Abstract
It is becoming increasingly recognized that post-traumatic stress disorder (PTSD) can be acquired vicariously from witnessing traumatic events. Recently, we published an animal model called the “Trauma witness model” (TWM) which mimics PTSD-like symptoms in rats from witnessing daily traumatic events (social defeat of cage mate) [15]. Our TWM does not result in any physical injury. This is a major procedural advantage over the typical intruder paradigm in which it is difficult to delineate the inflammatory response of tissue injury and the response elicited from emotional distress. Using TWM paradigm, we examined behavioral and cognitive effects in rats [15] however, the long-term persistence of PTSD-like symptoms or a time-course of these events (anxiety and depression-like behaviors and cognitive deficits) and the contribution of olfactory and auditory stress vs visual reinforcement were not examined. This study demonstrates that some of the features of PTSD-like symptoms in rats are reversible after a significant time lapse of the witnessing of traumatic events. We also have established that witnessing is critical to the PTSD-like phenotype and cannot be acquired solely due to auditory or olfactory stresses.
Keywords: Trauma witness model, anxiety, depression, stress
Introduction
Approximately 40% of the North American population is exposed to at least one traumatic event during their lifetime [11]. Exposure to traumatic events may be short-lived (e.g., a car accident) or sustained and repeated (e.g., sexual abuse). Several studies have reported strong association between experiencing of traumatic events and developing post-traumatic disorder (PTSD) [15, 21]. While the link between experiencing traumatic events and PTSD seems well established, the impact of witnessing traumatic events and acquiring PTSD is not clear. This is alarming as approximately 25% to 30% of individuals who witness a traumatic event may also develop PTSD and other forms of mental disorders including depression [22]. Approximately half of the individuals who develop PTSD continue to suffer from its effects several years later [11]. Recently, we published an animal model called the “Trauma witness model” (TWM) which mimics majority of PTSD-like symptoms in rats from witnessing daily traumatic events (social defeat of cage mate) [15]. Using TWM paradigm, we examined behavioral and cognitive effects in rats [15] however, the long-term persistence of PTSD-like symptoms (anxiety and depression-like behaviors and cognitive deficits) and the contribution of olfactory and auditory stress vs visual reinforcement were not examined.
Several studies have reported that PTSD-like behaviors last at least 3–4 weeks after experiencing traumatic events [1, 23] and that olfactory or auditory stress alone cause cognitive and behavioral impairments in animals [18, 19]. Limited information is however available regarding the witnessing aspects of trauma and persistence of PTSD-like behaviors [21]. Recently, Warren et al. had reported that deficits in social avoidance and sucrose preference are salient 1 month after emotional stress of witnessing traumatic events, perhaps indicating that neuronal adaptations underlying these behavioral effects build over time, in the absence of continued stress [8, 10, 21]. In the present study, we have addressed the question of whether the behavioral and cognitive deficits seen in our TWM model are long-lasting or a result of acute stress reaction due to witnessing traumatic events.
Methods and Materials
Animals
Male Sprague Dawley (SD) rats (225–250 g) were used as controls or intruders, and male Long-Evans (LE) retired breeders (400–500 g) served as residents (Charles River, Wilmington, MA). Rats were singly housed with a 12-h light, 12-h dark cycle (lights on at 0700 h) in a climate-controlled room with ad libitum food and water. All experiments were conducted in accordance with the NIH guidelines using approved protocols from the University of Houston Animal Care Committee.
Selection of aggressors
Successful application of chronic social defeat stress to SD rats was dependent on appropriate selection of LE rats with consistent levels of aggressive behaviors, as determined from the 3-d screening process as published by us [13, 14].
Trauma Witness Model (TWM)
The social defeat model used in the present study was modified from the resident-intruder model originally developed by Miczek [12]. As we previously published [15], this paradigm consisted of 7 encounters, carried out for 7 consecutive days, with an aggressive male Long Evans (LE) rat. Each intruder (Sprague Dawley) was defeated by 7 different resident LE rats [5, 7]. After defeat, a plexiglass partition with holes was placed in the cage to avoid direct physical contact between the LE and intruder. This partition allowed visual, auditory, and olfactory interactions for the remainder of the 30-min session (Fig. 1A). Social defeat was observed by the SD rat present outside the cage, initiating a freezing response. Two more bouts of social defeat were performed with 5-min separation, in order to reinforce the visual stress in the TW rat. Controls were placed behind a wire partition in a novel cage and another SD rat present outside the cage for 30 min daily. TW and SD rats were housed in separate cages after each social defeat exposure. Behavioral assessments were performed at 1, 2, 6 and 8 weeks post trauma witness. The experiments were conducted three times (experiment 1: n=4 rats/group, experiment 2: n=8 rats/group, experiment 3: n=10 rats/group).
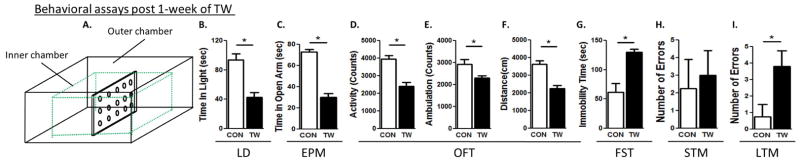
Figure 1. Examination of anxiety and depression-like behaviors and cognitive deficits 1 week after TWM.
A schematic representation of the TWM apparatus (A). Light-dark test (B), elevated plus maze test (C). The open-field test determined total (D), ambulatory (E) activities and distance travelled (F). Forced swim test (G) were conducted. Short-term (H) and long-term memory (I) tests were conducted in all rats. (*) significantly different from control rats, p<0.05. Bars represent means ± SEM, n=10 rats/group.
Auditory Stress
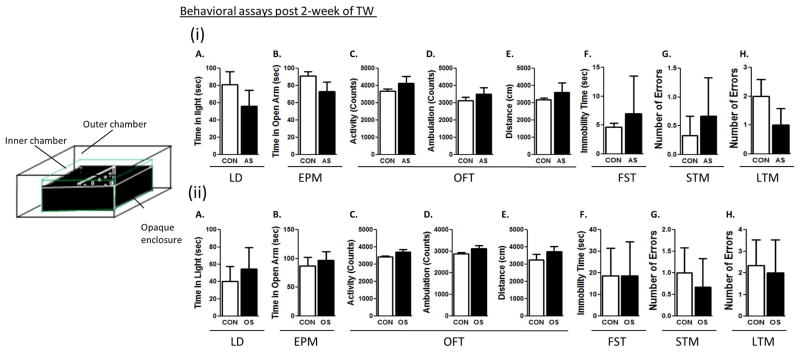
A Sprague-Dawley male rat (intruder rat) was put into the home cage of a large male LE rat (resident rat) resulting in social defeat of the intruder. During this process occasional vocalization sounds were made by the intruder. Brief sounds were also made when the intruder hit the walls of the central enclosure in an effort to escape attacks and avoid defeat. These sounds were audible to the cage mate (another small Sprague-Dawley male rat) of the intruder rat. The cage mate could hear the noise but not see the defeat process as the central enclosure was covered with opaque black paper (Fig. 5).
Figure 5. Examination of anxiety and depression-like behaviors and cognitive deficits 2 week after auditory or olfactory stress.
A schematic representation of the TWM apparatus, for auditory stress: in this set up the cage-mate (witness) could hear the noise but not see the social defeat process by the LE, as the central enclosure was covered with opaque black paper (i). For olfactory stress: the LE rat was placed in a blacked-out central enclosure, or its fur and urine were kept inside the enclosure. No social defeat was done (ii). Light-dark test (A), elevated plus maze test (B). The open-field test determined total (C), ambulatory (D) activities and distance travelled (E). Forced swim test (F) were conducted. Short-term (G) and long-term memory (H) tests were conducted. (*) significantly different from control rats, p<0.05. Bars represent means ± SEM, n=10 rats/group.
Olfactory Stress
Small Sprague-Dawley rat was allowed to smell but not see the LE rat. This was done either by placing the LE rats in a blacked-out central enclosure, or by putting LE fur and urine inside the enclosure. No social defeat was done (Fig. 5).
Anxiety-like behavior tests
First, open-field test was conducted followed by light-dark (LD) and elevated-plus maze (EPM) tests as published [16, 20].
Open Field (OF) activity
Rats were placed in the center of the OF (60×40 cm) and left free to explore the arena for 15 min and movement quantified using Opto-Varimax Micro Activity Meter v2.00 system (Optomax, Columbus Instruments; OH) [16, 20]. Total activity, ambulatory activity and distance covered were examined.
Light-Dark (LD) exploration
The light-dark box consisted of a light and a dark compartment separated with a single opening for passage from one compartment to the other and total time spent in the lit area was recorded [16, 20].
Elevated plus-maze (EPM)
A standard rat elevated plus-maze was obtained from Med Associates Inc., (St. Albans, VT). The rat’s movements were tracked manually. The observer was blinded to the group classification to avoid bias. Each session was started by placing the rat in the central area facing the open arms of the maze and lasted 5 min. The amount of time the rat spent in the open arms was noted [4].
Depression-like behavior tests
Forced Swim Test (FST)
Rats were individually introduced into a 25°C water tank for 5 min. During this time the rat assumes an immobile posture, marked by motionless floating and the cessation of struggling. The total time spent immobile was recorded [3, 6]. FST was performed 72 h after 1, 2, 6 and 8 week TW/AS/OS protocols.
Memory Function Test
Radial Arm Water Maze (RAWM)
A black circular water filled pool with six swim paths were used [15]. First set of six learning trials (trials # 1–6) were followed by a five min rest and another set of six learning trials (trials # 7–12). Rats were tested for short-term memory 30 min after the end of 12th trial and returned to their home cages and 24h later subjected to long-term memory test.
Data Analysis
Data are expressed as mean ± SEM. Significance was determined by unpaired student t-test (GraphPad Software, Inc. San Diego, CA). A value of p<0.05 was considered significant.
Results
Analysis of Anxiety-like behavior of rats
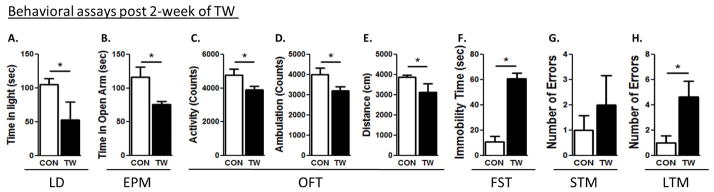
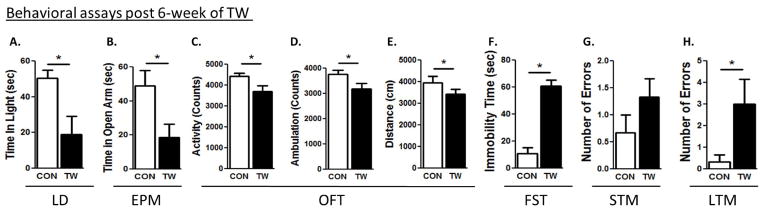
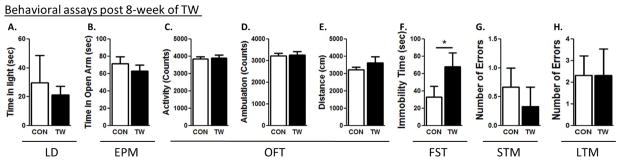
Control rats spent more time in the light compartment, when compared to TW rats 1 week (CON: 93 ± 8, TW: 43 ± 6, t=4.6, p=0.03), 2 weeks (CON: 105 ± 9, TW: 51 ± 14, t=3.1, p=0.001) and 6 weeks (CON: 61 ± 4, TW: 19 ± 8, t=3.1, p=0.04) after witnessing social defeat of their cage-mate, but after 8 weeks (CON: 29 ± 18, TW: 21 ± 5, t=0.4, p=0.69), anxiety-like behavior was not different from the controls (Fig. 1B, 2A, 3A, 4A). The amount of time control rats spent in the open arms of EPM test was significantly higher when compared to TW rats 1 week (CON: 72 ± 2, TW: 29 ± 3, t=7.3, p=0.04), 2 weeks (CON: 116 ± 14, TW: 76 ± 4, t=2.9, p=0.009) and 6 weeks (CON: 49 ± 9, TW: 18 ± 7, t=3.1, p=0.04) after witnessing social defeat of their cage-mate, but after 8 weeks (CON: 71 ± 8, TW: 63 ± 7, t=0.8, p=0.45), anxiety-like behavior was not different from the control group (Fig. 1C, 2B, 3B, 4B). In the open-field test, TW rats had lower total activity than their matched controls following 1 week (CON: 4785±344, TW: 3905±196, P=0.04, t=2.21, df=18), 2 weeks (CON: 3952±198, TW: 2414±212, P=0.002, t=4.31, df=18), and 6 weeks (CON: 4441±132, TW: 3708±173, P=0.04, t=2.41, df=18) of trauma witness protocol (Fig. 1D, 2C, 3C, 4C). TW rats also demonstrated lower ambulatory activity than their matched controls following 1 week (CON: 4008±318, TW: 3215±183, P=0.04, t=2.15, df=18), 2 weeks (CON: 2932±216, TW: 2308±100, P=0.017, t=2.73, df=18), and 6 weeks (CON: 3764±150, TW: 3174±122, P=0.03, t=2.19, df=18) of trauma witness (Fig. 1E, 2D, 3D, 4D). Less distance was travelled by TW rats in the open-field when compared to their matched controls following 1 week (CON: 3876±107, TW: 3100±116, P=0.04, t=2.19, df=15), 2 weeks (CON: 3629±209, TW: 2243±178, P=0.002, t=3.94, df=15), and 6 weeks (CON: 4295±49, TW: 3437±112, P=0.01, t=3.92, df=15) of trauma witness protocol (Fig. 1F, 2E, 3E, 4E). No difference was observed between TW rats and their matched controls in any of the parameters of the OFT after 8 week of trauma witness protocol. In the FST, TW rats spent more time being immobile when compared to their matched controls after 1 week (CON: 61 ± 15, TW: 129 ± 6, t=4.2, p=0.001), 2 weeks (CON: 11 ± 4, TW: 61 ± 4, t=8.0, p=0.005) and 6 weeks (CON: 32 ± 12, TW: 63 ± 22, t=3.1, p=0.04) of trauma witness protocol, but even after 8 weeks (CON: 33 ± 11, TW: 68 ± 12, t=3.1, p=0.05) the depression-like behavior existed in TW rats (Fig. 1G, 2F, 3F, 4F).
Figure 2. Examination of anxiety and depression-like behaviors and cognitive deficits 2 week after TWM.
Light-dark test (A), elevated plus maze test (B). The open-field test determined total (C), ambulatory (D) activities and distance travelled (E). Forced swim test (F) were conducted. Short-term (G) and long-term memory (H) tests were conducted in all rats. (*) significantly different from control rats, p<0.05. Bars represent means ± SEM, n=10 rats/group.
Figure 3. Examination of anxiety and depression-like behaviors and cognitive deficits 6 week after TWM.
Light-dark test (A), elevated plus maze test (B). The open-field test determined total (C), ambulatory (D) activities and distance travelled (E). Forced swim test (F) were conducted in all rats. Short-term (G) and long-term memory (H) tests were conducted. (*) significantly different from control rats, p<0.05. Bars represent means ± SEM, n=10 rats/group.
Figure 4. Examination of anxiety and depression-like behaviors and cognitive deficits 8 week after TWM.
Light-dark test (A), elevated plus maze test (B). The open-field test determined total (C), ambulatory (D) activities and distance travelled (E). Forced swim test (F) were conducted. Short-term (G) and long-term memory (H) tests were conducted in all rats. (*) significantly different from control rats, p<0.05. Bars represent means ± SEM, n=10 rats/group.
Analysis of memory deficits in rat
TW rats made comparable errors when compared to their matched controls in the STM test at 1, 2, 6 and 8 weeks after TWM (Fig. 1H, 2G, 3G, 4G). On the other hand, in the LTM, TW rats committed significantly higher number of errors when compared to their matched controls at 1 week (CON: 0.7 ± 0.7, TW: 3.8 ± 0.9, t=2.9, p=0.04), 2 weeks (CON: 1 ± 0.5, TW: 4.7 ± 1.2, t=2.9, p=0.04) and 6 weeks (CON: 0.3 ± 0.3, TW: 3 ± 1.1, t=3.2, p=0.04) after witnessing social defeat of their cage-mate, but after 8 weeks (CON: 2.3 ± 0.8, TW: 2.3 ± 1.2, t=0, p=0.96), the LTM of TW rats was not different from their matched controls (Fig. 1I, 2H, 3H, 4H). Thus, witnessing social defeat did not significantly affect STM but the LTM consolidation was affected in these rats until 6 weeks after witnessing social defeat.
In separate experiments, we addressed the issue of the visual experience as a key component in induction of majority of the PTSD-like symptoms. Our results suggest that auditory stress (Fig. 5(i)A–I) arising from hearing sounds (ultrasonic vocalizations) of defeat process or olfactory stress (Fig. 5(ii)A–I) from smelling resident odor, fur and urine do not lead to key PTSD-like behaviors, including anxiety and depression-like behaviors or STM or LTM in rats, suggesting that visually witnessing traumatic events is essential for development of PTSD-like behaviors in rats.
Discussion
We have previously shown that rats witnessing social defeat of cage-mates for 7 consecutive days developed majority of the PTSD-like behavioral (anxiety and depression-like) and cognitive deficits [15]. In this study, we have validated the robustness of this animal model and have established that some of the features of PTSD-like symptoms in rats are reversible after a significant time lapse of the witnessing of traumatic events. We also have established that witnessing is critical to the PTSD-like phenotype and cannot be acquired solely via application of auditory or olfactory stresses.
Our results suggest that anxiety-like behaviors persisted until after 6 weeks of termination of TWM but seemed to be reversible beyond this time period when examined after 8 weeks of TWM. Similarly, the LTM impairments lasted until 6 weeks post TWM but also were reversed after 8 weeks of TWM. These results seem to follow the “Dual–Branch Hypothesis of PTSD”, previously put forth by Kamprath et al who had suggested aversive encounters (physical or witness) lead to simultaneous formation of associative and non-associative memories [9,], and both memory components must be inhibited for maintenance of PTSD-like symptoms. Gradual desensitization and habituation processes are thought to slowly decrease the response to an aversive stimulus. Our 8 week TWM data clearly suggests an involvement of habituation and/or desensitization processes. Interestingly, depression-like behavior persisted 8 weeks after the trauma witness protocol. This is not surprising considering that depression affects several neural circuits and mechanisms as compared to anxiety or memory function, and hence could take more time to normalize. Perhaps, 8 week time lapse of TWM is not enough to cause depressive recovery.
Another attractive feature of this study is the that we have tested the importance of visually witnessing the traumatic effects rather than only hearing the social defeat (ultrasonic vocalizations, auditory stress) or smelling the predator odor and urine (olfactory stress). Both auditory as well as olfactory stress in itself had no effect on anxiety and depression-like behaviors or memory when tested 2 weeks after subjecting them to auditory or olfactory stress. This supports the fact that visually witnessing the traumatic event is an important aversive stimulus for the development of PTSD-like symptoms in rats.
Conclusions
In summary, witnessing traumatic events but not directly experiencing those lead to behavioral and cognitive deficits, and some of the deficits including anxiety and long-term memory impairment last at least up to 6 weeks after conclusion of TWM. Witnessing is an integral part of the TWM as auditory and olfactory stresses are not enough to cause anxiety and depression-like behaviors which are the key components of PTSD-like phenotype. Our results underline the importance of stress sensitization and associative learning as explained by the dual-branch hypothesis. Finally, we suggest that TWM is an excellent model for studying vicarious trauma.
Highlights.
Witnessing trauma causes behavioral deficits
Post-trauma witnessing deficits last at least up to 6 weeks
Auditory and olfactory stresses alone are not enough to cause PTSD-like phenotype
Acknowledgments
Funding was provided by 1R15MH093918-01A1 grant awarded to S.S. G.P and S.S conceived and designed the experiments and wrote the manuscript. G.P, A.S and H.L conducted research and analyzed the data.
Abbreviations
- LE
Long Evans
- SD
Sprague Dawley
- TWM
Trauma witness model
- LD
light-dark test
- OFT
Open field test
- EPM
Elevated plus maze
- RAWM
Radial Arm Water Maze
- FST
Forced swim test
Footnotes
All authors read and approved the final manuscript and declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adamec RE, Shallow T. Lasting effects on rodent anxiety of a single exposure to a cat. Physiology & behavior. 1993;54:101–109. doi: 10.1016/0031-9384(93)90050-p. [DOI] [PubMed] [Google Scholar]
- 2.Alam S, Bhatnagar S. Current status of anti-diarrheal and anti-secretory drugs in the management of acute childhood diarrhea. Indian journal of pediatrics. 2006;73:693–696. doi: 10.1007/BF02898447. [DOI] [PubMed] [Google Scholar]
- 3.Beck CT. Secondary traumatic stress in nurses: a systematic review. Archives of psychiatric nursing. 2011;25:1–10. doi: 10.1016/j.apnu.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Bert B, Fink H, Huston JP, Voits M. Fischer 344 and wistar rats differ in anxiety and habituation but not in water maze performance. Neurobiology of learning and memory. 2002;78:11–22. doi: 10.1006/nlme.2001.4040. [DOI] [PubMed] [Google Scholar]
- 5.Bhatnagar S, Vining C, Iyer V, Kinni V. Changes in hypothalamic-pituitary-adrenal function, body temperature, body weight and food intake with repeated social stress exposure in rats. Journal of neuroendocrinology. 2006;18:13–24. doi: 10.1111/j.1365-2826.2005.01375.x. [DOI] [PubMed] [Google Scholar]
- 6.Cougle JR, Resnick H, Kilpatrick DG. Does prior exposure to interpersonal violence increase risk of PTSD following subsequent exposure? Behaviour research and therapy. 2009;47:1012–1017. doi: 10.1016/j.brat.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nature protocols. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iniguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolanos-Guzman CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamprath K, Wotjak CT. Nonassociative learning processes determine expression and extinction of conditioned fear in mice. Learning & memory. 2004;11:770–786. doi: 10.1101/lm.86104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nature neuroscience. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 11.Meichenbaum D. A clinical handbook/practical therapist manual for assesing and treating adults with post-traumatic stress disorder. 1994 [Google Scholar]
- 12.Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- 13.Patki G, Solanki N, Atrooz F, Allam F, Salim S. Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain research. 2013;1539:73–86. doi: 10.1016/j.brainres.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patki G, Solanki N, Atrooz F, Ansari A, Allam F, Jannise B, Maturi J, Salim S. Novel mechanistic insights into treadmill exercise based rescue of social defeat-induced anxiety-like behavior and memory impairment in rats. Physiology & behavior. 2014;130:135–144. doi: 10.1016/j.physbeh.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patki G, Solanki N, Salim S. Witnessing traumatic events causes severe behavioral impairments in rats. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2014;17:2017–2029. doi: 10.1017/S1461145714000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behavioural brain research. 2010;208:545–552. doi: 10.1016/j.bbr.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Siegmund A, Wotjak CT. Toward an animal model of posttraumatic stress disorder. Annals of the New York Academy of Sciences. 2006;1071:324–334. doi: 10.1196/annals.1364.025. [DOI] [PubMed] [Google Scholar]
- 18.Spigel IM, McDonald T. Effects of auditory stress on grooming dominance in the rat. Animal Learning and Behavior. 1974;2:165–167. [Google Scholar]
- 19.Takahashi LK. Olfactory systems and neural circuits that modulate predator odor fear. Frontiers in behavioral neuroscience. 2014;8:72. doi: 10.3389/fnbeh.2014.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vollert C, Zagaar M, Hovatta I, Taneja M, Vu A, Dao A, Levine A, Alkadhi K, Salim S. Exercise prevents sleep deprivation-associated anxiety-like behavior in rats: potential role of oxidative stress mechanisms. Behavioural brain research. 2011;224:233–240. doi: 10.1016/j.bbr.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biological psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yehuda R, Resnick H, Kahana B, Giller EL. Long-lasting hormonal alterations to extreme stress in humans: normative or maladaptive? Psychosomatic medicine. 1993;55:287–297. doi: 10.1097/00006842-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Zoladz PR, Fleshner M, Diamond DM. Psychosocial animal model of PTSD produces a long-lasting traumatic memory, an increase in general anxiety and PTSD-like glucocorticoid abnormalities. Psychoneuroendocrinology. 2012;37:1531–1545. doi: 10.1016/j.psyneuen.2012.02.007. [DOI] [PubMed] [Google Scholar]