Abstract
Purpose
This study utilizes a survival model and clinical data with various radiation doses from prospective trials to determine radiation dose response parameters, such as radiosensitivity, and identify single-nucleotide-polymorphism (SNP) biomarkers that can potentially predict the dose response and guide personalized radiotherapy.
Methods
The study included 92 consecutive stage-III NSCLC patients with doses varied from 60 to 91 Gy. Logistic regression analysis of survival varying with SNP genotype and radiation dose was used to screen candidates for dose response analysis. The dose response parameter, represented by D50, was derived by fitting survival data into a model that takes into account both tumor control and treatment mortality. A candidate would be considered as a predictor if the 90% confident intervals (90%CIs) of D50 for the 2 groups stratified by the SNP genotype were separated.
Results
One SNP-signature (combining ERCC2:rs238406 and ERCC1:rs11615) was found to predict dose response. D50 values are 63.7 (90%CI: 53.5–66.3) Gy and 76.1 (90%CI: 71.3, 84.6) Gy for the 2 groups stratified by the genotypes. Using this biomarker-based model, a personalized dose prescription may be generated to improve 2-year survival from ~50% to 85% and ~3% to 73% for hypothetical sensitive and resistant patients, respectively.
Conclusions
We have developed a survival model that may be used to identify genomic markers, such as ERCC1/2 SNPs, to predict radiation dose response and potentially guide personalized radiotherapy.
Keywords: personalized radiotherapy, single-nucleotide-polymorphisms (SNPs), Biomarker, ERCC1, ERCC2, dose survival model, radiosensitivity
Introduction
Lung cancer is the leading cause of cancer death [1]. Although single-institution studies and secondary analysis of RTOG patients indicated that higher dose radiotherapy (RT) may increase local control and survival for patients with non-small cell lung cancer (NSCLC) [2–4], the RTOG 0617 study showed a contradictory result [5]. The overall survival (OS) in the high-dose arm (74-Gy) was poorer than that of the low-dose arm (60-Gy). These controversial results suggest a heterogeneous dose response of the patients, and the need of biomarkers to identify a patient’s sensitivity of dose response to RT for personalized medicine. For sensitive patients, 60-Gy may be sufficient to control the tumor, and increasing dose will only harm the patients by increasing treatment toxicity. On the other hand, for resistant patients, even 74-Gy may not be sufficient to control the tumor.
The radiation dose response, or radiosensitivity of a patient, depends on many factors, such as the DNA repair capability of tumor cells after radiation damage [6]. DNA repair genes play an important role in the processes of repairing these radiation damages through different pathways, and thus may correlate with a patient’s radiosensitivity. Recently, studies have shown that single nucleotide polymorphisms (SNPs) of DNA repair genes correlated with treatment response and OS for NSCLC patients with chemo-radiation treatment [7–9]. However, reports are often inconsistent [8]. One speculation of these inconsistencies is that OS or treatment outcome is the combined result of both tumor and normal tissue responses. A patient’s intrinsic DNA repair capability may affect both tumor and normal cells, so that its relation to treatment outcome is complicated by the heterogeneous chemo-radiation doses. Sensitive patients may have better outcome when the dose of a chemo-radiation regimen is low, while resistant patients may have better outcome when the dose is relatively high.
Correlation of radiosensitivity with biomarkers has not been successfully explored previously in patients, possibly because of its extraordinary challenges. Radiosensitivity is not an outcome parameter like OS. It cannot be measured for each individual patient. The average radiosensitivity of a group of patients, which is represented by a parameter D50, may be determined by fitting a sigmoid-shaped dose-response curve. However, a relatively large amount of patient data with various radiation doses, which are usually not clinically available, are required to determine the dose response curve. In addition, the tumor response is usually estimated by local tumor control, which may not be assessed objectively [4], especially for patients who died earlier from non-local tumor causes. Survival or death is objective, but confounded by many other factors, including (but not limited to) patient comorbidities, treatment toxicities and tumor metastasis. With a stage III NSCLC patient data set of various RT doses in prospective protocols, this study aims to evaluate the feasibility of utilizing OS as the surrogate endpoint for tumor response to derive the D50, and determine its association with a SNP marker by comparing the D50 values for the 2 groups of patients stratified by the marker. Specifically, we will 1) develop an OS model, which takes into account of not only the tumor control, but also other factors such as the patient comorbidities, treatment toxicities, and tumor metastasis, for deriving the D50 values; 2) identify SNP markers that are associated with the radiation dose response; and 3) presents a methodology of utilizing the SNP markers for personalized dose prescription to improve survival.
Materials and Methods
Study population
All stage-III NSCLC patients who enrolled in our institutional prospective protocols, and had blood samples when the SNP test was carried out, were eligible for the study. A total of 92 patients were eligible. The patients were mainly from 3 protocols, which have been all approved by IRB for biomarker correlation studies. The first is a dose escalation protocol using a PET-guided adaptive RT technique, with prescription dose limited by an iso-toxicity criterion, similar to that described in the RTOG 1106 study. The dose varied from 74–91 Gy in this protocol. The second is also a dose escalation study using a similar iso-toxicity criterion, but without advanced adaptive RT technique. The radiation dose was typically in the range of 66–80 Gy. The third is an imaging and biomarker protocol with standard RT. The radiation dose was typically 60–74 Gy (the high dose patients were treated before RTOG 0617 was completed). Dose tolerance criteria of organs-at-risk (OARs) similar to the iso-toxicity criterion were used for the patients in the third protocol. Those patients who did not finish treatments were also included in the study. Minimum follow-up was 2 years. Table 1 lists the basic properties of the patients.
Table 1.
Characteristics of the patients
| Characteristics | Number (%) | |
|---|---|---|
| Gender | Male | 75 (82) |
| Female | 17 (18) | |
| Race | Caucasian | 78 (85%) |
| Black | 4 (4%) | |
| Others and Unknown | 10 (11%) | |
| Age | <65 | 43 (47) |
| ≥65 | 49 (53) | |
| Histology | Squamous | 23 (25) |
| Adenocarcinoma | 19 (21) | |
| Others | 50 (54) | |
| Smoke | Yes | 88 (96) |
| No | 4 (4) | |
| Chemo (carboplatin + Paclitaxel) | Yes | 81 (88) |
| No | 11 (12) | |
| COPD | Yes | 39 (42) |
| No | 53 (53) | |
| Kps: Median (range) | 90 (50–100) | |
| GTV: Median (range) | 164.7 (2.4–921) CC | |
SNP/SNP-signature candidates
Blood samples were collected for each patient before RT. DNA was isolated from buffy coat, and a Sequenom MassArray System (Sequenom, Inc, San Diego, CA) in the Biomedical Research Core Facility at University of Michigan was used for SNP genotyping. A total of 24 functional SNPs in 11 DNA repair genes were tested. These include 7 ATM (rs1800057, rs189037, rs373759, rs609261, rs664677, rs664143 and rs4988044), 3 ERCC1 (rs11615, rs3212948 and rs3212961), 1 ERCC2 (rs238406), 2 ERCC5 (rs17655 and rs1047768), 1 XRCC1 (rs25489), 2 XRCC2 (rs3218536 and rs6464268), 3 XRCC4 (rs1478486, rs2075685 and rs9293329), 2 XPC (rs2228000 and rs2228001), 1 MGMT (rs12917), 1 NBN (rs1805794) and 1 TP53 (rs1625895) SNPs
The following 3 steps were used to screen SNP/SNP-signature candidates for dose response analysis. 1) All the SNPs were tested for the difference of OS rates of any 2 groups stratified by the SNP genotypes, and those with large differences (>15% and p<0.3) were advanced to the next step. 2) SNP-signatures were generated by combining any two of these SNPs using the “or” or “and” operator. However, usually only the “or” was used to increase the patient number for the group with less patients (usually the high-OS-rate group) so that patient numbers were more balanced for the 2 groups stratified by the SNP signature. SNPs and SNP-signatures were tested for the statistic correlation with the OS using Fisher’s Exact Test. Those significantly correlated with OS were advanced to the next step. (3) A conventional logistic model of OS with 2 variables, including the biomarker as a category variable, and the radiation dose as an explanatory variable, was fitted to the data to test if the biomarker and radiation dose were both statistically significant predictors of the OS. Those biomarkers that were significant were considered as candidates for the final OS model based dose response analysis.
OS model for dose response analysis
OS depends not only on local tumor control, but also many other factors. We divided these factors into 3 categories: (1) tumor response to RT; (2) normal tissue response to RT; and (3) all other factors not relating to RT. The factor for tumor response to a RT regimen can be modeled as tumor control probability (TCP) using a dose-dependent sigmoid-curve function. The factor for normal tissue response to the RT can be considered as treatment mortality and modeled as normal tissue complication probability (NTCP) using a similar sigmoid function. The other factors not relating to the RT can be patient factors, such as other diseases, aging, presence of micro-metastasis during treatment, susceptibility to metastasis, and so on, or other treatment factors such as chemotherapy. These non-RT related factors may be represented as a combined prognostic factor, P(B,t), which depends on many variables as well as biomarker status B and the follow-up time t. For simplicity, those variables were not specifically expressed in the formalism because the model was used for biomarker related dose response analysis. Thus, the OS rate for a group of patients is the multiplication of the contributions of the 3 sources, and can be expressed as:
| (1) |
where the 3 terms at the right correspond to contributions of combined prognosis, TCP and NTCP, respectively; B is the biomarker status; D50(B) and D(N)50(B) represent the radiosensitivity of the tumor and the normal structures, respectively; λ(B) and λ(N)(B) reflect the degree of their homogeneity in radiosensitivity and dictate the slope of the sigmoid curves; D(N) is the equivalent reference dose to the combined normal structures, including lung, heart, esophagus, and the immune system.
In this study, we used mean lung dose (MLD) as the equivalent reference dose for the combined normal structures to simplify the model, because MLD is significantly correlated to the esophagus, heart and other organ doses [10]. Considering iso-toxicity was generally applied for this group of patients, we assumed that D(N) was a constant. For convenience, 2-year OS was used in this study. Thus, Eq. (1) is simplified as:
| (2) |
where k(B) represents the multiplication of the combined prognosis and normal tissue response. It should be pointed out that tumor volume has been reported correlating to survival [11–12] and may indirectly relate to the dose. The effect of tumor volume to survival could be included in Eq. (1) for all 3 terms: (1) it may have a non-dose related prognosis included in P(B,t); (2) it may impact D50(B); (3) it impacts D(N)because large tumor volume usually results in high D(N). The iso-toxicity has normalized the impact of the tumor volume to D(N) in our data set. Again, because the correlation of tumor volume to survival was not the focus, it was not specifically expressed as a variable in P(B,t) and D50(B).
Eq. (2) presents the OS model to determine D50(B) for a group of patients. The patient data can be displayed in 2 different ways to fit the model. The first is to display calculated OS rates by dividing patients into several dose groups and calculating OS rate for each dose group. We used this approach to evaluate the goodness of fit (R2) of the model with patient data. However, due to the nature of retrospective study and limited number of patients, the division of the dose groups is often subjective. Thus, the approach is not statistically rigorous for deriving the fitting parameters. The second is to display OS rate as binary data (either 1 or 0) for each patient. This is a standard statistical approach and is also called binary approach. However, due to the nature of binary data, we are not able to illustrate how well the model fit with the data. We used this approach to determine the 3 parameters, D50(B), λ(B), and k(B), as well as their corresponding confidence intervals (CIs). An in-house computer program that utilizes the fitting functionality in the R software (a shared online statistical software platform) [13] was used to perform the fitting analyses. The model was first applied in the combined group (or mixed group) including all 92 patients, and then for the 2 groups stratified by each biomarker candidate. We considered that a biomarker candidate is associated with radiosensitivity if the 90% CIs of the D50 for the 2 groups stratified by the biomarker are not overlapped. The group with the lower D50 value was defined as the radiosensitive group.
OS model for personalized RT
The OS model has the potential to directly guide personalized RT if the radiosensitivity parameters can be determined. However, the simplified model of Eq. (2) does not include D(N)50(B), λ(N)(B) and D(N), the normal tissue related parameters due to the iso-toxicity prescription. We derive them by relating them to D50(B), λ(B) and D, the tumor related parameters. Because SNPs reflect genetic characteristics of an individual, both tumor and normal tissue cells would undergo the same degree of DNA repair and share similar sensitivity. Thus, we have m=D50(B)/D(N)50(B) and n=λ(B)/λ(N)(B) being constants not relating to “B”. In addition, the biomarker predicting radiosensitivity may not correlate to combined prognosis. Thus, P(B,t) = P(t=2-years) = P0. We further define SF = D/D(N) as an OAR sparing factor to reflect a RT plan’s capability of sparing the normal tissue. SF would depend on the tumor volume, tumor location and RT technique. Thus, the OS model of Eq. (1) becomes
| (3) |
And we have
| (4) |
Three sets of D50(B), λ(B) and k(B) values were obtained by fitting Eq. (2) for 3 different B statuses (mixed group, and 2 groups stratified by the biomarker). Plugging these values into Eq. (4), and assuming mean lung dose (MLD) = D(N), we had 3 equations with 3 unknowns, m, n and P0. The 3 unknowns were estimated by solving the 3 equations. Thus, Eq. (3) can predict OS for any patient with a given D, B, and SF.
Results
Four SNPs were considered as candidates in the first step due to their relatively large difference of OS rates between the 2 groups stratified by the candidates, as shown in Table 2. Among them, ERCC1:rs3212948 almost exactly correlated with ERCC1:rs11615. All 12 patients with the GG-type in rs3212948 were CC-type in rs11615, which has 13 patients. Therefore, the rs3212948 can be represented by the rs11615 and was not further analyzed. One SNP (ERCC2_rs238406), and 2 SNP-signatures (rs238406/rs11615 and rs238406/rs9293329) were significantly correlated with OS (p = 0.005, 0.006, and 0.01, respectively), as also shown in Table 2. The logistic regression analysis showed that the SNP (rs238406) and a SNP-signature (rs238406/rs11615) were dose-dependent significant predictor for the OS (p = 0.03 and 0.03 respectively), and were considered as the final candidates for dose response analysis.
Table 2.
The 4 SNP candidates advanced from Step-1 to Step-2 to form SNP-signatures, and the 2 SNP-signatures advanced from Step-2 to Step-3.
| SNPs or SNP-signatures | Genotypes | Patient number (% of patient) | P value | |
|---|---|---|---|---|
| Distribution | Two-year OS | |||
| ERCC2: rs238406 | GG | 30 (33%) | 6 (20%) | 0.005 |
| not GG (GT+TT+0) | 62 (67%) | 31 (50%) | ||
| ERCC1: rs11615 | CC | 13 (14%) | 3 (23%) | 0.07 |
| not CC (CT+TT+0) | 79 (86%) | 34 (43%) | ||
| ERCC1: rs3212948 | GG | 12 (13%) | 3 (25%) | 0.20 |
| not GG (CG+CC+0) | 80 (87%) | 34 (43%) | ||
| XRCC4 rs9293329 | AG | 11 (12%) | 3 (27%) | 0.28 |
| not AG (AA+GG+0) | 81 (88%) | 34 (42%) | ||
| Signature1:rs238406/rs11615 | GG in rs238406 or CC in rs11615 | 38 (41%) | 9 (24%) | 0.006 |
| The others | 54 (59%) | 28 (52%) | ||
| Signature2:rs238406/rs9293329 | GG in rs238406 or AG in rs9293329 | 36 (39%) | 9 (25%) | 0.01 |
| The others | 56 (61%) | 28 (50%) | ||
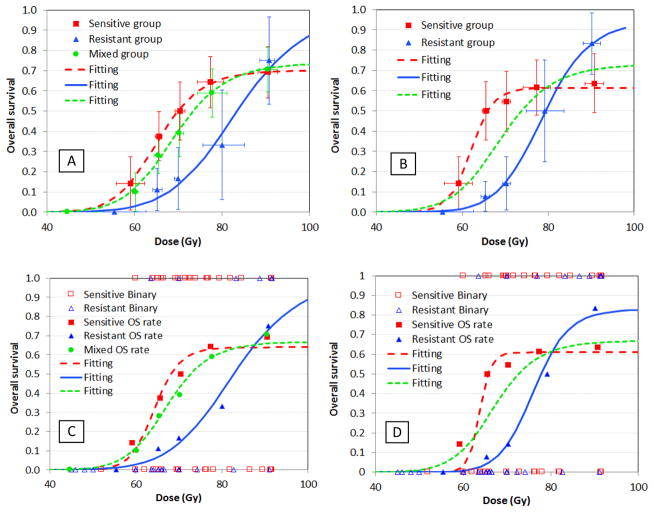
Fig. 1 shows the results of model-based dose response analyses for the two candidates. We first demonstrated that the model fits the clinical data very well for all situations using the dose-group approach, with R2 = 0.98–0.999, as shown in Figs. 1A and 1B. Using the binary approach, we derived the 3 parameters, D50(B), λ(B), and k(B), and their corresponding 90% CIs for the mixed group, as well as the two groups stratified by each biomarker candidate. The fitting curves are shown in Figs. 1C and 1D, and the parameters are listed in Table 3. We note that for the 2 groups stratified by the SNP-signature, their D50 values (and 90% CIs) are 63.7 (53.5–66.3) Gy and 76.1 (71.3, 84.6) Gy, respectively. The 90% CIs are not overlapped, suggesting that the SNP-signature is associated with radiosensitivity according to our criterion. On the other hand, for the 2 groups stratified by the single SNP candidate, the D50 values (and 90% CIs) are 64.3 (55.4–77.9) Gy and 82.7 (70.5, 95.1) Gy, respectively. Although the D50 values are very different, the 90% CIs are overlapped, suggesting our data is not sufficient to support that the SNP rs238406 is a biomarker that associates with radiosensitivity.
Fig 1.
Dose-survival relationship for the 2 SNP/SNP-signature candidates and their fits with the model for 2 approaches of displaying patient data: (A) Single SNP/Approach 1; (B) SNP-signature/Approach 1; (C) Single SNP/Approach 2; (D) SNP-signature/Approach 2. Approach 1 (Figs. A&B) displays 2-year OS in 5–6 data points by dividing patients into 5–6 dose groups; and the purpose is to illustrate how well the model fits to patient data. Approach 2 (Figs. C&D) plots OS as binary data (1/0) for each patient; and the purpose is to derive the fitting parameters. The patient OS rate data from approach 1 are also plotted in Figs. C&D for references.
Table 3.
Model Parameters and Goodness of Fit of the SNP and SNP-signature candidates.
| Biomarker Name | Biomarker Status | D50 (90% CI) (Gy) | λ (90% CI) | k (90% CI) | R2 |
|---|---|---|---|---|---|
| Single SNP rs238406 | Sensitive | 64.3 (55.4, 77.9) | 22.4 (4.6, ND) | 0.64 (0.49, ND) | 0.12 |
| Resistant | 82.7 (70.5, 95.1) | 10.9 (4.3, ND) | 1.0 (0.43, ND) | 0.37 | |
| SNP signature | Sensitive | 63.7 (53.5, 66.3) | 50 (6.8, ND) | 0.61 (0.48, ND) | 0.18 |
| Resistant | 76.1 (71.3, 84.6) | 19.5 (8.0, ND) | 0.83 (0.59, ND) | 0.49 | |
| Mixed group | 67.2 (62.2, 80.8) | 13.8 (4.8, 45) | 0.67 (0.51, ND) | 0.17 | |
ND = Not determined due to limited data points
Using the data in Table 3, we estimated that P0 ≅ 85%, m ≅ 3.64, and n ≅ 0.95. Thus, the model represented by Eq. (3) could be used to predict the 2-year OS and guide personalized RT for an individual patient. Fig. 2 shows examples of how to use the model to guide personalized RT by plotting OS varying with dose for different biomarker statuses, and for 2 typical hypothetical patients with different SF values (SF = 4.0 and 4.7). The optimal prescription dose and corresponding OS differ remarkably for different SNP-signature statuses and SF values. Two-year OS reaches 85% for the radiosensitivity, and 73% for radioresistant status for a patient with SF = 4.7 at optimal prescription dose. However, it is ~50% and ~3%, correspondingly, for use of a uniform prescription of ~64 Gy.
Fig. 2.
Dose-OS response curves predicted by the OS model, which varies with dose D, biomarker genotype B and OAR sparing factor SF. (A) for a virtual patient with SF =4.0; (B) for a virtual patient with SF =4.7.
Discussion
We have developed an OS model that can well fit to the clinical data and be used to derive the radiosensitivity parameters from a group of patients. Based on this model, we have also developed a methodology that can determine whether a biomarker candidate is associated with radiosensitivity. Using this methodology, we have identified a SNP-signature, a combination of ERCC2: rs238406 and ERCC1: rs11615, to be such a biomarker, because the D50 values and their 90% CIs are widely separated for the 2 groups of patients stratified by this biomarker. Logistic model based statistical analysis showed that this SNP-signature and the radiation dose are significant factors associated with OS, consisting with the result that the SNP-signature is associated with radiosensitivity. Finally, we have illustrated that a combination of this model and the biomarker data may be used to predict a patient’s survival and potentially guide personalized RT, as shown in Fig. 2. It should be pointed out that iso-toxicity is a key in deriving the sigmoid-shaped dose response model for the data analysis. We noted that MLDs were relatively uniform in these patients (16.8, 17.0, 16.0, 16.2, and 16.1 Gy, respectively, for the 5 dose groups), although with a large dose variation (60–91 Gy).
The ERCC1 and ERCC2 genes are well known for repairing ultraviolet-radiation induced DNA damage through the nucleotide excision repair (NER) pathway [14–16]. It has also been reported that they were correlated with platinum-based chemotherapy for treatment response, survival [7–8, 17] and treatment toxicities [18–20] due to their role in the NER pathway. Therefore, they are more likely to modulate chemo or ultraviolet-radiation response than RT response. However, these genes may be involved in transcription, and thus may contribute to repair of other types of damage, such as double strand breaks by ionizing radiation [15]. In addition, ionizing radiation also induces oxidative damage similar to ultraviolet radiation and chemotherapy. Therefore, it is understandable that these genes may be associated with radiosensitivity. Studies have shown that the ERCC1 gene was involved in double strand breaks [21] and correlated with radiosensitivity in glioma cell lines [22], and the SNPs of ERCC2 correlated with repair of x-ray induced DNA damages, such as chromatid aberrations [15] and chromosome dicentrics [16]. The ERCC2:rs238406 and ERCC1:rs11615, along with their highly associated tag-SNP [23], have also been reported to correlate with repair of Benzo[a]pyrene-diol-epoxide (BPDE)-induced DNA adducts in cultured primary lymphocytes [24–25]. It is also interesting to note that the genotype frequency of both rs238406 and rs11615 highly depends on race [26]. The GG type in rs238406, and CC type in rs11615, which correspond to radioresistance, have high frequency in Africans (>90%), intermediate in Asians (20–60%), and low in Europeans (7–20%) [26]. The majority of patients in this study were Caucasians.
The parameter k, which inversely relates to treatment mortality, is 0.61 for sensitive patients, and 0.83 for resistant patients stratified by the SNP signature. This suggests that patients with radiosensitive tumors may have radiosensitive normal tissue. We noted that at MLD ~17 Gy, treatment mortality reached >24% for radiosensitive patients. However, we did not observe such a large number of grade-5 toxicities of specific anatomic organs. One speculation is that the NTCP term in the model (Eq. 1) may also include radiation damage of the immune system. Recently, it has been reported that the immune system plays an important role in controlling the tumor [27–30]. Damage of the immune system may not directly cause conventional treatment mortality, but may affect tumor control and eventually patient death due to disease progression.
A major limitation of this study is the relative small patient data set. The patient number is not sufficient to separate them into a training and a validation cohort for statistical validation. Another caveat in this study is that this is a retrospective study including patients from 3 different protocols. Although all eligible Stage 3 patients were included, the enrollment criteria for the 3 protocols may be different, and different iso-toxicity criteria might be used for different protocols. In addition, the impacts of chemotherapy, tumor volume, and metastasis to the survival and the model were not systematically studied and not clearly understood. Due to the limited patient number, especially at the high radiation dose, it is possible that the correlation of SNP-signature with the dose response is false positive. Considering that 88% of patients underwent platinum-based chemotherapy, and the SNP-signature is well known for DNA repair in the NER pathway, the false positive could be a reflection of a prognostic predictor for chemotherapy. Further studies with a larger number of patients are needed to validate the SNP/SNP signature, and improve accuracy of the dose response parameters in the model.
In summary, we have developed an OS model that can fit to the clinical data well and can derive the radiosensitivity parameters from a group of patients. Based on this model, we have further developed a methodology that can identify whether a biomarker candidate, such as a SNP signature that combines ERCC2: rs238406 and ERCC1: rs11615, can predict radiosensitivity. This model, with inclusion of the biomarker, if validated, might be used for personalized RT to improve treatment outcome.
Acknowledgments
This work is partially supported by NIH/NCI grants R01 CA142840 and P01 CA59827.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ettinger DS, Akerley W, Borghaei H, et al. ; National comprehensive cancer network. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw. 2013 Jun 1;11(6):645–53. doi: 10.6004/jnccn.2013.0084. [DOI] [PubMed] [Google Scholar]
- 2.Kong FM, Ten Haken RK, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63(2):324–333. doi: 10.1016/j.ijrobp.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Rengan R, Rosenzweig KE, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60(3):741–747. doi: 10.1016/j.ijrobp.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Machtay M, Bae K, et al. Higher Biologically Effective Dose of Radiotherapy is Associated with Improved Outcomes for Locally Advanced Non-Small Cell Lung Carcinoma Treated with Chemoradiation: An Analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015 Feb;16(2):187–99. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall Eric J, Giaccia Amato J. Radiobiology for the Radiologist. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 7.Kalikaki A, Kanaki M, Vassalou H, Souglakos J, Voutsina A, Georgoulias V, Mavroudis D. DNA repair gene polymorphisms predict favorable clinical outcome in advanced non-small- cell lung cancer. Clin Lung Cancer. 2009 Mar;10(2):118–23. doi: 10.3816/CLC.2009.n.015. [DOI] [PubMed] [Google Scholar]
- 8.Xu TP, Shen H, Liu LX, Shu YQ. Association of ERCC1-C118T and -C8092A polymorphisms with lung cancer risk and survival of advanced-stage non-small cell lung cancer patients receiving platinum-based chemotherapy: a pooled analysis based on 39 reports. Gene. 2013 Sep 10;526(2):265–74. doi: 10.1016/j.gene.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Hu L, Wu C, Zhao X, et al. Genome-wide association study of prognosis in advanced non-small cell lung cancer patients receiving platinum-based chemotherapy. Clin Cancer Res. 2012 Oct 1;18(19):5507–14. doi: 10.1158/1078-0432.CCR-12-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006 Jul 15;65(4):1075–86. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 11.Soliman M, Yaromina A, Appold S, et al. GTV differentially impacts locoregional control of non-small cell lung cancer (NSCLC) after different fractionation schedules: subgroup analysis of the prospective randomized CHARTWEL trial. Radiother Oncol. 2013 Mar;106(3):299–304. doi: 10.1016/j.radonc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Ball DL, Fisher RJ, Burmeister BH, et al. The complex relationship between lung tumor volume and survival in patients with non-small cell lung cancer treated by definitive radiotherapy: a prospective, observational prognostic factor study of the Trans-Tasman Radiation Oncology Group (TROG 99.05) Radiother Oncol. 2013 Mar;106(3):305–11. doi: 10.1016/j.radonc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Ross Ihaka, Gentleman Robert. R: a language for data analysis and graphics. Journal of computational and graphical statistics. 1996;5(3):299–314. [Google Scholar]
- 14.Doig J, Anderson C, Lawrence NJ, Selfridge J, Brownstein DG, Melton DW. Mice with skin-specific DNA repair gene (Ercc1) inactivation are hypersensitive to ultraviolet irradiation- induced skin cancer and show more rapid actinic progression. Oncogene. 2006 Oct 12;25(47):6229–38. doi: 10.1038/sj.onc.1209642. [DOI] [PubMed] [Google Scholar]
- 15.Lunn RM, Helzlsouer KJ, Parshad R, Umbach DM, Harris EL, Sanford KK, Bell DA. XPD polymorphisms: effects on DNA repair proficiency. Carcinogenesis. 2000 Apr;21(4):551–5. doi: 10.1093/carcin/21.4.551. [DOI] [PubMed] [Google Scholar]
- 16.Au WW, Salama SA, Sierra-Torres CH. Functional characterization of polymorphisms in DNA repair genes using cytogenetic challenge assays. Environ Health Perspect. 2003 Nov;111(15):1843–50. doi: 10.1289/ehp.6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin M, Yan J, Martinez-Balibrea E, Graziano F, Lenz HJ, Kim HJ, Robert J, Im SA, Wang WS, Etienne-Grimaldi MC, Wei Q. ERCC1 and ERCC2 polymorphisms predict clinical outcomes of oxaliplatin-based chemotherapies in gastric and colorectal cancer: a systemic review and meta-analysis. Clin Cancer Res. 2011 Mar 15;17(6):1632–40. doi: 10.1158/1078-0432.CCR-10-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu W, Zhang W, Qiao R, Chen D, Wang H, Wang Y, Zhang S, Gao G, Gu A, Shen J, Qian J, Fan W, Jin L, Han B, Lu D. Association of XPD polymorphisms with severe toxicity in non-small cell lung cancer patients in a Chinese population. Clin Cancer Res. 2009 Jun 1;15(11):3889–95. doi: 10.1158/1078-0432.CCR-08-2715. [DOI] [PubMed] [Google Scholar]
- 19.Cortejoso L, García MI, García-Alfonso P, González-Haba E, Escolar F, Sanjurjo M, López-Fernández LA. Differential toxicity biomarkers for irinotecan- and oxaliplatin-containing chemotherapy in colorectal cancer. Cancer Chemother Pharmacol. 2013 Jun;71(6):1463–72. doi: 10.1007/s00280-013-2145-6. [DOI] [PubMed] [Google Scholar]
- 20.Damaraju S, Murray D, Dufour J, Carandang D, Myrehaug S, Fallone G, Field C, Greiner R, Hanson J, Cass CE, Parliament M. Association of DNA repair and steroid metabolism gene polymorphisms with clinical late toxicity in patients treated with conformal radiotherapy for prostate cancer. Clin Cancer Res. 2006 Apr 15;12(8):2545–54. doi: 10.1158/1078-0432.CCR-05-2703. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad A, Robinson AR, Duensing A, van Drunen E, Beverloo HB, Weisberg DB, Hasty P, Hoeijmakers JH, Niedernhofer LJ. ERCC1-XPF endonuclease facilitates DNA double-strand break repair. Mol Cell Biol. 2008 Aug;28(16):5082–92. doi: 10.1128/MCB.00293-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu ZG, Chen HY, Cheng JJ, Chen ZP, Li XN, Xia YF. Relationship between methylation status of ERCC1 promoter and radiosensitivity in glioma cell lines. Cell Biol Int. 2009 Oct;33(10):1111–7. doi: 10.1016/j.cellbi.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Topinka J, Hertz-Picciotto I, Dostal M, Chvatalova I, Yap PS, Herr CE, Greenfield T, Sram RJ. The DNA repair gene XPD/ERCC2 polymorphisms Arg156Arg (exon 6) and Lys751Gln (exon 23) are closely associated. Toxicol Lett. 2007 Jul 30;172(1–2):85–9. doi: 10.1016/j.toxlet.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 24.Zhao H, Wang LE, Li D, Chamberlain RM, Sturgis EM, Wei Q. Genotypes and haplotypes of ERCC1 and ERCC2/XPD genes predict levels of benzo[a]pyrene diol epoxide-induced DNA adducts in cultured primary lymphocytes from healthy individuals: a genotype-phenotype correlation analysis. Carcinogenesis. 2008 Aug;29(8):1560–6. doi: 10.1093/carcin/bgn089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X, Liu Y, Yu T, Xiao S, Bao X, Pan L, Zhu G, Cai Y, Liu Q, Jin C, Yang J, Wu S, An L, van der Straaten T. ERCC1 and ERCC2 haplotype modulates induced BPDE-DNA adducts in primary cultured lymphocytes. PLoS One. 2013 Apr 4;8(4) doi: 10.1371/journal.pone.0060006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.http://www.ncbi.nlm.nih.gov/snp
- 27.Gerber SA, Lim JY, Connolly KA, Sedlacek AL, Barlow ML, Murphy SP, Egilmez NK, Lord EM. Radio-responsive tumors exhibit greater intratumoral immune activity than nonresponsive tumors. Int J Cancer. 2014 May 15;134(10):2383–92. doi: 10.1002/ijc.28558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Draghiciu O, Walczak M, Hoogeboom BN, Franken KL, Melief KJ, Nijman HW, Daemen T. Therapeutic immunization and local low-dose tumor irradiation, a reinforcing combination. Int J Cancer. 2014 Feb 15;134(4):859–72. doi: 10.1002/ijc.28418. [DOI] [PubMed] [Google Scholar]
- 29.Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest. 2013 Jul 1;123(7):2756–63. doi: 10.1172/JCI69219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, Beckett MA, Lingen MW, Witt M, Weichselbaum RR, Fu YX. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013 Jun 1;190(11):5874–81. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]