Abstract
Context:
The Afirma gene expression classifier (GEC) is a molecular diagnostic test that has a high negative predictive value for ruling out malignancy in thyroid nodules with indeterminate cytology. Many patients with a cytologically indeterminate and GEC benign (Cyto-I/GEC-B) nodule undergo monitoring instead of diagnostic surgery, but few data describe their follow-up.
Objective:
The objective of the study was to determine the sonographic changes and clinical outcomes for patients with Cyto-I/GEC-B nodules compared with patients with cytologically benign (Cyto-B) nodules.
Design:
This was a retrospective analysis of consecutive Cyto-I/GEC-B nodules evaluated at Brigham and Women's Hospital compared with Cyto-B nodules.
Main Outcomes:
Nodule growth of 20% or greater in two dimensions or of 50% or greater in volume, change in sonographic features, and rates of repeat fine-needle aspiration, thyroidectomy, and malignancy.
Results:
Ninety-five Cyto-I/GEC-B nodules in 90 patients were identified. Five patients underwent primary surgical resection. Of the remaining 90 nodules, 58 (64.4%) had sonographic follow-up available at a median of 13 months (range 4–40 mo). Cyto-I/GEC-B nodules showed similar growth compared with 1224 Cyto-B nodules using either of the following criteria: 20% or greater in two dimensions (8.6% vs 8.3%, P = .80) or 50% or greater in volume (17.2% vs 13.8%, P = .44). Thyroidectomies were more frequent in the Cyto-I/GEC-B group (13.8% vs 0.9%, P < .0001), but cancer was found in only one patient, with no evidence of persistent disease after initial treatment.
Conclusions:
Cyto-I/GEC-B nodules demonstrate similar growth to Cyto-B nodules during follow-up. Although Cyto-I/GEC-B nodules were more frequently resected, only one malignancy was found. These data suggest that reassessment of Cyto-I/GEC-B nodules may be performed similarly to those with benign cytology.
Thyroid nodules are common and although usually benign, 10%–15% are malignant (1–3). Cytopathological evaluation by ultrasound (US)-guided fine-needle aspiration (FNA) is the principal means to evaluate malignancy risk (4, 5). Unfortunately, FNA yields an indeterminate result in 15%–25% of cases, with an overall cancer risk of 20%–30% in these nodules (6, 7). Surgical resection is often recommended but most prove benign (3, 6–8).
To standardize reporting and eliminate ambiguity, The Bethesda System for Reporting Thyroid Cytology (TBS) replaced the indeterminate category and instead defined and endorsed diagnostic categories of escalating malignancy risk (9). Regardless of category, however, the cancer risk remains such that surgical resection is frequently recommended (4, 5). For patients with benign disease, superfluous surgery carries unnecessary morbidity and cost (10, 11), whereas for those with malignancy, initial diagnostic surgery may be suboptimal (12, 13).
More recently, molecular testing to improve the diagnostic assessment of indeterminate nodules has become commercially available (14–16). The Afirma gene expression classifier (GEC) (Veracyte, Inc) analyzes the mRNA expression of 167 genes in aspiration material and provides a benign or suspicious result to improve preoperative risk stratification (17). In a prospective, multicenter, observational trial, the negative predictive values of the GEC were 95% and 94% for TBS categories of atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) and suspicious for follicular neoplasm/follicular neoplasm (SFN/FN), respectively (18), suggesting a malignancy rate low enough to allow surveillance rather than surgical resection. One subsequent investigation has shown that most patients with cytologically indeterminate-GEC benign (Cyto-I/GEC-B) nodules do not undergo surgery (19), but there are few data on the follow-up of such patients. To verify the appropriateness of conservative management for Cyto-I/GEC-B nodules, it is imperative to assess the outcomes of patients with these nodules.
The purpose of this study is to specifically assess the growth and change in sonographic appearance of Cyto-I/GEC-B nodules over time as well as the frequency of repeated FNA, surgical resection, and histologically proven malignancy (false negatives results). The assumption that these nodules are similar to those with benign cytology (Cyto-B) is tested by comparison with a large population of Cyto-B nodules with similar timing of sonographic follow-up.
Materials and Methods
Data for all adult patients (age ≥ 21 y) with a thyroid nodule 1 cm or greater in greatest dimension and a benign Afirma GEC result between November 23, 2010, and April 1, 2014, at the Brigham and Women's Hospital (BWH) Thyroid Nodule Clinic were retrospectively analyzed. The study period encompassed the earliest clinical use of the Afirma GEC at BWH and allowed 1 year or longer for subsequent US reassessment to be obtained.
The control population was obtained from the prospectively collected database of consecutive patients evaluated at the BWH Thyroid Nodule Clinic (3, 20, 21). Adult patients with a thyroid nodule 1 cm or greater, benign cytology (Cyto-B) at initial aspiration from January 1, 2001, to December 31, 2010, and subsequent sonographic assessment were included. This 10-year range did not overlap with the implementation of Afirma GEC testing to avoid nodule crossover between groups. Sonographic reassessment after 1 year was routinely recommended for Cyto-B nodules, although the exact interval varied. To more closely match the groups and facilitate the most relevant comparison, only Cyto-B nodules with initial US reassessment within the follow-up range of the Cyto-I/GEC-B nodule cohort (4–40 mo) were included. Additionally, because no Cyto-I/GEC-B nodules were predominantly cystic, Cyto-B nodules with a greater than 50% cystic component were excluded.
For all patients in either group, thyroid US evaluation was performed by a radiologist with expertise in thyroid sonography, using a 6- to 15-mHz transducer, to confirm the presence of a clinically relevant nodule. Nodule location and composition as solid or cystic (<25%, 25%–50%, 50%–75%, or >75% cystic) were reported, and size was measured in three dimensions. FNA was performed by a thyroidologist under US guidance, most often using a 25-gauge needle and involving two to four aspirations. All aspirates were processed using a liquid-based cytology preparation (ThinPrep; Hologic). In each case, one or two Thin Prep slides were prepared, with a selected minority of cases (<5%) also including preparation of a cell block. Each specimen was read by a cytopathologist experienced in thyroid cytopathology using TBS. Although many cytological assessments predated this system (circa 2009), the criteria and nomenclature used at BWH over the entire study period was similar to that later defined in TBS.
For nodules with indeterminate cytologies evaluated after the clinical introduction of the Afirma GEC, a separate secondary aspiration was performed and processed for Afirma GEC analysis at the discretion of the treating thyroidologist. For Cyto-I/GEC-B nodules, the timing of sonographic reassessment and clinical decisions regarding continued surveillance, repeat FNA biopsy, or surgical resection were made by the patient and treating thyroidologist.
For all included subjects, demographic, cytological, sonographic, and histopathological data were collected. Significant nodule enlargement was defined as a 20% or greater increase of two or more nodule dimensions or a 50% or greater increase in volume (4, 22), in which the nodule volume was calculated using the rotational ellipsoid formula (length × width × depth × π/6).
Assessment of US characteristics was performed by a single radiologist with expertise in thyroid sonography blinded to clinical and Afirma GEC results. Nodule characteristics evaluated included echogenicity (hyperechoic, isoechoic, hypoechoic, very hypoechoic), shape (round, taller than wide), calcifications (not present, rim, fine punctate), and relative color Doppler blood flow (none, minimal internal flow, peripheral flow, hypervascular internal flow). Suspicious characteristics were defined as hypo- or very hypoechogenicity, taller than wide shape, presence of any calcifications, increasing solid compared with cystic component, or hypervascular internal blood flow. All Cyto-I/GEC-B nodules were included irrespective of their baseline sonographic features and were evaluated for change. The appearance of any new suspicious sonographic feature was sufficient to categorize the nodule as suspicious during follow-up.
For cases that included surgical resection, the final diagnosis was based on histopathological analysis. False negatives were defined as malignant histopathology for the specified Cyto-I/GEC-B nodule. If present, the type and extent of thyroid cancer was recorded and available follow-up data collected to assess the implications of a false-negative result on patient outcome.
Data are presented as median (±range) as appropriate based on the normality test of D'Agostino and Pearson. Analyses were performed using the Mann-Whitney U test and χ2 test for continuous and dichotomous variables, respectively. Furthermore, the comparisons of growth between Cyto-I/GEC-B and Cyto-B nodules were corrected for covariates including age, nodule size, nodule composition, the presence of multinodularity, and duration of follow-up, using logistic regression analysis. Values of P < .05 were considered statistically significant. Analyses were performed with GraphPad Prism version 6.0c for Mac OS X, and SPSS version 22 (IBM) and figures produced using GraphPad Prism and Adobe Photoshop.
Investigational review board permission was granted for this study. This study did not receive financial support, approbation, or review by any commercial entity.
Results
During the study period (November 23, 2010, to April 1, 2014), there were 90 patients with 95 Cyto-I/GEC-B thyroid nodules. All nodules had a cytological diagnosis of either AUS/FLUS or SFN/FN. The rates of AUS/FLUS and SFN/FN cytology at BWH over this time were 9.7% and 2.9%, respectively. The suspicious for Hurthle cell neoplasm rate was 1.6%; however, no Cyto-I/GEC-B nodule had this cytological description. Five patients with Cyto-I/GEC-B nodules proceeded to surgical resection without further evaluation and these were performed due to patient preference (two cases) or cytological findings from a different nodule that necessitated surgical intervention at which time the Cyto-I/GEC-B nodule was also resected (three cases). These five nodules were all histologically benign. In the remaining cases, ultrasound follow-up was available for 58 of the 90 Cyto-I/GEC-B nodules (64.4%) in 56 patients.
For the comparison group, there were 1678 Cyto-B nodules evaluated from 2001 through 2010, of which 1379 had repeat US assessment from 4 to 40 months after initial cytological evaluation. There were 155 Cyto-B nodules with greater than 50% cystic component that were excluded, leaving a final cohort of 1224 nodules in 873 patients.
Patient and nodule data are shown in Table 1. For Cyto-I/GEC-B nodules, the median patient age was 58.4 (21–88) years, 46 of 56 patients (82.1%) were female, the median nodule size was 1.7 cm (1.0–5.3 cm), and the median follow-up was 13 months. These characteristics were similar when compared with the Cyto-B nodule group (Table 1). Multinodularity, defined as more than one nodule measuring 1 cm or greater, was present for 29 of 58 Cyto-I/GEC-B nodules (50%) compared with 806 of 1224 Cyto-B nodules (65.8%) (P = .02). Nodules were described as solid in 35 of 58 Cyto-I/GEC-B nodules (60.4%) compared with 449 of 1224 Cyto-B nodules (36.7%) (P < .001). For Cyto-I/GEC-B nodules, baseline patient and nodule characteristics were similar between those that had available sonographic follow-up and those that did not.
Table 1.
Patient and Nodule Characteristics for Cytologically Indeterminate/GEC Benign and Cytologically Benign Groups
| Cyto-I/GEC-B | Cyto-B | P Value | |
|---|---|---|---|
| Patient characteristics, n | 56 | 873 | |
| Median age (range), y | 58.4 (21–88) | 53.9 (21–95) | .19 |
| Female, n, % | 46 (82.1%) | 780 (89.3%) | .20 |
| Nodule characteristics, n | 58 | 1224 | |
| Median nodule size (range), cm | 1.7 (1.0–5.3) | 1.7 (1.0–8.4) | .50 |
| Multinodularity, n, % | 29/58 (50.0%) | 806/1224 (65.8%) | .02 |
| Composition, n, % | |||
| Solid | 35 (60.4%) | 449 (36.7%) | |
| Complex (<50% cystic) | 23 (39.6%) | 775 (63.3%) | <.001 |
| Cytologic classification, n, % | |||
| AUS/FLUS | 31 (53.5%) | n/a | |
| SFN/FN | 27 (46.5%) | n/a | |
| Nodule follow-up | |||
| Median (range), mo | 13.0 (4–40) | 14.3 (4–40) | .21 |
Abbreviation: n/a, not applicable.
Nodule growth
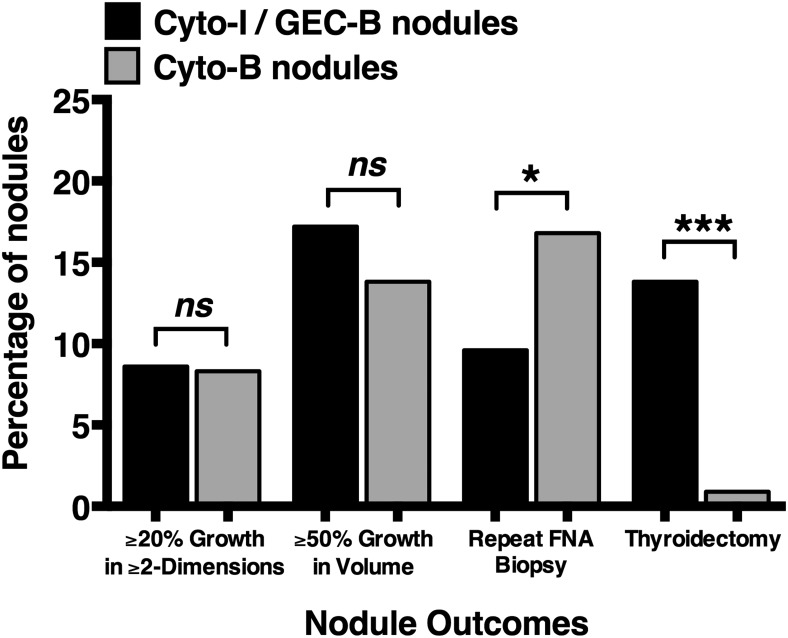
The growth of Cyto-I/GEC-B and Cyto-B nodules is shown in Figure 1. Using the growth criteria of 20% or greater in two or more dimensions, 5 of 58 Cyto-I/GEC-B nodules (8.6%) and 102 of 1224 Cyto-B nodules (8.3%) grew (P = .80). Using a 50% or greater volume increase to define growth, 10 of 58 Cyto-I/GEC-B nodules (17.2%) met this criterion compared with 169 of 1224 Cyto-B nodules (13.8%) (P = .44). When comparing Cyto-I/GEC-B nodule growth by cytological class, 4 of 31 AUS/FLUS nodules and 6 of 27 SFN/FN nodules demonstrated a 50% or greater volume growth during follow-up (P = .49).
Figure 1.
Nodule growth and outcomes for cytologically indeterminate/GEC benign nodules compared to cytologically benign nodules. Bar graphs representing the percentage of nodules meeting prespecified endpoints for 58 cytologically indeterminate and Afirma GEC benign (Cyto-I/GEC-B) nodules (black bars) compared with 1224 cytologically benign (Cyto-B) nodules (gray bars). Nodule growth of 20% or greater in two or more nodule dimensions and 50% or greater in nodule volume for these two groups was 8.6% vs 8.3% (P = .80) and 17.2% vs 13.8% (P = .44), respectively. Repeat assessment by FNA biopsy and thyroidectomy, for Cyto-I/GEC-B and Cyto-B nodule groups, occurred in 6.9% vs 16.8% (P = .05) and 13.8% vs 0.9% (P < .0001), respectively. Volume was defined as by ellipsoid formula (volume = length × width × depth × π/6). ns, not significant. *, P < .05; ***, P < .0001.
The comparison of nodule growth was further analyzed using a logistic regression analysis to adjust for differences between the groups. After adjusting for multinodularity, cystic content, and duration of follow-up, Cyto-I/GEC-B nodules showed a similar risk of growth compared with Cyto-B nodules using either 20% or greater in two or more dimensions (odds ratio 1.17 [0.45–3.10], P = .75) or 50% or greater volume (odds ratio 1.37 [0.67–2.82], P = .39). When extending this analysis to include additional covariates of age, sex, and initial nodule size, there remained no difference in growth risk between groups (data not shown).
Sonographic characteristics
There was no development of suspicious sonographic nodule features for 53 of the 58 Cyto-I/GEC-B nodules (91.4%). Three nodules were described as taller than wide at follow-up assessment, of which two were resected and were both histologically benign. Two additional nodules initially described as isoechoic were later described as hypoechoic. These nodules did not show the concurrent appearance of other suspicious features or interval growth. No nodules demonstrated new calcifications during monitoring. The technical settings for the color Doppler flow assessment frequently varied between US studies, precluding the evaluation of this parameter.
Repeat FNAs, thyroidectomies, and thyroid cancer diagnoses
To evaluate further the clinical course of Cyto-I/GEC-B nodules, occurrences of repeat FNA, thyroidectomy, and thyroid cancer were assessed. Repeat FNA biopsy was performed in four patients (6.9%) from 6 to 37 months after Afirma GEC assessment. In two cases, repeat cytology was benign, whereas in the other two cases, cytology remained indeterminate (SFN/FN for both). There were 206 repeat FNA assessments in the 1224 Cyto-B nodules (16.8%), (P < .05 compared with Cyto-I/GEC-B nodules; Figure 1). The cytological results from the repeated FNA biopsy of Cyto-B nodules were again benign for 190 of 206 nodules. In the remaining nodules, the cytological findings were nondiagnostic (n = 5), atypical (n = 6), suspicious for follicular neoplasm (n = 2), suspicious for papillary thyroid cancer (n = 2), and positive for papillary thyroid carcinoma (n = 1).
During follow-up, surgical resection was performed for 8 of 58 Cyto-I/GEC-B nodules (13.8%) (Table 2), at a median of 9 months (6–19 mo) post-Afirma GEC result. Thyroid malignancy was identified in one case (patient 8). This was a 3.1-cm minimally invasive follicular thyroid carcinoma (FTC) resected after sonographic assessment revealed an increase in maximal diameter from 3.0 to 3.5 cm (volume increase of 46%). Pathological evaluation showed a focal area of capsular invasion without extrathyroidal extension, lymphovascular invasion, or involved margins. Postoperative, post-131I therapy scanning was negative for iodine-avid metastases, and thyroglobulin was undetectable with a negative thyroglobulin antibody assessment 6 months postoperatively.
Table 2.
Characteristics and Histopathological Diagnosis of Cytologically Indeterminate/GEC Benign Nodules That Underwent Resection
| n | Age, y | Sex | Nodule Size (Initial), cm | Cytology | Time to Resection, mo | Nodule Size (final), cm | Reasons for Resection | Histopathology |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | 2.2 | AUS | 0 | n/a | Patient decision | Follicular adenoma |
| 2 | 56 | F | 1.7 | AUS | 0 | n/a | Second nodule requiring surgery | Nodular hyperplasia |
| 3 | 54 | F | 1.3 | AUS | 0 | n/a | Second nodule requiring surgery | Adenomatous nodule |
| 4 | 77 | F | 1.1 | SFN | 0 | n/a | Second nodule requiring surgery | Adenomatous nodule |
| 5 | 45 | M | 2.0 | SFN | 0 | n/a | History of EBRT; patient decision | Adenomatous nodule |
| 6 | 42 | F | 1.4 | AUS | 7 | 1.7 | History of EBRT with FDG-PET avidity | Adenomatous nodule |
| 7 | 58 | F | 2.7 | AUS | 12 | 2.9 | History of EBRT with nodule hypervascularity | Follicular adenoma |
| 8 | 52 | F | 3.0 | SFN | 14 | 3.5 | Nodule growth | Minimally invasive FTC |
| 9 | 21 | F | 2.2 | AUS | 7 | 2.8 | Nodule growth; repeat FNA benign | Nodular hyperplasia |
| 10 | 31 | F | 4.3 | SFN | 8 | 4.6 | Nodule growth | Nodular hyperplasia |
| 11 | 57 | M | 2.0 | AUS | 6 | 2.3 | Nodule growth; repeat FNA benign | Nodular hyperplasia |
| 12 | 59 | F | 2.7 | AUS | 9 | 2.9 | Nodule growth; repeat FNA SFN | Follicular adenoma |
| 13 | 42 | F | 2.3 | SFN | 19 | 2.6 | Nodule growth | Follicular adenoma |
Abbreviations: AUS, aytpia of undetermined significance; EBRT, external beam radiation treatment; FDG-PET, fluorodeoxyglucose positron emission tomography; F, female; M, male; n/a, not applicable; SFN, suspicious for follicular neoplasm.
Including the five Cyto-I/GEC-B nodules removed as initial management, thyroid malignancy was identified in 1 of 13 Cyto-I/GEC-B nodules (7.7%). During a similar follow-up interval, thyroidectomy was performed for only 12 of 873 patients with Cyto-B nodules (1.4%) (P < .0001; Figure 1). In this group, indications for surgery were abnormal repeat cytology (n = 7), compressive neck symptoms (n = 2), and nodule growth (n = 3). Of these 12, four (33%) were malignant (P = .16 compared with Cyto-I/GEC-B group). Eighty of the 90 patients with a Cyto-I/GEC-B nodule (89%) are still followed at our institution with no mortalities observed during up to 4 years of follow-up.
Discussion
The management of thyroid nodules with indeterminate cytology is rapidly evolving as the use of molecular markers to improve preoperative risk stratification gains wider use. For nodules with AUS/FLUS and SFN/FN cytologies, a benign Afirma GEC is suggested to have a sufficiently low malignancy risk to allow nonoperative monitoring (18, 19, 23). Data describing these nodules during observation, however, are lacking.
This study is the first to provide a comprehensive description of the growth and sonographic characteristics of Cyto-I/GEC-B nodules during follow-up. Previous reports assessing the outcomes of Afirma GEC-tested nodules have focused little on those with a benign result (19, 24–28). One previous study followed 71 AFirma GEC benign nodules, but the median follow-up was only 8.5 months and evaluation of nodule growth and sonographic features was not included (19).
In the current study, significant growth during surveillance was assessed by two widely accepted criteria: 20% or greater change in two or more dimensions or 50% or greater change in volume (4, 22). When comparing Cyto-I/GEC-B nodules with a control group of Cyto-B nodules over a similar follow-up duration, there was no observed difference in the proportion of nodules demonstrating growth. Multinodularity and cystic contents, previously shown to influence nodule growth (22, 29), were dissimilar between these groups, but a logistic regression analysis adjusting for these factors again indicated that the risk of nodule growth was similar.
The proportion of Cyto-I/GEC-B nodules with growth in this study is similar to previous studies evaluating growth of benign nodules. We observed that 8.6% of Cyto-I/GEC-B nodules grew 20% or greater in two or more dimensions, which is comparable with the 11.1% rate found for 1567 cytologically or sonographically benign nodules in a 5-year prospective study (22). Over that 5-year period, the average nodule growth was 4.9 mm (∼1 mm/y), which suggests a rate of growth that may be hard to assess after only 1 year of follow-up. A 50% or greater volume increase was found in 17.2% of Cyto-I/GEC-B nodules in this study, similar to the rate of 16.6% found in 249 benign nodules evaluated retrospectively, with the majority meeting this criterion within 36 months (30).
Repeat FNA cytology was performed in 6.9% of Cyto-I/GEC-B nodules but did not provide information to change clinical management in any case. Whereas more patients with Cyto-B nodules underwent repeat FNA, fewer had thyroidectomy performed during this period of follow-up. These differences likely convey the relatively low threshold for recommending thyroidectomy for patients with Cyto-I/GEC-B nodules, given the elevated concern for malignancy based on prior abnormal cytological results, whereas repeat FNA was preferred for those with previously benign cytology.
Of the 13 resected Cyto-I/GEC-B nodules, one false-negative result was found. The presence of thyroid cancer in Afirma GEC benign nodules that underwent resection was previously reported in two of five cases (both papillary cancer) by Harrell and Bimston (24) and one of four cases (an FTC exhibiting focal capsular and vascular invasion) by McIver et al (25). In contrast, no malignancies were found in the studies by Lastra et al (26), Marti et al (27), and Brauner et al (28), although these studies included very few resected Afirma GEC benign nodules. Together these studies suggest a small but persistent concern for false-negative Afirma GEC results. None of these studies, however, include further data on cancer treatment or outcome for these patients. In our study, complete cancer resection, 131I scanning without evident metastases, and an undetectable thyroglobulin are consistent with an excellent response and suggest that the potential delay in diagnosis was not harmful to this patient.
We recognize several limitations to the present study. The assessment of nodule growth over the relatively short follow-up time of 1 year is limited, given the slow growth of most benign nodules (22). However, because a common interval of follow-up for benign thyroid nodules is 6–18 months, our data report the change in Afirma GEC benign nodules during a clinically relevant monitoring period. The sample size in this study is small and encompasses 64% of the Cyto-I/GEC-B nodules not referred for initial surgery, introducing the possibility of selection bias. There may have been a less rigorous effort to follow up patients whose Cyto-I/GEC-B nodules were smaller or of lower risk, leading to an overestimation of risk in the study population. It is unlikely that patients at higher risk for a malignant nodule based on clinical or ultrasound findings were disproportionately excluded from follow-up at our institution.
The Cyto-B nodule control group was a retrospective cohort that is subject to potential confounding or bias, but this effect was likely limited because all patients were evaluated by the same physicians at the same institution, and most characteristics were similar between groups. Furthermore, subsequent correction for potential differences using logistic regression did not change our results. We were unable to evaluate the degree to which nodule growth was due to an increase in cystic component in Cyto-B nodules. Isolated cystic enlargement was likely rare, given that all nodules were predominantly solid, but we cannot exclude this possibility in some instances. We also did not evaluate every possible suspicious sonographic characteristic. Although suspicious features may predict thyroid malignancy despite a lack of nodule growth (31, 32), there is suboptimal reproducibility (33) and their usefulness has recently been questioned (34, 35). We attempted to mitigate these by focusing on higher-risk features (36) and using a single blinded evaluator. Similar sonographic review of all Cyto-B nodules was not feasible, but given the low rate of new suspicious features in the Cyto-I/GEC-B group, a statistical difference between groups is unlikely.
In conclusion, Cyto-I/GEC-B and Cyto-B nodules show similar growth during follow-up, suggesting comparable clinical behavior. Only one malignancy was detected, for which an excellent response was achieved despite any potential delay in diagnosis. These data provide a strong indication that treating Cyto-I/GEC-B nodules similarly to those with benign cytology is appropriate.
Acknowledgments
This research was supported by the National Institutes of Health T32 DK007529 training grant.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUS/FLUS
- atypia of undetermined significance/follicular lesion of undetermined significance
- BWH
- Brigham and Women's Hospital
- Cyto-B
- cytologically indeterminate benign cytology
- Cyto-I/GEC-B
- cytologically indeterminate-GEC benign
- FNA
- fine-needle aspiration
- FTC
- follicular thyroid carcinoma
- GEC
- gene expression classifier
- SFN/FN
- suspicious for follicular neoplasm/follicular neoplasm
- TBS
- Bethesda System for Reporting Thyroid Cytology
- US
- ultrasound.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. [DOI] [PubMed] [Google Scholar]
- 2. Popoveniuc G, Jonklaas J. Thyroid nodules. Med Clin North Am. 2012;96:329–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–516. [DOI] [PubMed] [Google Scholar]
- 4. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 5. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Invest. 2010;33:51–56. [PubMed] [Google Scholar]
- 6. Baloch ZW, Livolsi VA. Follicular-patterned lesions of the thyroid: the bane of the pathologist. Am J Clin Pathol. 2002;117:143–150. [DOI] [PubMed] [Google Scholar]
- 7. Nayar R, Ivanovic M. The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer. 2009;117:195–202. [DOI] [PubMed] [Google Scholar]
- 8. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol. 2012;56:333–339. [DOI] [PubMed] [Google Scholar]
- 9. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19:1159–1165. [DOI] [PubMed] [Google Scholar]
- 10. Sosa JA, Bowman HM, Tielsch JM, Powe NR, Gordon TA, Udelsman R. The importance of surgeon experience for clinical and economic outcomes from thyroidectomy. Ann Surg. 1998;228:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergenfelz A, Jansson S, Kristoffersson A, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393:667–673. [DOI] [PubMed] [Google Scholar]
- 12. Esnaola NF, Cantor SB, Sherman SI, Lee JE, Evans DB. Optimal treatment strategy in patients with papillary thyroid cancer: a decision analysis. Surgery. 2001;130:921–930. [DOI] [PubMed] [Google Scholar]
- 13. Hundahl SA, Cady B, Cunningham MP, et al. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996: an American College of Surgeons Commission on Cancer Patient Care Evaluation Study. Cancer. 2000;89:202–217. [DOI] [PubMed] [Google Scholar]
- 14. Bernet V, Hupart KH, Parangi S, Woeber KA. AACE/ACE Disease State Commentary: molecular diagnostic testing of thyroid nodules with indeterminate cytopathology. Endocr Pract. 2014;20:360–363. [DOI] [PubMed] [Google Scholar]
- 15. Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. [DOI] [PubMed] [Google Scholar]
- 16. Kim MI, Alexander EK. Diagnostic use of molecular markers in the evaluation of thyroid nodules. Endocr Pract. 2012;18:796–802. [DOI] [PubMed] [Google Scholar]
- 17. Chudova D, Wilde JI, Wang ET, et al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J Clin Endocrinol Metab. 2010;95:5296–5304. [DOI] [PubMed] [Google Scholar]
- 18. Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–715. [DOI] [PubMed] [Google Scholar]
- 19. Alexander EK, Schorr M, Klopper J, et al. Multicenter clinical experience with the Afirma gene expression classifier. J Clin Endocrinol Metab. 2014;99:119–125. [DOI] [PubMed] [Google Scholar]
- 20. Nou E, Kwong N, Alexander LK, Cibas ES, Marqusee E, Alexander EK. Determination of the optimal time interval for repeat evaluation after a benign thyroid nodule aspiration. J Clin Endocrinol Metab. 2014;99:510–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamran SC, Marqusee E, Kim MI, et al. Thyroid nodule size and prediction of cancer. J Clin Endocrinol Metab. 2013;98:564–570. [DOI] [PubMed] [Google Scholar]
- 22. Durante C, Costante G, Lucisano G, et al. The natural history of benign thyroid nodules. JAMA. 2015;313:926–935. [DOI] [PubMed] [Google Scholar]
- 23. Angell TE, Alexander EK. Validation and multicenter experience of the Afirma gene expression classifier. US Endocrinology. 2014;10:117–119. [DOI] [PubMed] [Google Scholar]
- 24. Harrell RM, Bimston DN. Surgical utility of Afirma: effects of high cancer prevalence and oncocytic cell types in patients with indeterminate thyroid cytology. Endocr Pract. 2014;20:364–369. [DOI] [PubMed] [Google Scholar]
- 25. McIver B, Castro MR, Morris JC, et al. An independent study of a gene expression classifier (Afirma™) in the evaluation of cytologically indeterminate thyroid nodules. J Clin Endocrinol Metab. 2014;99:4069–4077. [DOI] [PubMed] [Google Scholar]
- 26. Lastra RR, Pramick MR, Crammer CJ, LiVolsi VA, Baloch ZW. Implications of a suspicious afirma test result in thyroid fine-needle aspiration cytology: an institutional experience. Cancer Cytopathol. 2014;122:737–744. [DOI] [PubMed] [Google Scholar]
- 27. Marti JL, Avadhani V, Donatelli LA, et al. Wide inter-institutional variation in performance of a molecular classifier for indeterminate thyroid nodules. Ann Surg Oncol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brauner E, Holmes BJ, Krane JF, et al. Performance of the Afirma gene expression classifier in Hürthle cell thyroid nodules differs from other indeterminate thyroid nodules. Thyroid. 2015;25:789–796. [DOI] [PubMed] [Google Scholar]
- 29. Alexander EK, Hurwitz S, Heering JP, et al. Natural history of benign solid and cystic thyroid nodules. Ann Intern Med. 2003;138:315–318. [DOI] [PubMed] [Google Scholar]
- 30. Negro R. What happens in a 5-year follow-up of benign thyroid nodules. J Thyroid Res. 2014;2014:459791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kwak JY, Koo H, Youk JH, et al. Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology. 2010;254:292–300. [DOI] [PubMed] [Google Scholar]
- 32. Rosario PW, Purisch S. Ultrasonographic characteristics as a criterion for repeat cytology in benign thyroid nodules. Arq Bras Endocrinol Metab. 2010;54:52–55. [DOI] [PubMed] [Google Scholar]
- 33. Park SH, Kim SJ, Kim EK, Kim MJ, Son EJ, Kwak JY. Interobserver agreement in assessing the sonographic and elastographic features of malignant thyroid nodules. AJR Am J Roentgenol. 2009;193:W416–W423. [DOI] [PubMed] [Google Scholar]
- 34. Brito JP, Gionfriddo MR, Al Nofal A, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Samulski TD, Shutty C, LiVolsi VA, Montone K, Baloch Z. The reliability of thyroid nodule ultrasound features and size to predict malignancy in fine needle aspiration specimens: practical utility for the evaluating pathologist. Diagn Cytopathol. 2015;43:471–477. [DOI] [PubMed] [Google Scholar]
- 36. Remonti LR, Kramer CK, Leitão CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. 2015;25(5):538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]