Abstract
Context:
Animal studies indicate that glucocorticoids increase hepatic 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD-1) expression and activity.
Objective:
Our goal was to determine whether glucocorticoid excess increases cortisol production in the liver via 11β-HSD-1 enzyme pathway in humans.
Design:
A total of 1 mg each [4-13C] cortisone and [9,12,12-2H3] cortisol were ingested, and [1,2,6,7–3H] cortisol was infused to measure C13 cortisol (derived from ingested [4-13C] cortisone) turnover using the triple tracer technique, whereas glucose turnover was measured using isotope dilution technique following [6–62H2] glucose infusion during a saline clamp.
Setting:
This study took place at the Mayo Clinic Clinical Research Unit.
Participants:
Thirty nondiabetic healthy subjects participated.
Intervention:
Subjects were randomized to hydrocortisone (n = 15) or placebo 50 mg twice daily (n = 15) for 1 week.
Outcome Measures:
Hepatic cortisol production and endogenous glucose production were measured.
Results:
Plasma cortisol concentrations were higher throughout the study period in hydrocortisone group. Rates of appearance of C13 cortisol and hepatic C13 cortisol production were higher in hydrocortisone vs placebo group, indicating increased hepatic 11β-HSD-1 activity. Higher plasma cortisol and presumably higher intrahepatic cortisol was associated with impaired suppression of endogenous glucose production in hydrocortisone vs placebo group.
Conclusion:
Chronic glucocorticoid excess increases intrahepatic cortisone to cortisol conversion via the 11β-HSD-1 pathway. The extent to which this causes or exacerbates steroid induced hepatic insulin resistance remains to be determined.
Glucocorticoids are potent regulators of glucose, fat, and protein metabolism. Transgenic mice overexpressing 11 β-hydroxysteroid dehydrogenase 1 (11β-HSD-1) in adipose tissue (1, 2) develop obesity, insulin resistance, hyperlipidemia, and hypertension, whereas knockout of 11β-HSD-1 enzyme in mice attenuates glucocorticoid induced metabolic effects (3–5). Moreover, overexpression of 11β-HSD-1 in the liver (6) results in insulin resistance and hypertension. On the other hand, despite higher circulating glucocorticoids levels, there were no metabolic effects observed in liver-specific knockout 11β-HSD-1 mouse models (4, 5).
The role of glucocorticoids in modulation of insulin action in humans is an area of active investigation. Glucocorticoids induce glucose intolerance in humans in a dose- and duration-dependent manner (7–10) by inducing hepatic and extrahepatic insulin resistance (10–19). However, the exact mechanism of this effect remains unknown. In vitro studies have reported that glucocorticoid result in a time-dependent increase in 11β-HSD-1 enzyme activity and mRNA expression in rat liver cells (20, 21), which might vary with duration of exposure of cells to glucocorticoids. We have recently reported that liver cortisol production via the hepatic 11β-HSD-1 enzyme pathway is not altered in obese humans with or without diabetes (22–24) when compared to lean humans. Similarly, others have also demonstrated that liver 11β-HSD-1 activity is unchanged in obese individuals with diabetes (25). On the contrary, studies have also reported overexpression of 11β-HSD-1 in the liver of morbidly obese individuals with or without the metabolic syndrome (23, 26, 27). However, it is unknown whether exogenous glucocorticoids modulate this enzyme activity in humans.
That the liver is an important regulator of the diabetogenic effects induced by glucocorticoid use is borne out by the fact that liver-selective glucocorticoid receptor antagonists have been shown to reduce fasting glucose and improve hepatic insulin action during a hyperinsulinemic euglycemic clamp (28).
The present experiments sought to determine whether glucocorticoid excess increases hepatic conversion of cortisone to cortisol via 11β-HSD-1 enzyme pathway in humans. If so, this would provide valuable clinically relevant and translationally significant information regarding the mechanism of steroid induced hyperglycemia and the possible role of liver-specific 11β-HSD-1 inhibitors to mitigate this condition.
Research Design and Methods
Participants
After approval from the Radioactive Drug Research Committee, Food and Drug Administration, and Mayo Institutional Review Board, 30 nondiabetic nonobese individuals (body mass index [BMI] 19–28 kg/m2) provided written consent to participate in the study. All participants were in good health and had no chronic medical condition including overt cardiovascular, hepatic or renal disease, stroke, Alzheimer's disease, or any disorder that may have potentially impacted the outcome measures. Thyroid medications, statins, and antihypertensive medications that are metabolically neutral (eg, low-dose thiazides) were permitted. Subjects who were engaged in training for sports events, engaged in active sports, or actively losing weight were excluded. First-degree relatives of the participants did not have a history of diabetes mellitus. Individuals with a history of smoking or alcohol intake over and above American Diabetes Association guidelines as described previously (24) were excluded from participation in the study. All participants were instructed by a dietician to follow a weight maintenance diet (55% carbohydrate, 30% fat, and 15% protein) for at least 2 weeks before the study. Subjects were weighed at screening and at the time of admission for the in-patient study. Subjects were disqualified if their weight changed by more than 2% from the screening weight. Body composition (total fat and lean body mass) was measured in the Center for Clinical and Translational Science using Lunar iDXA, software version 11.4 (GE Healthcare Technologies), and waist–hip measurements were performed. Detailed baseline characteristics of participants are provided in Table 1.
Table 1.
Baseline Characteristics of Participants
| Characteristics | Hydrocortisone (n = 15) | Placebo (n = 15) |
|---|---|---|
| Age (years) | 44 ± 14 | 45 ± 13 |
| Gender (male/female) | 8/7 | 7/8 |
| Weight (kg) | 73 ± 8 | 73 ± 9 |
| BMI (kg/m2) | 24.6 ± 2.4 | 25.0 ± 2.3 |
| Lean body mass (kg) | 47.4 ± 7.9 | 45.6 ± 9.1 |
| Total body fat (%) | 30.3 ± 0.08 | 32.6 ± 9.3 |
| Waist hip ratio | 0.85 ± 0.08 | 0.82 ± 0.05 |
| Fasting plasma glucose (mM) | 4.9 ± 0.3 | 4.8 ± 0.04 |
Data are presented as mean ± sd.
After assessment of eligibility and recruitment, subjects were randomly allocated to receive either 50 mg of hydrocortisone or matching placebo containing an inactive ingredient twice a day for 1 week, including the morning of the inpatient study. A dose of 100 mg was chosen because it is approximately five times the customary replacement dose (ie, 15–20 mg/d) and therefore would result in considerable glucocorticoid excess. Extra tablets were provided to subjects in case they were dropped or misplaced. Both subjects and investigators were masked to the type of treatment.
Experimental design
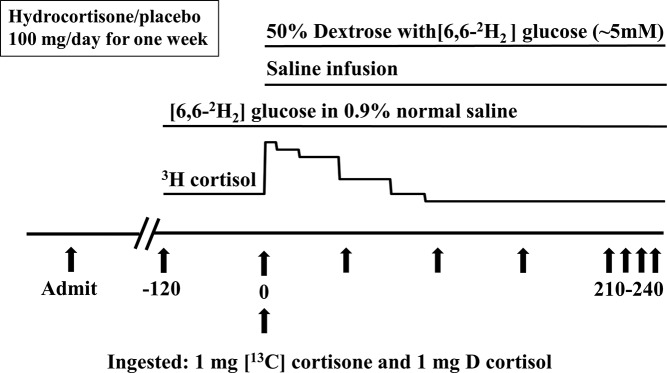
A schematic of the experimental design is shown in Figure 1. Participants were admitted to Mayo Clinic Clinical Research Unit at 5:00 pm on the evening before the study. A standard 10 kcal/kg meal (55% carbohydrate, 30% fat, and 15% protein) was eaten between 5:30 and 6:00 pm. Thereafter, participants remained fasting until the end of the study. Sips of water were permitted ad libitum. At 5:00 am on the morning of the study, an intravenous catheter was placed in a forearm vein for tracer infusion and another in the dorsum of the opposite hand placed in a heated box (∼55 C) to permit sampling of arterialized venous blood. A primed (20 μCi) continuous (0.20 μCi/min) infusion of [1,2,6,7–3H] cortisol (3H cortisol) was started at −120 minutes. At time 0, participants ingested 1.0 mg of [4-13C] cortisone (13C cortisone) and 1.0 mg of [9,12,12-2H3] cortisol (D cortisol) mixed in 38 ml of water over 15 minutes, as previously described (24). At time 0, the 3H cortisol infusion rate was changed to mimic the temporal pattern of change in cortisol concentrations, as previously described (24). A primed (33 μmol/kg) continuous (0.33 μmol/kg/min) infusion of [6,6-2H2] glucose was started at −120 minutes and continued until the end of study. A saline infusion (60 ml/min) was started at time 0 and continued until 240 minutes. Additionally, starting at time 0, 50% dextrose enriched with [6,6-2H2] glucose was infused at variable rates to maintain plasma glucose at ∼5 mM over the 4 hours of the study (29). The rate of the concurrent basal infusion of [6,6-2H2] glucose was also decreased at time 0, as previously described (29), to minimize the change in plasma tracer to tracee ratio.
Figure 1.
Schematic representation of the experimental design.
Arterialized venous blood was sampled at −30, −20, −10, 0, 10, 20, 30, 40, 50, 60, 75, 90, 120, 150, 180, 210, and 240 minutes for measurement of glucose, insulin, glucagon, C-peptide, D cortisol, D cortisone, 13C cortisone, C13 cortisol (derived from ingested 13C cortisone), [6,6-2H2] glucose enrichment, and 3H cortisol specific activity.
Analytical methods
All blood samples were immediately placed on ice, centrifuged at 4 C, separated and stored at −80 C until analyses. Plasma glucose was analyzed using YSI during the clamp portion of the study and GM9 Analox glucose analyzer (Analox Instruments) for routine testing by the Clinical Research Unit. Plasma insulin was measured using a chemiluminescence method with the Access Ultrasensitive Immunoenzymatic assay system (Beckman), and C-peptide and glucagon concentrations were measured by radioimmunoassay (Linco Research Inc.). Plasma cortisol, cortisone, D3 cortisol, D3 cortisone, 13C cortisone, and C13 cortisol enrichments were measured using liquid chromatography tandem mass spectrometry, as previously described (24, 30, 31). 3H cortisol radioactivity was measured using high-performance liquid chromatography followed by liquid scintillation counting (24, 32).
Calculations
Steele's steady-state equation was used to calculate glucose turnover and cortisol fluxes were calculated, as described previously (24); specific equations are provided in the Supplemental Materials. Nonsteady-state calculations were also done using Walker's method (33) (glucose volume of distribution and pool fraction), but were not included as steady-state calculations are more reliable and accurate.
Statistical analysis
Data in the text and figures are expressed as mean ± SD. Values from −30, −20, −10, and 0 minutes were averaged as basal and 210, 220, 230, and 240 minutes averaged as clamp for statistical analysis of glucose turnover and presentation in the figures and text. Student's nonpaired t test was used to test the hypothesis that following 1 week of placebo ingestion, hepatic 13C cortisone conversion to C13 cortisol over the 4 hours of the saline infusion is higher following 1 week of hydrocortisone ingestion than following 1 week of placebo. A P value <.05 was considered as statistically significant. The number of subjects in each group (n = 15) had greater than 80% power to detect a meaningful difference (∼25%) at a P value of .05.
Results
Plasma glucose, insulin, and cortisol concentrations
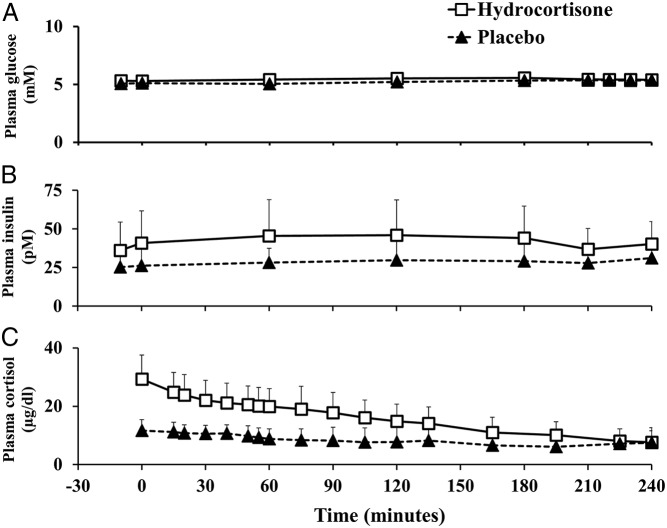
Plasma glucose concentrations were not different in the hydrocortisone vs placebo group both before (5.30 ± 0.36 vs 5.11 ± 0.34 mM; P = .14) and during a euglycemic saline clamp (5.42 ± 0.24 vs 5.35 ± 0.23 mM; P = .41) (Figure 2).
Figure 2.
Plasma glucose (A), insulin (B), and cortisol concentrations (C) before (−30 to 0 minutes) and during the euglycemic saline clamp (0–240 minutes) in the hydrocortisone (white rectangles) and placebo (black triangles) groups.
Plasma insulin concentrations were higher in the hydrocortisone vs placebo group before (38.42 ± 19.70 vs 25.74 ± 10.69 pM; P = .04), but were not different during a euglycemic saline clamp (38.59 ± 13.98 vs 29.50 ± 9.25 pM; P = .08).
Following 1 week of 100 mg/d hydrocortisone treatment, plasma cortisol concentrations were higher in the hydrocortisone vs placebo group (29.33 ± 8.24 vs 11.68 ± 3.74 μg/dl; P = .00001) at baseline and during the euglycemic saline clamp (incremental area under the curve, 0–240 minutes: 3299.18 ± 1382.36 vs 833.95 ± 1274.12 μg/dl/240 minutes; P = .0001).
Specific activity of 3H cortisol to C13 cortisol and 3H cortisol to D cortisol
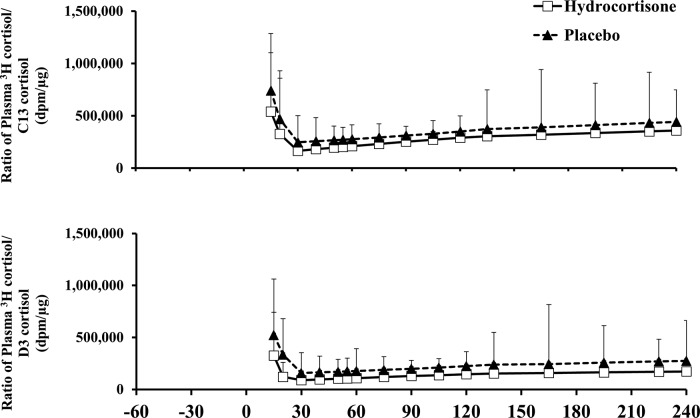
Except for minor perturbations during approximately first 30 minutes, ratios of plasma 3H cortisol to C13 cortisol and 3H cortisol to D cortisol concentrations remained constant in both groups throughout the study, thereby permitting accurate calculations of cortisol fluxes using steady-state equations 24 (Figure 3). Plasma 3H cortisol also did not differ on the hydrocortisone vs placebo study (data not shown), respectively, indicating no difference in clearance in the two study days.
Figure 3.
Ratios of plasma 3H cortisol/C13 cortisol and plasma 3H cortisol/D cortisol concentrations observed with the triple tracer cortisol technique in hydrocortisone (white rectangles) and placebo (black triangle) groups. A total of 1.0 mg of [4-13C] cortisone and 1.0 mg of D cortisol were ingested at time 0.
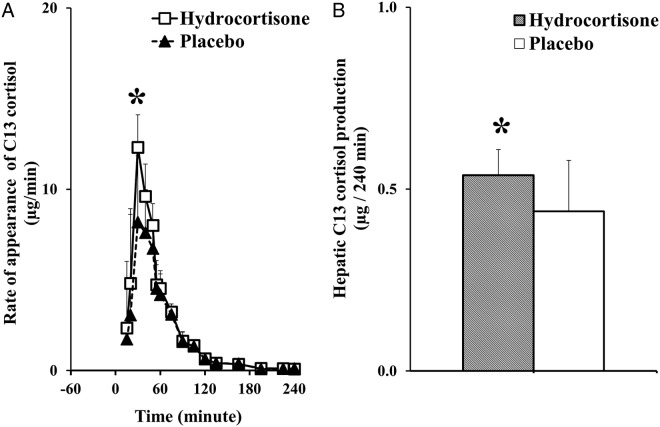
Rate of appearance of C13 cortisol and hepatic C13 cortisol production
The rate of appearance of C13 cortisol derived from ingested 13C cortisone provides an index of hepatic 11β-HSD-1 activity (24) (Figure 4). The rate of appearance of C13 cortisol was higher in the hydrocortisone vs placebo group (area under the curve: 538.17 ± 73.77 vs 439.09 ± 143.38 μg/240 minutes; P = .02), indicating that hydrocortisone stimulates conversion of cortisone to cortisol.
Figure 4.
Systemic rates of appearance of C13 cortisol (derived from ingested 13C cortisone) (A) calculated using Steele's steady-state equation in hydrocortisone (white rectangles) and placebo (black triangles) groups following the ingestion of 1.0 mg of 13C cortisone at time 0. *P = .02 vs placebo group. Hepatic C13 cortisol production (B) in hydrocortisone (shaded bars) and placebo (white bars) groups over 240 minutes following the ingestion of 1.0 mg of 13C cortisone at time 0. *P = .02 vs placebo group.
First-pass hepatic conversion of the ingested 13C cortisone to C13 cortisol was calculated using extraction of ingested D cortisol. Hepatic C13 cortisol production over 240 minutes was also higher in hydrocortisone vs placebo group (0.54 ± 0.07 vs 0.44 ± 0.14 μg/240 minutes; P = .02). This implies that hydrocortisone increases hepatic cortisol production via the hepatic 11β-HSD-1 enzyme pathway.
Glucose infusion rate, plasma tracer to tracee ratio of [6–62H2] glucose, rates of endogenous glucose production, and glucose disappearance
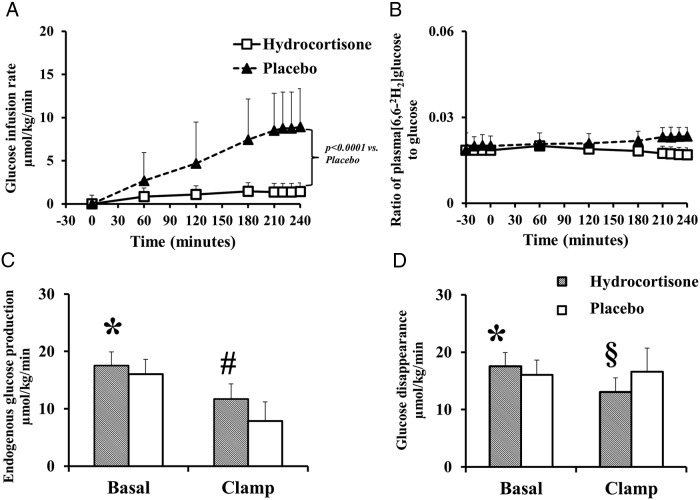
The glucose infusion rate required to maintain euglycemia during the saline clamp was lower in the hydrocortisone vs placebo group (1.38 ± 3.52 vs 8.73 ± 4.29 μmol/kg/min; P < .0001) (Figure 5).
Figure 5.
The glucose infusion rate (A) and plasma [6,62H2] glucose-specific activity (B) in hydrocortisone (white rectangles) and placebo (black triangles) groups. Rates of endogenous glucose production (C) and glucose disappearance (D) in hydrocortisone (shaded bars) and placebo groups (white bars). *P = .04 vs placebo group; #P = .001 vs placebo group; §P = .007 vs placebo group.
Plasma [6–62H2] glucose to glucose ratio remained constant throughout the study in both groups, enabling measurement of glucose fluxes using steady state equations. The glucose turnover calculations were based on data obtained from the last 30 minutes of the clamp.
The rates of endogenous glucose production (EGP) were higher in the hydrocortisone vs placebo group both before (17.53 ± 2.39 vs 15.75 ± 2.02 μmol/kg/min; P = .03) and during the euglycemic clamp (11.69 ± 2.60 vs 7.88 ± 3.31 μmol/kg/min; P = .001) indicating that hydrocortisone perhaps causes hepatic insulin resistance possibly due to increased hepatic conversion of cortisone to cortisol via hepatic 11β-HSD-1 enzyme pathway.
Basal rates of glucose disappearance were higher in the hydrocortisone group as compared to placebo (17.53 ± 2.39 vs 15.75 ± 2.02 μmol/kg/min; P = .03) but lower during the euglycemic clamp (13.04 ± 2.47 vs 16.61 ± 4.13 μmol/kg/min; P = .007) indicating extrahepatic insulin resistance in hydrocortisone vs placebo group.
Discussion
The present data indicate that, compared to placebo, 50 mg twice daily of oral hydrocortisone for 7 days increases hepatic cortisone to cortisol conversion via hepatic 11β-HSD-1 enzyme pathway. This implies that the resultant glucocorticoid excess increases intrahepatic 11β-HSD-1 enzyme activity causing an increase in intrahepatic cortisol concentrations, which may be a possible mechanism to induce or exacerbate glucocorticoid associated hepatic insulin resistance.
Studies have reported increased endogenous glucose production in healthy volunteers following pharmacological doses of glucocorticoids (11, 12, 34), but the mechanism for glucocorticoid-induced hepatic insulin resistance was unknown. It was postulated that glucocorticoids increase endogenous glucose production by stimulating hepatic gluconeogenic enzymes and augmenting supply of substrates to liver for gluconeogenesis by peripheral lipolysis and proteolysis (35, 36). Although it has been reported that insulin decreases 11β-HSD1 mRNA expressions in rat hepatocytes (21), hyperinsulinemia has been reported to increase 11β-HSD1 activity in human adipose tissue (37). Therefore, glucocorticoid induced hyperinsulinemia could account in whole or in part for increase in hepatic conversion of cortisone to cortisol via 11β-HSD1 enzyme pathway. To our knowledge, the present experiments are the first to identify the intrahepatic enzymatic pathway responsible, at least in part, for glucocorticoid-induced hepatic insulin resistance. We also confirm that glucocorticoid use causes peripheral insulin resistance, as observed by lower rates of glucose infusion required to maintain euglycemia and lower rates of glucose disappearance during the clamp in comparison to placebo.
The triple tracer methodology previously established by us (24), coupled with the isotope dilution technique, provides a robust measurement of intrahepatic cortisol metabolism. We have previously established that although intrahepatic cortisol uptake is increased in obese compared to lean nondiabetic individuals, intrahepatic cortisol production does not differ in lean nondiabetic individuals, obese nondiabetic, and obese subjects with type 2 diabetes (24). The data demonstrate that short-term glucocorticoid use can further augment intrahepatic cortisol production by stimulating 11β-HSD-1 activity in the liver, resulting in higher endogenous glucose production when compared to placebo.
The metabolic features of Cushing's syndrome, namely hyperglycemia, hypertension, visceral obesity, and hyperlipidemia, are all thought to be caused by overproduction of cortisol. A recent review has elegantly highlighted the utility of using specific 11β-HSD-1 inhibitors to target cortisol overproduction in the liver without any concerns about inducing adrenocortical insufficiency by inhibiting this enzyme (38). The current experiments provide a mechanism by which glucocorticoid can cause dysregulation of glucose metabolism by further increasing hepatic conversion of cortisone to cortisol, thus aggravating the metabolic effects of circulating glucocorticoids on hepatic glucose metabolism. On the other hand, inhibition of 11β-HSD-1 and therefore local regeneration of cortisol from cortisone in nonhepatic tissues (eg, the immune system) could blunt the anti-inflammatory effect of glucocorticoids. Hence, careful attention will need to be paid to the beneficial vs deleterious effects of tissue-specific 11β-HSD-1 inhibition.
Our study has limitations. We chose to study individuals with a BMI in the range of 19–28 kg/m2 because it was possible that more obese individuals may have generalized insulin resistance, thereby making it difficult to interpret our observations. Subjects were treated with 50 mg of hydrocortisone twice daily for 7 days. We chose 100 mg/d because this equals ∼10 times the daily endogenous cortisol production rate (39, 40) and therefore resulted in substantial glucocorticoid excess. As a result, additional studies will be required to determine if a lesser degree of glucocorticoid excess and states of endogenous cortisol excess (eg, Cushing's syndrome) also stimulates hepatic 11β-HSD-1 activity. We did not measure free fatty acid release from adipose tissue. Therefore increased free fatty acid availability resulting from a glucocorticoid-induced increase in lipolysis also could contribute to hepatic insulin resistance. Furthermore, the relatively short-term (1 week) exposure to high-dose glucocorticoids per design does not permit us to determine the effects of shorter or longer term steroid exposure on this pathway. Studies are necessary to determine the effects of varying duration of steroid exposure on this pathway in humans. Future studies testing a lesser degree of glucocorticoid excess alone or in combination with specific intrahepatic 11β-HSD-1 inhibitors would be of considerable interest to determine whether and if so the extent to which systemic glucocorticoid excess stimulates intrahepatic cortisone to cortisol conversion thereby exacerbating fasting endogenous glucose production.
In summary, the current study provides novel insight that glucocorticoid excess in the liver increases intrahepatic cortisone to cortisol conversion via the 11β-HSD-1 enzyme pathway. This is associated with increased endogenous glucose production in the fasting state. Therefore, liver-specific inhibitors of this enzyme pathway may ameliorate the hepatic insulin resistance that occurs in people who are obese and/or have type 2 diabetes.
Acknowledgments
We are deeply indebted to the research participants. Our sincere thanks to all of the staff of the Mayo Center for Clinical and Translational Science (CCaTS), the Clinical Research Unit (CRU), the CCaTS Immunochemical Core Laboratory, and the CCaTS Metabolomics Core facility (Mai Persson). We thank Barbara Norby (research nurse), Cheryl Shonkwiler (research nurse), Pamela Reich (research assistant), and Betty Dicke (laboratory technician) for technical assistance; Brent McConahey (laboratory technician) for technical assistance and graphic design; and Wendy Siewert (administrative assistant) for assistance with submission of the manuscript.
This study was supported by National Institutes of Health Grant R01 DK29953 and UL1 TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health.
Author Contributions: S.D. assisted in the conduct of the study, data handling, data analyses and manuscript writing, review, and editing. A.B. and R.B. contributed to the study design; conduct of study, data analyses; and manuscript writing, review, and editing. R.A.R contributed to the study design, manuscript review, and editing. M.Q.S assisted in the data analyses, manuscript review, and editing.
Disclosure Summary: R.B. is the guarantor of this work, had full access to all the data, and takes full responsibility for the integrity of data and the accuracy of data analysis. There are no conflicts of interest, financial or otherwise, to declare for any of the authors.
Footnotes
- 11β-HSD-1
- 11β-hydroxysteroid dehydrogenase type 1
- BMI
- body mass index
- 13C cortisone
- [4-13C] cortisone
- D cortisol
- [9,12,12-2H3] cortisol
- 3H cortisol
- [1,2,6,7–3H] cortisol.
References
- 1. Masuzaki H, Paterson J, Shinyama H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. [DOI] [PubMed] [Google Scholar]
- 2. Masuzaki H, Yamamoto H, Kenyon CJ, et al. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest. 2003;112:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kotelevtsev Y, Holmes MC, Burchell A, et al. 11 β-hydroxysteroid dehydrogenase type 1 knockout mice show attenuated glucocorticoid-inducible responses and resist hyperglycemia on obesity or stress. Proc Natl Acad Sci U S A. 1997;94:14924–14929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Harno E, Cottrell EC, Keevil BG, et al. 11-Dehydrocorticosterone causes metabolic syndrome, which is prevented when 11beta-HSD1 is knocked out in livers of male mice. Endocrinology. 2013;154:3599–3609. [DOI] [PubMed] [Google Scholar]
- 5. Morgan SA, McCabe EL, Gathercole LL, et al. 11beta-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc Natl Acad Sci U S A. 2014;111:E2482–E2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paterson JM, Morton NM, Fievet C, et al. Metabolic syndrome without obesity: Hepatic overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in transgenic mice. Proc Natl Acad Sci U S A. 2004;101:7088–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blackburn D, Hux J, Mamdani M. Quantification of the risk of corticosteroid-induced diabetes mellitus among the Elderly. J Gen Intern Med. 2002;17:717–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uzu T, Harada T, Sakaguchi M, et al. Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract. 2007;105:c54–c57. [DOI] [PubMed] [Google Scholar]
- 9. Hoes JN, van der Goes MC, van Raalte DH, et al. Glucose tolerance, insulin sensitivity and beta-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70:1887–1894. [DOI] [PubMed] [Google Scholar]
- 10. Petersons CJ, Mangelsdorf BL, Jenkins AB, et al. Effects of low-dose prednisolone on hepatic and peripheral insulin sensitivity, insulin secretion, and abdominal adiposity in patients with inflammatory rheumatologic disease. Diabetes Care. 2013;36:2822–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rizza R, Mandarino L, Gerich J. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a post-receptor defect of insulin action. J Clin Endocrinol Metab. 1982;54:131–138. [DOI] [PubMed] [Google Scholar]
- 12. van Raalte DH, Brands M, van der Zijl NJ, et al. Low-dose glucocorticoid treatment affects multiple aspects of intermediary metabolism in healthy humans: a randomised controlled trial. Diabetologia. 2011;54:2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dirlewanger M, Schneiter PH, Paquot N, Jequier E, Rey V, Tappy L. Effects of glucocorticoids on hepatic sensitivity to insulin and glucagon in man. Clin Nutr. 2000;19:29–34. [DOI] [PubMed] [Google Scholar]
- 14. Nicod N, Giusti V, Besse C, Tappy L. Metabolic adaptations to dexamethasone-induced insulin resistance in healthy volunteers. Obes Res. 2003;11:625–631. [DOI] [PubMed] [Google Scholar]
- 15. Larsson H, Ahren B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab. 1996;81:4428–4432. [DOI] [PubMed] [Google Scholar]
- 16. Hazlehurst JM, Gathercole LL, Nasiri M, et al. Glucocorticoids fail to cause insulin resistance in human subcutaneous adipose tissue in vivo. J Clin Endocrinol Metab. 2013;98:1631–1640. [DOI] [PubMed] [Google Scholar]
- 17. Geer EB, Islam J, Buettner C. Mechanisms of glucocorticoid-induced insulin resistance: focus on adipose tissue function and lipid metabolism. Endocrinol Metab Clin North Am. 2014;43:75–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zarkovic M, Beleslin B, Ciric J, et al. Glucocorticoid effect on insulin sensitivity: a time frame. J Endocrinol Invest. 2008;31:238–242. [DOI] [PubMed] [Google Scholar]
- 19. van Raalte DH, Diamant M. Steroid diabetes: from mechanism to treatment? Neth J Med. 2014;72:62–72. [PubMed] [Google Scholar]
- 20. Voice MW, Seckl JR, Edwards CRW, Chapman KE. 11 β-hydroxysteroid dehydrogenase type 1 expression in 2S FAZA hepatoma cells is hormonally regulated: a model system for the study of hepatic glucocorticoid metabolism. Biochem J. 1996;317:621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jamieson PM, Chapman KE, Edwards CRW, Seckl JR. 11ß-hydroxysteroid dehydrogenase is an exclusive 11ß-reductase in primary cultures of rat hepatocytes: effect of physicochemical and hormonal manipulations. Endocrinology. 1995;136:4754–4761. [DOI] [PubMed] [Google Scholar]
- 22. Basu R, Singh RJ, Basu A, et al. Obesity and type 2 diabetes do not alter splanchnic cortisol production in humans. J Clin Endocrinol Metab. 2005;90:3919–3926. [DOI] [PubMed] [Google Scholar]
- 23. Basu R, Basu A, Grudzien M, et al. Liver is the site of splanchnic cortisol production in obese non-diabetic humans. Diabetes. 2009;58:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dube S, Norby B, Pattan V, Lingineni RK, Singh RJ, Carter RE, Basu A, Basu R. Hepatic 11beta-hydroxysteroid dehydrogenase type 1 activity in obesity and type 2 diabetes using a novel triple tracer cortisol technique. Diabetologia. 2014;57:1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stimson RH, Andrew R, McAvoy NC, Tripathi D, Hayes PC, Walker BR. Increased whole-body and sustained liver cortisol regeneration by 11beta-hydroxysteroid dehydrogenase type 1 in obese men with type 2 diabetes provides a target for enzyme inhibition. Diabetes. 2011;60:720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baudrand R, Carvajal CA, Riquelme A, et al. Overexpression of 11beta-hydroxysteroid dehydrogenase type 1 in hepatic and visceral adipose tissue is associated with metabolic disorders in morbidly obese patients. Obes Surg. 2010;20:77–83. [DOI] [PubMed] [Google Scholar]
- 27. Torrecilla E, Fernandez-Vazquez G, Vicent D, et al. Liver upregulation of genes involved in cortisol production and action is associated with metabolic syndrome in morbidly obese patients. Obes Surg. 2012;22:478–486. [DOI] [PubMed] [Google Scholar]
- 28. Zinker B, Mika A, Nguyen P, et al. Liver-selective glucocorticoid receptor antagonism decreases glucose production and increases glucose disposal, ameliorating insulin resistance. Metabolism. 2007;56:380–387. [DOI] [PubMed] [Google Scholar]
- 29. Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes. 2005;54:1942–1948. [DOI] [PubMed] [Google Scholar]
- 30. Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography-tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem. 2003;48:1511. [PubMed] [Google Scholar]
- 31. Basu R, Singh RJ, Basu A, et al. Splanchnic cortisol production occurs in humans: evidence for conversion of cortisone to cortisol via the 11-β hydroxysteroid dehydrogenase (11-β HSD) type 1 pathway. Diabetes. 2004;53:2051–2059. [DOI] [PubMed] [Google Scholar]
- 32. Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes. 2000;49:272–283. [DOI] [PubMed] [Google Scholar]
- 33. Andrew R, Westerbacka J, Wahren J, Yki-jarvinen H, Walker BR. The contribution of visceral adipose tissue to splanchnic cortisol production in healthy humans. Diabetes. 2005;54:1364–1370. [DOI] [PubMed] [Google Scholar]
- 34. Wajngot A, Giacca A, Grill V, Vranic M, Efendic S. The diabetogenic effects of glucocorticoids are more pronounced in low- than in high-insulin responders. Proc Natl Acad Sci U S A. 1992;89:6035–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kraus-Friedmann N. Hormonal regulation of hepatic gluconeogenesis. Physiol Rev. 1984;64:170–259. [DOI] [PubMed] [Google Scholar]
- 36. Vander Kooi BT, Onuma H, Oeser JK, et al. The glucose-6-phosphatase catalytic subunit gene promoter contains both positive and negative glucocorticoid response elements. Mol Endocrinol. 2005;19:3001–3022. [DOI] [PubMed] [Google Scholar]
- 37. Wake DJ, Homer NZ, Andrew R, Walker BR. Acute in vivo regulation of 11beta-hydroxysteroid dehydrogenase type 1 activity by insulin and intralipid infusions in humans. J Clin Endocrinol Metab. 2006;91:4682–4688. [DOI] [PubMed] [Google Scholar]
- 38. Stomby A, Andrew R, Walker BR, Olsson T. Tissue-specific dysregulation of cortisol regeneration by 11betaHSD1 in obesity: has it promised too much? Diabetologia. 2014;57:1100–1110. [DOI] [PubMed] [Google Scholar]
- 39. Crown A, Lightman S. Why is the management of glucocorticoid deficiency still controversial: a review of the literature. Clin Endocrinol. 2005;63:483–492. [DOI] [PubMed] [Google Scholar]
- 40. Esteban NV, Yergey AL. Cortisol production rates measured by liquid chromatography/mass spectrometry. Steroids. 1990;55:152–158. [DOI] [PubMed] [Google Scholar]