Abstract
Context and Objective:
Effects of prenatal exposure to bisphenol A (BPA) on gestational and birth outcomes are controversial. The aim of the study was to evaluate the relationship between prenatal exposure to BPA and birth and gestational outcomes.
Design, Setting, Participants, and Outcome:
Levels of unconjugated (uBPA) and BPA glucuronide in 80 matching samples of pregnant women during the first trimester of pregnancy and at delivery and matching term cord blood obtained from a prospective study conducted at the University of Michigan Hospitals were determined using a methodology validated in the National Institutes of Environmental Health Sciences funded Round Robin study and related to pregnancy outcomes.
Results:
Highest levels of uBPA were found in maternal term samples followed by first trimester maternal (M1) samples and cord blood. A 2-fold increase in M1 uBPA was associated with 55-g less birth weight when male and female pregnancies were combined and 183-g less birth weight with only female pregnancies. A 2-fold increase in maternal term uBPA was associated with an increased gestational length of 0.7 days for all pregnancies and 1.1 days for only female pregnancies.
Conclusion:
Higher uBPA exposure levels during first trimester and term are associated with sex-specific reduction in birth weight and increase in gestational length, respectively. Race, parity, and employment have an effect on BPA exposure. Because low birth weight is associated with adverse health outcomes, effect of early pregnancy BPA levels on reducing birth weight highlights the risk posed by developmental exposure to BPA.
Bisphenol A (BPA), an endocrine-disrupting chemical, is used in the manufacturing of polycarbonate plastics and epoxy resins and commonly found in consumer products such as water bottles, medical equipment, consumer electronics, lining of food cans, cosmetics, and dental sealants. BPA is so widespread that it is detected in drinking water and indoor dust (1). The ubiquity of BPA is also evident from biomonitoring studies that document the presence of BPA in urine in greater than 90% of subjects studied (2) and circulation (3, 4). This is of concern given that BPA has estrogenic, antiandrogenic, and antithyroid activities and can disrupt insulin homeostasis (5).
Developmental exposure to BPA leads to later life metabolic and reproductive dysfunctions in animal models (5, 6). Epidemiological studies also point to an association between BPA exposure and metabolic syndrome, obesity, and diabetes in adults (7, 8). BPA is found in maternal circulation, amniotic fluid, and umbilical cord samples (9), suggesting the exposure of the developing fetus to BPA. It is critical to assess internal exposure levels of BPA in maternal and fetal circulation in humans for determining translatable relevance for studies in animal models. Accurate internal measures of BPA is faced with challenges that relate to the efficiency and specificity of the detection methods and the sample collection system (1). In addition, considerable concerns exist relative to contamination from external sources during sample collection and preparation (10). A National Institutes of Environmental Health Sciences (NIEHS) –funded Round Robin study validated the reliability of BPA measurement across several laboratories (11) with only one study addressing the internal exposure levels of BPA during gestation (3) by one of the participating laboratories being published since completion of the Round Robin study.
Because critical periods of developmental susceptibility to insults vary depending on the organ system being studied and it is unclear how much BPA is transferred from mother to fetus, it is important to gain an understanding of BPA exposure throughout gestation to establish the relationship between maternal and fetal exposure levels and to accurately assess developmental exposure to BPA. This is the first longitudinal study that addresses the exposure risk to unconjugated BPA (uBPA) in the first trimester, term-matched maternal samples, and matched term cord blood samples that relates BPA exposure levels during two different windows of exposure to pregnancy outcomes.
Materials and Methods
Validation study
To address the potential release of BPA from the iv catheterization, jugular veins from three adult female sheep were aseptically catheterized (BD Angiocath, Beckton Dickinson) and connected to an iv infusion set. Ringer lactate solution was administered at 25 mL/h for 48 hours. Plasma samples were collected and processed using a complete plastic-free sample collection procedure before and 48 hours after the infusion set was placed.
Population studies
Study population and sample collection
The study was comprised of two phases.
Phase 1 (pre-Round Robin study)
Following institutional review board approval, first trimester (pregnancy wk 8–14) maternal (n = 61) and maternal and umbilical cord samples at time of delivery were collected at the University of Michigan Von Voigtlander Women's Hospital (n = 61). All samples were processed avoiding any contact with plastic and stored in glass vials.
Phase 2 (post-Round Robin study)
Following institutional review board approval and written informed consent, women were recruited at the University of Michigan Von Voigtlander Women's Hospital. First trimester maternal (M1) samples (pregnancy wk 8–14) and maternal (MT) and umbilical cord (UC) samples at the time of delivery were collected (n = 80). Women were contacted during their first trimester prenatal clinic visit and were included in the study if they fit the inclusion criteria: 18 years or older, natural conception, and singleton pregnancy. Supplemental Table 1 summarizes details of samples collected and nature of BPA measurements undertaken for both phases.
BPA analyses
All BPA samples were measured in one of the laboratories that participated in the Round Robin study. uBPA and total BPA (tBPA) from validation and phase 1 studies were measured prior to the Round Robin study. For phase 2 samples, uBPA and BPA glucuronide (gBPA) were measured using methods validated in the Round Robin study.
Validation and phase 1 BPA analyses
Measurement details of uBPA and tBPA in phase 1 have been described previously (4). In brief, free and tBPA (after digestion with β-glucuronidase) levels in plasma were quantified using Agilent 1100 series HPLC interfaced with an Applied Biosystems API 2000 electrospray tandem mass spectroscopy (MS/MS). The recovery of spiked d16 BPA was 81 ± 19%; the limit of BPA detection (LOD) was 0.05 ng/mL, which was calculated as twice that of the valid lowest acceptable calibration standard.
Phase 2 BPA analyses
Plasma samples were extracted following methods described elsewhere, with some modifications (12). Briefly, after thawing, plasma (0.5 mL) was transferred to a 15-mL glass tube, and internal standards (d6-BPA and 13C12-gBPA), ammonium acetate buffer, formic acid, and milli-Q water were added to a total volume of 3 mL. An Oasis MCX cartridge (60 mg/3 cc; Waters) preconditioned with methanol and water was used for extraction and clean-up. After loading the sample, the cartridge was washed with 15% methanol and then eluted with methanol. The eluate was concentrated to 0.5 mL. uBPA and gBPA levels were quantified using an Agilent 1100 series HPLC interfaced with an Applied Biosystems API 5500 electrospray MS/MS (Applied Biosystems). Then, 10 μL of the extract was injected onto an analytical column (Betasil C18, 100 × 2.1 mm column; Thermo Electron Corporation) connected to a Javelin guard column (Betasil C18, 20 × 2.1 mm). The mobile phase comprised of methanol and 10mM ammonium acetate in water. The MS/MS was operated in the electrospray negative ion mode. Data were acquired using multiple reaction monitoring for the transitions of 227 > 212 for BPA, 233 > 215 for d6-BPA, 403 > 113 for gBPA, and 415 > 113 for 13C12-gBPA.
Quality control parameters include method validation by spiking internal standards into the sample matrices and passing through the entire analytical procedure to calculate recoveries of target analytes. A procedural blank was analyzed with the samples. LOD was 0.02 ng/mL for uBPA and gBPA. The recovery of d6-BPA and 13C12-gBPA spiked into samples was 95 ± 29% (mean ± SD) and 103 ± 37%, respectively. Reported concentrations were corrected for the recoveries of the surrogate standard. The native standards (made available through NIEHS) spiked into procedural blank and selected sample matrices and passed through the entire analytical procedure yielded recoveries of 105 ± 5% and 102 ± 7% for BPA, and 107 ± 19% and 95 ± 20% for gBPA. An external calibration curve was prepared by injecting 10 μL of 0.01–100 ng/mL standards, and the calibration coefficient was > 0.99.
Statistical analysis
Phase 1 and validation study
Values below the LOD were replaced with a value (0.035 ng/mL) equal to the LOD divided by the square root of 2, as previously described (13). Paired t test was used to analyze BPA levels before and after iv infusion of ringer lactate. ANOVA was used to compare uBPA and tBPA across time points, and Pearson correlation assessed the association between uBPA and tBPA levels in maternal and umbilical cord samples.
Phase 2
Values below the LOD were replaced with 0.014 ng/mL, equal to the LOD divided by the square root of 2. Spearman correlation coefficients were calculated to study the correlation between pairs of BPA measurements among sample series. ANOVA with repeated measures was used to study differences in BPA levels and subject characteristics between all uBPA and gBPA measures across the three sample series. To determine whether subject characteristics affect the pattern of uBPA and gBPA, these variables were added as covariates, and interactions terms between these characteristics and type of measurements were tested. Multiple linear regression analysis was used to study BPA effect on birth weight, maternal body mass index (BMI) change, and gestational length, adjusting for potential confounders with and without stratification by newborn sex. All BPA measures were log transformed prior to the regression analyses. ANOVA and Kruskal-Wallis analyses followed by appropriate post-hoc test with Tukey adjustment was used to test association between demographic variables and gestation-related variables. Kernal density estimation with Gaussian kernel was used to compare BPA measures and generate distribution plots.
For all analyses Predictive Analysis Software Statistics for Windows release 18.0.1 and SAS for Windows release 9.1.3 (SAS Institute) were used, and appropriate transformations were applied with significance defined as P < .05.
Results
Validation study
Circulating levels of uBPA and tBPA measured before and after the iv infusion with ringer lactate for 48 hours were not significantly different (Supplemental Figure 1).
Phase 1 studies
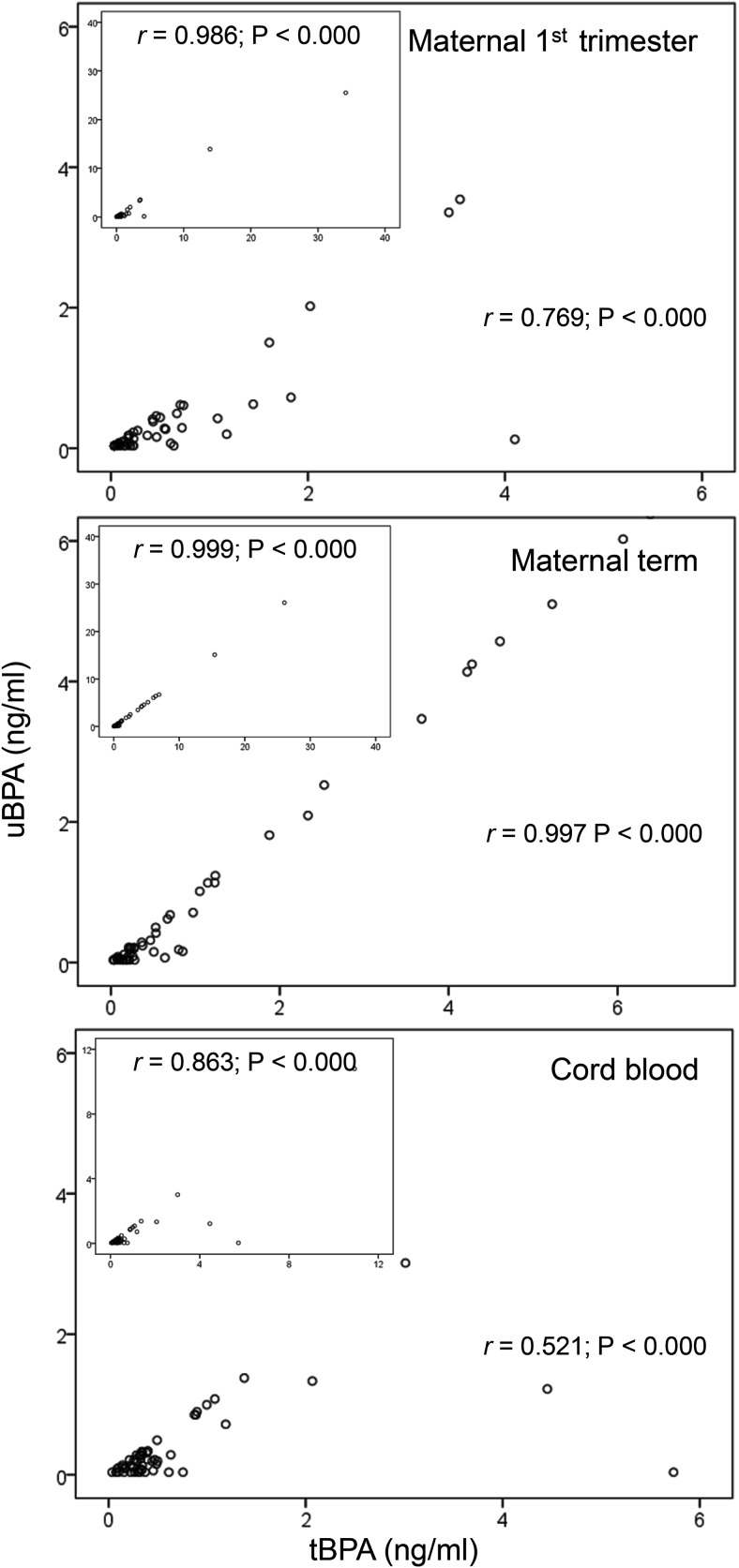
Table 1 shows uBPA and tBPA levels in M1, MT, and UC samples. Variability in uBPA levels was evident in all three sample sets (M1, MT, and UC). The highest uBPA and tBPA mean levels were found in MT samples followed by M1 and UC without any significant differences. Before and after excluding five outliers (two M1, two MT, and one UC), a strong correlation between uBPA and tBPA was found in all three sample sets (Figure 1).
Table 1.
Phase 1 (Pre-Validation Study): Levels of BPA Analytes in Maternal and Umbilical Cord Plasma
| Sample Origin | BPA Analyte | Mean ± sem (ng/mL) | Min | Percentiles |
Max | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||||
| Maternal 1st trimester | uBPA | 1.0 ± 0.5 | <LOD | <LOD | <LOD | 0.09 | 0.38 | 3.36 | 25.51 |
| tBPA | 1.3 ± 0.6 | <LOD | <LOD | <LOD | 0.20 | 0.64 | 3.54 | 34.14 | |
| Maternal term | uBPA | 1.7 ± 0.5 | <LOD | <LOD | <LOD | 0.20 | 1.14 | 6.70 | 26.06 |
| tBPA | 1.8 ± 0.5 | <LOD | <LOD | 0.15 | 0.28 | 1.23 | 6.92 | 26.06 | |
| Umbilical cord | uBPA | 0.5 ± 0.2 | <LOD | <LOD | 0.07 | 0.19 | 0.32 | 1.38 | 10.79 |
| tBPA | 0.8 ± 0.2 | <LOD | 0.08 | 0.25 | 0.34 | 0.50 | 4.45 | 10.93 | |
LOD for uBPA and tBPA, 0.05 ng/mL.
Figure 1.
Correlation between the uBPA (ng/mL) and tBPA (ng/mL) in M1 (top), MT (middle), and UC (bottom) samples with outliers excluded (five samples: two maternal first trimester, two maternal term, and one cord). Indents in each panel shows the correlation with all samples included. Pearson correlation coefficient is shown as r.
Phase 2 studies (post-Round Robin validation study)
Demographics
Most women were Caucasian (77.2%), between 30 and 35 years old (51.2%), with a normal BMI (62.3%), and with a bachelor's degree and above (78.1%). Table 2 shows subject characteristics, namely age, race, age, BMI, education, income, parity, offspring sex distribution, gestational length, and birth weight. In this cohort, mothers were younger if they had lower education (P < .0001), had lower income (P < .01), were single (P < .0001), had smoking exposure (P < .04), and had access to fast food (P < .05). In addition, when the effect of subject characteristics on gestational age, pre and end weight, pre and end BMI, and mother's age was tested, prepregnancy weight was found to be higher in those subjects with gestational diabetes (P < .02).
Table 2.
Characteristics of Study Subjects of Phase 2
| Characteristics | N (%); Mean ± sem |
|---|---|
| Mother | |
| Age | |
| <30 y | 23 (28.7) |
| 30–35 y | 41 (51.2) |
| ≥35 y | 16 (20.0) |
| Race | |
| Caucasian | 61 (77.2) |
| African-American | 7 (8.9) |
| Other | 11 (13.9) |
| BW/BMI | |
| BW prepregnancy, kg | 69.9 ± 1.9 |
| BMI at prepregnancy | 25.5 ± 0.6 |
| <18.5 | 1 (1.3) |
| 18.5–24.9 | 48 (62.3) |
| 25–29.9 | 14 (18.2) |
| >30 | 14 (18.2) |
| BW end of pregnancy, kg | 83.0 ± 2.0 |
| BMI end of pregnancy | 30.3 ± 0.6 |
| <18.5 | 0 (0) |
| 18.5–24.9 | 10 (13.0) |
| 25–29.9 | 33 (42.9) |
| >30 | 34 (44.2) |
| Education | |
| High school or college | 16 (21.9) |
| Bachelors and above | 57 (78.1) |
| Income, USD | |
| < 25 000 | 15 (19.5) |
| 25 000–49 000 | 11 (14.3) |
| 50 000–74 000 | 17 (22.1) |
| 75 000–99 999 | 13 (16.9) |
| 100 000–125 000 | 10 (13.0) |
| >125 000 | 11 (14.3) |
| Offspring | |
| Sex | |
| Female | 34 (42.5) |
| Male | 46 (57.5) |
| Parity | |
| 0 | 31 (38.7) |
| ≥1 | 49 (61.3) |
| Gestational age, d | 276.9 ± 0.8 |
| Birth weight, kg | 3440 ± 59.5 |
Abbreviation: BW, body weight; BMI, body mass index.
Maternal vs cord blood changes in uBPA and gBPA
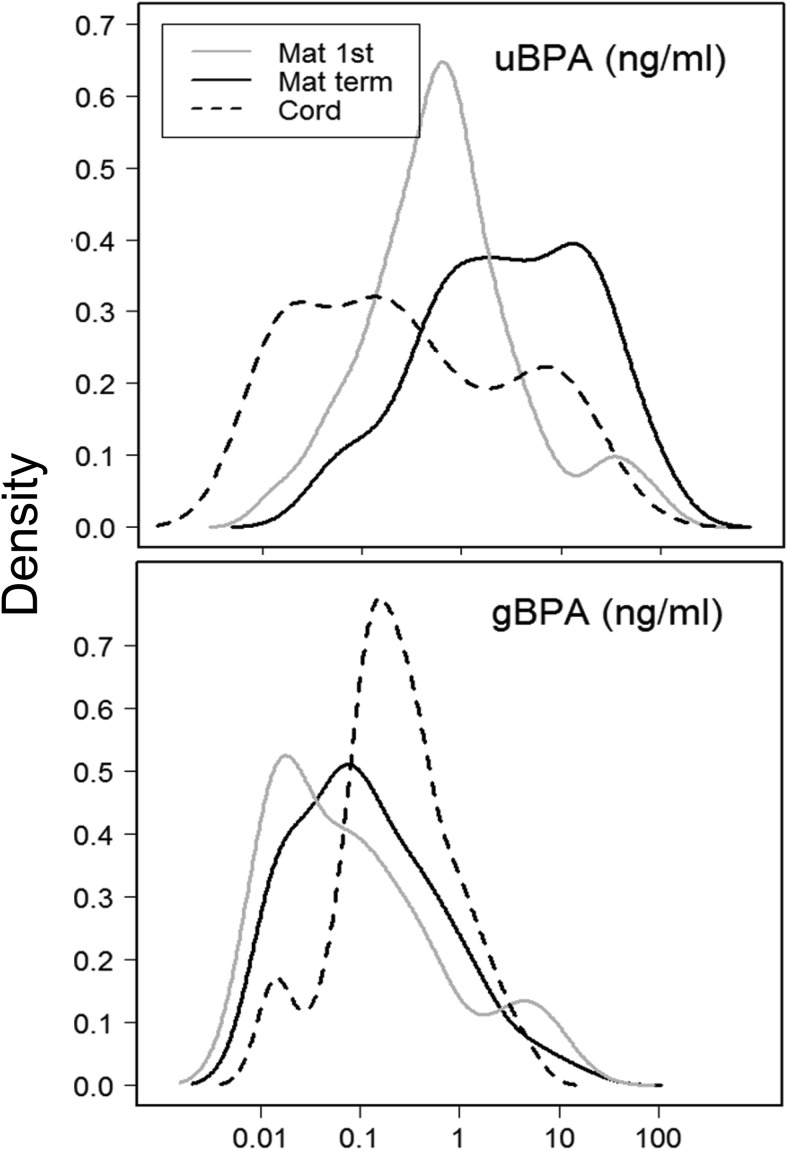
Both uBPA and gBPA were detectable in all three sample series (M1, MT, and UC). Table 3 and Figure 2 show mean, range, and percentile distribution for both uBPA and gBPA. Repeated measures analyses without controlling for any covariate revealed that uBPA levels differed significantly across the three sample series (P < .02) with levels in MT being highest followed by M1 and UC being the lowest. Across the sample series uBPA levels were not significantly correlated. Without controlling for any covariate, gBPA levels across the three samples (M1, MT, and UC) differed significantly (P < .001). UC gBPA levels were lower compared with both maternal measures (P < .01). Spearman correlation analyses revealed that gBPA was positively correlated between M1 and MT (r = 0.330; P < .01) and MT and UC (r = 0.380; P < .001) but not M1 and UC (r = 0.180; P = .11). No correlation was observed between uBPA and gBPA within each sample series.
Table 3.
Phase 2 (Post-Validation Study): Levels of BPA Analytes in Maternal and Umbilical Cord Plasma
| BPA Analyte | Sample Origin | Mean ± sem, ng/mL | Min | Percentiles |
Max | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||||
| uBPA | M1 | 4.8 ± 1.6a | <LOD | <LOD | 0.23 | 0.66 | 1.51 | 31.91 | 96.43 |
| MT | 11.9 ± 2.2b | <LOD | 0.08 | 0.7 | 3.03 | 16.57 | 58.74 | 89.60 | |
| UC | 3.1 ± 0.7c | <LOD | <LOD | <LOD | 0.19 | 2.81 | 14.83 | 41.83 | |
| gBPA | M1 | 0.8 ± 0.2a | <LOD | <LOD | <LOD | 0.07 | 0.33 | 6.21 | 10.04 |
| MT | 0.6 ± 0.2a | <LOD | <LOD | <LOD | 0.10 | 0.37 | 2.76 | 15.46 | |
| UC | 0.5 ± 0.1b | <LOD | <LOD | <LOD | 0.19 | 0.47 | 2.27 | 4.85 | |
LOD for uBPA and gBPA, 0.02 ng/mL.
Different letter superscripts represent significant differences at P < .05 within uBPA and gBPA measures.
Figure 2.
Distribution plots of uBPA (top) and gBPA (bottom) for M1 (continuous gray), MT (continuous black), and UC (discontinuous black).
Association of subject characteristics on BPA levels
Subject characteristics had an effect on both uBPA and gBPA levels during pregnancy (Table 4). M1 (both uBPA and gBPA) and MT gBPA were affected by race. M1 uBPA levels were higher in all other races combined relative to Caucasians. MT gBPA levels were higher in Caucasians compared with African-Americans. Race had no effect on UC uBPA and gBPA. UC uBPA levels were higher in women pregnant for the first time (P < .0001). UC uBPA levels were also higher in pregnancies of women exposed to dental fillings in the first trimester (P < .05), although only four subjects fit into this category (not shown). M1 uBPA levels were lower in working mothers compared with those who were unemployed.
Table 4.
Mean (±sem) uBPA and gBPA Levels (ng/mL) in Maternal First Trimester, Maternal Term, and Term Cord Blood as it Relates to Subject Characteristics
| Characteristics | N | Maternal 1st Trimester |
Maternal Term |
Umbilical Cord |
|||
|---|---|---|---|---|---|---|---|
| uBPA | gBPA | uBPA | gBPA | uBPA | gBPA | ||
| Race | |||||||
| Caucasian | 61 | 3.0 ± 1.2d | 0.7 ± 0.2d,e | 11.0 ± 2.2 | 0.5 ± 0.2d,e | 3.8 ± 0.9 | 0.5 ± 0.1 |
| African-American | 7 | 1.5 ± 0.8d,e | 0.07 ± 0.05d | 10.9 ± 3.5 | 0.07 ± 0.05d | 0.08 ± 0.03 | 0.4 ± 0.2 |
| Othera | 11 | 17.1 ± 9.1e | 1.8 ± 1.0e | 18.3 ± 10.1 | 1.7 ± 1.4e | 1.8 ± 1.0 | 0.8 ± 0.4 |
| Marital status | |||||||
| Single/separated | 18 | 1.0 ± 0.4 | 0.3 ± 0.2 | 9.4 ± 2.7 | 0.2 ± 0.1 | 3.6 ± 2.3 | 0.7 ± 0.2 |
| Marriedb | 62 | 5.9 ± 2.1 | 0.9 ± 0.3 | 12.6 ± 2.7 | 0.8 ± 0.3 | 3.0 ± 0.7 | 0.5 ± 0.1 |
| Education | |||||||
| College, <1–2 y | 16 | 2.8 ± 2.0 | 0.7 ± 0.5 | 10.8 ± 5.1 | 1.1 ± 1.0 | 3.7 ± 2.6 | 0.6 ± 0.3 |
| >Bachelors | 57 | 5.9 ± 2.2 | 0.7 ± 0.3 | 11.3 ± 2.7 | 0.5 ± 0.2 | 3.3 ± 0.8 | 0.5 ± 0.1 |
| Sex | |||||||
| Boy | 46 | 6.2 ± 2.7 | 0.7 ± 0.3 | 11.9 ± 3.2 | 0.3 ± 0.1 | 3.1 ± 0.9 | 0.4 ± 0.1 |
| Girl | 34 | 2.8 ± 1.2 | 0.9 ± 0.3 | 11.9 ± 2.8 | 1.1 ± 0.5 | 3.2 ± 1.3 | 0.6 ± 0.2 |
| Smoking | |||||||
| Nonsmoker | 70 | 5.4 ± 1.9 | 0.9 ± 0.2 | 12.0 ± 2.5 | 0.7 ± 0.3 | 3.3 ± 0.8 | 0.5 ± 0.1 |
| Smoker | 6 | 0.8 ± 0.4 | 0.1 ± 0.04 | 13.7 ± 4.7 | 0.1 ± 0.02 | 1.8 ± 1.5 | 0.2 ± 0.04 |
| Quit <1 mo | 3 | 0.2 ± 0.05 | 0.2 ± 0.1 | 9.0 ± 5.2 | 0.2 ± 0.2 | 2.2 ± 1.2 | 0.3 ± 0.1 |
| Gravida | |||||||
| 1 | 21 | 5.7 ± 4.5 | 0.7 ± 0.4 | 12.2 ± 4.8 | 0.4 ± 0.2 | 6.3 ± 2.3d | 0.6 ± 0.2 |
| 2–7 | 59 | 4.5 ± 1.5f | 0.8 ± 0.3 | 11.8 ± 2.4f | 0.7 ± 0.3 | 2.0 ± 0.5e,F | 0.5 ± 0.1 |
| Parity | |||||||
| 0 | 31 | 6.9 ± 3.7 | 0.8 ± 0.3 | 10.3 ± 3.3 | 0.5 ± 0.2 | 5.0 ± 1.6d | 0.5 ± 0.2 |
| 1−6 | 49 | 3.5 ± 1.3 | 0.8 ± 0.3 | 12.9 ± 2.9 | 0.7 ± 0.3 | 2.0 ± 0.6e | 0.5 ± 0.1 |
| Employment | |||||||
| Noc | 16 | 11.9 ± 4.9d | 2.2 ± 0.9 | 11.9 ± 5.2 | 1.3 ± 1.0 | 4.3 ± 2.6 | 0.5 ± 0.3 |
| Yes | 58 | 3.3 ± 1.7e | 0.3 ± 0.1 | 10.9 ± 2.6 | 0.5 ± 0.1 | 3.0 ± 0.7 | 0.5 ± 0.1 |
| Apgar | |||||||
| ≤7 | 20 | 4.3 ± 3.3 | 1.5 ± 0.6 | 10.4 ± 2.3 | 0.7 ± 0.3 | 3.3 ± 1.2 | 0.4 ± 0.1 |
| 8 or 9 | 60 | 5.0 ± 1.9 | 0.6 ± 0.2 | 12.4 ± 2.8 | 0.6 ± 0.3 | 3.1 ± 0.9 | 0.6 ± 0.1 |
| Delivery | |||||||
| C/S | 10 | 4.0 ± 2.5 | 0.1 ± 0.1 | 10.7 ± 5.1 | 0.2 ± 0.1 | 3.9 ± 1.6 | 0.5 ± 0.2 |
| NSVD | 70 | 4.9 ± 1.8 | 0.9 ± 0.2 | 12.1 ± 2.4 | 0.7 ± 0.3 | 3.0 ± 0.8 | 0.5 ± 0.1 |
| Income (USD) | |||||||
| <25 000 | 15 | 3.4 ± 2.1 | 1.5 ± 0.8 | 18.4 ± 5.3 | 1.3 ± 1.0 | 0.8 ± 0.3 | 0.7 ± 0.3 |
| 25 000–49 000 | 11 | 3.6 ± 2.9 | 0.4 ± 0.3 | 7.1 ± 2.7 | 0.6 ± 0.6 | 4.3 ± 3.8 | 0.5 ± 0.2 |
| 50 000–74 000 | 17 | 2.6 ± 1.4 | 0.1 ± 0.03 | 6.9 ± 2.4 | 0.5 ± 0.3 | 2.5 ± 0.9 | 0.5 ± 0.3 |
| 75 000–99 000 | 13 | 14.5 ± 8.4 | 1.2 ± 0.7 | 11.9 ± 6.1 | 0.3 ± 0.1 | 3.4 ± 1.6 | 0.4 ± 0.1 |
| 100 000–125 000 | 10 | 0.5 ± 0.1 | 0.4 ± 0.3 | 24.1 ± 11.3 | 0.5 ± 0.2 | 5.8 ± 2.1 | 0.5 ± 0.2 |
| >125 000 | 11 | 0.9 ± 0.4 | 1.4 ± 0.7 | 7.2 ± 2.7 | 0.7 ± 0.3 | 4.1 ± 2.6 | 0.4 ± 0.1 |
| Landfill exposure | |||||||
| No | 76 | 5.0 ± 1.7 | 0.8 ± 0.2 | 12.0 ± 2.3 | 0.7 ± 0.2 | 3.2 ± 0.8 | 0.5 ± 0.1 |
| Yes | 3 | 0.4 ± 0.3 | 0.2 ± 0.1 | 12.7 ± 12.2 | 0.2 ± 0.1 | 3.8 ± 2.7 | 0.2 ± 0.0 |
| Canned food | |||||||
| No | 30 | 5.8 ± 2.7 | 1.1 ± 0.5 | 8.4 ± 2.8 | 0.8 ± 0.5 | 2.3 ± 0.7 | 0.5 ± 0.2 |
| Yes | 49 | 4.2 ± 2.1 | 0.6 ± 0.2 | 14.3 ± 3.1 | 0.6 ± 0.2 | 3.7 ± 1.1 | 0.5 ± 0.1 |
| Fast food | |||||||
| No | 48 | 2.8 ± 1.0 | 0.8 ± 0.3 | 12.0 ± 3.0 | 0.7 ± 0.3 | 3.7 ± 1.1 | 0.5 ± 0.1 |
| Yes | 31 | 8.0 ± 3.9 | 0.8 ± 0.3 | 12.1 ± 3.3 | 0.6 ± 0.2 | 2.3 ± 1.0 | 0.5 ± 0.1 |
Abbreviation: C/S, Cesarean section; NSVD, normal spontaneous vaginal delivery.
All significant differences are indicated in bold.
N < 80 for each variable represents missing data in that variable.
Asian, native Hawaiian or Pacific Islander, Hispanic, or multiracial,
Married or significant other,
Unemployed, student, or homemaker.
d ≠ e Significant differences (P < .05) within a time point (M1, maternal term, or term cord blood).
Significant differences (P < .05) across the three time points.
Pattern of uBPA in the thre sample series were affected by gravida (P = .027) with dental filling exposure affecting only UC levels (P = .04). For women with gravida at least 2, uBPA levels were different across sample series (P < .0001) with MT uBPA being the highest, M1 the second highest, and UC the lowest. UC uBPA levels were lower compared with both maternal measures. No significant differences were found in those women with gravida of 1. For women exposed to dental fillings (n = 4), uBPA levels across sample series did not differ. None of the tested covariates affected the pattern of the gBPA measures.
Relationship of BPA with outcome variables
Regression analyses revealed that a 2-fold increase in M1 uBPA was associated with 55-g less birth weight (P < .05) (Table 5). Covariates included in this analysis were race, education, marital status, occupation, smoking exposure, and maternal age. This effect was sex specific and present in female but not male pregnancies; a 2-fold increase in M1 uBPA was associated with 183-g less female birth weight (P < .005). A 2-fold increase of MT uBPA was associated with an increase in birth weight of 96 g in females (P < .05). Similar analyses with gestational length revealed that a 2-fold increase in MT uBPA was associated with 0.7 days of a longer gestation (P < .05). This effect was also driven by female pregnancies where a 2-fold increase in MT uBPA tended to be associated with 1.1 days of longer gestation (P = .06). BPA did not have an effect on maternal BMI change.
Table 5.
Association Between BPA and Pregnancy Outcomes
| Birth Weighta
|
Gestational Ageb |
|||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| uBPA | ||||
| M1 | −81.0 (n.s.) | −607.2 (P = .0093) | −0.4 (n.s.) | −2.4 (n.s.) |
| MT | 50.7 (n.s.) | 318.0 (P = .034) | 0.9 (n.s.) | 3.7 (P = .06) |
| UC | 114.6 (n.s.) | 4.9 (n.s.) | 0.9 (n.s.) | 2.9 (n.s.) |
| gBPA | ||||
| M1 | −25.1 (n.s.) | 93.7 (n.s.) | −1.2 (n.s.) | 1.5 (n.s.) |
| MT | 128.3 (n.s.) | −32.4 (n.s.) | 1.3 (n.s.) | 4.1 (n.s.) |
| UC | 41.1 (n.s.) | −95.1 (n.s.) | 0.2 (n.s.) | 1.5 (n.s.) |
Abbreviation: n.s., not significant.
Values from regression analysis represent the effect of the uBPA exposure on birth weight. For example, a 10-fold increase in M1 uBPA was associated with 607.2 g less birth weight in female.
Negative signs before the association value indicates inverse relationship between BPA exposure and outcome (birth weight or gestational age).
Significant differences are indicated in bold.
Change in g based on a 10-fold change in BPA levels.
Change in days based on a 10-fold change in BPA levels.
Discussion
This is the first longitudinal study documenting levels of uBPA and gBPA in maternal first trimester and term samples and the matching term cord blood sample and their relationship to pregnancy outcomes, namely gestational length and birth weight. Findings from this study provide evidence in support of 1) existence of measurable levels of uBPA in maternal and umbilical cord samples; 2) high degree of correlation in gBPA, but not uBPA levels among M1, MT, and UC samples; 3) influence of race, employment, gravida/parity, and dental fillings on exposure levels of BPA; and 4) sex-specific effect of higher exposure levels of BPA during early gestation with birth weight and term BPA with gestational length.
Relationship between maternal and cord uBPA levels
Results from both phases found highest levels of uBPA in MT samples followed by M1 samples with UC showing the lowest. MT uBPA was nearly twice as high as that found in M1 and 3–4 times that of the UC. Barring one report that reported similar levels across pregnancy (14) using the less-sensitive ELISA, most human studies found lower uBPA (15) or tBPA (16–20) in UC compared with MT. A high degree of variability (2–30-fold difference) in the relationship between maternal and fetal levels was evident across studies (3, 16–20). Although 78% of UC samples in the present study had detectable uBPA levels, detection of BPA in samples in other studies ranged between 47 and 100% for uBPA (3, 15, 21) and 27–100% for tBPA (16, 17, 19, 20). These differences may relate to sensitivity and specificity of measurement approach or population characteristics (11).
The higher MT uBPA levels in both phases suggest an increased exposure to BPA. In phase 2, in which the same subjects were studied during first trimester and term, higher levels of uBPA were found in 68% mothers at term. uBPA differences across pregnancy may reflect food intake prior to sampling or reduced BPA conjugation during the later stages of pregnancy; differences in BPA pharmacokinetics across pregnancy has been demonstrated in rhesus monkeys (22).
Despite the similarities in the direction of changes across time points, uBPA levels in phase 1 were 5–10 times lower compared with phase 2. The differences in absolute levels of uBPA measured between the two phases may reflect higher detection capability (11) of the post Round Robin approach, which likely stem in part from differences in standards used.
Relationship between maternal and cord gBPA levels
gBPA was measurable in 67, 81, and 92% of M1, MT, and UC samples, respectively (phase 2). Considering metabolized BPA cannot reflect atmospheric contamination, differences in gBPA levels must represent the differing exposure levels; gBPA levels were lower in UC compared with the maternal levels, but the magnitude of change was within 0.3 ng/mL. The ratio of gBPA/uBPA (BPA conjugation) was lower in MT compared with M1 (M1, 0.16 vs MT, 0.05). This ratio was considerably lower than that reported for rhesus monkeys (50.9 vs 81.4 at gestational d 50 and 95, respectively; term, ∼164 days). Given that gBPA accounts for most of the BPA conjugation (1), the higher levels of uBPA seen at term likely represent a higher BPA exposure closer to term. Alternatively, because the research coordinator was not at the bedside during MT collection, whether the nurses used the collection needle provided in the package or resorted to butterfly system is unclear. If the latter, there is a potential for external contamination. This argument does not hold true for the phase 1 studies in which both M1 and MT samples were collected by the nurses using similar approaches. Furthermore, the validation studies involving similar collection approach as phase 1 provide no evidence of BPA leaching from the iv system. Additional studies are required to unequivocally rule out any outside airborne contamination in MT samples.
The high percentage of samples in which gBPA was detected in the current study (92%) is similar to another US cohort that evaluated the levels of gBPA in umbilical cord in second trimester pregnancy terminations [76%; (3)]. Maximum concentrations of both uBPA (this study: 41.8; Gerona et al: 52.2 ng/mL) and gBPA (this study: 4.85; Gerona et al: 5.41 ng/mL) (3) detected are similar with both studies using validated Round Robin methods (11). The similarity in BPA levels detected in both studies at different gestational time points suggest that the fetus is likely exposed to uBPA throughout gestation.
Relationship between BPA exposure and sociodemographic characteristics
Available evidence points to African-Americans and ethnic minorities to be disproportionately exposed to environmental pollutants (23). This premise is supported by findings of higher BPA in maternal, but not cord blood of African-Americans (15). In the present study, uBPA levels were similar between African-Americans and Caucasians, although higher maternal uBPA were found in the category that included Asian, native Hawaiian, Pacific Islander, Hispanic, or multiracial. Another study also found no effect of race on urinary levels of BPA in pregnant women (24). Differences in outcomes between these studies may relate to factors that are independent of ethnicity such as income and consumer habits (variables not controlled for in these studies). A likely contributor to this disparity is the location of the cohort (15) and dietary habits dictated by food accessibility (25).
The largest predictor of BPA exposure seems to be income, not race (26). The lack of association between income and BPA exposure in the present study suggests that maternal unemployment is not predictive of overall household income. These findings must be interpreted with caution as the unemployment category includes not only unemployed individuals, but also students and homemakers, who may be staying at home because they are in a higher socioeconomic stratum. A significant effect of exposure to dental fillings on higher UC uBPA levels is based only on four subjects and needs to be tested in larger cohorts.
Relationship between BPA exposure and pregnancy outcomes
The negative association found between high M1 uBPA and reduced birth weight points to the vulnerability of the fetus to insults during early development. The relationship between maternal BPA exposures at different gestational time points with birth weight are inconsistent with some studies reporting a reduction (19, 27) and others an increase (17), a U-shaped response curve (28), or lack of an effect (4, 29). However, in one study (28), pregnancy outcomes were assessed in relation to maternal BPA levels at term. In the Philippat study, BPA measurements included a broad range of gestational ages (6–30 wk) without controlling for the effect of gestational age. Differences among studies suggest how the timing of BPA measurement is a critical variable in relating BPA exposure to newborn outcomes. This is substantiated in this study from the opposing finding of a negative association of birth weight with M1 uBPA levels, contrasting the positive association of birth weight with MT uBPA. The lack of association between term uBPA and birth weight in our earlier study (4), as opposed to the positive relationship in this study, may be driven by 1) the measurement approach used, 2) the smaller sample size in previous study, and 3) the lack of consideration of covariates in previous study. Differences in birth weight outcomes reported in all earlier studies may arise not only from variables discussed above but also the location of the study cohorts (China, France, Mexico, United States) and/or BPA measurement methods.
Overall, the finding of a negative association between M1 uBPA levels and birth weight is a concern. To what extent this contributes to small-for-gestational-age babies remains to be determined. The greater decrease seen in female compared with male pregnancies points to sex-specific effects of M1 BPA exposure. Gender-specific effects on birth outcomes have been reported relative to BPA (17, 19) and other endocrine disruptors (28), although such associations were based on BPA measures from maternal and/or cord blood at term. Furthermore, the association between BPA measures at term with pregnancy outcome has been inconsistent across cohorts with some reporting a reduction (19), whereas others an increase (17) in male but not female birth weight as a function of tBPA measured. These differences may also relate to the type of BPA measured, assay methodology, and/or racial/ethnic background of the cohort.
Even fewer studies have investigated the association between BPA exposures and gestational age with most studies reporting no effect (4, 19, 29) and a few reporting reduced gestational age and prematurity (30, 31). In the Tang study (31), the reduction in gestational length was restricted to male pregnancies in the low and middle exposure groups. Contrary to this, in our cohort higher MT uBPA levels were associated with a longer gestational length and restricted to female pregnancies. Given that most maternal variables were comparable between Tang et al (31) and the present study, the outcome differences may relate to racial distribution (Tang, not defined; this study, 77% Caucasian) and sample source (Tang, urine; this study, maternal blood). Longer gestational length is associated with reduced morbidity, mortality (32), and improved cognitive function (33). Gender-specific reduction in gestational length has been reported earlier with other endocrine disruptors (34).
The associations of BPA with pregnancy outcomes relate to uBPA, the biologically active form, and not gBPA. No information is available as to whether gBPA is biologically active, accumulates in tissues depots, and/or releases uBPA via deconjugation at later time points. Evidence supports the presence of tBPA in adipose tissues (35) and association of higher BPA levels with obesity (36).
Considering that lower birth weight has been correlated with adult disease outcomes (37), the association of lower birth weight with higher M1 uBPA is of concern. We recently found that higher M1 uBPA are associated with higher oxidative stress (38), a risk factor for metabolic disorders.
Conclusion
This is the first documentation of exposure levels of uBPA and gBPA in subject-matched M1, MT, and UC samples and the association that exists between higher maternal uBPA exposure levels with pregnancy outcomes, reduced sex-specific birth weight with higher M1 uBPA levels, and increased gestational length with higher MT uBPA levels. The effect of race, gravida, parity, and employment on BPA exposure highlights how genetic susceptibility, sex, and the environment may interact in influencing vulnerability to BPA exposure. Controlling for contamination during sample collection and using a validated post-Round Robin BPA measurement approach, this study represents the first step toward assessment of risks posed by maternal BPA exposure to offspring health. The recent risk assessment report by the European Food Safety Authority (EFSA) states that there is no consumer health risk from BPA exposure, although they reduced the tolerable daily intake (TDI) of BPA from 50 to 4 μg/kg of body weight per day factoring in the “Uncertainties surrounding potential health effects of BPA on the mammary gland, reproductive, metabolic, neurobehavioural and immune systems” (39). The EFSA report concludes, “the TDI is temporary pending the outcome of a long-term study in rats” (39). Because 1) uBPA levels in carefully collected M1 samples of several subjects in the present study exceed the TDI reported in the recent EFSA report, 2) of the concerns expressed about the widespread use of altricial rodent models for risk assessments of endocrine disruptors in precocial human (40), 3) higher M1 uBPA are associated with lower birth weight (this study) and increased oxidative stress (38), and 4) evidence points to an association between lower birth weight and adverse health outcomes (37), additional studies involving larger cohorts are required to validate these findings, investigate whether this association holds true in newborns that fall outside the normative weight range such as small-for-gestational-age babies, and assess postnatal consequences in children exposed to higher BPA levels during early pregnancy.
Acknowledgments
We thank Jann Howell and Lucy Allbaugh for their support during human subjects' recruitment, Samantha Milewski for the meticulous clinical data verification, and Jacob Moeller for careful language editing of the manuscript.
Current Address for Almudena Veiga-Lopez: Department of Animal Sciences, Michigan State University, East Lansing, MI, 48824.
This work was supported by an American Recovery and Reinvestment Act supplement to National Institute for Environmental Health Sciences (NIEHS) Grant R01 ES017500, NIEHS Grant P01 ES022844, and the United States Environmental Protection Agency (US EPA) Grant RD 83543601. Analytical support for this research was also provided by the NIEHS Grant P30ES017885. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. The effort spent by A.V.-L. during manuscript writing was supported by AgBioResearch and the USDA National Institute of Food and Agriculture.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- BPA
- bisphenol A
- gBPA
- BPA glucuronide
- LOD
- limit of BPA detection
- M1
- first trimester maternal
- MS/MS
- tandem mass spectroscopy
- MT
- maternal at time of delivery
- NIEHS
- National Institutes of Environmental Health Sciences
- tBPA
- total BPA
- TDI
- tolerable daily intake
- uBPA
- unconjugated BPA
- UC
- umbilical cord.
References
- 1. Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gerona RR, Woodruff TJ, Dickenson CA, et al. Bisphenol-A (BPA), BPA glucuronide, and BPA sulfate in midgestation umbilical cord serum in a northern and central California population. Environ Sci Technol. 2013;47:12477–12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Padmanabhan V, Siefert K, Ransom S, et al. Maternal bisphenol-A levels at delivery: A looming problem? J Perinatol. 2008;28:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alonso-Magdalena P, Vieira E, Soriano S, et al. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118:1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunt PA, Lawson C, Gieske M, et al. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A. 2012;109:17525–17530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. [DOI] [PubMed] [Google Scholar]
- 8. Teppala S, Madhavan S, Shankar A. Bisphenol A and metabolic syndrome: Results from NHANES. Int J Endocrinol. 2012;2012:598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS. Human exposures to bisphenol A: Mismatches between data and assumptions. Rev Environ Health. 2013;28:37–58. [DOI] [PubMed] [Google Scholar]
- 10. Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environ Health Perspect. 2013;121:283–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vandenberg LN, Gerona RR, Kannan K, et al. A round robin approach to the analysis of bisphenol A (BPA) in human blood samples. Environ Health. 2014;13:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao C, Kannan K. Determination of free and conjugated forms of bisphenol A in human urine and serum by liquid chromatography-tandem mass spectrometry. Environ Sci Technol. 2012;46:5003–5009. [DOI] [PubMed] [Google Scholar]
- 13. Christensen KL, Lorber M, Ye X, Calafat AM. Reconstruction of bisphenol A intake using a simple pharmacokinetic model. J Expo Sci Environ Epidemiol. 2015;25(3):240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Human Reprod. 2002;17:2839–2841. [DOI] [PubMed] [Google Scholar]
- 15. Unal ER, Lynn T, Neidich J, et al. Racial disparity in maternal and fetal-cord bisphenol A concentrations. J Perinatol. 2012;32:844–850. [DOI] [PubMed] [Google Scholar]
- 16. Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee YJ, Ryu HY, Kim HK, et al. Maternal and fetal exposure to bisphenol A in Korea. Reprod Toxicol. 2008;25:413–419. [DOI] [PubMed] [Google Scholar]
- 18. Wan Y, Choi K, Kim S, et al. Hydroxylated polybrominated diphenyl ethers and bisphenol A in pregnant women and their matching fetuses: Placental transfer and potential risks. Environ Sci Technol. 2010;44:5233–5239. [DOI] [PubMed] [Google Scholar]
- 19. Chou WC, Chen JL, Lin CF, Chen YC, Shih FC, Chuang CY. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: A birth cohort study in Taiwan. Environ Health. 2011;10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang T, Sun H, Kannan K. Blood and urinary bisphenol A concentrations in children, adults, and pregnant women from china: Partitioning between blood and urine and maternal and fetal cord blood. Environ Sci Technol. 2013;47:4686–4694. [DOI] [PubMed] [Google Scholar]
- 21. Fénichel P, Dechaux H, Harthe C, et al. Unconjugated bisphenol A cord blood levels in boys with descended or undescended testes. Human Reprod. 2012;27:983–990. [DOI] [PubMed] [Google Scholar]
- 22. Vom Saal FS, VandeVoort CA, Taylor JA, Welshons WV, Toutain PL, Hunt PA. Bisphenol A (BPA) pharmacokinetics with daily oral bolus or continuous exposure via silastic capsules in pregnant rhesus monkeys: Relevance for human exposures. Reprod Toxicol. 2014;45:105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gee GC, Payne-Sturges DC. Environmental health disparities: A framework integrating psychosocial and environmental concepts. Environ Health Perspect. 2004;112:1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mortensen ME, Calafat AM, Ye X, et al. Urinary concentrations of environmental phenols in pregnant women in a pilot study of the National Children's Study. Environ Res. 2014;129:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morland K, Filomena S. Disparities in the availability of fruits and vegetables between racially segregated urban neighbourhoods. Public Health Nutr. 2007;10:1481–1489. [DOI] [PubMed] [Google Scholar]
- 26. Nelson JW, Scammell MK, Hatch EE, Webster TF. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003–2006. Environ Health. 2012;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Miao M, Yuan W, Zhu G, He X, Li DK. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod Toxicol. 2011;32:64–68. [DOI] [PubMed] [Google Scholar]
- 28. Philippat C, Mortamais M, Chevrier C, et al. Exposure to phthalates and phenols during pregnancy and offspring size at birth. Environ Health Perspect. 2012;120:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolff MS, Engel SM, Berkowitz GS, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cantonwine D, Meeker JD, Hu H, et al. Bisphenol a exposure in Mexico City and risk of prematurity: A pilot nested case control study. Environ Health. 2010;9:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang R, Chen MJ, Ding GD, et al. Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut. 2013;178:115–120. [DOI] [PubMed] [Google Scholar]
- 32. MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Natl Vital Stat Rep. 2012;60:1–22. [PubMed] [Google Scholar]
- 33. Espel EV, Glynn LM, Sandman CA, Davis EP. Longer gestation among children born full term influences cognitive and motor development. PLoS One. 2014;9:e113758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang P, Tian Y, Wang XJ, et al. Organophosphate pesticide exposure and perinatal outcomes in Shanghai, China. Environ Int. 2012;42:100–104. [DOI] [PubMed] [Google Scholar]
- 35. Wang L, Asimakopoulos AG, Kannan K. Accumulation of 19 environmental phenolic and xenobiotic heterocyclic aromatic compounds in human adipose tissue. Environ Int. 2015;78C:45–50. [DOI] [PubMed] [Google Scholar]
- 36. Wang T, Li M, Chen B, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab. 2012;97:E223–E227. [DOI] [PubMed] [Google Scholar]
- 37. Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–624. [DOI] [PubMed] [Google Scholar]
- 38. Veiga-Lopez A, Pennathur S, Kannan K, et al. Impact of gestational bisphenol A on oxidative stress and free fatty acids: human association and interspecies animal testing studies. Endocrinology. 2015;156:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. EFSA. 2015. Available from: http://www.efsa.europa.eu/en/press/news/150121.htm Accessed: March 18, 2015.
- 40. Habert R, Muczynski V, Grisin T, et al. Concerns about the widespread use of rodent models for human risk assessments of endocrine disruptors. Reproduction. 2014;147:R119–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]