Abstract
Rapastinel (GLYX-13) is a NMDA receptor modulator with glycine-site partial agonist properties. It is a robust cognitive enhancer and shows rapid and long-lasting antidepressant properties in both animal models and in humans. Contextual fear extinction (CFE) in rodents has been well characterized and used extensively as a model to study the neurobiological mechanisms of post-traumatic stress disorder (PTSD). Since CFE is NMDA receptor modulated and neural circuitry in the medial prefrontal cortex (MPFC) regulates both depression and PTSD, studies were undertaken to examine the effects of rapastinel for its therapeutic potential in PTSD and to use rapastinel as a tool to study its underlying glutamatergic mechanisms. A 21-day chronic mild unpredictable stress (CUS) rat model was used to model depression and PTSD. The effects of CUS alone compared to No CUS controls, and the effects of rapastinel (3 mg/kg IV) on CUS-treated animals were examined. The effect of rapastinel was first assessed using CUS-treated rats in three depression models, Porsolt, sucrose preference, and novelty-induced hypophagia tests, and found to produce a complete reversal of the depressive-like state in each model. Rapastinel was then assessed in a MPFC-dependent positive emotional learning paradigm and in CFE and again a reversal of the impairments induced by CUS treatment was observed. Both synaptic plasticity and metaplasticity, as measured by the induction of long-term potentiation in rat MPFC slice preparations, was found to be markedly impaired in CUS-treated animals. This impairment was reversed when CUS-treated rats were administered rapastinel and tested 24 hrs later. Transcriptomic analysis of MPFC mRNA expression in CUS-treated rats corroborated the link between rapastinel’s behavioral effects and synaptic plasticity. A marked enrichment in both the LTP and LTD connectomes in rapastinel-treated CUS rats was observed compared to CUS-treated controls. The effects of rapastinel on depression models, PEL, and most importantly on CFE demonstrate the therapeutic potential of rapastinel for the treatment of PTSD. Moreover rapastinel appears to elicit its therapeutic effects through a NMDA receptor-mediated, LTP-like, metaplasticity process in the MPFC.
Keywords: NMDA Receptor, GLYX-13, Depression, Medial Prefrontal Cortex, LTP
INTRODUCTION
Rapastinel (GLYX-13) is a NMDA receptor modulator with glycine-site partial agonist properties. It is an amidated tetrapeptide (threonine-proline-proline-threonine-amide) derived by cloning a hypervariable region of a monoclonal antibody, that was found to be a NMDAR-specific glycine site partial agonist [1]. In rat hippocampal slices, rapastinel preferentially enhanced conductance of NR2B-containing NMDARs at rat Schaffer collateral-CA1 synapses in vitro [2], and also enhanced the magnitude of long-term potentiation (LTP) of synaptic transmission, while simultaneously reducing that of long-term depression (LTD) [2, 3]. In whole animal studies, rapastinel enhanced performance in a variety of hippocampal-dependent learning tasks, including trace eyeblink conditioning and the Morris water maze, in both young adult and learning-impaired aging rats [3]. Rapastinel has also gone through extensive non-clinical studies and human clinical trials that showed it to have rapid and long-lasting antidepressant properties [4]. Thus, rapastinel is both a cognitive enhancer and a rapid acting, long lasting antidepressant.
Post-traumatic stress disorder (PTSD) is a major anxiety disorder that has been reported to affect as many as 30% of individuals that have experienced psychologically traumatic events [5]. PTSD is considered a memory disorder in which cues associated with the traumatic event form powerful sensory memories [6], hence context is a critical variable in fear conditioning and fear extinction. As such, contextual fear extinction (CFE) paradigms, used most often with rats, have been developed to mirror the effects of PTSD in humans and substantial validating data have been generated for them [7]. CFE in rodents is also considered to be a learning modulated process. Noteworthy here are data showing that inhibition of NMDA receptors inhibit fear extinction and that the regulation of amygdala output, perhaps the key brain structure in CFE, is modulated in part by infralimbic structures in the medial prefrontal cortex (MPFC) [8]. In addition, the neural circuits responsible for fear extinction are very well preserved across species including humans [9, 10]. The NMDA receptor glycine site partial agonist d-cycloserine (DCS) has shown efficacy in both preclinical models of contextual fear extinction and clinical trials for PTSD [7, 11–13].
The goal of the studies reported here was to evaluate the effects of rapastinel for its therapeutic potential in PTSD and as a tool to further understand glutamatergic mechanisms associated with PTSD. Rats were exposed to a chronic mild and unpredictable stressor (CUS) paradigm as the basis of the CFE model, because CUS induces a depressive-like phenotype that models clinical features of depression in humans and has been well characterized at the behavioral, physiological, cellular, and molecular levels. [14, 15].
MATERIALS AND METHODS
Animals
Adult male (2–3 month old) Sprague-Dawley (SD) rats were purchased from Harlan (USA). Rats were housed in Lucite cages with aspen wood chip bedding, maintained on a 12:12 light:dark cycle (lights on at 5 AM), and given ad libitum access to Purina lab chow (USA) and tap water throughout the study. All experiments were approved by the Northwestern University or New York Medical College Animal Care and Use Committees.
Drugs
Rapastinel was synthesized in free base form by Sai Life Sciences (India), and was administered in 1 ml/kg 0.9% sterile saline vehicle.
Chronic Unpredictable Stress (CUS) Procedure
Rats were exposed to a CUS protocol previously shown to elicit depression-like symptoms [16]. Animals received 21 days of CUS before dosing, and continued to receive CUS until the animals were sacrificed 2 days after the last behavioral test (total of 37 days of CUS). A total of 9 different CUS stressors were used (2 stressors per day). The stressors (days) included rotation on a shaker for 1 hr (days 3, 9, 13, 19, 24, 28, 33, 37) placement in a 4°C ambient chamber for 1 hr (days 1, 5, 12, 14, 18, 22, 26, 30, 36) lights off for 3 hours from 10:00 AM to 1:00 PM (days 2, 10, 17, 23, 31, 34, 37) lights on overnight (days 1, 5, 8, 13, 16, 22, 32, 34), strobe light overnight (days 3, 6, 9, 14, 17, 20, 23, 28, 31, 33), 45° tilted cages overnight (days 4, 7, 11, 15, 18, 21, 25, 29, 35), food and water deprivation overnight (days 2, 6, 10, 15, 19, 26, 27, 30), crowded housing overnight (days 4, 7, 11, 16, 21, 25, 29, 35), and isolation housing overnight (days 8, 12, 20, 24, 27, 32, 36). Animals in both the CUS and No CUS group were weighed every 7 days and received behavioral testing without additional stressors. Animals in the CUS groups received a single optimal dose of rapastinel (3 mg/kg iv) previously shown to produce a robust antidepressant-like response [4] or 1 ml/kg 0.9% sterile saline vehicle. For the transcriptomic analysis, animals did not receive any behavioral testing before sacrifice. The antidepressant-like effects of rapastinel both in non-stressed rats [4], and CUS-treated rats reported here did not habituate during the duration of the studies, hence, only a single administration of drug was used.
Porsolt Forced Swim Test
Porsolt forced swim testing was conducted as described in [4], and occurred 1 hr, 1 day, 7 days, and 14 days post-dosing. Animals were placed in a 46 cm tall × 20 cm in diameter clear glass tube filled to 30 cm with tap water (23 ± 1 °C) for 15 min on the first day (habituation) and 5 min on the subsequent test days (1 hr, 24 hrs, 1 week, 2 weeks post-dosing). Water was changed after every other animal. Animals were videotaped, and floating time was defined as the minimal amount of effort required to keep the animals head above water. Tapes were scored offline by a blind experimenter with high inter-rater reliability (Pearson’s r > .9).
Sucrose Preference Test
Sucrose preference testing was conducted as described previously [16], and occurred 3 days postdosing. Rats were exposed to a palatable sucrose solution (1%; Sigma, USA) for 48 hours, followed by 4 hours of water deprivation and a 1 hour exposure to two identical bottles, one filled with sucrose solution and the other with tap water. Sucrose preference was defined as (volume of sucrose solution consumed) / (volume of sucrose consumed + volume of water consumed), expressed as a percentage.
Novelty-induced Hypophagia (NIH) Test
Novelty-induced hypophagia testing was conducted as described previously [4], and occurred 2 days post-dosing. Animals were food deprived on the night before testing, and lab chow was placed into the center chamber of the open field (40 × 40 × 20 cm) for 10 min under dim-red lighting. Latency (in seconds) for the animal to take the first bite of food, as well as locomotor activity (line crosses) was scored offline from video recordings by an experimenter blinded to the treatment condition. Between animals, feces and urine were removed from the apparatus. Immediately after NIH testing, the latency to eat in the animal’s home cage was determined as a control. In a separate group of animals, food intake was determined in their home cage 24 hrs after food depravation.
Positive Emotional Learning (PEL) Test
Positive emotional learning measures the acquisition of hedonic/aversive ultrasonic vocalizations (USVs) to a social stimulus (heterospecific rough-and-tumble play) during a single 3 min test session. This assay captures both the positive (anhedonic) and negative symptoms (increased negative affect) of depression. Antidepressants have been shown to decrease rates of the aversive rat ultrasonic vocalization [17].
Heterospecific rough-and-tumble play was conducted as previously described [18], and testing occurred 3 hr and 2 weeks post-dosing. Heterospecific rough-and-tumble play stimulation was administered by the experimenter's right hand. Animals received 3 min of heterospecific rough-and-tumble play that consisted of alternating 15 sec blocks of heterospecific play and 15 sec of no-stimulation. High frequency ultrasonic vocalizations (USVs) were recorded and analyzed by sonogram with Avasoft SASlab Pro (Germany) as previously described [18]. Animals were not habituated to play stimulation before dosing and testing, and the experimenter was blind to treatment condition of the animals.
Contextual Fear Conditioning Test
Contextual fear conditioning and extinction testing was conducted as previously described [19], and the first extinction tests occurred 1 hr post-dosing. On the contextual fear training day (D0), animals were placed in a Coulbourn instruments (USA) shock chamber (40 × 40 × 40 cm) for 400 seconds and received three 0.5 mA 1 sec footshocks delivered to the floor bars at 90, 210, and 330 second timepoints. During extinction, rats were subjected to daily 5 min non-reinforced (no shock) extinction trials for the first 6 days after training, and on day 14 post-training (consolidation trial). Freezing was quantified via FreezeFrame software (Actimetrics, USA); at baseline (30–60 sec) on D0, and during the last 3 min of each extinction trial.
Medial Prefrontal Cortex (MPFC) Slice Electrophysiology
Slice LTP recording experiments were conducted as described previously [4], modified for MPFC slices [20]. For ex vivo studies, animals were dosed with rapastinel (3 mg/kg IV) or 0.9% sterile saline vehicle (1 ml/kg IV), then, 24 hr post-dosing, they were deeply anesthetized with isoflurane and decapitated. Brains were removed rapidly, submerged in ice-cold artificial cerebrospinal fluid (aCSF, 2–4 °C) which contained (in mM): 160 sucrose, 25 NaCl, 2.5 KCl, 4 MgCl2, 0.5 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 20 glucose; at pH 7.4, and gassed continuously with 95% O2/5% CO2). For in vitro studies, rapastinel (20–1000 nM) or aCSF vehicle was bath applied to slices 30 min prior to induction of LTP. MPFC slices were prepared as described in [20]. Four hundred µm slices were cut using a vibratome (Leica VT1200S) in a modified coronal orientation containing both prelimbic and infralimbic regions of the MPFC that are targets (via the fornix) of the hippocampal-MPFC pathway. MPFC slices were then transferred to an interface holding chamber which contained oxygenated cutting solution (in mM: 150 Sucrose, 25 NaCl, 2.5 KCl, 4 MgCl2, 1 CaCl2, 1.25 NaH2PO4, 10 Glucose, 26 NaHCO3) for incubation at 30°C for 30 minutes and then transferred to another interface holding chamber which contained oxygenated artificial cerebrospinal fluid (aCSF) solution at room temperature for additional 30 minutes before electrophysiological recording. Artificial cerebrospinal fluid (aCSF) contained (in mM: 126 NaCl, 2.5 KCl, 1.3 MgCl2, 2.5 CaCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose; at pH 7.4, and gassed continuously with 95% O2/5% CO2). For in vitro studies, rapastinel (20–1000 nM) or aCSF vehicle was bath applied to slices 30 min prior to induction of LTP.
Slices were transferred to a Haas-style interface recording chamber continuously perfused at 3 ml/min with oxygenated aCSF plus picrotoxin (10µM) and heated to 32 ± 0.5°C. Low resistance patch recording electrodes were made from thin-walled borosilicate glass (1–2 MΩ after filling with aCSF) with a Flaming/Brown micropipette puller (P97, Sutter Instruments) and inserted into layer III/IV of the prelimbic MPFC slices, and monosynaptically-evoked field excitatory postsynaptic potentials (fEPSPs) from layer V pyramidal neurons were recorded. A bipolar tungsten stimulating electrode (FHC, Bowdoin, ME) was placed on MPFC deep white matter inputs that contain afferents from multiple regions including the hippocampus, and constant current stimulus intensity adjusted to evoke approximately half-maximal fEPSPs once each 30 sec (50–100 pA; 100 µs duration from an ISO-flex isolater triggered by a Master 8 pulse generator, AMPI, Jerusalem, Israel). fEPSP slopes were measured before and after induction of LTP by linear interpolation from 20 to 80% of maximum initial negative deflection, and slopes confirmed to be stable to within ± 10% for at least 15 min before starting an experiment. LTP was induced by stimulation of input axons with 3 high frequency theta burst stimulus trains of 10 × 100 Hz/5 pulse bursts each, applied at an inter-burst interval of 200 ms. Each train was 2 sec in duration, and trains were applied 3 min apart. Electrophysiological data were digitized and sampled with an A/D board (National Instruments, Austin, TX) and a model 1700 differential AC amplifier (A-M Systems, Carlsborg, WA) controlled by SciWorks software (Datawave Technologies, Loveland, CO).
Whole cell patch clamp recordings were performed as previously described [4] using a MultiClamp 700B amplifier (Molecular Devices, Union City, CA). Patch pipette resistance ranged from 6 to 6.5 MΩ when filled with intracellular solution that contained (in mM): 135 CsMeSO2, 8 NaCl, 10 HEPES, 0.2 EGTA, 2 Mg-ATP, 0.3 Na-GTP, and 1 QX-314 [N-(2,6-dimethylphenylcarbamoylthyl)-triethylammonium bromide], 275 mOsm, pH 7.25 adjusted with Cs(OH)2. Neurons in layer V of prelimbic MPFC were visualized by infrared imaging and patched using a 60× water-immerged objective mounted to a Zeiss microscope (Axioskop 2 Fs plus). After whole-cell voltage clamp configuration was established, access resistance was carefully monitored, and only cells with stable access resistance (<5% change) were included in analyses. Electric signals were filtered at 3kHz and digitized at 10 kHz with a Digidata 1322A controlled by a Clampex (v9.2) (Molecular Devices, Union city, CA). A bipolar tungsten stimulating electrode (FHC, Bowdoin, ME) was placed in the MPFC deep white matter input, at least 200 µm from the patched neuron, and stimulus pulses (800 µS duration) were delivered at 15 sec intervals. Neurons were voltage clamped at −70 mV to record EPSCs to assess input-output relations and paired-pulse facilitation. Neurons were clamped at −40 mV for recording NMDA currents, to relieve voltage-dependent magnesium block, and slices were perfused with ACSF containing 0 added magnesium, 3 mM calcium, 10 µM picrotoxin, and 10 µM CNQX, to isolate NMDA conductances.
All aCSF and internal pipette solutions were made with deionized distilled water (resistance > 18 MΩ cm−2; Milli-Q system; Millipore, USA), using salts purchased from Sigma-Aldrich (St. Louis, MO). All data were further processed and presented with Origin 6.1 (Originlab Corp, Northhampton, MA), CorelDRAW 12 (Corel Corp, Ottawa) and GraphPad Prism 6 (Graphpad Software, San Diego, CA) programs.
Transcriptome Profiling
Microarray and data analyses were conducted as previously described [4,18, 21–24]. Triplicate microarray analyses were performed using the MPFC isolated from rats treated with CUS and rapastinel (3 mg/kg IV; CUS + rapastinel), CUS and saline vehicle (1 ml/kg IV; CUS + vehicle), or No CUS and saline vehicle (No CUS) (n = 5–8 per group). At 24 hr post-dosing, their brains were rapidly removed, frozen on dry ice, and the MPFC was dissected and stored at −80°C until assay. Individual 45-mer oligonucleotides complementary to sequences of 1283 cloned rat CNS mRNAs were synthesized on a PolyPlex™ 96-well oligonucleotide synthesizer (GeneMachines®, USA) and spotted in triplicate onto epoxy coated slides (Telechem, USA) using an OmniGrid™ robotic microarrayer (GeneMachines®). Total RNA was extracted (RNeasy, Qiagen, USA) and used as the substrate for RNA amplification and labeling using the Eberwine protocol (Van Gelder et al, 1990). Two micrograms of Cy5-labeled (experimental) and Cy3-labeled (universal rat reference, Stratagene, USA) amplified RNA (aRNA) were cohybridized on individual arrays at 46°C for 16 hr. Arrays were scanned using two lasers (633 nm and 543 nm) at 5 µm resolution on the ScanArray 4000XL (Packard Biochip Technologies, USA). Raw image files were normalized using locally weighted scatter plot smoothing (LOWESS) curve-fitting (GeneSpring, USA). Data were analyzed using the Significance Analysis of Microarrays (SAM, v2.23a, Stanford University, USA) algorithm with a 10% false discovery rate (FDR) threshold. Balancing the level of stringency (FDR) and the accompanying level of acceptable false positives with the size of the final dataset is central to the interpretation of subsequent post hoc ontological analyses that are dependent upon this dataset. While higher stringencies generate smaller, higher confidence gene sets, this strategy will potentially miss biologically relevant ontological correlations.
Ontological Analyses
The genes identified as differentially expressed by SAM analysis were examined for their biological association to gene ontology (GO) categories [25] as defined by the GO Consortium [25]. Analyses were performed using the ontological mapping software GoMiner (Zeeberg et al., 2003, available at (http://discover.nci.nih.gov/gominer). This algorithm calculated the enrichment or depletion of individual ontological categories with genes that had changed expression and identified cellular pathways potentially relevant to rapastinel-associated reversal of CUS-induced synaptic deficits. Pathways within three independent functional hierarchies, namely biologic process, molecular function, and cellular compartment, were queried. Statistical analysis (via Fisher’s exact test) highlighted the total number of genes represented in each GO pathway, as well as the proportion of those up- and down-regulated and changed. Importantly, GoMiner ascribed a level of significance to these changes within each individual GO annotation and potential biological characteristics of a given coregulated gene set. A P<0.05 was considered significant.
As an adjunct to GoMiner-based ontological analyses, and to gain more insight into enrichment of gene groups functionally related to long-term potentiation (LTP) and long-term depression (LTD), we examined select segments of the microarray datasets using DAVID (Database for Annotation, Visualization and Integrated Discovery, v6.7; NIH, USA) ([26, 27], available at http://david.abcc.ncifcrf.gov). Like GoMiner, DAVID takes into account the frequencies of genes belonging to a particular ontological group among the genes found to be regulated and among all of the genes studied in the experiment. However, unlike GoMiner, DAVID utilizes biological classifications based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways that, unlike the analogous GoMiner categories, optimally capture the glutamatergic component of the LTP and LTD pathways.
Statistical Analyses
Behavioral and electrophysiological data were analyzed by ANOVA, followed by Fisher’s PLSD post hoc test using StatView (SAS institute Inc., USA). Microarray data were analyzed by SAM, DAVID, and GoMiner as described above. The level of statistical significance was preset to P < .05.
RESULTS
A Single IV Dose of Rapastinel Produces a Long-Lasting Antidepressant-Like Effect in the Porsolt, Sucrose Preference, and NIH Tests in Rats Exposed to CUS
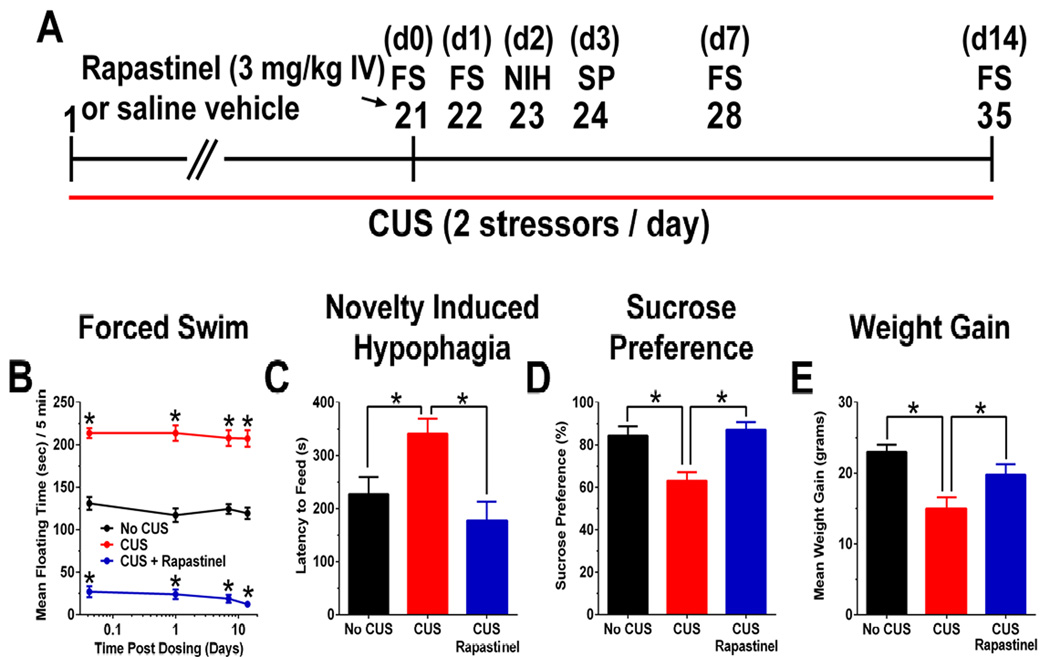
As shown in Figure 1, rats treated with CUS + rapastinel (3 mg/kg, IV) showed a significantly reduced floating time in the Porsolt forced swim test (1 hr, 1 day, 1 week, and 2 weeks post-dosing), when compared with rats treated with CUS + vehicle, and CUS + vehicle rats showed increased floating times compared to No CUS rats [F(2, 113) = 774.6, P < .05; Fisher’s PLSD post hoc test rapastinel vs. vehicle, or vehicle vs. No CUS control, P < .05].
Figure 1. Rapastinel (3 mg/kg IV) produces a rapid-acting and long-lasting antidepressant-like effect in a CUS paradigm.
(A) Schematic demonstrating the timeline for CUS exposure, drug administration, and behavioral testing. Numbers in parentheses represent days after drug administration. Rats were exposed to CUS and administered a single dose of rapastinel (3 mg/kg IV) or 0.9% sterile saline vehicle (1 ml/kg IV) on day 21. Mean ± SEM floating time in the (B) Porsolt forced swim test (FS) in CUS treated rats treated with a single dose of Rapastinel (3 mg/kg IV; n = 10), saline vehicle (n = 10), or No CUS exposed control rats (n = 9) tested 1 hr, 1 day, 1 week, and 2 weeks post-dosing. Mean ± SEM (C) latency to feed in the novelty induced hypophagia (NIH) test (2 days post-dosing), (D) sucrose preference (SP) in the sucrose preference test, 3 days post-dosing, and (E) change in body weight (2 weeks post-dosing compared to CUS day 0). * P < .05 Fisher’s PLSD post hoc test vs. No CUS group or CUS vehicle group.
Rapastinel increased sucrose preference scores in CUS + rapastinel rats (4 days post-dosing) when compared with CUS + vehicle rats. CUS + vehicle rats showed significantly decreased sucrose preference scores compared to No CUS rats [F(2, 26) = 10.8, P < .05; Fisher’s PLSD post hoc test rapastinel vs. vehicle, vehicle vs. No CUS control, P < .05].
Rapastinel treatment significantly decreased feeding latency in a novel environment in the NIH test (2 days post-dosing) compared to CUS + vehicle, while CUS + vehicle rats showed longer feeding latencies compared to No CUS rats [F(2, 26) = 7.0, P < .05; Fisher’s PLSD post hoc test rapastinel vs. vehicle, vehicle vs. No CUS control, P < .05], but no changes were seen in latency to eat in the home cage [F(2, 26) = 0.1, P > .05; Supplemental figure 1] or line crosses in the novel environment [F(2, 26) = 2.4, P > .05; Supplemental figure 1]. Rapastinel did not alter total food consumption in the home cage measured 1 hr and 24 hrs [F(2, 24) = 0.2, P < .05] after testing (Supplemental figure 1).
Rapastinel treatment also increased weight gain (2 weeks post-dosing) compared to CUS + vehicle rats, while CUS + vehicle rats showed decreased weight gain compared to No CUS rats [F(2, 26) = 8.1, P < .05; Fisher’s PLSD post hoc test rapastinel vs. vehicle, vehicle vs. No CUS control, P < .05].
Rapastinel Increased Positive Emotional Learning (PEL) in Rats Exposed to CUS
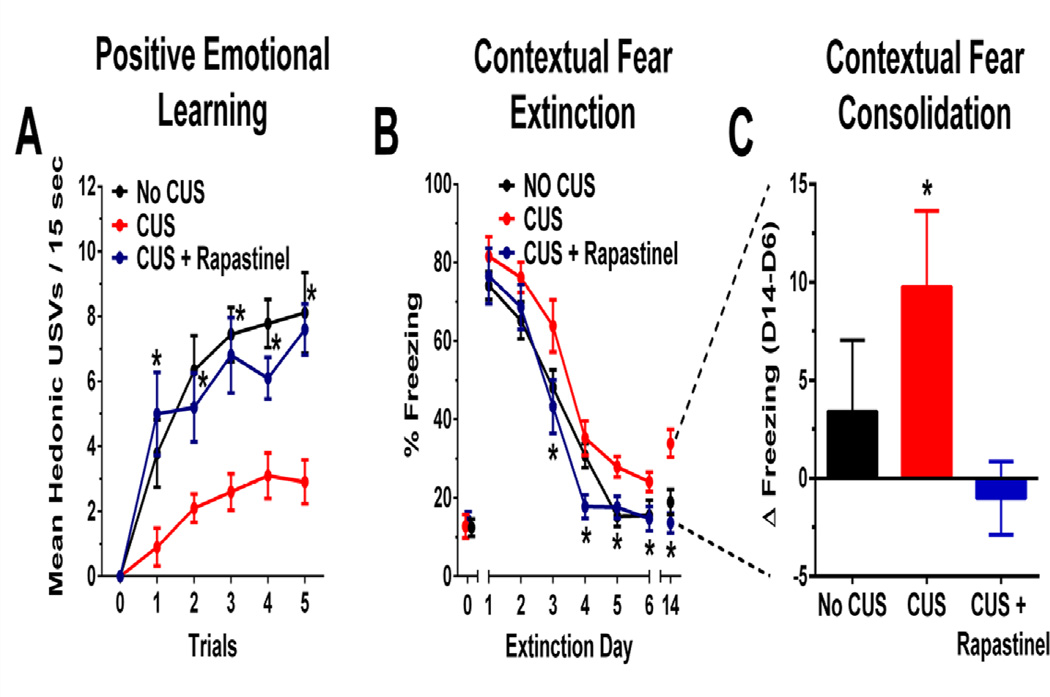
As shown in Figure 2, CUS + rapastinel (3 hr and 2 weeks post-dosing) increased rates of hedonic USVs in response to a conditioned stimulus (CS) that predicted heterospecific play when compared with CUS + vehicle treatment, and CUS + vehicle rats showed decreased CS-elicited hedonic USVs compared to No CUS rats [F(2, 26) = 11.2 (3 hrs), 5.5 (2 week), P < .05 Fisher’s PLSD post hoc test rapastinel vs. vehicle, vehicle vs. No CUS control, P < .05].
Figure 2. Rapastinel (3 mg/kg IV) facilitates MPFC-dependent positive emotional learning and fear extinction in CUS-treated rats.
(A) Mean ± SEM hedonic USVs in response to a conditioned stimuli that predicts heterospecific play 3 hrs post-dosing with Rapastinel (3 mg/kg IV) or 0.9% sterile saline vehicle (1 ml/kg IV) in 2–3 month old adult male SD rats exposed to a depressogenic regimen of chronic unpredictable stress (CUS; 2 stressors / day for 21 consecutive days) or non-stressed control rats (No CUS). A similar increase in PEL was seen in animals tested 2 weeks post-dosing (results section). (B–C) Mean ± SEM % time freezing in CUS treated animals dosed with rapastinel (3 mg/kg IV) or sterile saline vehicle (1 ml/kg IV) 1 hr before extinction day 1. Three shocks (0.5 mA, 1 s) were administered 90, 210, and 330 sec after animals were placed in the conditioning chamber on D0. During extinction, rats were submitted to a 5 min non-reinforced test trial every 24 hrs for 6 consecutive days (D1-6), and on day 14 post-conditioning (consolidation trial). Freezing was quantified via FreezeView software and was measured at baseline (30–60 sec) on D0, and during the last 3 min of each extinction trial. N = 9 – 14 rats per group. * P < .05 Fisher’s PLSD post hoc test vs. No CUS group (A–B), * P < .05 within subjects t-test, 2 tailed (C).
In addition, CUS + rapastinel (3 hr and 2 weeks post-dosing) increased rates of hedonic USVs and reduced rates of aversive 20-kHz compared to CUS + vehicle, while CUS + vehicle rats showed decreased hedonic USVs and increased aversive USVs when compared with No CUS rats [Hedonic USVs F(2, 26) = 65.7 (3 hr), 61.7 (2 week), P < .05; Aversive USVs F(2, 26) = 9.9 (3 hr), 8.9 (2 week), P < .05 Fisher’s PLSD post hoc test rapastinel vs. vehicle, vehicle vs. No CUS control, P < .05; data not shown]. CUS + rapastinel increased both running speed to self-administer heterospecific play and center crosses when compared with CUS + vehicle, while CUS + vehicle rats showed decreased running speed and center crosses when compared with No CUS rats [Running Speed F(2, 26) = 9.9 (3 hr), 7.2 (2 week), P < .05; Center Crosses F(2, 26) =9.9 (3 hr), 27.4 (2 week), P < .05 Fisher’s PLSD post hoc test rapastinel vs. vehicle, vehicle vs. No CUS control, P < .05; data not shown], but did not alter locomotor activity as measured by line crosses [F(2, 26) =0.6 (3 hr), 0.7 (2 week), P > .05; data not shown].
Rapastinel Facilitated Contextual Fear Extinction and Prevented Fear Re-Consolidation in Rats Exposed to CUS
As shown in Figure 2, CUS + rapastinel (3–14 days post-dosing) rats showed decreased freezing levels as compared to CUS + vehicle rats, and CUS+ vehicle rats showed increased freezing levels compared to No CUS rats [F(2, 29) = 4.4, P < .05; Fisher’s PLSD post hoc test rapastinel vs. vehicle, vehicle vs. No CUS control, P < .05]. CUS + vehicle treatment increased freezing levels on the consolidation retrieval day (D14) when compared to D14 (t(8) = 2.5, P < .05), whereas CUS + rapastinel and No CUS rats did not show this effect (all P’s > .05), indicating that rapastinel reversed the CUS-induced enhancement of fear conditioning.
Rapastinel Persistently Enhances the Magnitude of Long-term Potentiation (LTP) of Synaptic Strength in the MPFC
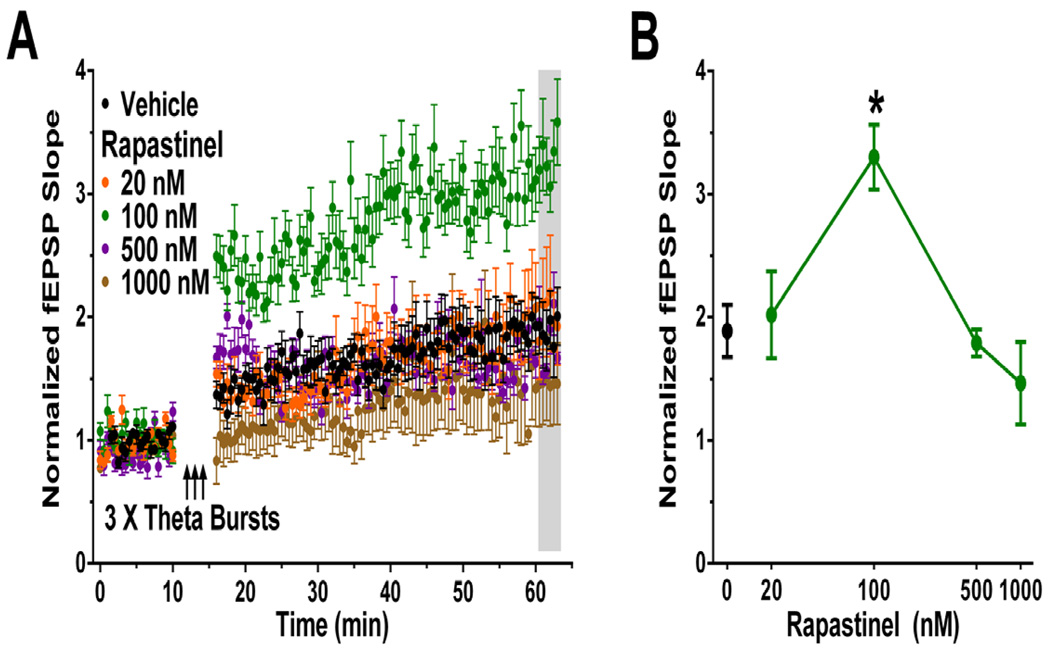
As shown in Figure 3, acute bath application of rapastinel (100nM) increased the magnitude of LTP elicited by 3 submaximal high-frequency theta burst stimulus trains in MPFC slices [F(4,37) = 7.3, P < 0.05; Fisher’s PLSD post hoc test vehicle vs. rapastinel, P < 0.05].
Figure 3. In vitro application of Rapastinel (100 nM) enhances the magnitude of LTP in the MPFC.
(A) Time course, and (B) Mean ± SEM 45 min post-theta burst stimulation, of MPFC layer II/III evoked normalized field EPSP slopes in rat slices recorded from MPFC layer IV. Three submaximal bouts of high-frequency layer II/III stimulation (10 × 100 Hz / 5 pulse bursts each, applied at an inter-burst interval of 200 ms) were applied at 3 minute intervals, and LTP was measured 45–47 min post-tetanus (shaded). Rapastinel (20 – 1,000 nM) was bath applied starting 10 min before tetanus, and remained in the bath throughout the experiment. N = 5–11 slices per group. * P < .05 Fisher’s PLSD post hoc test vs. aCSF control group.
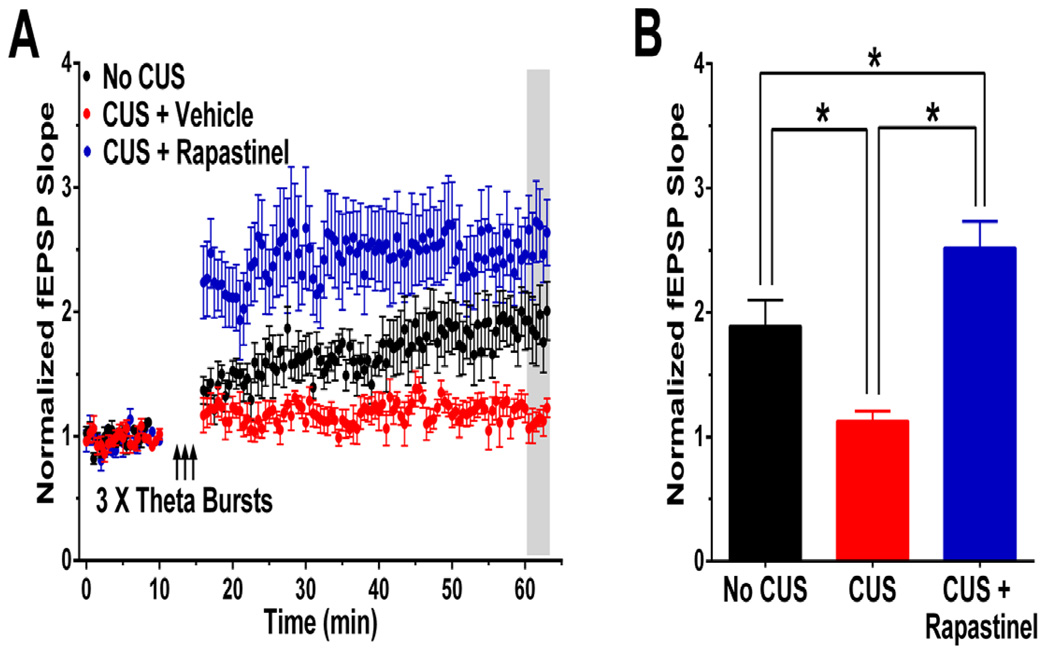
As shown in Figure 4, a single in vivo dose of rapastinel (3 mg/kg iv) administered 24 hrs prior to MPFC slice preparation also increased the magnitude of LTP elicited by 3 submaximal high-frequency theta burst trains in MPFC slices [F(2,24) = 12.6, P < 0.05; Fisher’s PLSD post hoc test for each possible comparison, P < 0.05]. The NMDAR antagonist D-AP5 (25 µM) eliminated LTP in these MPFC synapses [F(2,17) = 23.2, P < 0.05; Fisher’s PLSD post hoc test for CUS + rapastinel vs. CUS + rapastinel + AP5, P < 0.05; Supplemental Figure 2]. These data demonstrate that rapastinel both acutely enhanced the magnitude of LTP, and elicited a long-lasting metaplastic increase in the sensitivity of mechanisms for inducing NMDAR-dependent LTP. In addition, rapastinel reversed CUS-induced increases in the NR2B/NR2A NMDAR current ratio in layer V pyramidal cells in the MPFC [F(2,27) = 22.5, P < 0.05; Fisher’s PLSD post hoc test for each possible comparison, P < 0.05], while not altering basal synaptic transmission as measured by either input/output relations [F(1,13) = 0.1, P > 0.05], or paired-pulse facilitation [F(1,13) = 0.1, P > 0.05], in layer V pyramidal cells in the MPFC (Supplemental figure 2).
Figure 4. Rapastinel (3 mg/kg IV) rescues CUS-induced deficits in LTP in the MPFC 24 hrs post-dosing.
(A) Time course, and (B) Mean ± SEM 45 min post-theta burst stimulation of normalized field EPSP slopes evoked in layer II/III and recorded in layer IV of rat MPFC slices from 2–3 month old adult male SD rats exposed to a depressogenic regimen of 21 days of Chronic Unpredictable stress (CUS; 2 stressors / day for 21 consecutive days; red circles and bar), versus control animals (No CUS; black circles and bar). CUS-treated animals were dosed with rapastinel (3 mg/kg IV; blue circles and bar) or 0.9% sterile saline vehicle (1 ml/kg IV) 24 hrs before ex-vivo LTP testing. LTP was measured 45–47 min post-tetanus (shaded). N = 8–10 slices per group. * P < .05 Fisher’s PLSD post hoc test.
Rapastinel Reversed CUS-induced Transcriptome Changes Associated with Synaptic Plasticity in MPFC
Exposure to CUS resulted in robust cortical transcriptomic changes (Supplementary Table 1). Ontological analysis of these changes demonstrated that a wide variety of cellular pathways were significantly affected (Supplementary Table 3). Up-regulation of protein, carbohydrate, and lipid metabolism were among the most significant changes observed, as exemplified both by the large number of genes affected in each of these categories and the high level of significance ascribed to these changes. In addition to substantial changes in cellular metabolism, exposure to CUS resulted in significant down-regulation of gene expression within biochemical pathways directly related to synaptic plasticity; including synapse structure/function and glutamate receptor-mediated cell signaling, as well as cytoskeletal-associated genes potentially related to neuronal morphology, synapse remodeling and stabilization. Importantly, significant enrichment of GoMiner pathways localized within the synaptic and post-synaptic compartments was also demonstrated (Table 1).
Table 1. Rapastinel leads to the reversal of significant CUS-induced deficits in pathways related to synaptic plasticity-associated gene expression in the MPFC.
The genes identified as differentially expressed when comparing (A) CUS-treated and No CUS samples and (B) rapastinel -treated CUS and CUS-treated samples were examined for their biologic association to Gene Ontology (GO) categories (detailed in the text). The significance and directionality of the calculated enrichment in each GO category was calculated as a p-value using Fisher's Exact Test (2-tailed) and is represented by a different color for each category: the ontological categories significantly down-regulated in green and those upregulated in red.
| A. CUS vs. No CUS | |
|---|---|
| GO Term | p-value |
| Cellular Metabolismh | |
| cellular protein metabolic process | 0.004 |
| cellular lipid metabolic process | 0.019 |
| cellular carbohydrate metabolic process | 0.016 |
| Synaptic Transmission | |
| synaptic transmission | 0.021 |
| transmission of nerve impulse | 0.005 |
| Signaling | |
| cell-cell signaling | 0.032 |
| cell surface receptor-linked signaling | 0.034 |
| glutamate signaling pathway | 0.017 |
| protein serine/threonine kinase activity | 0.023 |
| GTPase activity | 0.014 |
| Receptor/Channel Activity | |
| glutamate-gated ion channel activity | 0.021 |
| NMDA-selective glutamate receptor activity | 0.050 |
| Synapse Structure/Function | |
| regulation of postsynaptic membrane potential | 0.018 |
| negative regulation of cytoskeleton organization | 0.008 |
| Synaptic Protein Complexes | |
| receptor complex | 0.024 |
| protein kinase CK2 complex | 0.020 |
| Synaptic Compartment | |
| synapse | 0.026 |
| synaptosome | 0.019 |
| synaptic vesicle | 0.024 |
| postsynaptic membrane | 0.049 |
| B. CUS (+rapastinel) vs. CUS (+vehicle) | |
|---|---|
| GO Term | p-value |
| Cellular Metabolism | |
| cellular protein metabolic process | 0.015 |
| nucleic acid metabolic process | 0.023 |
| Synaptic Transmission | |
| positive regulation of synaptic transmission | 0.005 |
| positive regulation of transmission of nerve impulse | 0.008 |
| Signaling | |
| cell-cell signaling | 0.010 |
| protein kinase C activity | 0.020 |
| protein serine/threonine kinase activity | 0.025 |
| calmodulin-dependent protein kinase activity | 0.011 |
| Receptor/Channel Activity | |
| ionotropic glutamate receptor activity | 0.003 |
| AMPA-selective glutamate receptor activity | 0.006 |
| kainate selective glutamate receptor activity | 0.011 |
| Synapse Structure/Function | |
| regulation of synapse structure and activity | 0.038 |
| cytoskeleton-dependent intracellular transport | 0.004 |
| Synaptic Protein Complexes | |
| Ca++ and calmodulin-dependent protein kinase complex | 0.022 |
| ionotropic glutamate receptor complex | 0.001 |
| Synaptic Compartment | |
| synapse | 0.027 |
| synaptosome | 0.044 |
| endocytic vesicle | 0.001 |
| dendritic spine | 0.001 |
Treatment of animals exposed to CUS with a single dose of rapastinel also led to a robust transcriptomic response, with synaptic changes in the opposite direction consistent with the reversal of CUS-induced deficits (Table 1 and Supplementary Table 2). Along with significant down-regulation of processes associated with protein metabolism, treatment with rapastinel also led to significant down-regulation of nucleic acid (DNA/RNA) metabolism. In addition, the up-regulation of cytoskeletal-associated processes, including intracellular vesicular transport; again, is consistent with the reversal of CUS-induced changes. The most robust effects of rapastinel treatment were the up-regulation of biological processes associated with the positive regulation of synaptic transmission, ionotropic glutamate receptor activity and intracellular signaling, kinase-mediated changes, and synaptic remodeling. All of these changes were reflective of significant enrichment of biological activity in synaptic compartments (Supplementary Tables 2 and 4; summarized in Table 1).
In order to augment the GoMiner-based analyses, and to specifically characterize CUS-induced deficits in LTP/LTD pathways, we evaluated the CUS-induced down-regulated gene set (i.e., reflective of the CUS-induced deficits) using DAVID analysis (Table 2). CUS treatment led to a significant, albeit modest, enrichment in LTD-associated transcripts (KEGG pathway rno04730) that were down-regulated in the medial prefrontal cortex, compared to vehicle treated rats (1.75-fold enrichment, P < 0.05). No CUS-induced deficits in LTP-associated transcripts (KEGG pathway rno04720) were observed.
Table 2. Rapastinel leads to the rescue of significant CUS-induced deficits in pathways related to synaptic plasticity-associated gene expression patterns in the MPFC.
Rapastinel-treated CUS, CUS, and No CUS treated samples were examined for their biologic association to Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways associated with synaptic plasticity (LTP and LTD). The significance and directionality of the calculated enrichment in each KEG pathway was calculated as a p-value using Fisher's Exact Test (2 tailed) and is represented by a different color for each category: the ontological categories significantly down-regulated in green and those upregulated in red.
| CUS vs. No CUS | ||
|---|---|---|
| KEGG Term | Fold Enrichment | P value |
| rno04720:Long-term potentiation (LTP) | 1.38 | n/s |
| rno04730:Long-term depression (LTD) | 1.75 | 0.049 |
| CUS (+rapastinel) vs. CUS (+vehicle) | ||
|---|---|---|
| KEGG Term | Fold Enrichment | P value |
| rno04720:Long-term potentiation (LTP) | 2.94 | 9.16E-6 |
| rno04730:Long-term depression (LTD) | 2.70 | 0.001 |
To determine the effects of rapastinel on CUS-induced deficits in LTP and LTD pathways, we similarly evaluated the rapastinel-upregulated gene set using DAVID. A single administration of rapastinel to CUS-treated rats produced a marked enrichment of LTP-associated transcripts (KEGG pathway rno04720, 2.94-fold enrichment, P < 0.00001) that were up-regulated compared to CUS-treated rats administered vehicle. Rapastinel administration also led to a significant enrichment in up-regulated LTD-associated transcripts (KEGG pathway rno04730, 2.70-fold enrichment, P < 0.001).
DISCUSSION
The studies presented here were undertaken to evaluate rapastinel for its therapeutic potential for the treatment of post-traumatic stress disorder (PTSD), and to use it as a tool to further investigate glutamatergic mechanisms shared by learning and memory, depression, and PTSD. A mild chronic unpredictable stress (CUS) paradigm was used as the foundation for these studies because it captures both the positive and negative symptoms of depression and is well suited for contextual fear extinction studies that were used here to model PTSD.
NMDA receptor modulators, in particular DCS and ketamine, have shown therapeutic efficacy in PTSD patients [11, 28]. However, DCS appears to be limited in that it shows relatively poor efficacy in depressed patients [29, 30] and ketamine displays marked dissociative effects and is considered a substance of abuse, making repeat dosing problematic [31, 32]. Rapastinel, on the other hand, has been shown to have robust rapid acting and long lasting antidepressant properties and shows none of the side effects of ketamine and other NMDA receptor antagonists [33].
Richter-Levin and Maroun coined the term “behavioral metaplasticity” based on their observations that an external, acute behavioral stressor led to marked suppression of LTP in MPFC [34–36]. The electrophysiological studies reported here showed that rapastinel reversed the CUS-induced: (1) depressive effects; (2) reduction in PEL; and (3) induction of contextual fear. Furthermore, the ability to suppress NMDAR-dependent LTP in the MPFC was completely reversed by rapastinel treatment. In fact, the magnitude of LTP in MPFC slices of CUS plus rapastinel rats was significantly greater than even in control, No CUS rats. Further, rapastinel also rescued CUS-induced increases in NR2B/NR2A NMDAR current ratios in the MPFC, without affecting basal synaptic transmission. Thus, a metaplasticity-type mechanism appears to be playing a key role in the long-term antidepressant properties of rapastinel, and could also account for the effects of rapastinel in facilitating CUS-induced contextual fear extinction.
Transcriptomic analyses reinforced the behavioral metaplasticity hypothesis by showing that rapastinel treatment restored CUS-induced deficits in gene expression patterns in the MPFC associated with synaptic plasticity. And consistent with rapastinel acting as an NMDAR functional partial agonist, CUS-induced deficits in glutamatergic receptor activity and related signaling pathways were also reversed by rapastinel. The most significantly enriched molecular pathway affected by rapastinel treatment of CUS animals was that associated with the formation of LTP.
It is noteworthy that the individual transcript changes were at most two-fold, begging the question as to the linkage between fold-change and biological significance. Many studies have reported that biologically relevant transcriptomic expression patterns can involve groups of transcripts with relative abundance changes considerably less than two-fold. Additionally, it has been argued that interpreting the meaning of quantitative changes in individual transcripts alone, without the additional statistical rigor provided by the concomitant analysis of ontological interaction networks [37], can severely limit critical insights into relevant biologic processes. As such, to identify gene families and significantly affected biochemical pathways involved in treatment-dependent transcriptome responses, bioinformatics tools such as GoMiner, DAVID, Gene Set Enrichment Analysis (GSEA), or HiMAP-based interactome analysis are included as a key element of the focused oligonucleotide microarray platform used in the studies reported here [4, 18, 21, 22, 24, 38–41]. These tools extend the interpretation of large-scale experiments from the identification of single genes to the identification of linked biochemical pathways and processes, and focus on gene sets that tend to be more reproducible and interpretable. Single gene methods are powerful only when the individual gene effect is marked and the variance is small across samples- atypical of complex drug responses. Ontological analyses are instrumental if complex phenotypes result from modest variation in the expression or activity of multiple members of the same pathway. In other words, as the single-gene analyses revealed only modest changes and did not fully illuminate globally affected pathways, GoMiner analysis provided additional statistical power to discriminate gene sets modified in complex disease paradigms (like CUS) and complex drug responses (e.g., following rapastinel treatment) from large datasets.
In conclusion, rapastinel induced changes in behavior (the reversal of CUS-induced deficits in MPFC-dependent positive emotional learning and contextual fear extinction), associated metaplasticity (the reversal of CUS-induced inhibition of LTP and reversal of CUS-Induced increases in NR2B/NR2A NMDAR current ratio), and MPFC-associated gene expression patterns (restoration of CUS-induced inhibition of LTP-associated transcripts suppressed by CUS), all substantiate the argument for an NMDAR-triggering mechanism akin to LTP-like activity-dependent long-term synaptic plasticity associated with learning and memory. Rapastinel appears to exert its antidepressant effects, cognitive enhancing effects, and its ability to facilitate contextual fear extinction, at least in part, by a shared glutamatergic-based mechanism and, as such, rapastinel may have therapeutic potential for PTSD.
Supplementary Material
Mean ± SEM (A) line crosses in the NIH test, (B) latency to take the first bite of food in the home cage following the NIH test (Feeding latency in the novel cage is reported in figure1), and food intake in the home cage (C) 1 hr and (D) 24 hrs after overnight food deprivation in CUS treated rats treated with a single dose of rapastinel (3 mg/kg IV; saline vehicle, or No CUS exposed control rats tested 3 days post-dosing. N = 9–10 per group. All P values > .05
Mean ± SEM (A) NR2B current / total NMDA current in slices from control (No CUS, black bar), CUS (red bar) and CUS + rapastinel (blue bar) rats. (B) Effects of CUS (red bar), CUS + rapastinel (dark blue bar), and the NMDAR antagonist D-AP5 following CUS + rapastinel (light blue bar), on the magnitude of LTP, with normalized slope of 1.0 being no LTP, (C) EPSC Peak Current as a function of stimulus intensity in slices from CUS (red circles) and CUS + rapastinel (blue circles) rats. (D) Paired-Pulse Facilitation (PPF) ratio in CUS treated rats treated with a single dose of rapastinel (red circles, 3 mg/kg IV, saline vehicle), versus No CUS exposed control rats (blue circles). Ex vivo electrophysiological studies in layer V MPFC were performed 24 hrs post-dosing. N = 3–12 per group. * P < .05 Fisher’s PLSD post hoc test.
Highlights.
Chronic unpredictable stress (CUS) induced a PTSD-like state in rats
Rapastinel reversed CUS-induced PTSD-like behavior
Rapastinel reversed CUS-induced deficits in MPFC-dependent learning and memory
Rapastinel reversed CUS-induced suppression of MPFC LTP
ACKNOWLEDGEMENTS
The authors wish to thank Mr. Derek Small and Dr. Jeffrey Sprouse for their helpful discussions. We thank the Northwestern University Behavioral Phenotyping Core for their assistance.
FUNDING
JR Moskal was further supported by grants from The Ralph and Marian Falk Medical Research Trust (Chicago, IL), JS Burgdorf was supported by NIH grant MH094835, PK Stanton by NIH grant NS044421, C Weiss by NIH grant NS059879, and JF Disterhoft by NIH grant MH47340.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
JR Moskal is the founder of Naurex, Inc., has founders' shares of stock in the company, and also receives financial compensation as a consultant. PK Stanton, JS Burgdorf, and JF Disterhoft are consultants for Naurex, Inc., and have received financial compensation and stock. RM Burch, AL Gross and RA Kroes are employees of Naurex, Inc., and have received financial compensation and stock. JD Leander is a paid consultant for Naurex, Inc., and also has stock in the company. Over the last 3 years he has received financial compensation and/or stock with the following companies: AgeneBio, Nektar, and CoLucid. XL Zhang and C Weiss receive salary support from a grant from Naurex, Inc., to PK Stanton and JF Disterhoft respectively.
REFERENCES
- 1.Moskal JR, Kuo AG, Weiss C, Wood PL, O'Connor Hanson A, Kelso S, et al. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49:1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Zhang XL, Sullivan JA, Moskal JR, Stanton PK. A NMDA receptor glycine site partial agonist, GLYX-13, simultaneously enhances LTP and reduces LTD at Schaffer collateral-CA1 synapses in hippocampus. Neuropharmacology. 2008;55:1238–1250. doi: 10.1016/j.neuropharm.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burgdorf J, Zhang XL, Weiss C, Matthews E, Disterhoft JF, Stanton PK, et al. The N-methyl-d-aspartate receptor modulator GLYX-13 enhances learning and memory, in young adult and learning impaired aging rats. Neurobiol Aging. 2011;32:698–706. doi: 10.1016/j.neurobiolaging.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgdorf J, Zhang X-I, Nicholson KL, Balster RL, Leander JD, Stanton PK, et al. GLYX-13, an NMDA Receptor Glycine-Site Functional Partial Agonist, Induces Antidepressant-Like Effects Without ketamine-Like Side Effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of general psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 6.Parsons RG, Ressler KJ. Implications of memory modulation for post-traumatic stress and fear disorders. Nature neuroscience. 2013;16:146–153. doi: 10.1038/nn.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- 9.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biological psychology. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:218–228. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 12.Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, et al. A randomized, double-blind evaluation of D-cycloserine or alprazolam combined with virtual reality exposure therapy for posttraumatic stress disorder in Iraq and Afghanistan War veterans. The American journal of psychiatry. 2014;171:640–648. doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, et al. D-cycloserine augmentation of exposure therapy for post-traumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology. 2014;39:1052–1058. doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18:391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 15.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 16.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez C, Meier E. Behavioral profiles of SSRIs in animal models of depression, anxiety and aggression. Are they all alike? Psychopharmacology. 1997;129:197–205. doi: 10.1007/s002130050181. [DOI] [PubMed] [Google Scholar]
- 18.Burgdorf J, Kroes RA, Weiss C, Oh MM, Disterhoft JF, Brudzynski SM, et al. Positive emotional learning is regulated in the medial prefrontal cortex by GluN2B-containing NMDA receptors. Neuroscience. 2011;192:515–523. doi: 10.1016/j.neuroscience.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akirav I, Segev A, Motanis H, Maroun M. D-cycloserine into the BLA reverses the impairing effects of exposure to stress on the extinction of contextual fear, but not conditioned taste aversion. Learning & memory. 2009;16:682–686. doi: 10.1101/lm.1565109. [DOI] [PubMed] [Google Scholar]
- 20.Parent MA, Wang L, Su J, Netoff T, Yuan LL. Identification of the hippocampal input to medial prefrontal cortex in vitro. Cerebral cortex. 2010;20:393–403. doi: 10.1093/cercor/bhp108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroes RA, Panksepp J, Burgdorf J, Otto NJ, Moskal JR. Modeling depression: social dominance-submission gene expression patterns in rat neocortex. Neuroscience. 2006;137:37–49. doi: 10.1016/j.neuroscience.2005.08.076. [DOI] [PubMed] [Google Scholar]
- 22.Burgdorf J, Kroes RA, Beinfeld MC, Panksepp J, Moskal JR. Uncovering the molecular basis of positive affect using rough-and-tumble play in rats: a role for insulin-like growth factor I. Neuroscience. 2010;168:769–777. doi: 10.1016/j.neuroscience.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 23.Corcoran ME, Kroes RA, Burgdorf JS, Moskal JR. Regional Changes in Gene Expression after Limbic Kindling. Cell Mol Neurobiol. 2011 doi: 10.1007/s10571-011-9672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crosson LA, Kroes RA, Moskal JR, Linsenmeier RA. Gene expression patterns in hypoxic and post-hypoxic adult rat retina with special reference to the NMDA receptor and its interactome. Mol Vis. 2009;15:296–311. [PMC free article] [PubMed] [Google Scholar]
- 25.Harris MA, Clark J, Ireland A, Lomax J, Ashburner M, Foulger R, et al. The Gene Ontology (GO) database and informatics resource. Nucleic acids research. 2004;32:D258–D261. doi: 10.1093/nar/gkh036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 27.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA psychiatry. 2014;71:681–688. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- 29.Heresco-Levy U, Gelfin G, Bloch B, Levin R, Edelman S, Javitt DC, et al. A randomized add-on trial of high-dose D-cycloserine for treatment-resistant depression. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:501–506. doi: 10.1017/S1461145712000910. [DOI] [PubMed] [Google Scholar]
- 30.Heresco-Levy U, Javitt DC, Gelfin Y, Gorelik E, Bar M, Blanaru M, et al. Controlled trial of D-cycloserine adjuvant therapy for treatment-resistant major depressive disorder. Journal of affective disorders. 2006;93:239–243. doi: 10.1016/j.jad.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Therapeutic advances in chronic disease. 2015;6:97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgan CJ, Curran HV, Independent Scientific Committee on D. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- 33.Moskal JR, Burch R, Burgdorf JS, Kroes RA, Stanton PK, Disterhoft JF, et al. GLYX-13, an NMDA receptor glycine site functional partial agonist enhances cognition and produces antidepressant effects without the psychotomimetic side effects of NMDA receptor antagonists. Expert opinion on investigational drugs. 2014;23:243–254. doi: 10.1517/13543784.2014.852536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richter-Levin G, Maroun M. Stress and amygdala suppression of metaplasticity in the medial prefrontal cortex. Cerebral cortex. 2010;20:2433–2441. doi: 10.1093/cercor/bhp311. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt MV, Abraham WC, Maroun M, Stork O, Richter-Levin G. Stress-induced metaplasticity: from synapses to behavior. Neuroscience. 2013;250:112–120. doi: 10.1016/j.neuroscience.2013.06.059. [DOI] [PubMed] [Google Scholar]
- 36.Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emmett MR, Kroes RA, Moskal JR, Conrad CA, Priebe W, Laezza F, et al. Integrative biological analysis for neuropsychopharmacology. Neuropsychopharmacology. 2014;39:5–23. doi: 10.1038/npp.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moskal JR, Burgdorf J, Kroes RA, Brudzynski SM, Panksepp J. A novel NMDA receptor glycine-site partial agonist, GLYX-13, has therapeutic potential for the treatment of autism. Neuroscience and biobehavioral reviews. 2011;35:1982–1988. doi: 10.1016/j.neubiorev.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Corcoran ME, Kroes RA, Burgdorf JS, Moskal JR. Regional changes in gene expression after limbic kindling. Cellular and molecular neurobiology. 2011;31:819–834. doi: 10.1007/s10571-011-9672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kroes RA, Burgdorf J, Otto NJ, Panksepp J, Moskal JR. Social defeat, a paradigm of depression in rats that elicits 22-kHz vocalizations, preferentially activates the cholinergic signaling pathway in the periaqueductal gray. Behavioural brain research. 2007;182:290–300. doi: 10.1016/j.bbr.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moskal JR, Kroes RA, Otto NJ, Rahimi O, Claiborne BJ. Distinct patterns of gene expression in the left and right hippocampal formation of developing rats. Hippocampus. 2006;16:629–634. doi: 10.1002/hipo.20198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean ± SEM (A) line crosses in the NIH test, (B) latency to take the first bite of food in the home cage following the NIH test (Feeding latency in the novel cage is reported in figure1), and food intake in the home cage (C) 1 hr and (D) 24 hrs after overnight food deprivation in CUS treated rats treated with a single dose of rapastinel (3 mg/kg IV; saline vehicle, or No CUS exposed control rats tested 3 days post-dosing. N = 9–10 per group. All P values > .05
Mean ± SEM (A) NR2B current / total NMDA current in slices from control (No CUS, black bar), CUS (red bar) and CUS + rapastinel (blue bar) rats. (B) Effects of CUS (red bar), CUS + rapastinel (dark blue bar), and the NMDAR antagonist D-AP5 following CUS + rapastinel (light blue bar), on the magnitude of LTP, with normalized slope of 1.0 being no LTP, (C) EPSC Peak Current as a function of stimulus intensity in slices from CUS (red circles) and CUS + rapastinel (blue circles) rats. (D) Paired-Pulse Facilitation (PPF) ratio in CUS treated rats treated with a single dose of rapastinel (red circles, 3 mg/kg IV, saline vehicle), versus No CUS exposed control rats (blue circles). Ex vivo electrophysiological studies in layer V MPFC were performed 24 hrs post-dosing. N = 3–12 per group. * P < .05 Fisher’s PLSD post hoc test.