Abstract
Context:
Without antiresorptive therapy, postmenopausal women lose bone mass after teriparatide (TPTD) discontinuation; estrogen treatment prevents bone loss in this setting. It is not known whether premenopausal women with regular menses lose bone mass after teriparatide discontinuation.
Objective:
This study aimed to test the hypothesis that normally menstruating premenopausal women with idiopathic osteoporosis (IOP) will maintain teriparatide-associated bone mineral density (BMD) gains after medication cessation.
Design:
Twenty-one premenopausal IOP women previously enrolled in an open-label pilot study of teriparatide (20 mcg for 18–24 mo), had substantial BMD increases at the lumbar spine (LS; 10.8 ± 8.3%), total hip (TH; 6.2 ± 5.6%), and femoral neck (7.6 ± 3.4%). For this study, BMD was remeasured 2.0 ± 0.6 years after teriparatide cessation.
Participants:
Fifteen women, who had gained 11.1 ± 7.2% at LS and 6.1 ± 6.5% at TH and were premenopausal at teriparatide completion, were followed without antiresorptive treatment.
Results:
Two years after completing teriparatide, BMD declined by 4.8 ± 4.3% (P = .0007) at the LS. In contrast, BMD remained stable at the femoral neck (−1.5 ± 4.2%) and TH (−1.1 ± 3.7%). Those who sustained LS bone loss >3% (−7.3 ± 2.9%; n = 10), did not differ from those with stable LS BMD (0.1 ± 1.1%; n=5) with regard to baseline body mass index, BMD at any site, or duration of followup, but were significantly older at re-evaluation (46 ± 3 vs 38 ± 7; P = .046), had larger increases in LS BMD during teriparatide treatment and higher cancellous bone remodeling on transiliac biopsy at baseline and completion of teriparatide treatment. Serum bone turnover markers did not differ at baseline or teriparatide completion, but tended to be higher at the re-evaluation timepoint in those with post-teriparatide bone loss.
Conclusions:
These findings lead us to conclude that premenopausal women with IOP, particularly those over 40, may require antiresorptive treatment to prevent bone loss after teriparatide.
Idiopathic osteoporosis (IOP) is an uncommon disorder that affects young, otherwise-healthy individuals with intact gonadal function and no secondary cause of bone loss (1). Using high-resolution peripheral quantitative computed tomography (HR-pQCT) of the distal radius and tibia (2), central QCT of the lumbar spine (LS), and proximal femur (3) and microCT of iliac bone biopsies (obtained in 64 subjects and 40 controls) (4), we have reported that compared with healthy premenopausal controls, premenopausal women with IOP have substantial microstructural deficits at both central and peripheral skeletal sites: thinner cortices; fewer, thinner, more widely separated, and heterogeneously distributed trabeculae; and reduced bone stiffness by finite element analysis (2–4).
To address these structural deficits, in 2008, we initiated an open-label pilot study of teriparatide (TPTD) for premenopausal IOP. The study included 21 women treated for 18–24 months with teriparatide, 20 μg daily. Although response was variable, on average we observed impressive 24-month gains in areal bone mineral density (BMD) (aBMD) by dual energy x-ray absorptiometry (DXA): BMD increased by 10.8 ± 6.4% at the spine, 6.2 ± 5.7% at the total hip (TH), 7.6 ± 3.4% at the femoral neck (FN), and did not change significantly at the distal radius (5). Longitudinal HR-pQCT assessment documented significant increases in radial and tibial volumetric BMD, plate bone volume fraction, and whole bone stiffness (6). Paired transiliac bone biopsies performed before and after treatment in 19 participants revealed improvements in trabecular structure as well as a 71% increase in trabecular stiffness assessed by finite element analysis of microCT images (5).
We hypothesized that BMD would remain stable after teriparatide cessation in these premenopausal women because they had normal gonadal status and no known condition that would predispose to bone loss. This hypothesis was based upon data from prior studies documenting maintainance of teriparatide-associated BMD gains after teriparatide cessation in healthy postmenopausal women on hormone replacement therapy (7), postmenopausal women on glucocorticoids treated with hormone replacement therapy (8), and premenopausal women with return of menses after GnRH analog (and teriparatide) cessation (9). Therefore, after treatment with teriparatide for 18–24 months, the participants were followed without additional antiresorptive treatment.
Materials and Methods
For the original pilot, teriparatide treatment study, premenopausal women, age 20–48 years, with documented low trauma fractures and/or very low spine or hip aBMD by DXA (z score ≤−2.0) were recruited at Columbia University Medical Center, New York, NY and Creighton University, Omaha, NE. Fractures were ascertained by review of radiographs or reports and categorized as low trauma (equivalent to a fall from a standing height or less) by physician panel (E.S., A.C., R.R.R.). All had regular menses off hormonal contraception and early follicular phase FSH levels less than 20 mIU/mL. Women with secondary osteoporosis related to estrogen deficiency, eating disorders, endocrinopathies, celiac or other gastrointestinal diseases, abnormal mineral metabolism, marked hypercalciuria (>300 mg/g creatinine), serum 25-hydroxyvitamin D levels less than 20 ng/mL, and drug exposures were excluded (5). All provided informed consent and the institutional review boards of both institutions approved this study.
At the end of the teriparatide treatment study, two women (ages 48 and 50 y) were perimenopausal and began antiresorptive treatment (oral bisphosphonate) to prevent bone loss. The remaining 19 participants were invited for annual BMD and laboratory followup after teriparatide cessation. Four declined followup or were lost to follow up. Herein we present data from 15 women who had BMD measured 2.0 ± 0.6 years after teriparatide cessation on no bone active therapy other than oral contraceptives (in 5/15 participants).
aBMD
aBMD was measured by DXA (Discovery, Hologic Inc.) at the spine, femur, and radius. Short-term coefficient of variation is 0.7% (spine) and 1.4% (FN) at Columbia and 1.5% (spine and FN) at Creighton. Phantoms were circulated at 6-month intervals. z scores were generated using the manufacturer's sex-specific databases.
Transiliac bone biopsy
Double tetracycline-labeled, transiliac biopsies were obtained from opposite iliac crests before and after 18 months of teriparatide as previously described (5). Biopsy specimens were embedded, sectioned, and stained using established procedures (10). Histomorphometry was performed with a digitizing image-analysis system (OsteoMeasure, Version 4.00C, OsteoMetrics, Inc.). All variables were calculated according to the American Society for Bone and Mineral Research recommendations (11). Bone biopsy was not performed at the follow-up timepoint after teriparatide cessation.
Laboratory testing: Serum bone turnover markers and mineral metabolism
Fasting serum was collected at treatment study visits and stored at −80°C for batch analyses of baseline PTH by immunoradiometric assay (Scantibodies Laboratory, Inc.) and 25 hydroxyvitamin D (25 vitD) by RIA (Diasorin), and baseline and treatment end N-terminal propeptides of procollagen type 1 (P1NP) by RIA (Immunodiagnostic Systems) and C-telopeptide (CTX) by chemiluminescent immunoassay (Immunodiagnostic Systems) in Columbia University's Irving Institute for Clinical and Translational Research Biomarkers Core Laboratory. Additional samples collected at followup were stored at −80°C for batch analysis of CTX and P1NP by the same methods, and anti-Müllerian hormone, a marker of ovarian reserve (12) by ELISA (Beckman Coulter), at a later timepoint.
Statistical analysis
Statistical analyses were performed using SAS software (SAS Institute). We used ANOVA to present BMD change over 18–24 months of treatment in this subset of the original teriparatide treatment study. Paired t tests were used to compare change from teriparitide cessation to the follow-up timepoint. For between-group comparisons, those with LS BMD loss less than 3% were defined to have stable BMD whereas those with LS loss greater than 3% were defined as the BMD loss group. Students t tests were used for between-group comparisons of those with BMD loss vs those with stable BMD. Results for age within groups were not normally distributed, and Wilcoxon rank-sum tests are reported for age as well. All table data are expressed as mean ± SD and graphs show mean ± SE. Correlation analyses were used to describe relationships between bone loss and age or bone remodeling variables. Because not all variables were normally distributed, Spearman correlations were used. Results were considered significant with P < .05.
Results
Fifteen women had BMD measured 2.0 ± 0.6 years after teriparatide cessation while on no specific therapy other than oral contraception. Five women used hormonal contraception (combination oral contraceptives) in the interim period. There were no interim pregnancies. Their baseline characteristics at the time of entry to the treatment study are shown in Table 1. At entry, they ranged in age from 26–46 years. The majority (11/15) were included on the basis of low trauma fracture history; four were included on the basis of very low BMD alone. Bone remodeling rate was variable based on both serum markers and biopsies at baseline (Table 1).
Table 1.
Baseline Characteristics
| Characteristic | At Study Entry, TPTD Initiation ± sd |
|---|---|
| n = 15 | |
| Age, y | 39 ± 6 |
| BMI, kg/m2 | 21.9 ± 3.9 |
| BMD z score | |
| LS | −1.9 ± 0.8 |
| TH | −1.7 ± 0.5 |
| FN | −1.9 ± 0.7 |
| Distal radius | 0.1 ± 0.7 |
| No. of adult fractures | 2.0 ± 1.8 |
| CTXa, ng/mL | 0.319 ± 0.137 |
| P1NPb, μg/L | 48 ± 17 |
| PTHc, pg/mL | 34 ± 13 |
| 25 Vit Dd, ng/mL | 41 ± 18 |
| Bone biopsy: Cancellous | |
| BV/TV, % | 21.3 ± 7.1 |
| Bone formation rate, mm2/mm/y | 0.008 ± 0.007 |
| Mineral apposition rate, μm/d | 0.64 ± 0.13 |
| Mineralized perimeter, % | 3.5 ± 2.8 |
Abbreviations: TPTD, teriparatide.
Normal range, 0–0.700 ng/mL in premenopausal women.
Normal range, 19–83 μg/L in premenopausal women.
Normal range, 14–66 pg/mL.
Normal range > 20–30 ng/mL.
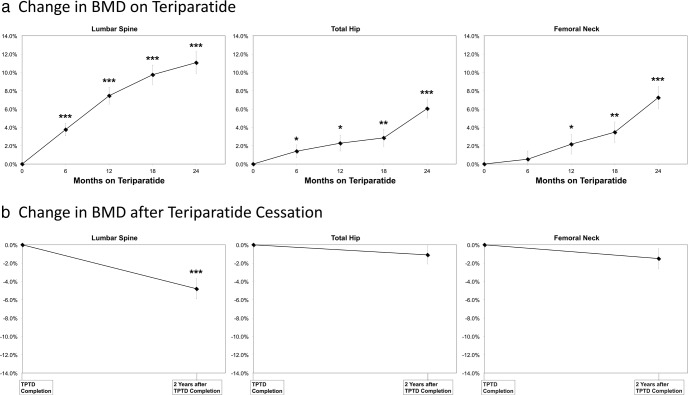
The majority (13/15) were treated with teriparatide for 24 months. Two women chose to stop teriparatide after 18 months. As described in the prior paper (5), substantial, significant increases in BMD were seen at the LS, TH, and FN over the course of treatment (Figure 1A). No significant change was seen at the distal radius (data not shown).
Figure 1.
A, Change in BMD on teriparatide for the 15 women included in this study. B, Change in BMD 2.0 ± 0.6 y after teriparatide cessation. Graphs are shown ± SE. For percent change vs baseline: *, P < .05; **, P < .01; ***, P < .001.
Compared with the time of teriparatide completion, BMD measured at the follow-up timepoint 2.0 ± 0.6 years after teriparatide cessation had declined significantly at the LS (−4.8 ± 4.3%; P = .0007) but remained stable at the FN (−1.5 ± 4.2%), TH (−1.1 ± 3.7%), and 1/3 radius (+0.2 ± 2.5%); Figure 1B.
Two of the fifteen women reported that they became menopausal over the course of the follow-up period. After excluding these two women, the BMD decrease at the spine after teriparatide cessation was lower but still significant (−3.9 ± 3.9%; P = .004).
BMD decline at the LS was quite variable (Figure 2). Ten women had BMD decreases of more than 3% (the least significant change based on densitometer precision), whereas five women had BMD decreases of less than 3%. Thus, the ten with BMD loss more than 3% were compared with the remaining five, considered to have stable BMD (Table 2). Those who sustained significant spine bone loss (mean spine BMD change, −7.3 ± 2.9%; n = 10), did not differ from those with stable spine BMD (mean spine BMD change, 0.1 ± 1.1%; n = 5) with regard to baseline body mass index (BMI), BMD at any site, or duration of followup. Of the five using hormonal contraception, three were in the bone loss group and two in the stable group. Those with spine bone loss tended to be older at baseline and teriparatide completion, and were significantly older at re-evaluation. Using between-group comparisons based on medians for age (Wilcoxon rank sum), those with spine bone loss tended to be older at baseline (P = .07) and were significantly older at teriparatide completion and re-evaluation (both P = .03). Those with spine BMD loss had larger increases in spine BMD during teriparatide treatment and had higher cancellous bone remodeling (mineralized perimeter) on transiliac biopsy at baseline (P = .07) and completion of teriparatide treatment (P < .05). Serum bone turnover markers did not differ at baseline or teriparatide completion. However, at re-evaluation, serum CTX tended to be higher (P = .07) in those with post-teriparatide bone loss. Table 2 also includes results after excluding the two women who became menopausal during the follow-up period (both in the bone loss group). Comparisons of this more limited group to the stable group are shown, and do not substantively change the overall interpretation.
Figure 2.
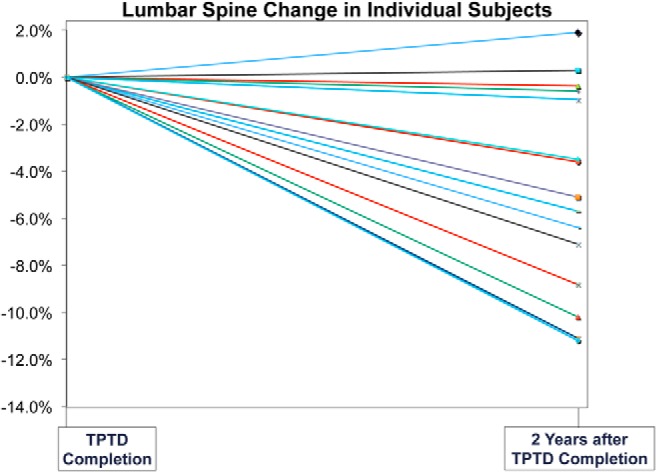
BMD loss at the LS 2.0 ± 0.6 y after teriparatide cessation was quite variable.
Table 2.
Characteristics of Women who Experienced Significant Bone Loss
| BMD Loss (n = 10) | Stable BMD (n = 5) | P | Limited to Subjects Who Remained Premenopausal |
||
|---|---|---|---|---|---|
| BMD Loss (n = 8) | P Versus Stable | ||||
| Before TPTD treatment | |||||
| Age at baseline, y | 40.8 ± 2.8 | 33.8 ± 7.4 | .1 | 40.9 ± 3.0 | .1 |
| BMI, kg/m2 | 22.6 ± 3.9 | 20.3 ± 3.9 | .3 | 21.8 ± 3.5 | .5 |
| BMD z score | |||||
| LS | −1.9 ± 0.8 | −1.8 ± 1.0 | .7 | −1.7 ± 0.7 | .9 |
| TH | −1.9 ± 0.4 | −1.5 ± 0.5 | .1 | −1.9 ± 0.3 | .07 |
| FN | −2.0 ± 0.7 | −1.6 ± 0.6 | .3 | −2.2 ± 0.5 | .1 |
| Cancellous bone remodeling | |||||
| Bone formation rate, mm2/mm/y | 0.010 ± 0.007 | 0.005 ± 0.004 | .1 | 0.010 ± 0.008 | .2 |
| Mineral apposition rate, μm/d | 0.63 ± 0.08 | 0.65 ± 0.2 | .8 | 0.61 ± 0.08 | .7 |
| Mineralized perimeter, % | 4.4 ± 2.9 | 1.7 ± 1.3 | .07 | 4.2 ± 3.2 | .1 |
| Serum bone turnover markers | |||||
| CTX, ng/mL | 0.320 ± 0.121 | 0.317 ± 0.178 | .9 | 0.316 ± 0.138 | .9 |
| P1NP, μg/L | 51.6 ± 16.0 | 41.8 ± 19.3 | .3 | 49.4 ± 13.5 | .4 |
| At TPTD treatment completion | |||||
| Age, y | 43.9 ± 2.8 | 36.0 ± 7.2 | .07 | 43.9 ± 3.2 | .02 |
| BMD, % change from baseline | |||||
| LS | 12.7 ± 6.4 | 5.8 ± 4.2 | .047 | 11.9 ± 6.8 | .1 |
| TH | 6.4 ± 7.2 | 3.7 ± 3.7 | .4 | 3.5 ± 3.1 | .9 |
| FN | 5.7 ± 4.7 | 7.1 ± 6.0 | .6 | 4.6 ± 4.7 | .4 |
| Cancellous bone remodeling | |||||
| Bone formation rate, mm2/mm/y | 0.008 ± 0.006 | 0.002 ± 0.002 | .08 | 0.006 ± 0.003 | .04 |
| Mineral apposition rate, μm/d | 0.61 ± 0.13 | 0.54 ± 0.04 | .4 | 0.60 ± 0.14 | .5 |
| Mineralized perimeter, % | 3.39 ± 2.16 | 0.90 ± 0.80 | .049 | 2.86 ± 1.64 | .05 |
| Serum bone turnover markers | |||||
| CTX, ng/mL | 0.363 ± 0.226 | 0.308 ± 0.204 | .7 | 0.320 ± 0.154 | .9 |
| P1NP, μg/L | 44.5 ± 20.7 | 37.8 ± 10.0 | .5 | 46.1 ± 23.2 | .5 |
| At re-evaluation | |||||
| Age, y | 45.9 ± 3.1 | 37.6 ± 6.7 | .046 | 45.8 ± 3.5 | .01 |
| Time since TPTD completion, y | 2.1 ± 0.5 | 1.7 ± 0.8 | .2 | 2.1 ± 0.6 | .3 |
| BMD, % change after TPTD | |||||
| LSa | −7.3 ± 2.9 | 0.06 ± 1.1 | .0001 | −6.4 ± 2.6 | .0003 |
| TH | −1.2 ± 4.2 | −0.7 ± 1.4 | .8 | 0.02 ± 3.5 | .8 |
| FN | −2.0 ± 4.5 | 0.3 ± 2.9 | .4 | −0.8 ± 3.5 | .6 |
| Serum bone turnover markers | |||||
| CTX, ng/mL | 0.387 ± 0.107 | 0.270 ± 0.107 | .07 | 0.378 ± 0.120 | .1 |
| P1NP, μg/L | 40.1 ± 17.2 | 32.1 ± 11.6 | .4 | 37.3 ± 15.3 | .5 |
Bold indicates statistical significance. Italics indicates trend. TPTD, teriparatide.
Variable used for group determination.
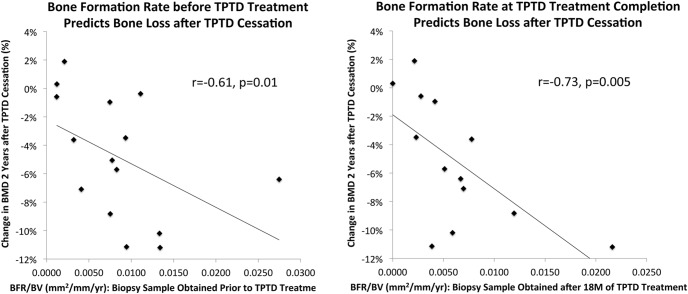
Bone remodeling variables and age were also examined in correlation analyses in the group as a whole. LS BMD percent change at re-evaluation was inversely associated with bone formation rate (r = −0.61; P = .01) and mineralized perimeter (r = −0.69; P = .005) on the baseline biopsy, and even more strongly with bone formation rate (r = −0.73; P = .005) and mineralized perimeter (r = −0.76; P = .003) on the 18-month biopsy (Figure 3). These results were unchanged by exclusion of the two women who became menopausal (bone formation rate, r = −0.71, P = .01; mineralized perimeter, r = −0.75; P = .007). Serum bone turnover markers at baseline and teriparatide completion did not predict post-teriparatide LS bone loss (P = .3-.9). However, correlations with LS BMD percent change were significant or near significant for CTX (r = −0.62; P = .01) and P1NP (−0.46; P = .09) measured at re-evaluation. CTX results were unchanged and P1NP changed only slightly after exclusion of the two women who became menopausal (CTX, r = −0.62; P = .03; P1NP, r = −0.38; P = .2). Older age at both the end of teriparatide treatment and the re-evaluation time point tended to correlate with LS BMD loss (r = 0.4; P = .1 for both). However, at re-evaluation, only two subjects were less than 40 years of age. These two subjects, age 30 and 31 years, had stable BMD at all sites at the post teriparatide re-evaluation time point. In those greater than 40 years, BMD loss was quite variable and not predicted by age (r = −0.12; P = .7).
Figure 3.
LS BMD percent change at re-evaluation was inversely associated with bone formation rate (r = −0.61; P = .01) on the baseline biopsy, and even more strongly with bone formation rate (r = −0.73; P = .005) on the 18-mo biopsy.
We also examined ovarian reserve, estrogen exposure, and proximity to menopause to determine whether these predicted bone loss. Estrogen and gonadotropin levels were not assayed because visits were not timed to the menstrual cycle and some used hormonal contraception. We assessed anti-Müllerian hormone (AMH) levels at the follow-up time point, as an index of ovarian reserve. Although AMH levels correlated well with age (r = −0.84; P = .0002) and were lower in the bone-loss group (0.70 ± 1.1 vs 1.7 ± 2.7 ng/mL), the difference was not statistically significant (P = .5). Results were unchanged by exclusion of the two women who became menopausal. Five women were on combination oral contraceptives containing a wide range of ethinyl estradiol (EE) doses (10–35 mcg/d). Of note, two women, ages 42 and 48 years, who took pills containing 10–20 mcg EE during the follow-up period, were both in the bone-loss group. Three women, ages 40–44 years, took pills containing 30–35 mcg EE; two were in the stable BMD group, whereas one was in the bone-loss group. Most participants continue to report regular menses and no menopausal symptoms at this time, approximately 4 years after teriparatide cessation. Timing of menopause is available in three of the 15 participants who have become menopausal (two before the follow-up time point and one additional patient after this time point). All three were in the bone-loss group. At the follow-up time point, CTX was significantly higher in those who were menopausal or close to menopause (the three women described above compared with the other 12): 0.461 ± 0.067 vs 0.310 ± 0.112 ng/mL; P = .048. Even after excluding all three women who went through menopause within 4 years of completing teriparatide, LS loss was still statistically significant: −3.7 ± 3.9%; P = .009 by paired t test in the group as a whole.
Discussion
By an average of 2 years after completing an 18–24-month course of teriparatide, a majority of premenopausal women with IOP sustained partial loss of LS BMD. In contrast, the gains at the hip were maintained. Those with significant bone loss were older, had had a more robust response to teriparatide, had higher bone remodeling rates on transiliac biopsy, and tended to have higher serum bone turnover markers.
It is well known that postmenopausal women lose bone mass after discontinuing teriparatide or PTH (1–84), and that this bone loss is prevented by antiresorptive therapy. In the PaTH study (13), 119 postmenopausal women treated with PTH (1–84) alone during the first study year were randomly assigned 1:1 to receive alendronate or placebo during year 2. Although those randomly assigned to alendronate gained aBMD (4.9%; P < .001) at the LS in year 2, those randomly assigned to placebo lost 1.7% (P = .002) at this site. In the European Study of Forsteo (EUROFORS) (14), more than 500 postmenopausal women who had received teriparatide for 1 year were randomly assigned to continue teriparatide, raloxifene, or no active treatment. During the second year, LS BMD decreased significantly (−2.7%; 95% confidence interval, −3.6–−1.8%) in the no-active-treatment group, increased significantly in the teriparatide group and did not change significantly in the raloxifene group.
In other studies of postmenopausal women, hormone replacement with estrogen seemed to provide sufficient antiresorptive effect to prevent bone loss after teriparatide cessation. In 27 postmenopausal women continuing established hormone replacement therapy for 1 year after cessation of PTH (1–34), BMD remained stable at both the spine and hip (7). In 28 postmenopausal women on established glucocorticoid treatment and hormone replacement therapy (Premarin, 0.625 mg/d or equivalent), BMD also remained stable or increased further by 1 year after cessation of PTH (1–34) (8). The only prior data on BMD after cessation of PTH treatment in premenopausal women comes from a study of women who had received PTH (1–34) for 6–12 months while also receiving nafarelin to suppress menstrual cycles for the treatment of endometriosis, and had gained bone mass (15). After cessation of nafarelin and PTH (1–34), and resumption of normal menses, a significant increase in spine BMD was documented after 1 year of followup (9).
Based on these data, we hypothesized that normal premenopausal gonadal status would be sufficient to maintain teriparatide-associated BMD gains after teriparatide cessation in the women with IOP who participated in this pilot treatment study. Our results document partial bone loss at the spine and thus do not support this hypothesis. Moreover, we found that BMD loss after teriparatide cessation was quite variable and difficult to predict. Those who lost bone tended to be older, had more robust increases in BMD while on treatment, and had evidence of higher bone turnover on biopsies. However, at the two most useful timepoints for the purpose of clinical decision making (baseline and teriparatide cessation), bone turnover markers did not differ significantly between those who lost bone and those whose BMD remained stable.
Limited data were available regarding ovarian reserve, estrogen exposure, and proximity to menopause. We found that all women who entered menopause during or shortly after the follow-up period were in the bone loss group. However, the majority remained premenopausal and still exhibited significant LS bone loss. AMH levels did not predict bone loss. Two women taking very low-dose (EE, 10–20 mcg/d) oral contraceptives were in the bone-loss group, whereas the bone-loss group varied among women on EE, 30–35 mcg/d. This potential dose effect requires further investigation.
This study has several limitations. The sample size was quite small and limited our ability to define predictors of bone loss. The duration of followup varied among subjects and may have affected the results. Use of hormonal contraceptives varied over the follow-up period, and definitive studies of estrogen status were not available at the final evaluation. Because most women were over 40 years of age at the final evaluation, these results may not be generalizable to women under 35 years of age. No fractures occurred during the follow-up period, and this sample size and study duration do not allow us to analyze the effect of either teriparatide treatment or teriparatide cessation on fracture risk.
These findings lead us to conclude that antiresorptive treatment should be considered for all premenopausal women with IOP who are completing a course of teriparatide treatment, particularly those over age 40 years and those who have had dramatic bone gain while on teriparatide. However, because of this study's small sample size, additional larger-scale prospective studies are needed to make firm conclusions regarding post-teriparatide treatment recommendations. Further investigation is required to define the safety and efficacy, and most beneficial duration of the different antiresorptive treatment options in this clinical situation.
Acknowledgments
This study was registered in ClinicalTrials.gov as trial number NCT01440803.
These treatment and follow-up studies were investigator initiated. The principal investigators designed and conducted these studies.
Eli Lilly supplied teriparatide and provided financial support for the treatment study. These studies were also supported by Grants R01 AR49896 (to E.S.), 2K24 AR052665 (to E.S.), and 1K23 AR054127 (to A.C.); the National Center for Research Resources and the National Center for Advancing Translational Sciences; National Institutes of Health through Grant UL1 RR024156; and the Thomas L. Kempner Jr. and Katheryn C. Patterson Foundation.
Disclosure Summary: E.S. is the Principal Investigator of a grant from Eli Lilly to Columbia University that provided partial funding for this study. R.R.R. and D.W.D. have consulted for Eli Lilly and their total compensation exceeds $5000. D.W.D. has received grant support from Eli Lilly. A.C., M.K.-K., J.M.L., H.Z., S.C., M.B., J.S. have nothing to declare.
Footnotes
- aBMD
- areal bone mineral density
- AMH
- anti-Müllerian hormone
- BMD
- bone mineral density
- BMI
- body mass index
- CTX
- C-telopeptide
- DXA
- dual energy x-ray absorptiometry
- EE
- ethinyl estradiol
- EUROFORS
- European Study of Forsteo
- FN
- femoral neck
- HR-pQCT
- high-resolution peripheral quantitative computed tomography
- IOP
- idiopathic osteoporosis
- LS
- lumbar spine
- P1NP
- N-terminal propeptides of procollagen type 1
- TH
- total hip.
References
- 1. Heshmati HM, Khosla S. Idiopathic osteoporosis: A heterogeneous entity. Ann Med Interne (Paris). 1998;149:77–81. [PubMed] [Google Scholar]
- 2. Cohen A, Liu XS, Stein EM, et al. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2009;94:4351–4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen A, Lang TF, McMahon DJ, et al. Central QCT reveals lower volumetric BMD and stiffness in premenopausal women with idiopathic osteoporosis, regardless of fracture history. J Clin Endocrinol Metab. 2012;97:4244–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen A, Dempster DW, Recker RR, et al. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2011;96:3095–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen A, Stein EM, Recker RR, et al. Teriparatide for idiopathic osteoporosis in premenopausal women: A pilot study. J Clin Endocrinol Metab. 2013;98:1971–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishiyama KK, Cohen A, Young P, et al. Teriparatide increases strength of the peripheral skeleton in premenopausal women with idiopathic osteoporosis: A pilot HR-pQCT study. J Clin Endocrinol Metab. 2014;99:2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cosman F, Nieves J, Woelfert L, et al. Parathyroid hormone added to established hormone therapy: Effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16:925–931. [DOI] [PubMed] [Google Scholar]
- 8. Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Bone mass continues to increase at the hip after parathyroid hormone treatment is discontinued in glucocorticoid-induced osteoporosis: Results of a randomized controlled clinical trial. J Bone Miner Res. 2000;15:944–951. [DOI] [PubMed] [Google Scholar]
- 9. Finkelstein JS, Arnold AL. Increases in bone mineral density after discontinuation of daily human parathyroid hormone and gonadotropin-releasing hormone analog administration in women with endometriosis. J Clin Endocrinol Metab. 1999;84:1214–1219. [DOI] [PubMed] [Google Scholar]
- 10. Dempster DW, Shane E. Bone quantification and dynamics of bone turnover. In: Becker KL, ed. Principles and practice of endocrinology and metabolism. Philadelphia, PA: Lippincott Co, 2002;475–479. [Google Scholar]
- 11. Dempster DW, Compston JE, Drezner MK, et al. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-Müllerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Black DM, Bilezikian JP, Ensrud KE, et al. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. 2005;353:555–565. [DOI] [PubMed] [Google Scholar]
- 14. Eastell R, Nickelsen T, Marin F, et al. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: Final results of the randomized, controlled European Study of Forsteo (EUROFORS). J Bone Miner Res. 2009;24:726–736. [DOI] [PubMed] [Google Scholar]
- 15. Finkelstein JS, Klibanski A, Arnold AL, Toth TL, Hornstein MD, Neer RM. Prevention of estrogen deficiency-related bone loss with human parathyroid hormone-(1–34): A randomized controlled trial. JAMA. 1998;280:1067–1073. [DOI] [PubMed] [Google Scholar]