) | (202 ± 1) | |
| Triglycerides, mmol/L, mg/dLa | 0.90 ± 0.02 | 1.33 ± 0.03 | 1.03 ± 0.03 | 1.75 ± 1.03 |
| (80 ± 2) | (118 ± 3) | (91 ± 3) | (155 ± 3) | |
| LDL-C, mmol/L, mg/dL | 2.82 ± 0.08 | 3.21 ± 0.08 | 3.16 ± 0.16 | 3.39 ± 0.05 |
| (Calculated) | (109 ± 3) | (124 ± 3)c | (122 ± 6) | (131 ± 2) |
| HDL-C, mmol/L, mg/dL | 1.45 ± 0.03 | 1.01 ± 0.03 | 1.42 ± 0.03 | 0.98 ± 0.3 |
| (56 ± 1) | (39 ± 1) | (55 ± 1) | (38 ± 0.3) | |
| Adiponectin, μg/mLb | 11.6 ± 0.3 | 9.9 ± 0.2c | 10.1 ± 0.5 | 8.8 ± 0.1g |
| MHNO (n = 93) | MUNO (n = 286) | MHO (n = 34) | MUO (n = 520) | |
| TNF-α, pg/mLb | 4.20 ± 0.27 | 4.38 ± 0.17 | 5.28 ± 0.44 | 5.55 ± 0.21 |
| IL-6, pg/mLb | 4.46 ± 0.93 | 5.43 ± 0.96 | 4.30 ± 0.81 | 8.02 ± 1.01d |
| IL-1b, pg/mLb | 0.41 ± 0.07 | 0.38 ± 0.05 | 0.66 ± 0.21 | 0.58 ± 0.07 |
P values were not calculated as these variables were part of the definition. The values show distribution of these variables. All P values were adjusted for pairwise comparisons between the groups.
SI units not available.
Among nonobese, c P ≤ .001; d P ≤ .01.
Among obese, e P ≤ .01; f P ≤ .001; g P ≤ .05.
Subsequent pairwise analyses were performed among obese and nonobese groups separately. In nonobese group, metabolically healthy individuals were younger, had lower BMI, WHR, and HOMA-IR scores compared with their metabolically unhealthy counterparts (MHNO vs MUNO). Among the obese cohort, metabolically healthy individuals were younger and had lower BMI and HOMA-IR compared with metabolically unhealthy individuals (MHO vs MUO). Although WHR was also lower among MHO individuals compared with MUO individuals, the difference was not statistically significant after adjustment for multiple comparisons. In both groups, ADPNs were significantly higher in metabolically healthy individuals than in metabolically unhealthy individuals (P < .01). These results were significant even after adjustment for age, sex, BMI, and use of HT.
When all four groups were analyzed after excluding overweight individuals (BMI > 25 and ≤ 30 kg/m2), those on lipid-lowering medications, or those on HT separately, ADPNs continued to be higher among metabolically healthy individuals belonging to both obese and nonobese groups (P < .05) compared with metabolically unhealthy individuals. All results were adjusted for age, sex (when men were included), HT (when individuals on HT were included) and multiple comparisons. Further, we performed sensitivity analyses in women only and found that the ADPNs continue to be higher among both MHO (P = .005) and nonobese (P = .003) individuals compared with their metabolically unhealthy counterparts. However, these differences were significant only between MHNO and MUO among women over the age of 55 years (presumed to be postmenopausal). In nonobese women at least 55 years of age, ADPNs were 13.48 ± 1.08 μg/mL among metabolically healthy group compared with 12.24 ± 0.78 μg/mL (P = not significant [NS]). In obese women at least 55 years of age, ADPNs were 11.89 ± 1.13 μg/mL among metabolically healthy group compared with 10.29 ± 0.29 μg/mL (P = NS). These results were adjusted for age, BMI, and HT. Sample sizes were much smaller in this analyses of women over the age of 55 years (MHNO, 42; MHO, 23; MUNO, 187; MUO, 475).
The nonobese and obese individuals were further stratified by the presence of HA (Table 4). Twenty-six percent of the nonobese and 15% of the obese had HA. In both the nonobese and obese groups, individuals with HA were slightly older, and had a lower BMI. WHR was lower among nonobese individuals with HA compared with nonobese individuals without HA.
Table 4.
Distribution of Anthropometric and Metabolic Characteristics by HA and BMI (Adjusted Means ± se)
| Characteristic | Nonobese |
Obese |
||
|---|---|---|---|---|
| Without HA (n = 699) | With HA (n = 243) | Without HA (n = 1308) | With HA (n = 236) | |
| Age, y | 43.2 ± 15.9 | 49.4 ± 17.5b | 47.4 ± 13.1 | 52.9 ± 13.5e |
| Women, % | 59.7 | 65.4 | 77.8 | 81.4 |
| BMI, kg/m2 | 25.1 ± 3.2 | 23.8 ± 3.2b | 39.2 ± 7.6 | 37.6 ± 6.5e |
| Adjusted for age, sex, BMI, and HT | ||||
| WHR | 0.89 ± 0.01 | 0.86 ± 0.01c | 0.92 ± 0.01 | 0.91 ± 0.01 |
| Hypertension, % | 41 ± 3 | 34 ± 4 | 47 ± 2 | 38 ± 4 |
| SBP, mm Hg | 128 ± 1 | 126 ± 1 | 129 ± 1 | 130 ± 1 |
| DBP, mm Hg | 80 ± 1 | 77 ± 1d | 81 ± 0.5 | 80 ± 1 |
| Type 2 diabetes mellitus, % | 7 ± 1 | 3 ± 1 | 10 ± 1 | 10 ± 2 |
| HOMA-IR | 12.02 ± 1.9 | 8.9 ± 1.3b | 15.6 ± 1.2 | 8.6 ± 1.4f |
| Dyslipidemia, % | 76 ± 3 | 56 ± 5c | 86 ± 1 | 67 ± 4f |
| Total cholesterol, mmol/L, mg/dL | 4.97 ± 0.05 | 4.87 ± 0.08 | 5.31 ± 0.05 | 5.28 ± 0.08 |
| (192 ± 2) | (188 ± 3) | (205 ± 2) | (204 ± 3) | |
| Triglycerides, mmol/L, mg/dL | 1.42 ± 0.06 | 1.20 ± 0.06 | 1.49 ± 0.05 | 1.83 ± 0.06e |
| (126 ± 5) | (106 ± 5)c | (132 ± 4) | (162 ± 5)c | |
| LDL-C, mmol/L, mg/dL | 3.13 ± 0.10 | 3.11 ± 0.10 | 3.44 ± 0.10 | 3.37 ± 0.13 |
| (Calculated) | (121 ± 4) | (120 ± 4) | (133 ± 4) | (130 ± 5) |
| HDL-C, mmol/L, mg/dL | 1.06 ± 0.03 | 1.19 ± 0.03 | 0.98 ± 0.03 | 1.17 ± 0.03 |
| (40 ± 1) | (46 ± 1)c | (38 ± 1) | (45 ± 1)f | |
| Adiponectin, μg/mLa | 7.5 ± 2.6 | 16.7 ± 5.4 | 7.1 ± 2.6 | 16.6 ± 4.5 |
| Without HA (n = 284) | With HA (n = 95) | Without HA (n = 469) | With HA (n = 85) | |
| TNF-α, pg/mLa | 4.19 ± 0.18 | 4.60 ± 0.26 | 5.46 ± 0.21 | 5.80 ± 0.40 |
| IL-6, pg/mLa | 5.00 ± 1.07 | 5.53 ± 0.85 | 7.52 ± 1.06 | 9.04 ± 1.57 |
| IL-1b, pg/mLa | 0.39 ± 0.05 | 0.37 ± 0.07 | 0.57 ± 0.08 | 0.67 ± 0.18 |
Means are adjusted for age, BMI, sex, HT, and all P values were adjusted for pairwise comparisons between the groups.
SI units not available and are expected to be statistically significantly different.
Among nonobese, b P ≤ .05; c P ≤ .001; d P ≤ .01.
Among obese, e P ≤ .05; f P ≤ .001.
Hyperadiponectinemia (HA) was also associated with a higher HDL-C levels and lower prevalence of dyslipidemia in both the nonobese and obese individuals. In addition, HOMA-IR levels were lower among obese and nonobese individuals with HA compared with those without HA. Even though the prevalence of type 2 diabetes mellitus and hypertension was lower among individuals with HA in both nonobese and obese groups, it did not reach statistical significance after adjustment for multiple comparisons. There were no differences in the inflammatory markers between individuals with and without HA.
Discussion
The concept of “all obesity is not created equal” has gained momentum in recent years, leading investigators to examine various factors that could contribute to the pathogenicity of adipose tissue accumulation (20–23). Adipose tissue secretes a number of adipokines, which can greatly influence metabolic effects (24–27). In this study, we sought to investigate the association of adiponectin, one such adipokine, with metabolic health and made important observations. First, we found that ADPNs were highest among MHNO individuals and lowest among MUO individuals. Secondly, WHR (a surrogate marker for peripheral adiposity) was lowest among MHNO (the group with highest ADPNs) and highest among MUO individuals (the group with lowest ADPNs). Thirdly, ADPNs were higher in individuals with a metabolically healthy phenotype compared with individuals with a metabolically unhealthy phenotype, among both obese and nonobese individuals. In addition, those with HA had lower insulin resistance and dyslipidemia than those without HA, among both obese and nonobese individuals. Higher ADPNs were previously reported in MHO individuals (10–12, 28). However, this is the first report of higher ADPNs seen among MHNO individuals compared with metabolically unhealthy. Furthermore, this is the first large study among North American white population and confirms what has been previously noted in African American, Hispanic, and Estonian obese individuals (10–12, 28). The results of our study remained significant even after adjustment for age, sex, BMI, and HT. Although these observations must be confirmed in additional studies, our results point to a robust association between circulating ADPNs and metabolic health.
Our data also show that ADPNs were inversely correlated with BMI and WHR, and individuals with HA have a lower BMI and WHR confirming previous observations (5–8, 29, 30). These findings are contradictory to the fact that adiponectin is exclusively secreted by adipose tissue in adult humans (31, 32). One would expect higher ADPNs with increase in adiposity, however ADPNs are lower in the obese, especially those with central adiposity (or higher WHR) (33). Adding to the intrigue, a metabolically healthy phenotype was described in some obese individuals (as many as 20–30% in some estimates) and they were noted to have paradoxically higher ADPNs (10, 11). Taken together, these studies suggest at least two distinct types of obesity, based on their association with circulating ADPNs, one with central adiposity associated with lower ADPNs and adverse metabolic implications (34), and the other with higher ADPNs and fewer metabolic implications (10, 11). These studies raise the possibility that ADPN secretion may differ based on adiposity distribution, rather than total adiposity. To this effect, we have recently shown that higher abdominal subcutaneous adipose tissue (SAT) to visceral adipose tissue (VAT) ratios (as measured by the computerized tomography scan) were associated with higher circulating ADPNs (13). We also noted that SAT-to-VAT ratios were one of the two positive predictors of plasma ADPNs, the others being sex, age, and waist circumference. These findings raise the possibility that these HA individuals are individuals with peripheral adiposity distribution and a higher SAT (ie, pear-shaped or peripheral obesity) (10–12, 28).
We sought to evaluate the above-mentioned association in the current study by using WHR as a surrogate marker for adiposity distribution. Among both the nonobese and obese groups, one common characteristic of the individuals who were metabolically healthy and those who had HA was a lower WHR, although it reached statistical significance only in the nonobese individuals. Although these data are not robust, possibly due to WHR being just a surrogate marker for adiposity distribution, they do support the idea that adiposity distribution into various adipose tissue depots (peripheral vs central) does play a role in determining circulating ADPNs. In a small study of nine individuals, Motoshima et al (35) showed that VAT secretion of adiponectin was lower with higher BMI, whereas no such decrease was noted with SAT. In an another study that included 39 individuals with metabolic syndrome and 26 controls, Bremer et al (36) showed that even though circulating ADPNs were lower among individuals with metabolic syndrome, SAT levels of adiponectin was not significantly different between the two groups suggesting preserved secretion of adiponectin from SAT despite lower circulating levels.
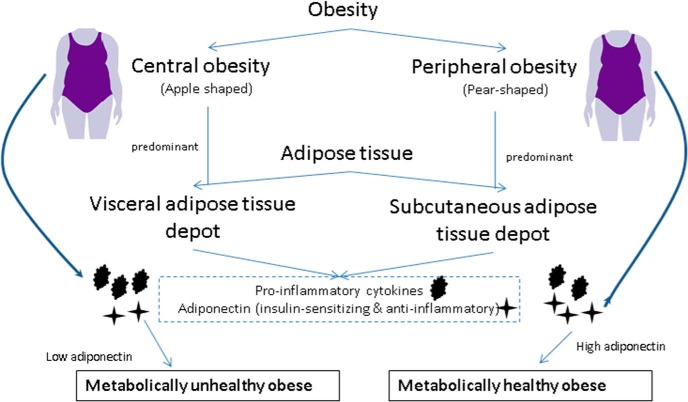
According to epidemiologic studies, individuals with predominantly SAT accumulation are less prone to metabolic complications compared with individuals with VAT (central adiposity) (37). It is possible that adipose tissue depot–dependent secretion of adiponectin can mediate metabolic protection seen in individuals with peripheral adiposity (or MHO). Findings of our study are biologically plausible. Adiponectin activates two different seven-transmembrane receptors, adiponectin receptor 1 and adiponectin receptor 2, and stimulation of either receptor activates numerous pathways including adenosine monophosphate (AMP)-protein kinase and MAPK pathways (38, 39). Adiponectin-receptor knockout studies have shown detrimental effects on metabolism with increases in adiposity, decreased glucose tolerance, and resistance to diet-induced obesity; all showing a significant role for adiponectin in obesity related metabolic events and diseases (40, 41). In addition, adiponectin suppresses proinflammatory actions and inhibits transformation of macrophages to foam cells (42–44). Combined evidence from epidemiologic and in vitro studies makes a robust case for the need for further exploration of roles of adiponectin and adiposity distribution in metabolic health (Figure 2).
Figure 2.
Higher SAT is associated with higher circulating ADPN, which is anti-inflammatory and insulin sensitizing.
These findings also highlight the vulnerability experienced by MUNO. Even though the definitions of metabolic health have varied across studies, MUNO individuals are at a higher risk for BP (45, 46) compared with MHNO. In our study, we show that these individuals are at a disadvantage due to lower ADPNs and higher WHR (lower peripheral adiposity) compared with their metabolically healthy counterparts in the same BMI category.
The relationship between circulating inflammatory markers and ADPNs is not as robust as we expected. The markers of inflammation are significantly higher among obese individuals compared with nonobese individuals. Although trends suggestive of higher levels of inflammatory markers were noted in metabolically unhealthy cohort compared with metabolically healthy cohort, the results were not statistically significant. We believe that the sample size was too small to detect significant differences when the sample was further stratified into four groups based on metabolic health and ADPNs.
Our study has several limitations. The cross-sectional nature of the study makes it only possible to determine the association between adiponectin, adiposity distribution, and metabolic health, and cannot determine cause–effect relationship. It is entirely possible that adiponectin is a mere marker of another adipokine or substance that coexists or is present downstream or upstream in the pathway. Adiponectin may even be the determinant of adiposity distribution rather than the result. The individuals in the study were white and predominantly women; however, these results are consistent with previous reports in other ethnic groups (10, 11). The percentage of MHO was smaller in our subject population (7%) than was previously reported (20–30%), perhaps due to the rigorous definition of metabolic health we used. In addition, BP levels were measured only on one occasion on the day of the study visit, and may have allowed a misclassification of some of the individuals. We may have overestimated the prevalence of type 2 diabetes by classifying all individuals taking metformin as having type 2 diabetes, even though some of them may be taking it for other conditions such as polycystic ovarian disease. We also acknowledge that an a priori power analysis was not performed. We performed post-hoc analyses of the data already collected for a different study to test our current hypothesis. In addition, given that many of these individuals were members of a weight loss club or relatives of the weight loss club's member, the metabolically unhealthy group may have been overrepresented by self-selected individuals with metabolic abnormalities trying to lose weight by self motivation or provider advice. Participation in the study itself may have changed subject's attitudes toward lifestyle modifications, diet, and exercise. However, smaller numbers of metabolically healthy individuals should not interfere with observations in the comparison groups, thus making these observations valid. WHR was considered a surrogate marker for adiposity distribution (although not a gold standard) because it was the most feasible method due to the large sample size. In addition, HOMA-IR was used as a surrogate for insulin resistance measured by euglycemic clamp technique, again due to the large sample size we studied. We did not control for activity level, smoking status, menopausal status, or dietary variations, which may have affected ADPNs or the metabolic parameters that were measured. However, all the parameters were collected on individuals when their weight was stable for at least 6 months and in a fasting state. Lastly, there are different isoforms of adiponectin that may dictate different effects but these were not analyzed in this study.
In conclusion, this study confirms previous associations of ADPN with metabolic health indicators in obese individuals and for the first time in nonobese individuals as well. Association of adiponectin secretion with adiposity distribution is demonstrated by low WHR in individuals with HA in the current study, association of ADPN with abdominal SAT-to-VAT ratio in our previous report, and reports of reduced VAT secretion of ADPN with increase in BMI (13, 35). Obese individuals in the population who do not fit the usual phenotype with metabolic abnormalities (ie, MHO) are of particular interest and understanding why certain obese individuals remain free of metabolic disarray could help us better recognize who is susceptible to obesity-related diseases. Although HA is associated with better metabolic health in the current study, the observations are cross sectional and hence no cause–effect conclusions can be reached. Future studies are necessary to confirm these findings and possible mechanisms behind this association.
Acknowledgments
We thank the staff and local membership of TOPS Club, Inc. in the recruitment and ascertainment process and finally to all the members and their families who volunteered for this study.
Dr Ahmed Kissebah, the principal investigator behind the body of this work, was fully involved in all aspects of the TOPS Family Study up to his death on May 17, 2012. We are saddened by his loss and deeply respectful of his long and distinguished career in obesity research.
This work was supported by grants from the National Institutes of Health (NIH) (DK071895-03 and DK65598-01) (to A.K.) and by Take Off Pounds Sensibly Club, Inc. This project was also supported by the National Center for Advancing Translational Sciences, NIH, through Grant No. 8UL1TR000055. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
S.K. is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADPN
- adiponectin level
- BMI
- body mass index
- BP
- blood pressure
- DBP
- diastolic blood pressure
- GEE
- generalized estimating equation
- HA
- hyperadiponectinemia
- HDL-C
- high-density lipoprotein cholesterol
- HOMA-IR
- homeostasis model assessment–insulin resistance index
- HT
- hormone therapy
- LDL-C
- low-density lipoprotein cholesterol
- MHNO
- metabolically healthy nonobese
- MHO
- metabolically healthy obese
- MUNO
- metabolically unhealthy nonobese
- MUO
- metabolically unhealthy obese
- NS
- not significant
- SAT
- subcutaneous adipose tissue
- SBP
- systolic blood pressure
- TOPS
- “Take Off Pounds Sensibly”
- VAT
- visceral adipose tissue
- WHR
- waist-to-hip ratio.
References
- 1. Clearfield M, Pearce M, Nibbe Y, Crotty D, Wagner A. The “new deadly quartet” for cardiovascular disease in the 21st century: Obesity, metabolic syndrome, inflammation and climate change: How does statin therapy fit into this equation? Curr Atheroscler Rep. 2014;16(1):380. [DOI] [PubMed] [Google Scholar]
- 2. McGown C, Birerdinc A, Younossi ZM. Adipose tissue as an endocrine organ. Clin Liver Dis. 2014;18(1):41–58. [DOI] [PubMed] [Google Scholar]
- 3. Robinson K, Prins J, Venkatesh B. Clinical review: Adiponectin biology and its role in inflammation and critical illness. Crit Care. 2011;15(2):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13(2):84–89. [DOI] [PubMed] [Google Scholar]
- 5. Heidemann C, Sun Q, van Dam RM, et al. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149(5):307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hivert MF, Sullivan LM, Fox CS, et al. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008;93(8):3165–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trujillo ME, Scherer PE. Adiponectin—Journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257(2):167–175. [DOI] [PubMed] [Google Scholar]
- 8. Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930–1935. [DOI] [PubMed] [Google Scholar]
- 9. Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J. Serum total adiponectin in nonalcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism. 2011;60(3):313–326. [DOI] [PubMed] [Google Scholar]
- 10. Doumatey AP, Bentley AR, Zhou J, Huang H, Adeyemo A, Rotimi CN. Paradoxical hyperadiponectinemia is associated with the metabolically healthy obese (MHO) phenotype in African Americans. J Endocrinol Metab. 2012;2(2):51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aguilar-Salinas CA, García EG, Robles L, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93(10):4075–4079. [DOI] [PubMed] [Google Scholar]
- 12. Morrison JA, Glueck CJ, Daniels S, Wang P, Horn P, Stroop D. Paradoxically high adiponectin and the healthy obese phenotype in obese black and white 16-year-old girls. Transl Res. 2010;156(5):302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guenther M, James R, Marks J, Zhao S, Szabo A, Kidambi S. Adiposity distribution influences circulating adiponectin levels. Transl Res. 2014;164(4):270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74(4):761–811. [DOI] [PubMed] [Google Scholar]
- 15. Kissebah AH, Sonnenberg GE, Myklebust J, et al. Quantitative trait loci on chromosomes 3 and 17 influence phenotypes of the metabolic syndrome. Proc Natl Acad Sci U S A. 2000;97(26):14478–14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 17. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 18. Comuzzie AG, Tejero ME, et al. The genes influencing adiponectin levels also influence risk factors for metabolic syndrome and type 2 diabetes. Hum Biol. 2007;79(2):191–200. [DOI] [PubMed] [Google Scholar]
- 19. Natarajan S, Lipsitz S, Parzen M, Lipshultz S. A measure of partial association for generalized estimating equations. Stat Modelling. 2007;7(2):175–190. [Google Scholar]
- 20. Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. [DOI] [PubMed] [Google Scholar]
- 21. Snijder MB, Dekker JM, Visser M, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: The Hoorn Study. Am J Clin Nutr. 2003;77(5):1192–1197. [DOI] [PubMed] [Google Scholar]
- 22. Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: A protective fat depot? Diabetes Care. 2009;32(6):1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arner P. Not all fat is alike. Lancet. 1998;351(9112):1301–1302. [DOI] [PubMed] [Google Scholar]
- 24. Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11(8):327–332. [DOI] [PubMed] [Google Scholar]
- 25. Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ, Burrell MA. The adipocyte: A model for integration of endocrine and metabolic signaling in energy metabolism regulation. Am J Physiol Endocrinol Metab. 2001;280(6):E827–E847. [DOI] [PubMed] [Google Scholar]
- 26. Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity-related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444(7121):847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eglit T, Ringmets I, Lember M. Obesity, high-molecular-weight (HMW) adiponectin, and metabolic risk factors: Prevalence and gender-specific associations in Estonia. PLoS One. 2013;8(9):e73273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20(6):1595–1599. [DOI] [PubMed] [Google Scholar]
- 30. Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes. 2005;54(2):534–539. [DOI] [PubMed] [Google Scholar]
- 31. Turer AT, Scherer PE. Adiponectin: Mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. [DOI] [PubMed] [Google Scholar]
- 32. Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: Lessons from translational research. Am J Clin Nutr. 2010;91(1):258S–261S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: A review of current evidence. Endocr Rev. 2012;33(4):547–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses' Health Study. Am J Epidemiol. 1997;145(7):614–619. [DOI] [PubMed] [Google Scholar]
- 35. Motoshima H, Wu X, Sinha MK, et al. Differential regulation of adiponectin secretion from cultured human omental and subcutaneous adipocytes: Effects of insulin and rosiglitazone. J Clin Endocrinol Metab. 2002;87(12):5662–5667. [DOI] [PubMed] [Google Scholar]
- 36. Bremer AA, Devaraj S, Afify A, Jialal I. Adipose tissue dysregulation in patients with metabolic syndrome. J Clin Endocrinol Metab. 2011;96(11):E1782–E1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96(11):E1756–E1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vansaun MN. Molecular pathways: Adiponectin and leptin signaling in cancer. Clin Cancer Res. 2013;19(8):1926–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–769. [DOI] [PubMed] [Google Scholar]
- 40. Yamauchi T, Nio Y, Maki T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–339. [DOI] [PubMed] [Google Scholar]
- 41. Bjursell M, Ahnmark A, Bohlooly-Y M, et al. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes. 2007;56(3):583–593. [DOI] [PubMed] [Google Scholar]
- 42. Arita Y, Kihara S, Ouchi N, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105(24):2893–2898. [DOI] [PubMed] [Google Scholar]
- 43. Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–1301. [DOI] [PubMed] [Google Scholar]
- 44. Shibata R, Sato K, Pimentel DR, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11(10):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kwon BJ, Kim DW, Her SH, et al. Metabolically obese status with normal weight is associated with both the prevalence and severity of angiographic coronary artery disease. Metabolism. 2013;62(7):952–960. [DOI] [PubMed] [Google Scholar]
- 46. Kim S, Lee H, Lee DC, Lee HS, Lee JW. Predominance of small dense LDL differentiates metabolically unhealthy from metabolically healthy overweight adults in Korea. Metabolism. 2014;63(3):415–421. [DOI] [PubMed] [Google Scholar]