Abstract
Context:
Significant gaps remain in the understanding of genetic and environmental risk factors, as well as related mechanisms that contribute to gestational diabetes mellitus (GDM).
Objectives:
This study aimed to investigate early pregnancy maternal serum metabolites and subsequent risk of GDM.
Design:
Information on participant characteristics and GDM diagnosis was collected using in-person interviews and medical record abstraction, respectively. Early pregnancy serum samples were used for nontargeted metabolite profiling using a gas chromatography–mass spectrometry platform. Lasso regression was used to select a set of metabolites that are jointly associated with GDM case-control status. We evaluated the predictive performance of the set of selected metabolites using a receiver operating characteristics curve and area under the curve.
Participants:
A total of 178 GDM cases and 180 controls participated in a pregnancy cohort study.
Results:
A set of 17 metabolites (linoleic acid, oleic acid, myristic acid, d-galactose, d-sorbitol, o-phosphocolamine, l-alanine, l-valine, 5-hydroxy-l-tryptophan, l-serine, sarcosine, l-pyroglutamic acid, l-mimosine, l-lactic acid, glycolic acid, fumaric acid, and urea) differentiated GDM cases from controls. Fold changes of relative abundance of these metabolites among GDM cases compared with controls ranged from 1.47 (linoleic acid) to 0.78 (5-hydroxy-l-tryptophan). Addition of these selected metabolites to a set of well-known GDM risk factors improved the area under the curve significantly from 0.71 to 0.87 (P = 3.97E-07).
Conclusions:
We identified combinations of metabolites in early pregnancy that are associated with subsequent risk of GDM. Replication of findings may improve understanding of GDM pathogenesis and may have implications for the design of GDM prevention and early diagnosis protocols.
Gestational diabetes mellitus (GDM), a common pregnancy-related carbohydrate metabolism disorder first recognized in pregnancy, is associated with significant maternal and offspring morbidity and mortality (1). Similar to type 2 diabetes, the central disturbances in GDM involve increased insulin resistance and decreased insulin secretion (1). However, significant gaps remain in the understanding of genetic and environmental risk factors, as well as related mechanisms that contribute to GDM (1–3). Population-level research on assessment of definitive causal associations between these putative risk factors and GDM will benefit from investigations of potential mechanisms, including metabolomics investigations.
Metabolomics involves profiling of a broad range of low-molecular-weight (<1 kDa) endogenous and exogenous metabolites (eg, lipids, amino acids, and organic acids) (4) representing cellular functions at the intersection of genetic and environmental effects (4). Metabolomics investigations have provided important information on risk factors, mechanisms, and pathogenesis of type 2 diabetes while highlighting the promise of using these metabolites as biomarkers in clinical settings (5–6). Only a handful of studies have been conducted on metabolomics of GDM or hyperglycemia during pregnancy (7–13). Findings from these metabolomic studies of GDM and maternal hyperglycemia during pregnancy have generally been inconsistent, although alterations in amino acid levels, particularly alterations of branched-chain amino acids (BCAAs), free fatty acids, fatty acid oxidation products, and gluconeogenic precursors have been reported by several studies. Previous studies were sparse, and relatively small, with the number of GDM (or hyperglycemia) cases included in the studies ranging from 20 to 80 (7–13). Few assessed metabolites during early pregnancy, a critical time period for GDM pathogenesis with clinical implications for GDM prediction and early diagnosis. Importantly, earlier studies have not assessed combination of metabolites in relation to risk of GDM, limiting the opportunity to understand joint effects of metabolites and the systemic disturbances these metabolite profiles represent. Therefore, we investigated early pregnancy maternal serum metabolites and subsequent risk of GDM among participants of a pregnancy cohort. We also examined relationships between identified metabolites and putative GDM risk factors, as well as the extent to which identified metabolites improve GDM risk prediction.
Research Design and Methods
Study setting and study population
This nested case-control study was conducted among participants of the Omega study. The Omega study is a prospective cohort study designed to examine maternal dietary and lifestyle risk factors of pregnancy complications (14). The study population comprised of women attending prenatal care clinics affiliated with Swedish Medical Center (SMC) Seattle, WA and Tacoma General Hospital (TGH), Tacoma, WA. Women eligible for inclusion into the study were those who initiated prenatal care prior to 20 weeks' gestation. Women were ineligible if they were less than 18 years of age, did not speak and read English, did not plan to carry the pregnancy to term, or did not plan to deliver at either of the two research hospitals. The procedures used in this study were approved by the institutional review boards of the SMC and TGH. All participants provided written informed consent.
Data collection
Participants were invited to participate in an in-person interview and provided blood samples at or shortly after enrollment (16 weeks' gestation, on average). From structured questionnaires and medical records, we obtained information on maternal sociodemographic characteristics, lifestyle habits (eg, physical activity), family medical history, and reproductive and medical histories. Prepregnancy body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). After delivery, maternal and infant records were reviewed for information on course and outcomes of pregnancy including GDM case control status.
GDM case and control selection
The diagnosis of GDM was made using the Carpenter and Coustan American Diabetes Association 2004 guidelines (15). In our study settings, all perinatal care clinic attendants are screened at 24–28 weeks' gestation using a 1-hour oral glucose load (50 g) screening test. Women who have postload glucose concentrations greater than 140 mg/dL were then followed up within 1–2 weeks with a 3-hour oral glucose (100 g) tolerance test. Women were diagnosed with GDM if at least two of the four diagnostic glucose concentration measurements met or exceeded the following: fasting, at least 95 mg/dL; 1-hour postchallenge, at least 180 mg/dL; 2-hour postchallenge, at least 155 mg/dL; and, 3-hour postchallenge, at least 140 mg/dL. We identified and sampled all 191 women who developed GDM. We also randomly sampled 191 control women who did not develop GDM. None of the GDM cases or selected controls had pregestational diabetes. There were 13 GDM cases and 11 controls who did not have adequate serum samples for metabolite profiling. The final analytic population included 178 GDM cases and 180 controls.
Sample preparation, metabolite extraction, and quality control
Protein precipitation was performed by adding 1 mL of working solution composed of acetonitrile, isopropanol, and water (3:3:2) containing isotope-labeled internal standards at a concentration of 1.25 μg/mL (l-Tyrosine, 3,3-d2; l-glutamic acid, 2,3,3,4,4-d5; l-alanine, 2,3,3,3-d4; l-phenylalanine-phenyl-d5–2,3,3,-d3, glycine-d5, myristic 2 acid d27) to 30 μL of plasma. After mixing by vortex, samples were centrifuged at 14 500 × g for 15 minutes at room temperature. The supernatant was then concentrated to dryness in speedvac. The dried samples were stored at −20°C until derivatization and subsequent analysis by gas chromatography–mass spectrometry (GC-MS). A pooled quality control (QC) sample was analyzed for each batch by taking an equal volume from each prepared biological sample within the batch. The QC was then run multiple times at the beginning of each batch, in between runs, and at the end of each batch. The data from the QC sample were used for quality assessment. Also, a “blank” sample containing only the derivatization agents was run at the beginning of each batch to assess the background ions introduced by sample derivatization. A mixture of standards was also run at the beginning and the end of each batch for retention index (RI) calibration.
GC-MS data acquisition and preprocessing
We used an Agilent 7890A GC coupled to the LECO Pegasus HT time of flight MS for nontargeted GC-MS profiling. We derivatized the dried samples in each batch prior to injection following the steps described before. Briefly, 20 μL of 20 mg/mL methoxyamine hydrochloride in pyridine was added to the dried extracts, vortexed, and incubated at 80°C for 20 minutes. After returning the samples to room temperature, 80 μL of MSTFA was added at 80°C for 20 minutes. Samples were then centrifuged for 15 minutes at 14 500 rpm, and 60 μL of reaction mixture was transferred into 250-μL clear glass autosampler vials. A retention index (RI) standard was prepared by mixing fatty acid methyl esters with alkanes and injected at the beginning and the end of each batch for calibration of the RI in each batch. The raw GC-MS data were then preprocessed using the MetaboliteDetector software (http://md.tu-bs.de/node/3) yielding measurements on a total of 268 analytes (16). The preprocessing included outlier screening, peak detection, and deconvolution, RI calibration, metabolite identification (library search), and alignment. Alignment was done based on the spectral matching for neighbor peaks (in terms of retention time) across different runs. In both identification and alignment steps, a combined score based on spectral matching and RI similarity was used. The National Institute of Standards and Technology and Fiehn libraries were used to identify metabolite IDs by spectral matching.
Metabolites with more than 50% missing values were removed leaving a total of 218 metabolites for further evaluation. Log transformation was applied to make the data normally distributed for subsequent statistical tests. Imputations were performed separately for GDM cases and controls using the function impute.knn of the R Bioconductor package impute. This function uses k-nearest neighbors in the space of analytes to impute missing expression values. For each analyte with missing values, the k-nearest neighbors using a Euclidean metric were identified based on samples for which that analyte is not missing (used k = 10). The ComBat approach (17) implemented in the R function ComBat was applied to remove batch effects. This method uses an empirical Bayes method to remove known batch effects by fitting a regression model of the preprocessed GC-MS data with group effect, along with additive and multiplicative batch effects. The latter are estimated by their posterior means, which are then used to adjust the raw data.
Statistical analyses
Characteristics of the study population were examined by assessing distributions, mean (SD) for continuous variables, and number (percentage) for categorical variables, among GDM cases and controls. We used partial least squares discriminant analyses to determine metabolite profile patterns that discriminate between GDM cases and controls. This was conducted using information from unidentified (previously not described) metabolites and identified (described) metabolites.
A penalized logistic regression method (lasso regression) was applied to select sets of metabolites that are jointly associated with the GDM case-control status among the identified metabolites. The method allows the selection of relevant variables and the estimation of their regression coefficients (18). The number of selected variables is guided by a penalty parameter: the larger the parameter, the smaller the selected subset. A 20-fold cross-validation approach was performed and the penalty parameter value yielding the smallest prediction error was used. We then fitted a multivariable logistic regression model using the metabolites selected by the lasso regression. These analyses were adjusted for maternal age, prepregnancy BMI, family history of diabetes, and maternal race.
We examined the cluster structure between the metabolites selected by lasso regression using 1) a weighted gene coexpression network analysis (WGCNA) (19), and 2) Gaussian Graphical Models (GGM) (20). For the WGCNA, a metabolite coexpression adjacency matrix encoding the connection strength and defining a measure of dissimilarity among metabolites was obtained using Pearson correlation coefficients. This similarity matrix was then used to perform hierarchical clustering. The WGCNA package in R was used for this analysis. GGM is based on the concept of conditional independence: a direct relation between two variables exists if the two variables are conditionally dependent given all the remaining variables. This conditional dependence is represented by edges between nodes/metabolites. The GGMselect package in R was used for this analysis. In addition, Pearson correlation coefficients between the metabolites were calculated among GDM cases and controls, separately, and represented using heat maps.
Correlation coefficients (Pearson's correlation coefficients for continuous variables and Spearman correlation coefficients for categorical variables) were calculated to evaluate correlations between the lasso-selected metabolites and a subset of clinical covariates that include known GDM risk factors and biomarkers closely related to GDM risk. We constructed receiver operating characteristic (ROC) curves to evaluate the predictive performance of different sets of indicators, comprised of known participant characteristics (maternal age, family history of diabetes, and prepregnancy BMI), biomarkers (serum ferritin, C-reactive protein, hepcidin, and total 25[OH]D) and our set of selected metabolites. The area under the curve (AUC) corresponding to these ROC curves were calculated. Differences between AUCs were compared using a test statistic implemented in the R package pROC.
Finally, we examined functions and functional evaluations of identified metabolites using pathway analyses tools: Integrated Molecular Pathway Level Analysis (IMPaLA) and Ingenuity Pathway Analyses.
Results
GDM cases were older, less likely to be non-Hispanic White, have higher prepregnancy BMI, be physically inactive, and have a family history of diabetes, compared with controls (Table 1). Gestational age at blood collection and hours from last meal to blood collection were similar for GDM cases and controls. In partial least squares discriminant analyses, we observed some, but not distinct, clustering of samples by GDM case control status or samples that were clear outliers (Supplemental Figure 1).
Table 1.
Selected characteristics of GDM (GDM) cases and controls
| GDM (n = 178) |
Controls (n = 180) |
P Value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Maternal age, ya | 34.0 ± 4.8 | 33.1 ± 4.3 | .06 | ||
| <25 | 7 | 3.9 | 4 | 2.2 | .06 |
| 25–29 | 17 | 9.6 | 33 | 18.3 | |
| 30–34 | 70 | 39.3 | 73 | 40.6 | |
| ≥35 | 84 | 47.2 | 70 | 38.9 | |
| Non-Hispanic white | 127 | 71.4 | 154 | 85.6 | .01 |
| Maternal education ≤ HS | 7 | 3.9 | 4 | 2.2 | .38 |
| Multiparous | 79 | 44.4 | 77 | 42.8 | .76 |
| Family history of diabetes | 53 | 29.8 | 27 | 15.0 | <.001 |
| Multivitamin intake in pregnancy | 175 | 98.3 | 175 | 97.2 | .48 |
| Cigarette smoking history | |||||
| Never | 138 | 77.5 | 134 | 74.4 | .31 |
| Former | 27 | 15.2 | 37 | 20.6 | |
| Current | 13 | 7.3 | 9 | 5.0 | |
| Chronic hypertension | 18 | 10.1 | 5 | 2.8 | .01 |
| Worked during pregnancy | 143 | 80.3 | 146 | 81.1 | .85 |
| Inactive in pregnancy | 40 | 22.5 | 27 | 15.0 | .07 |
| Multiple pregnancy | 11 | 6.2 | 10 | 5.6 | .80 |
| Prepregnancy BMI, kg/m2a | 26.7 ± 7.2 | 23.4 ± 5.3 | <.001 | ||
| <18.5 | 5 | 2.8 | 7 | 3.9 | <.001 |
| 18.5–24.9 | 89 | 50.0 | 126 | 70.0 | |
| 25–29.9 | 44 | 24.7 | 37 | 20.5 | |
| ≥30 | 40 | 22.5 | 10 | 5.6 | |
| Gestational age at blood collection, wka | 15.6 ± 2.9 | 15.6 ± 2.9 | .93 | ||
| Time from last meal to blood collection, ha | 2.92 ± 2.58 | 2.67 ± 2.09 | .32 | ||
Abbreviation: HS: high school.
P value from student t test or χ2 test/Fisher's exact test.
Mean ± sd, otherwise Number (percentage).
Using lasso regression, we identified a total of 17 metabolites whose joint expression differentiated GDM cases from controls (Table 2 and Supplemental Figure 2). These included fatty acids (linoleic acid, oleic acid, myristic acid), sugars and alcohols (d-galactose, d-sorbitol, o-phosphocolamine), amino acids (l-alanine, l-valine, 5-hydroxy-l-tryptophan, l-phenylalanine-phenyl, l-serine, sarcosine, l-pyroglutamic acid, and l-mimosine), and organic acids (l-lactic acid, glycolic acid, fumaric acid, and urea). Of these metabolites, linoleic acid, oleic acid, myristic acid, d-galactose, d-sorbitol, l-alanine, l-valine, l-lactic acid, and fumaric acid were more abundant among GDM cases whereas o-phosphocolamine, 5-hydroxy-l-tryptophan, l-phenylalanine-phenyl, l-serine, sarcosine, l-pyroglutamic acid, l-mimosine, glycolic acid and urea were more abundant among controls. Fold changes of relative abundance among GDM cases compared with controls for these metabolites ranged from 1.47 (linoleic acid) to 0.78 (5-hydroxy-l-tryptophan).
Table 2.
List of Known Metabolites Associated With Gestational Diabetes Mellitus Case Control Status in Lasso Logistic Regression
| Metabolites | Fold Changea |
Multivariable Analysisb |
Univariate Analysisc |
||
|---|---|---|---|---|---|
| Coefficient |
Coefficient |
||||
| Mean | Median | (P-Value) | (P-Value) | (FDR P-Value) | |
| Fatty acids | |||||
| Linoleic acid | 1.47 | 1.57 | 2.18 | 1.29 | 1.29 |
| (8.65E-08) | (6.27E-08) | (1.25E-06) | |||
| Oleic acid | 1.30 | 1.35 | −1.04 | 0.50 | 0.50 |
| (9.97E-03) | (9.43E-03) | (5.63E-02) | |||
| Myristic acid | 1.00 | 0.99 | −0.96 | −0.61 | −0.61 |
| (5.37–01) | (5.42E-01) | (6.38E-01) | |||
| Sugars and alcohols | |||||
| d-galactose | 1.31 | 1.38 | 1.38 | 1.52 | 1.52 |
| (4.19E-03) | (1.48E-05) | (1.48E-04) | |||
| d-sorbitol | 1.07 | 1.07 | 1.88 | 1.89 | 1.89 |
| (1.50E-01) | (1.97E-02) | (5.63E-02) | |||
| o-phosphocolamine | 0.95 | 0.95 | −0.39 | −0.19 | −0.19 |
| (1.97E-01) | (4.16E-01) | (5.54E-01) | |||
| Amino acids and their derivatives | |||||
| l-alanine | 1.03 | 1.03 | 1.34 | 0.62 | 0.62 |
| (1.51E-01) | (2.46E-01) | (4.47E-01) | |||
| l-valine | 1.04 | 1.04 | 1.18 | 0.29 | 0.29 |
| (1.19E-01) | (5.13E-01) | (6.38E-01) | |||
| 5-hydroxy-L-tryptophan | 0.78 | 0.71 | −0.16 | −0.28 | −0.28 |
| (2.50E-01) | (1.58E-02) | (5.63E-02) | |||
| l-serine | 0.92 | 0.96 | −2.58 | −1.01 | −1.01 |
| (2.57E-04) | (1.46E-02) | (5.63E-02) | |||
| Sarcosine | 0.94 | 1.01 | −0.87 | −0.46 | −0.46 |
| (1.37E-01) | (3.17E-01) | (5.27E-01) | |||
| l-pyroglutamic acid | 0.98 | 0.99 | −0.42 | −0.04 | −0.04 |
| (4.49E-01) | (9.02E-01) | (9.02E-01) | |||
| l-mimosine | 0.98 | 0.98 | −0.40 | −0.53 | −0.53 |
| (6.14E-01) | (4.00E-01) | (5.54E-01) | |||
| Organic acids | |||||
| l-lactic acid | 1.10 | 1.13 | 1.60 | 1.13 | 1.13 |
| (2.16E-02) | (1.76E-02) | (5.63E-02) | |||
| Glycolic acid | 0.98 | 1.00 | −0.89 | −0.95 | −0.95 |
| (5.10E-01) | (3.43E-01) | (5.27E-01) | |||
| Fumaric acid | 1.11 | 1.14 | 0.47 | 0.36 | 0.36 |
| (6.55E-02) | (7.81E-02) | (1.83E-01) | |||
| Urea | 0.88 | 0.93 | −0.28 | −0.40 | −0.40 |
| (3.64E-01) | (8.24E-02) | (1.83E-01) | |||
Abbreviation: FDR, false discovery rate.
Lasso logistic regression (adjusted for maternal age, pre-pregnancy BMI, family history of diabetes, and maternal race) performed to identify sets of metabolites jointly associated with case-control status.
Fold change (GDM case/control).
Multivariable logistic regression model based on lasso-selected metabolites adjusting for maternal age, pre-pregnancy BMI, family history of diabetes, and maternal race.
Univariate logistic regression of each lasso-selected metabolite adjusted for maternal age, pre-pregnancy BMI, family history of diabetes, and maternal race.
Overall, we observed stronger correlations in relative abundance of metabolites in the fatty acid and amino acid groups (compared with correlations in relative abundance of metabolites in the sugar and alcohol, and organic acid groups) among both GDM cases and controls (Supplemental Figure 3). In GGM-WGCNA analyses, we identified a network that comprised of all but one metabolite identified using lasso regression (Supplemental Figure 4). Prepregnancy BMI was correlated with relative abundance of metabolites belonging to fatty acids (oleic acid and myristic acid) and sugar/alcohol (d-galactose and d-sorbitol) groups among GDM cases. With the exception of d-galactose and prepregnancy BMI correlations, these correlations were not observed among controls (Supplemental Tables 1 and 2). In contrast, parity was correlated with relative abundance of fatty acids (linoleic acid and oleic acid) and amino acids and their derivatives (l-alanine, l-valine, 5-hydroxy-l-tryptophan, and l-pyroglutamic acid) among controls. None of these correlations were observed among GDM cases.
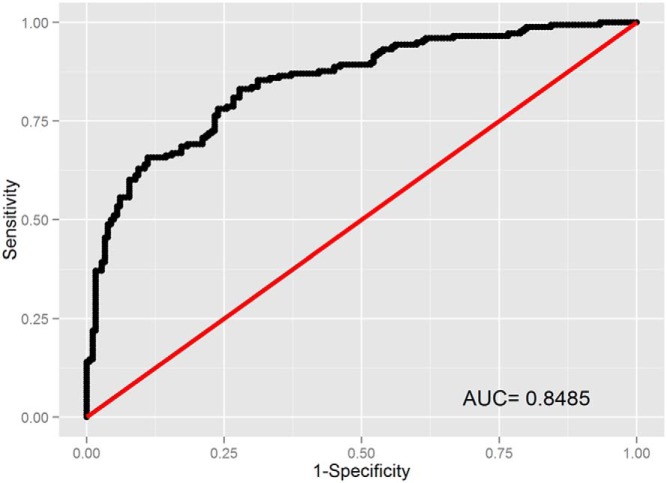
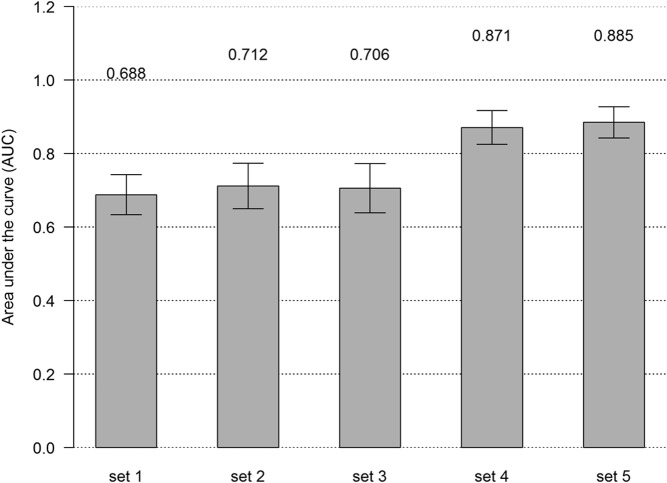
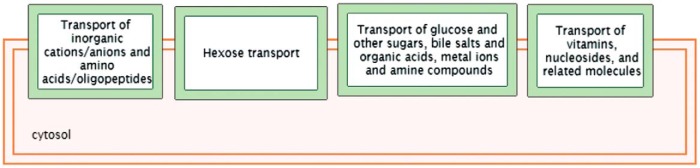
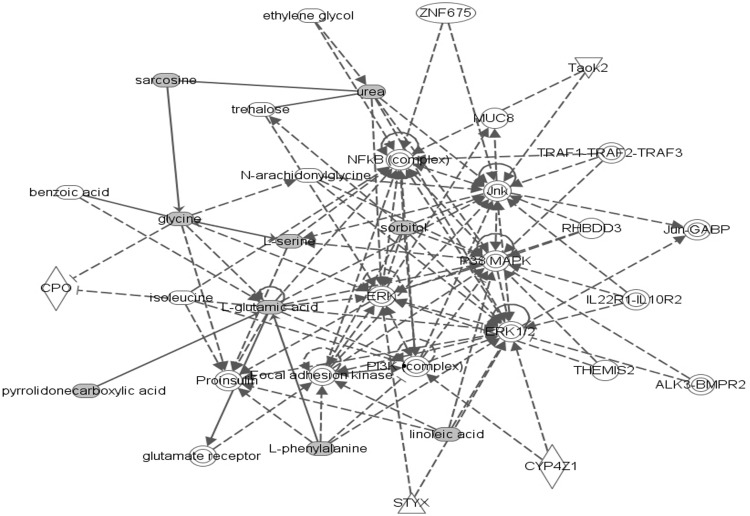
In ROC analyses, addition of lasso-selected metabolites to the set that included other GDM risk factors (maternal age, family history, prepregnancy BMI, ferritin, CRP, hepcidin, and total vitamin D) improved the AUC from 0.71 to 0.87 (P = 3.97E-07) (Figures 1 and 2). In pathway analyses, using IMPaLA, we observed that selected metabolites participate in solute-carrier (SLC)-mediated transmembrane transport including transport of inorganic amino acids, cations/anions, hexose, glucose, and other sugars, bile salts, organic acids, amine compounds, vitamins, nucleosides, and related molecules (Figure 3). A similar network comprised of metabolites representing amino acid metabolism, molecular transport, and small molecule chemistry was identified in Ingenuity Pathway Analysis (Figure 4).
Figure 1.
ROC curves for GDM prediction using metabolites with putative IDs.
Figure 2.
AUC of ROCs corresponding to increasing subsets* of clinical factors and subgroups of metabolites. *, set1: Maternal age, family history, and prepregnancy BMI; set2: addition of ferritin and CRP to set1; set3: addition of hepcidin and total vitamin D to set2; set4: addition of selected metabolites to set3; and set5: addition of nonselected metabolites with putative IDs to set4.
Figure 3.
Top networks represented by selected metabolites that are associated with GDM using IMPaLA.
Figure 4.
Top networks represented by selected metabolites that are associated with GDM using Ingenuity Pathway Analysis.
Discussion
In the current study, we identified a set of 17 early pregnancy serum metabolites (fatty acids, sugars and alcohols, amino acids, and organic acids) that are jointly associated with subsequent risk of GDM. Abundance of several of these metabolites correlated with prepregnancy BMI among GDM cases and parity among controls. The AUC from ROC curve for GDM prediction was significantly improved by addition of these metabolites to well-known GDM risk factors. The selected metabolites represented SLC-mediated transmembrane transport, small molecule transport, and amino acid metabolism pathways.
Previous findings of GDM-related metabolomic studies, conducted using serum, plasma, urine, breast milk, and amniotic fluid specimen, reported inconsistent findings (7–13). Several (7–11), but not all (12–13) studies found significant metabolic profile differences between GDM cases and controls. Similar to previous reports, our set of metabolites that distinguished GDM cases from controls included a branched-chain amino acid (BCAA) (l-valine), l-alanine, l-serine, 5-hydroxy-l-tryptophan, linoleic acid, oleic acid, and myristic acid. Recent studies have reported independent associations of plasma or serum BCAA in midpregnancy (24–28 weeks' gestation) with risk of GDM or higher fasting plasma glucose among pregnant women (11, 24). BCAAs are involved in several pathways (eg, fatty acid oxidation, mTOR, JNK, and IRS1pathways) of insulin resistance (25–26). BCAAs have also been shown to reduce insulin secretion through their effect on mitochondria of pancreatic beta cells (25–26). Serine phosphorylation of the insulin receptor substrate-1 is a well-described mechanism for insulin resistance (27). The amino acid 5-hydroxy-l-tryptophan is a precursor of serotonin with demonstrated functions that include increased insulin release by serotonylation of GTPases within pancreatic β-cells (28). Beside amino acids, fatty acid metabolism deregulation has also been related to insulin resistance and related disorders (7, 29, 30). Elevated free fatty acids inhibit insulin signaling in muscle, contribute to hyperglycemia by antagonizing effects of insulin on endogenous glucose production in the liver, and affect insulin secretion in the pancreas (7, 29, 30). Fatty acids, including oleic acid, linoleic acid, and myristic acid have been associated with different components of the insulin resistance and glucose metabolism, and shown to differ between groups defined by different severity of hyperglycemia or GDM (7, 30).
In addition to previously reported metabolites, we identified metabolites such as d-galactose and urea, which were previously not related to GDM risk or hyperglycemia during pregnancy. Our observation of higher d-galactose among women who later developed GDM, in accordance with its reported involvement in remodeling of oxidative energy metabolism to improve glucose handling, is indicative of glucose metabolism abnormalities that are present well before GDM diagnosis (31). In contrast, our observations of reduced serum urea in early pregnancy among pregnancies that were complicated by subsequent GDM is unexpected. Although serum urea is generally expected to be lower in pregnancy, serum urea levels usually correlate with amino acid metabolism and are generally expected to be higher in diabetes and diabetes-related states. It is not clear whether any of the other conditions identified with lower serum urea such as liver disease, malnutrition, or overhydration played a role in these pregnancies. This warrants further investigation.
In line with previous (7–13) and our current findings, our pathway analyses suggested that metabolites that differentiated GDM and control pregnancies over-represented a broad set of small molecule transport mechanisms and amino acid metabolism. The SLC family of transporters comprises a large family (>384 unique proteins functionally grouped into 52 families) of membrane transporters responsible for the transmembrane transport of a wide variety of substrates such as inorganic ions, nucleotides, amino acids, neurotransmitters and sugars, purines, fatty acids, and drugs (32). A number of genes (eg, SLC16A11, SLC5A2, and SLC2A1, also known as GLUT1) have been identified for these family of proteins or their interactions, some of which (particularly SLC5A2) are being targeted in diabetes-related drug development (32).
In contrast, our set of metabolites did not include other metabolites that were previously associated with GDM and type 2 diabetes. Notable ones among these include other BCAAs (isoleucine and leucine), aromatic amino acids (tyrosine and phenylalanine), acylcarnitines, and choline-containing phospholipids, 3-hydroxybutryate, and tryglcerides (7–13, 33). This is in line with observed inconsistencies between findings of studies in this area. Differences between findings from the studies could be from differences in GDM or hyperglycemia diagnostic criteria, differences in metabolome profiling platforms, timing of metabolite profiling, differences in specimen, as well as differences in inherent characteristics (eg, overweight or obesity status of study participants) of the study populations. Future studies that follow strict guidelines and same recommendations across studies may help improve replication of findings (34).
Importantly, our study evaluated combinations of metabolites that differentiated GDM cases and controls, in contrast with evaluations of independent single metabolite abundance differences, as in similar previous studies (7–13). This is a particular strength of our study because joint effects of metabolites underlie GDM pathogenesis. Further, the widespread effort to identify single metabolites that contribute to GDM or type 2 diabetes and are significant disease predictors has largely been unsuccessful. Increasingly, investigators are using combined indicators (eg, genetic risk scores) in risk stratification in other research fields. Although AUC under different models for prediction of type 2 diabetes have been evaluated, we are aware of only one such model in GDM (35–36). A previous study investigated newborn meconium and urine and identified 14 meconium metabolites and three urinary metabolite markers associated with GDM using ROC analyses (36). Our study improved upon this by evaluating metabolites in early pregnancy. It was also different from the previous study because it evaluated metabolites in tandem. The improved AUC by including selected metabolites, over and above the predictive capacity of well-known risk factors such as prepregnancy BMI, supported the diagnostic ability (improved discrimination) of the metabolite profile to predict GDM risk.
Few studies have evaluated correlations between metabolites and GDM risk factors. Benteley-Lewis (37) identified metabolite changes, related to history of GDM, were associated with BMI, fasting glucose, race, adiponectin, and parity. Among women (N = 38) with history of GDM, amino acid metabolites (N = 22) were associated with response to an oral glucose tolerance test and the clinical covariates that were related to these metabolites were race, BMI, breast feeding, and parity. Interestingly, in our study alanine was related to parity among controls and prepregnancy BMI was associated with a number of metabolites among GDM cases. Similar studies have the potential to enhance our understanding of mechanistic relationships between risk factors and GDM risk.
Our study has other notable strengths. To our knowledge, this is the largest, most statistically powerful GDM metabolomics study to date. Serum metabolite profiling has advantage over urine because it reflects multiple tissue/organ metabolites and serum metabolites are less likely to be affected by diurnal changes (38). We conducted early pregnancy metabolite profiling, which has implications for prevention and early diagnosis. In contrast, several limitations of our study deserve mention. First, we performed nontargeted metabolite profiling and did not do targeted profiling or follow-up investigations. Although several of the metabolites we identified have also been reported before, others need independent validation in future studies. We did not use training and testing sets to evaluate the discriminating potentials of the identified metabolites. To mitigate this limitation, we conducted a leave-one-out cross-validation procedure in the ROC analyses. In these analyses, addition of lasso-selected metabolites the set that included other GDM risk factors (maternal age, family history, prepregnancy BMI, ferritin, hepcidin, and total vitamin D) improved the AUC from 0.69 to 0.79 (P = 2.18E-05). Fold changes of relative abundance of metabolites were small causing potential concerns of false-positive findings. However, small changes may not necessarily mean low biologic significance (39). This is particularly true in our analysis given that we investigated joint abundance (and possibly systemic disturbances). Samples evaluated in the current study were nonfasting blood samples and we did not have specific information on time interval between last meal and blood collection, as well as the type of last meal consumed prior to blood collection. However, consistency of some of our findings with previous similar studies (7–13) is reassuring. We also did not evaluate effects of metabolites on insulin sensitivity and signaling. Finally, additional similar studies are needed among other study populations to confirm generalizability of our study findings.
In sum, in this study, we identified combinations of metabolites in early pregnancies that are associated with subsequent risk of GDM. We also showed that these metabolites significantly increased our diagnostic ability to predict GDM. Although the observed statistically significant improvement in the AUC and relevance of functions of identified metabolites in GDM pathogenesis is encouraging, clinical implications of using these metabolites as predictors of GDM needs additional investigation. Future similar investigations that evaluate joint abundance of metabolites, integrate clinical characteristics with metabolites, use multiple platforms, and analyses of multiple specimen types can advance our understanding of metabolite perturbations in GDM.
Acknowledgments
We thank the participants of the Omega study for their cooperation. They are also grateful for the technical expertise of staffs of the Center for Perinatal Studies; Swedish Medical Center; and Ressom Lab, Georgetown University.
This work was supported by grants from the National Institutes of Health (R01HD032562, R01HD034543, and K01HL103174).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BCAA
- branched-chain amino acid
- BMI
- body mass index
- GC-MS
- gas chromatography–mass spectrometry
- GDM
- gestational diabetes mellitus
- GGM
- Gaussian Graphical Model
- IMPaLA
- Integrated Molecular Pathway Level Analysis
- QC
- quality control
- RI
- retention index
- ROC
- receiver operating characteristic
- SLC
- solute carrier
- SMC
- Swedish Medical Center
- TGH
- Tacoma General Hospital
- WGCNA
- weighted gene coexpression network analysis.
References
- 1. Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med. 2004;21:103–113. [DOI] [PubMed] [Google Scholar]
- 2. Kwak SH, Jang HC, Park KS. Finding genetic risk factors of gestational diabetes. Genomics Inform. 2012;10:239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang C, Ning Y. Effect of dietary and lifestyle factors on the risk of gestational diabetes: Review of epidemiologic evidence. Am J Clin Nutr 2011;94:1975S–1979S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patti GJ, Yanes O, Siuzdak G. Innovation: Metabolomics: the apogee of the omics trilogy. Nat Rev Mol Cell Biol. 2012;13:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedrich N. Metabolomics in diabetes research. J Endocrinology. 2012;215:29–42. [DOI] [PubMed] [Google Scholar]
- 7. Chen X, Scholl TO, Leskiw M, Savaille J, Stein TP. Differences in maternal circulating fatty acid composition and dietary fat intake in women with gestational diabetes mellitus or mild gestational hyperglycemia. Diabetes Care. 2010;33:2049–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diaz SO, Pinto J, Graça G, et al. Metabolic biomarkers of prenatal disorders: An exploratory NMR metabonomics study of second trimester maternal urine and blood plasma. J Proteome Res. 2011;10:3732–3742. [DOI] [PubMed] [Google Scholar]
- 9. de Seymour JV, Conlon CA, Sulek K, et al. Early pregnancy metabolite profiling discovers a potential biomarker for the subsequent development of gestational diabetes mellitus. Acta Diabetol. 2014;51:887–890. [DOI] [PubMed] [Google Scholar]
- 10. Dudzik D, Zorawski M, Skotnicki M, et al. Metabolic fingerprint of gestational diabetes mellitus. J Proteomics. 2014;103:57–71. [DOI] [PubMed] [Google Scholar]
- 11. Scholtens DM, Muehlbauer MJ, Daya NR, et al. Metabolomics reveals broad-scale metabolic perturbations in hyperglycemic mothers during pregnancy. Diabetes Care. 2014;37:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sachse D, Sletner L, Mørkrid K, et al. Metabolic changes in urine during and after pregnancy in a large, multiethnic population-based cohort study of gestational diabetes. PLoS One. 2012;7:e52399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graça G, Goodfellow BJ, Barros AS, et al. UPLC-MS metabolic profiling of second trimester amniotic fluid and maternal urine and comparison with NMR spectral profiling for the identification of pregnancy disorder biomarkers. Mol Biosyst. 2012;8:1243–1254. [DOI] [PubMed] [Google Scholar]
- 14. Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes Res Clin Pract. 2005;70:134–142. [DOI] [PubMed] [Google Scholar]
- 15. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–S20. [DOI] [PubMed] [Google Scholar]
- 16. Hiller K, Hangebrauk J, Jäger C, Spura J, Schreiber K, Schomburg D. MetaboliteDetector: Comprehensive analysis tool for targeted and nontargeted GC/MS based metabolome analysis. Anal Chem. 2009;81:3429–3439. [DOI] [PubMed] [Google Scholar]
- 17. Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical bayes methods. Biostatistics. 2007;8:118–127. [DOI] [PubMed] [Google Scholar]
- 18. Hoggart CJ, Whittaker JC, De Iorio M, Balding DJ. Simultaneous analysis of all SNPs in genome-wide and re-sequencing association studies. PLoS Genet. 2008;4:e1000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Langfelder P, Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marchetti GM. Independencies induced from a graphical Markov model after marginalization and conditioning: The R package ggm. J Stat Software. 2006;15:1–15. [Google Scholar]
- 21. Graca G, Duarte IF, Barros AS, et al. Impact of prenatal disorders on the metabolic profile of second trimester amniotic fluid: A nuclear magnetic resonance metabonomic study. J Proteome Res. 2010;9:6016–6024. [DOI] [PubMed] [Google Scholar]
- 22. Dani C, Bresci C, Berti E, et al. Metabolomic profile of term infants of gestational diabetic mothers. J Matern Fetal Neonatal Med. 2014;27:537–542. [DOI] [PubMed] [Google Scholar]
- 23. Dessì A, Marincola FC, Fanos V. Metabolomics and the great obstetrical syndromes—GDM, PET, and IUGR. Best Pract Res Clin Obstet Gynaecol. 2015;29:156–164. [DOI] [PubMed] [Google Scholar]
- 24. Park S, Park JY, Lee JH, Kim SH. Plasma levels of lysine, tyrosine, and valine during pregnancy are independent risk factors of insulin resistance and gestational diabetes. Metab Syndr Relat Disord. 2015;13:64–70. [DOI] [PubMed] [Google Scholar]
- 25. Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Copps KD, White MF. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Draznin B. Molecular mechanisms of insulin resistance: Serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: The two sides of a coin. Diabetes. 2006;55:2392–2397. [DOI] [PubMed] [Google Scholar]
- 28. Paulmann N, Grohmann M, Voigt JP, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32 Suppl 3:14–23. [DOI] [PubMed] [Google Scholar]
- 30. Ebbesson SO, Tejero ME, López-Alvarenga JC, et al. Individual saturated fatty acids are associated with different components of insulin resistance and glucose metabolism: The GOCADAN study. Int J Circumpolar Health. 2010;69:344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kase ET, Nikolic N, Bakke SS, et al. Remodeling of oxidative energy metabolism by galactose improves glucose handling and metabolic switching in human skeletal muscle cells. PLoS One. 2013;8:e59972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rask-Andersen M, Masuram S, Fredriksson R, Schiöth HB. Solute carriers as drug targets: Current use, clinical trials and prospective. Mol Aspects Med. 2013;34:702–710. [DOI] [PubMed] [Google Scholar]
- 33. Lowe WL, Jr, Karban J. Genetics, genomics and metabolomics: New insights into maternal metabolism during pregnancy. Diabet Med. 2014;31:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tzoulaki I, Ebbels TM, Valdes A, Elliott P, Ioannidis JP. Design and analysis of metabolomics studies in epidemiologic research: A primer on -omic technologies. Am J Epidemiol. 2014;180:129–139. [DOI] [PubMed] [Google Scholar]
- 35. Walford GA, Porneala BC, Dauriz M, et al. Metabolite traits and genetic risk provide complementary information for the prediction of future type 2 diabetes. Diabetes Care. 2014;37:2508–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng S, Zhang J, Liu L, et al. Newborn meconium and urinary metabolome response to maternal gestational diabetes mellitus: A preliminary case-control study. J Proteome Res. 2015;14:1799–1809. [DOI] [PubMed] [Google Scholar]
- 37. Bentley-Lewis R, Xiong G, Lee H, Yang A, Huynh J, Kim C. Metabolomic analysis reveals amino acid responses to an oral glucose tolerance test in women with prior history of gestational diabetes mellitus. J Clin Transl Endocrinol. 2014;1:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang A, Sun H, Wang P, Han Y, Wang X. Recent and potential developments of biofluid analyses in metabolomics. J Proteomics. 2012;75:1079–1088. [DOI] [PubMed] [Google Scholar]
- 39. Suhre K. Genetics meets metabolomics: From experiment to systems biology. Springer Science and Business Media, LLC; 2012. [Google Scholar]