ABSTRACT
A recent phase 3 trial with soluble herpes simplex virus 2 (HSV-2) glycoprotein D (gD2t) in adjuvant failed to show protection against genital herpes. We postulated that live attenuated HSV-2 would provide more HSV antigens for induction of virus-specific antibodies and cellular immunity than would gD2t. We previously reported an HSV-2 mutant, HSV2-gD27, in which the nectin-1 binding domain of gD2 is altered so that the virus is impaired for infecting neural cells, but not epithelial cells, in vitro and is impaired for infecting dorsal root ganglia in mice (K. Wang, J. D. Kappel, C. Canders, W. F. Davila, D. Sayre, M. Chavez, L. Pesnicak, and J. I. Cohen, J Virol 86:12891–12902, 2012, doi:10.1128/JVI.01055-12). Here we report that the mutations in HSV2-gD27 were stable when the virus was passaged in cell culture and during acute infection of mice. HSV2-gD27 was attenuated in mice when it was inoculated onto the cornea, intramuscularly (i.m.), intravaginally, and intracranially. Vaccination of mice i.m. with HSV2-gD27 provided better inhibition of challenge virus replication in the vagina than when the virus was used to vaccinate mice intranasally or subcutaneously. Comparison of i.m. vaccinations with HSV2-gD27 versus gD2t in adjuvant showed that HSV2-gD27 induced larger reductions of challenge virus replication in the vagina and reduced latent viral loads in dorsal root ganglia but induced lower serum neutralizing antibody titers than those obtained with gD2t in adjuvant. Taken together, our data indicate that a live attenuated HSV-2 vaccine impaired for infection of neurons provides better protection from vaginal challenge with HSV-2 than that obtained with a subunit vaccine, despite inducing lower titers of HSV-2 neutralizing antibodies in the serum.
IMPORTANCE Genital herpes simplex is one of the most prevalent sexually transmitted diseases. Though HSV-2 disease is usually mild, it can be life threatening in neonates and immunocompromised persons. In addition, genital herpes increases the frequency of HIV infection and transmission. HSV-2 maintains a latent infection in sensory neurons and cannot be cleared with antiviral drugs. The virus frequently reactivates, resulting in virus shedding in the genital area, which serves as a source for transmission. A prophylactic vaccine is needed to prevent disease and to control the spread of the virus. Previous human trials of subunit vaccines have been unsuccessful. Here we report the results of vaccinating mice with a new type of live attenuated HSV-2 vaccine that is impaired for infection of neurons and provides better protection of mice than that obtained with a subunit vaccine. The strategy of altering the cell tropism of a virus is a new approach for a live attenuated vaccine.
INTRODUCTION
Genital herpes simplex is one of the most prevalent sexually transmitted diseases. In 2012, more than 417 million persons worldwide were reported to be infected with herpes simplex virus 2 (HSV-2), with more than 19.2 million new persons infected in that year alone (1). While HSV-2 disease is mild in most cases, infection can be severe and life threatening in immunocompromised patients and neonates. In addition, genital herpes increases the risk of acquisition of HIV infection 3-fold (2). While HSV-2 traditionally has been the principal cause of genital herpes, more recent studies have shown that HSV-1 may be replacing HSV-2 as the most frequent cause of genital herpes in the United States (3, 4). While both HSV-1 and HSV-2 cause primary and recurrent genital herpes, genital recurrences are much more frequent with HSV-2 than with HSV-1 (5).
HSV-2 infects epithelial cells in the vaginal mucosa, replicates in the cells, and enters the nerve endings innervating the mucosa. Viral capsids travel retrograde in axons to the cell body in sensory ganglia, where HSV establishes a latent infection in the nuclei of neurons. Latent HSV can be reactivated by multiple stimuli, such as stress, fever, and exposure to UV light. Viral capsids travel anterograde from the ganglion down the axons to the mucosa, where the virus replicates and is shed in the presence or absence of symptomatic genital lesions. This allows the virus to spread to uninfected persons. Licensed drugs for treatment of HSV, such as acyclovir, are effective at inhibiting virus replication, reducing the severity and duration of lesions, and lowering the rate of transmission. However, these drugs do not clear the latent virus or completely block transmission. Thus, the most effective method to prevent the spread of HSV will be an effective vaccine.
Despite over 30 years of basic research and clinical trials, an HSV-2 vaccine has not been licensed for use in humans. Most preclinical and clinical trials have focused on subunit vaccines due to their safety and simplicity. The most extensively tested subunit vaccines have been recombinant soluble HSV-2 glycoprotein D (gD2t) and glycoprotein B (gB2t) expressed in CHO cells. HSV-2 gD2t and gB2t reduced genital disease in guinea pigs after intravaginal (i.vag.) challenge with HSV-2 but did not prevent HSV infection (6–8). HSV-2 gD2t and gB2t in MF59 adjuvant showed very transient efficacy in clinical trials (9, 10). gD2t in alum and monophosphoryl lipid A (MPL) initially showed promising results in preventing HSV-2 infection in women who were seronegative for both HSV-1 and HSV-2 (11); however, in a phase 3 study involving over 8,000 women who were seronegative for HSV-1 and HSV-2, the vaccine showed no efficacy for preventing HSV-2 genital herpes (12). The ineffectiveness of this vaccine has increased interest in other approaches for HSV-2 vaccines, including replication-defective (13–15) and live attenuated (16–19) vaccines.
Live attenuated vaccines present most, if not all, viral proteins to the immune system to induce antibodies and CD4 and CD8 T cell responses, as opposed to the limited antigens presented in subunit vaccines, such as gD2t, which induce antibodies and CD4 T cell responses to one viral glycoprotein (12). However, live attenuated vaccines present additional safety concerns, since they have the potential to establish latency in the nervous system and to reactivate. Thus, live attenuated virus vaccines need to be avirulent while still retaining potent immunogenicity. An HSV-2 mutant with reduced or absent neurotropism might meet this goal.
HSV-2 infects a wide range of cells, including epithelial cells, fibroblasts, lymphocytes, and neurons. Virus receptors on cells are one of the important determinants of the susceptibility of cells to infection. The two principal receptors for HSV, HVEM (also known as HveA, TNFRSF14, LIGHTR, and CD270) and nectin-1 (also known as HveC, PVRL1, and CD111), are differentially expressed on various cell types. Neurons express abundant nectin-1 (20, 21) and little to no HVEM (22–24), while lymphocytes express HVEM (22) and epithelial cells express both HVEM and nectin-1 (25). HSV gD binds to both HVEM and nectin-1, and this step is essential for HSV infection of cells. The HVEM and nectin-1 binding domains of HSV gD are located in different regions of the glycoprotein: the HVEM binding domain is at the N terminus (26–28), and the nectin-1 domain is in the middle of HSV gD (28–30). Mutations in one domain can impair the ability of HSV gD to use one receptor but not the other receptor (28, 31, 32). Accordingly, we constructed an HSV-2 gD mutant virus, HSV2-gD27, in which the nectin-1 binding domain was mutated and the virus was impaired for infecting neuronal cells in vitro and in vivo (33). Here we report the stability and attenuation of the HSV2-gD27 virus and its efficacy in protecting mice from genital herpes after intravaginal challenge.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were purchased from ATCC and maintained in Dulbecco's modified Eagle's medium (DMEM) (Gibco) containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 U/ml streptomycin at 37°C. ARPE-19 human epithelial cells were purchased from ATCC and maintained in DMEM/F-12 (1:1) with 10% fetal bovine serum and antibiotics. Mouse melanoma B78H1 cells are resistant to HSV infection; B78H1-A10 and B78H1-C10 cells, which stably express human HVEM and nectin-1, respectively, were provided by Gary Cohen and Roselyn Eisenberg (University of Pennsylvania). B78H1-A10 and B78H1-C10 cells were maintained in DMEM containing 250 μg/ml and 500 μg/ml G418, respectively, as well as 5% fetal bovine serum and antibiotics.
HSV-2 R519 is a plaque-purified clone from HSV-2 333 that does not cause syncytia in Vero cells. R519 was propagated and titrated in Vero cells. HSV-2 H312-1BB− (HSV2-ΔBAC38) is a derivative of the HSV-2 bacterial artificial chromosome (BAC) clone bHSV2-BAC38; the BAC cassette was removed when the virus was rescued in Vero cells, and its phenotype is similar to that of R519 in mouse models (33). HSV-2 H312-1BB− was propagated and titrated in B78H1-A10 cells unless otherwise specified. HSV2-gD27, also a derivative of BAC clone bHSV2-BAC38, has point mutations (D215G, R222N, and F223I) in gD that impair the ability of gD to interact with the HSV-2 cellular receptor nectin-1, and therefore it is unable to infect cells that do not express functional HVEM (33). HSV2-gD27 was propagated and titrated in B78H1-A10 cells. HSV-2 H312-1BB− served as a wild-type (WT) control in experiments testing the HSV2-gD27 mutant, since both viruses were derived from the same BAC; the former has a wild-type gD sequence, while the latter has gD mutations. Cell-free vaccine virus was produced by freezing and thawing infected cells three times, removing cellular debris by centrifugation at 1,940 × g, and storing the supernatant at −80°C. Mock vaccine was produced by freezing and thawing uninfected B78H1-A10 cells and was processed as described for cell-free virus.
Testing the stability of HSV2-gD27 in cell culture.
Six-well plates were seeded with ARPE-19 cells at 8 × 105 cells/well 1 day before infection. ARPE-19 cells were infected at a multiplicity of infection (MOI) of ∼0.01 with HSV2-gD27 that had been propagated in B78H1-A10 cells. Two days later, the infected cells were harvested, cell-free virus was obtained by freezing and thawing the cells, and cellular debris was removed by centrifugation. The supernatant, containing HSV2-gD27 ARPE-19 passage 1 (HSV2-gD27 RPE-P1), was used to infect ARPE-19 cells, resulting in HSV2-gD27 RPE-P2, and serial passages were performed up to passage 19 (HSV2-gD27 RPE-P19). HSV2-gD27 RPE-P5, -P11, and -P19 were titrated in both ARPE-19 and B78H1-C10 cells to determine if these viruses could infect B78H1-C10 cells that express nectin-1 but not HVEM.
Soluble HSV-2 gD2t/alum/MPL vaccine.
The recombinant soluble HSV-2 gD2t vaccine consists of the ectodomain of gD2 secreted from CHO cells (7) (a gift from Chiron Corporation). For each mouse, 3 μg of gD2t was incubated with Imject alum (Thermo Scientific) for 30 min at room temperature, and 7.5 μg of MPL (Avanti Polar Lipids) was then added, resulting in the HSV-2 gD2t/alum/MPL vaccine.
Animal studies.
All mouse studies were performed under a protocol approved by the Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases. Female BALB/c mice were purchased from Harlan Laboratories and were inoculated at 6 weeks of age; 4-week-old mice were used for intracranial (i.c.) inoculations.
Mouse ocular model.
Mice were anesthetized with ketamine/xylazine (125/20 mg/kg of body weight), and corneas were scarified by using a 25-gauge needle to scratch the surface of the cornea 16 times in a crisscross fashion. Five microliters of HSV-2 was applied to each eye, bilaterally. Mouse eyes and mortality were monitored daily.
Mouse i.n., s.c., and i.m. models.
Mice were inoculated intranasally (i.n.) by gentle instillation with 5 μl of HSV2-gD27 containing 4,000 PFU into each nostril with a P20 pipette. Animals were inoculated subcutaneously (s.c.) and intramuscularly (i.m.) in the right thigh with 100 μl of virus containing 16,000 PFU. All mice were monitored daily for local lesions and systemic signs of illness, such as lethargy and a reduced response to stimuli; animals were euthanized when moribund.
Mouse intracranial model.
Four-week-old mice were anesthetized with ketamine/xylazine (125/20 mg/kg). The injection site on the head of a mouse was shaved and swabbed with iodine and 70% ethanol, and a 5-mm incision of the skin on top of the head was made with a scalpel. Ten microliters of virus was inoculated with a needle to a depth of 3 to 4 mm beneath the skull. The skin incision was glued together, and mice were monitored for illness. When animals become moribund, they were euthanized and autopsied.
Mouse vaginal model.
Five days before intravaginal inoculation of virus (HSV2-gD27, H312-1BB−, or R519), mice were injected s.c. with 2 mg/mouse of medroxyprogesterone acetate injectable suspension (Depo-Provera; USP, Pharmacia & Upjohn) diluted in phosphate-buffered saline (PBS). HSV-2 (10 μl) was instilled gently into the vagina by use of a P20 pipette. To detect the level of virus replication in the vagina, vaginal swabs were obtained on various days after infection. A polyester swab (MicroPur swab; AMD-Ritmed Inc.) was gently inserted into the mouse vagina, to a depth of about 1 to 1.5 cm (until resistance was felt), and then dipped into a vial with 0.5 ml of ARPE-19 cell growth medium with 2.5 μg/ml amphotericin B, and the vial was frozen at −80°C for later titration.
To determine the ability of HSV2-gD27 to reach the dorsal root ganglia (DRG) after intravaginal inoculation, 5 mice from each group were euthanized on days 2, 3, 4, and 5 postinoculation, and their DRG were harvested, added to 0.5 ml of ARPE-19 growth medium with zirconium oxide beads (Omni International), homogenized (Percellys Minilys homogenizer) twice at 4,000 rpm for 20 s at room temperature, and the homogenates were added to wells containing ARPE-19 cells for plaque assays.
To determine the ability of HSV2-gD27 and WT virus to establish latent infection, mice infected with WT virus (H312-1BB−) were given 0.5 ml of intravenous immune globulin (IVIG) (Gamunex-C; Talecris Biotherapeutics) diluted in PBS (1:13.3 [vol:vol]) intraperitoneally (i.p.) 1 day after infection to allow more mice to survive acute infection. On day 36 after infection, mice were euthanized, DRG were harvested, and DNAs were extracted with DNeasy blood and tissue kits (Qiagen). Latent viral DNA was quantified by real-time PCR, using primers and a probe for the HSV-2 gG gene, as described previously (33).
To determine if the mutations in HSV2-gD27 were stable during virus replication in mice, HSV2-gD27 recovered from DRG of 5 mice on days 4 and 5 postinfection was passaged once in ARPE-19 cells, and cell-free virus was prepared and used for titration and sequencing. Virus was titrated in ARPE-19 cells and used to infect B78H1-C10 cells. The HSV-2 gD gene was amplified by PCR and sequenced with primers gDF103, gDF106, gDR103, and gDR105 (Table 1). A portion of the HSV-2 gB gene that includes codons D280 (equivalent to HSV-1 gB codon D285) and A546 (equivalent to HSV-1 gB codon A549) was amplified by PCR with primers gBF53.5 and gBR54 (Table 1) and sequenced with primers gBF33 and gBR40 (Table 1).
TABLE 1.
Primers used for PCR and sequencing of the gD and gB regions
| Primer name | Sequence (5′ to 3′) | Usage |
|---|---|---|
| gDF103 | CATAAGCTGCATTGCGAACGACTA | PCR and sequencing |
| gDR103 | CCACACGGGCCCAGAGGTA | PCR and sequencing |
| gDF106 | GGACGAGGCCCGAAAGCACAC | Sequencing |
| gDR105 | GCGGGGGCGTGCATCAGGAA | Sequencing |
| gBF53.5 | CGGCCAAGTACGTGCGGAACAACA | PCR and sequencing |
| gBR54 | TAGCCCCCGCCGAAGATGAAGTAGC | PCR and sequencing |
| gBF33 | CGTCCGTGGAGCGCATCAAGA | Sequencing |
| gBR40 | CTTGAAGCGGTCGGCGGCGTAGC | Sequencing |
Virus challenge.
About 3 weeks after the second vaccination, mice were injected s.c. with 2 mg of medroxyprogesterone acetate. Five days later, mice were challenged intravaginally with 4 × 104 PFU (10 μl) of HSV-2 strain R519. Vaginal swabs were taken on days 1, 2, 3, 5, and 7 after challenge, stored at −80°C, and later titrated in Vero cells. Mice were monitored for survival for 30 days.
Plaque assays.
Serial dilutions (500 μl) of virus, vaginal swabs, or DRG homogenates were added to ARPE-19 or Vero cell monolayers in 6-well plates and incubated at 37°C for 1 h. The inoculum was removed, and 2.5 ml of medium containing 0.3% Gamunex-C (3:1,000 [vol:vol]) was added. About 2 days later, cells were stained with a solution containing 10% (vol/vol) formaldehyde, 5% (vol/vol) acetic acid, 60% (vol/vol) methanol, and 1% (wt/vol) crystal violet and plaques were counted.
Assays for gD-binding and neutralizing antibodies.
HSV-2 gD antibody was quantified using a luciferase immunoprecipitation system (LIPS) assay (34). Briefly, a Renilla luciferase–HSV-2 gD fusion protein was expressed in COS-1 cells, lysates were prepared by sonication, and cellular debris was cleared by centrifugation. The luciferase activity (light units [LU]/ml) in the lysate was determined by using a luminometer, with coelenterazine as the substrate. HSV-2 gD antibody titers in mouse sera were determined by adding 10 μl of a 1:10 dilution of mouse serum to 1 × 106 LU of cell lysate containing the Renilla luciferase–HSV-2 gD fusion protein, followed by adding protein A/G beads. After extensive washing, the coelenterazine substrate was added, and the number of LU was measured in a luminometer.
Neutralizing antibody was quantified by adding serial dilutions of heat-inactivated (56°C for 30 min) mouse serum in PBS to HSV-2 R519 for 1 h at room temperature and then adding the serum-virus mixture to Vero cell monolayers in 6-well plates for 1 h at 37°C. The inoculum was removed, and medium containing 0.3% Gamunex-C (3:1,000 [vol:vol]) was added. Cells were stained as described above, and the 50% plaque inhibition titers were calculated with Prism software, using nonlinear regression.
DNA isolation, real-time PCR, and immunohistochemistry.
Lumbar-sacral DRG from each mouse were pooled and digested in 0.8 ml of ATL buffer (QIAgene DNeasy blood and tissue kit) with 40 μl of proteinase K at 56°C overnight. Eight hundred microliters of an AL buffer-ethanol (1:1) mixture was added to the tissue lysate and mixed, and the remainder of the procedure was based on the manufacturer's instructions. HSV-2 DNA copies in DRG DNA were quantified by real-time PCR, using HSV-2 gG primers and a gG probe (33), 150 ng of DRG DNA, and 0.5% bovine serum albumin (BSA) per 25 μl of reaction mixture.
Immunohistochemistry for HSV-2 antigens.
Immunohistochemistry was carried out on a Benchmark XT immunostaining platform (Ventana Medical Systems, Tucson, AZ) according to the manufacturer's instructions. Briefly, mouse brain sections were stained with 1:200-diluted rabbit anti-HSV polyclonal antibody (Dako, Carpinteria, CA) for 30 min, and detection was carried out using an UltraView Universal DAB detection kit (Ventana). Slides were read by a pathologist who was blinded to the experimental design.
RESULTS
The genotype and phenotype of HSV2-gD27 are stable when the virus is passaged in human epithelial cells in vitro and during acute infection of mice.
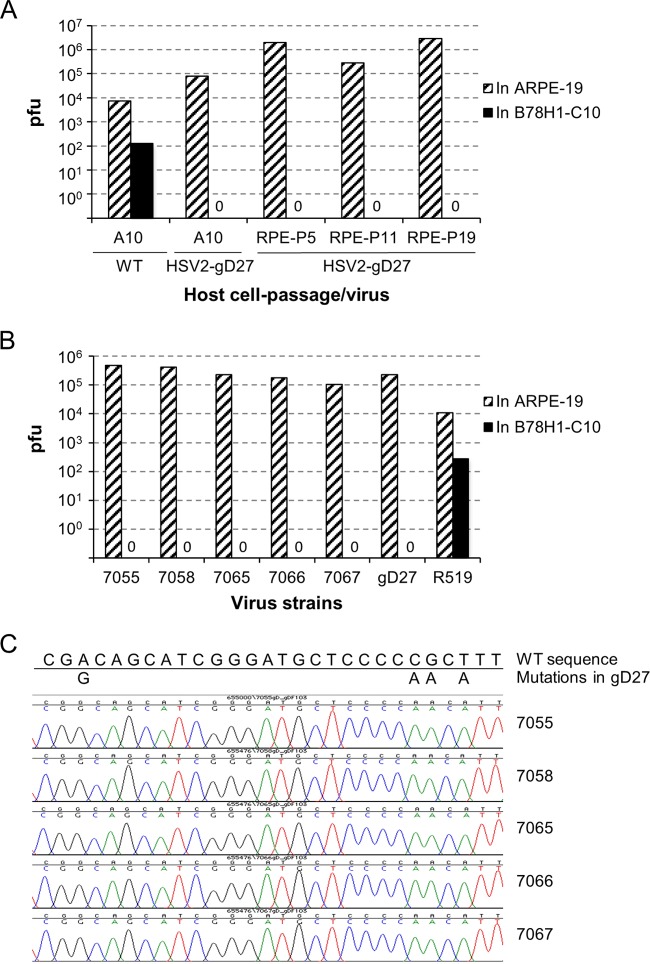
HSV2-gD27 was propagated in B78H1-A10 mouse cells, which express human HVEM but not human nectin-1. HSV2-gD27 was not able to infect B78H1-C10 mouse cells, which express human nectin-1 but not HVEM, since the mutation in gD prevents its interaction with nectin-1 (33). To determine the sensitivity of the assay to detect WT virus mixed with HSV2-gD27, we infected B78H1-C10 cells with 400 PFU of WT virus (titrated in ARPE-19 cells) and 106 PFU of HSV2-gD27 (also titrated in ARPE-19 cells), either together or separately, and assayed the number of plaques on B78H1-C10 cells, which support replication of WT virus but not HSV2-gD27. Coinfection of B78H1-C10 cells with the two viruses resulted in a mean of 6.5 plaques, infection with WT virus alone yielded 5.5 plaques, and infection with HSV2-gD27 yielded no plaques. These data indicate that HSV2-gD27 does not inhibit infection of cells with WT virus. To determine if the mutation in gD is stable when HSV2-gD27 is passaged in cells expressing both human HVEM and nectin-1, cell-free virus was passaged 19 times in human ARPE-19 epithelial cells, and the ability of the progeny virus to infect B78H1-C10 cells was tested. While HSV2-gD27 progeny viruses derived from multiple passages in ARPE-19 cells yielded >105 to 106 plaques in ARPE-19 cells, the same amount of virus yielded no plaques in B78H1-C10 cells (Fig. 1A), indicating that the virus was stable after passage and remained unable to utilize nectin-1 as an entry receptor.
FIG 1.
The phenotype of HSV2-gD27 is stable when the virus is passaged in human epithelial cells and during infection in mice. (A) HSV2-gD27 and WT HSV-2 were propagated in B78H1-A10 mouse melanoma cells (A10). HSV2-gD27 was then passaged in ARPE-19 human epithelial cells (RPE) for 19 passages, and the titers of viruses from passages 5 (P5), 11 (P11), and 19 (P19) were determined in B78H1-C10 mouse melanoma cells (C10) expressing human nectin-1 or in ARPE-19 cells, as a control. (B) Twenty mice were inoculated with 8,000 PFU of HSV2-gD27 intravaginally 5 days after being treated with medroxyprogesterone acetate s.c. (2 mg/mouse). Five mice each were euthanized on days 2, 3, 4, and 5 postinoculation, and DRG were harvested, homogenized, and cultured in ARPE-19 cells. Virus was detected in cultures of DRG from mice 7055, 7058, 7065, 7066, and 7067, passaged an additional time in ARPE-19 cells, and then titrated in ARPE-19 or B78H1-C10 cells. HSV-2 R519, which uses both HVEM and nectin-1 to enter cells, was used as a control. (C) The entire HSV-2 gD open reading frame (ORF) and part of the gB ORF were amplified by PCR from viruses recovered from infected mouse DRG and then sequenced. The portion of gD that encompasses the mutations in HSV2-gD27 is shown.
The stability of HSV2-gD27 was also tested during acute infection of mice. Five mice each were euthanized at 2, 3, 4, and 5 days after intravaginal inoculation, and DRG were removed, homogenized, and plated onto ARPE-19 cells. Nine of 20 mouse DRG cultures showed a cytopathic effect (CPE); viruses were harvested from the 5 cultures with the most CPE, passaged once more in ARPE-19 cells, and used to infect B78H1-C10 and ARPE-19 cells. Viruses that resulted in >106 plaques in ARPE-19 cells failed to produce any plaques in B78H1-C10 cells (Fig. 1B), indicating that the viruses recovered from mouse DRG were dependent on HVEM for entry and could not use nectin-1 as a receptor.
To determine if the point mutations in HSV2-gD27 were stable after passage in vivo, the gD gene of virus recovered from DRG was sequenced. A previous study reported that an HSV-1 gD mutant, K26-gD:R222N/F223I (with R222N/F223I mutations in gD), that was unable to use the nectin-1 receptor developed compensatory changes in gB (D285N and A549T mutations) which allowed the virus to use nectin-1 (35). Therefore, we also determined the sequence of the portion of gB that includes the corresponding codons, D280 and A546, of HSV-2 gB from virus recovered from DRG. No nucleotide changes were found in the sequence of gD (Fig. 1C) or the relevant portion of gB (data not shown) for all 5 clones recovered from DRG of 5 mice.
HSV2-gD27 is attenuated in mice.
We previously reported that HSV2-gD27 is attenuated in mice when inoculated intramuscularly, with no evidence of disease and no infectious virus detectable in DRG after acute infection (33). Here we tested the virulence of HSV2-gD27 by inoculating the virus by other routes. HSV2-gD27 and WT HSV-2 (H312-1BB−) were propagated and titrated in B78H1-A10 cells. WT HSV-2 yielded lower titers when it was titrated in B78H1-A10 cells than when it was titrated in Vero cells; 1 PFU in B78H1-A10 cells was equivalent to 14 PFU in Vero cells.
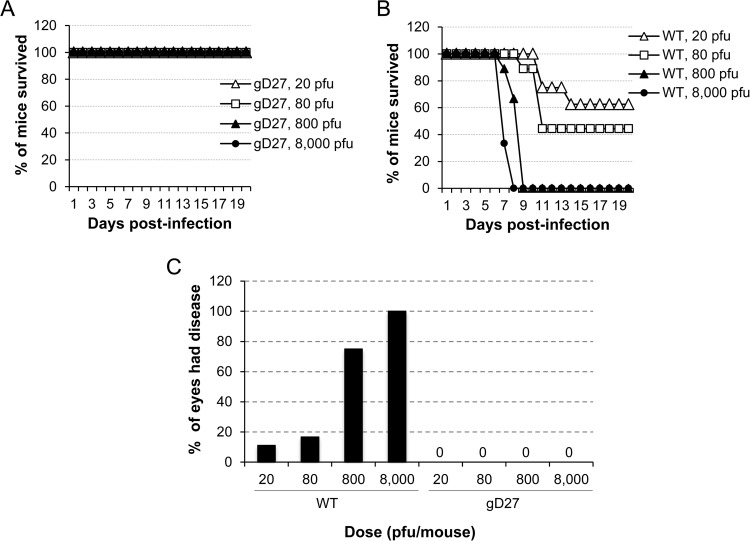
HSV2-gD27 was initially tested in a mouse ocular model. BALB/c mice (6 to 10 per group) were inoculated with 20, 80, 800, or 8,000 PFU of WT HSV-2 (H312-1BB−) or HSV2-gD27 that had been titrated in B78H1-A10 cells. While none of the mice that received HSV2-gD27 died (Fig. 2A), about 50% of the mice that received 20 to 80 PFU of WT HSV-2 died, and all the mice that received 800 or 8,000 PFU of WT HSV-2 died on day 8 or 9 after infection (Fig. 2B); the difference in survival between the WT HSV-2 and HSV2-gD27 groups was statistically significant (P < 0.0001; log rank test). Mice inoculated with HSV2-gD27 showed no signs of ocular disease or inflammation (Fig. 2C), while 75 to 100% of mice infected with 800 to 8,000 PFU of WT virus showed signs of ocular infection. Similarly, mice inoculated i.n. with 8,000 PFU or s.c. with 16,000 PFU of HSV2-gD27 showed no clinical signs of infection.
FIG 2.
HSV2-gD27 is attenuated in the mouse ocular model. Corneas of mice (6 to 10 animals per group) were scarified and infected with HSV2-gD27 (gD27) (A) or WT HSV-2 (B) at the doses indicated (the PFU was based on the virus titer in B78H1-A10 cells), and animals were monitored for survival. (C) Animals used for the experiments in panels A and B were examined for ocular disease, and the percentage of eyes with disease for each group of mice is shown.
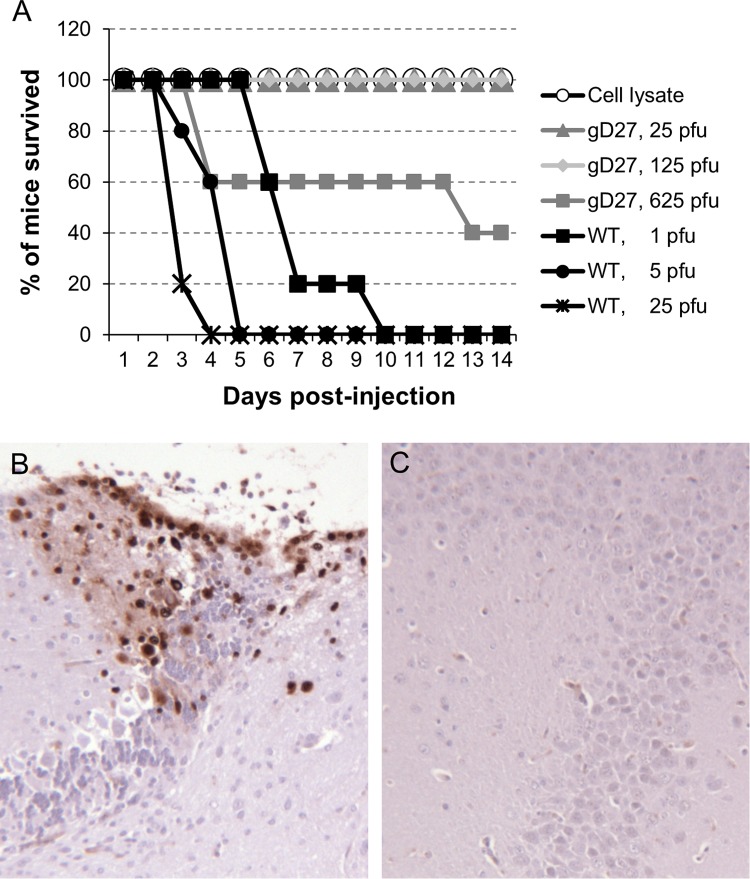
The neurovirulence of HSV2-gD27 was tested by injecting the virus intracranially. Five mice in each group received 25, 125, or 625 PFU of HSV2-gD27, and 5 mice each received 1, 5, or 25 PFU of WT (H312-1BB−) HSV-2 titrated in B78H1-A10 cells. All mice injected with WT virus died by day 10 postinjection, while 40% of mice injected with 625 PFU of HSV2-gD27 and all mice that received lower doses of HSV2-gD27 survived (Fig. 3A); the difference in survival between the WT HSV-2 and HSV2-gD27 groups was statistically significant (P < 0.0001; log rank test). Based on analysis of the hazard ratio between HSV2-gD27 and WT HSV-2, HSV2-gD27 was at least 5,000-fold less virulent than WT virus in the mouse intracranial model. To confirm that animals inoculated intracranially with HSV-2 died due to virus infection and not due to trauma from the inoculation, animals were euthanized when moribund, and the brain was removed and sections examined by histopathology. Minimal to mild focal meningitis was found in the brains of HSV2-gD27-infected mice, while mild multifocal meningitis was found in the brains of WT HSV-2 (H312-1BB−)-infected mice. Brain sections were stained with rabbit anti-HSV antibody, and HSV staining was observed in sections from the brains of all mice infected with WT virus, while no staining was detected in sections from brains of mice infected with HSV2-gD27 (Fig. 3B and C).
FIG 3.
HSV2-gD27 is >5,000-fold less neurovirulent than WT HSV after intracranial inoculation. (A) Groups of 4-week-old mice (5 mice per group) were anesthetized, 10-μl aliquots of various titers of HSV2-gD27 (gD27), WT HSV-2 (WT), or cell lysate were injected into the brain, and animals were monitored for survival. Brains from euthanized morbid mice were fixed in 10% formalin overnight, sectioned, stained with rabbit anti-HSV-1 antibody as described in Materials and Methods, and visualized using DAB chromogen. Representative sections from a WT HSV-infected mouse (B) and an HSV2-gD27-infected mouse (C) are shown. Magnification, ×100. Brown staining indicates HSV-2-positive cells.
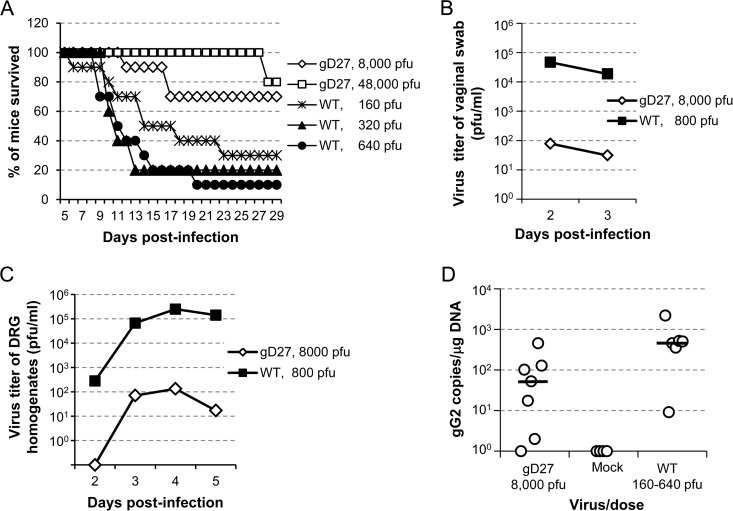
The virulence of HSV2-gD27 was also tested in the mouse vaginal model. Mice were treated with medroxyprogesterone acetate (2 mg) s.c. to synchronize their estrous cycles 5 days before i.vag. inoculation with HSV-2. Groups of mice (10 animals per group) were injected i.vag. with 8,000 or 48,000 PFU of HSV2-gD27 (based on the maximal volume that we could inoculate) or with 160, 320, or 640 PFU of WT HSV-2 (H312-1BB−). After inoculation with 160, 320, or 640 PFU of WT HSV-2, 30%, 20%, or 10% of the mice survived, respectively. In contrast, after inoculation with 8,000 PFU or 48,000 PFU of HSV2-gD27, 70% or 80% of mice survived, respectively (Fig. 4A). Thus, HSV2-gD27 is ≥300-fold less virulent than WT HSV-2 in the mouse vaginal model (P < 0.0001; log rank test).
FIG 4.
HSV2-gD27 is attenuated when inoculated intravaginally. Groups of 10 mice each received medroxyprogesterone acetate s.c. (2 mg/animal) and 5 days later were inoculated intravaginally with various doses of HSV2-gD27 (gD27) or WT HSV-2 (WT) in a volume of 10 μl. (A) The percentage of mice surviving in each group was determined. (B) Groups of 40 or 20 mice were inoculated with 8,000 PFU of HSV2-gD27 or 800 PFU of WT HSV-2, respectively, vaginal swabs were obtained 2 and 3 days later, and virus titers in ARPE-19 cells were determined. (C) DRG from groups of 5 mice were obtained 2, 3, 4, and 5 days after intravaginal infection and then homogenized, and homogenates were titrated in ARPE-19 cells. (D) Groups of 10 mice each were infected intravaginally with 8,000 PFU of HSV2-gD27 (gD27) or with 160, 320, or 640 PFU of WT HSV-2 (WT). Thirty-six days after infection, lumbosacral DRG were harvested, and DNAs were extracted from DRG. Real-time PCR for detection of the HSV-2 gG gene was performed with 150 ng DRG DNA per 25-μl reaction mix. PCR data for the groups receiving 3 different doses of WT HSV-2 were combined into one group, because only 5 of 30 mice survived acute infection. Each open circle represents one mouse.
To determine the ability of HSV2-gD27 to replicate in the vagina, 20 mice were infected with 800 PFU of WT HSV-2 (H312-1BB−), 20 mice were infected with 8,000 PFU of HSV2-gD27, vaginal swabs were obtained 2 and 3 days later to assess virus replication, and viruses from swabs were titrated on ARPE-19 cells. The group mean swab titers for WT virus- and HSV2-gD27-infected mice were 470,000 PFU/ml and 78 PFU/ml, respectively, for day 2 (P < 0.0001; t test) and 18,767 PFU/ml and 31 PFU/ml, respectively, for day 3 (P = 0.0001; t test) (Fig. 4B). Thus, the ability of HSV2-gD27 to replicate in the vagina is >600-fold less than that of the WT virus.
To determine the ability of HSV2-gD27 to infect DRG when inoculated i.vag., 20 mice were infected with 8,000 PFU of HSV2-gD27 and 20 mice were infected with 800 PFU of WT virus. Two, 3, 4, and 5 days after inoculation, 5 mice from each group were euthanized, and DRG were harvested, homogenized, and plated on ARPE-19 cell monolayers. The group mean virus titers for DRG from HSV2-gD27-infected mice were 0, 71, 134, and 17 PFU/ml for days 2, 3, 4, and 5, respectively (Fig. 4C). In contrast, the group mean virus titers for DRG from WT virus-infected mice were 280, 67,300, 250,000, and 143,000 PFU/ml for days 2, 3, 4, and 5, respectively; the differences were statistically significant (P = 0.0001; t test). The ratios of virus titers for DRG infected with WT virus to those infected with HSV2-gD27 were >280, 945, 1,860, and 8,300 for days 2, 3, 4, and 5, respectively, even though animals received WT virus at 1/10 the dose of HSV2-gD27. While 80% of mice inoculated with WT HSV-2 had virus detected in DRG on day 2, and 100% had virus detected in DRG on days 3, 4, and 5, 0% of mice inoculated with HSV2-gD27 at 10 times the dose of WT virus had detectable virus in DRG on day 2, and 60% had detectable virus in DRG on days 3, 4, and 5. Since infectious HSV2-gD27 was detected in DRG during acute infection, we examined the latent viral DNA loads in DRG. Because most mice infected with 800 PFU of WT HSV-2 i.vag. died during acute infection, the dose of the WT virus was lowered to 160, 320, or 640 PFU/mouse, and 0.5 ml of 0.75% human intravenous immune globulin (IVIG; has neutralizing antibody to HSV-2) was given i.p. 1 day after infection to increase the survival rate of the mice. However, even with this intervention, only 6 of the 30 mice receiving WT virus survived. Mice were euthanized 36 days after infection, DRG were harvested, and DNAs were extracted for real-time PCR to determine the number of copies of HSV-2 DNA. The group mean was 108 HSV-2 copies/μg DRG DNA (median, 52.5 copies/μg DRG DNA) for mice infected with HSV2-gD27 and 675 HSV-2 copies/μg DRG DNA (median, 482 copies/μg DRG DNA) for animals infected with WT HSV-2 at infection doses ranging from 160 to 640 PFU/mouse (Fig. 4D). Due to the much lower dose of WT virus than that of HSV2-gD27, treatment with IVIG for animals receiving WT virus, and increased mortality for animals infected with WT virus (80% for mice receiving WT virus versus none for mice receiving HSV2-gD27), we could not precisely compare the latent viral DNA copy numbers for HSV2-gD27 and WT HSV-2; however, there was much less latent HSV2-gD27 DNA than WT HSV-2 DNA in mouse DRG.
Intramuscular vaccination with HSV2-gD27 is more effective than intranasal or subcutaneous vaccination to protect mice from HSV-2 intravaginal challenge.
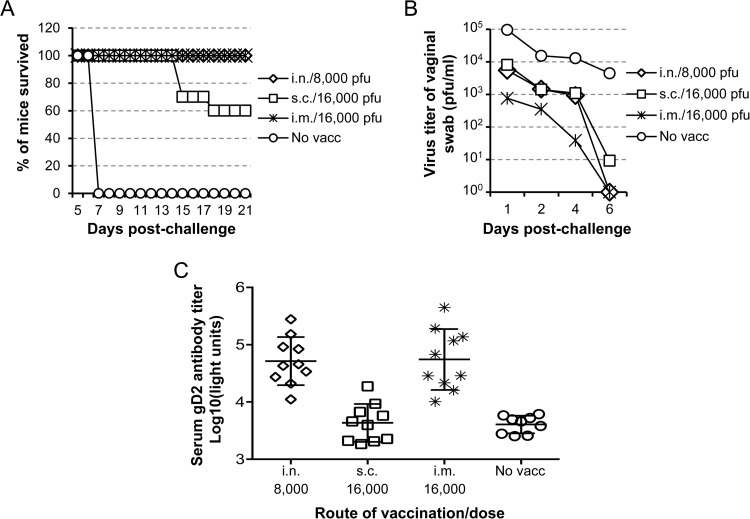
To determine which vaccination route provides the best protection against intravaginal challenge, mice were vaccinated with 8,000 PFU of HSV2-gD27 i.n. or i.vag. or with 16,000 PFU s.c. or i.m. or injected with uninfected B78H1-A10 cell lysate as a mock vaccine control. Mice received a second dose of vaccine 21 days later. Three to 4 weeks after the second vaccination, mice were treated with medroxyprogesterone acetate, and 5 days later they were challenged with 4 × 104 PFU of HSV-2 R519 intravaginally. To monitor virus replication in the genital tract, vaginal swabs were obtained 1, 2, 3, 5, and 7 days after challenge and titrated in Vero cells. While all mice that received the mock vaccine died by day 7 after challenge, no animals in the groups vaccinated with HSV2-gD27 i.n. or i.m. died (P < 0.0001 for mock vaccine versus other groups; log rank test) (Fig. 5A). Sixty percent of mice vaccinated s.c. survived the challenge, which was significantly lower than the survival rates of the i.n. and i.m. groups (P = 0.0006; log rank test). The virus titers in vaginal swabs after challenge of mice vaccinated i.n. and s.c. were about 10-fold lower than those for the mice that were mock vaccinated (P = 0.018 for i.n. versus mock groups and P = 0.0104 for s.c. versus mock groups; t test). Mice vaccinated i.m. had vaginal swab titers that were about 100-fold lower than those for mock-vaccinated animals (P = 0.0008; t test) and about 10-fold lower than those for mice that were vaccinated i.n. or s.c. (P = 0.51 or 0.08, respectively; t test) (Fig. 5B).
FIG 5.
Intramuscular vaccination with HSV2-gD27 is more effective than intranasal or subcutaneous vaccination to reduce shedding after intravaginal challenge. Mice were vaccinated twice 28 days apart with 8,000 PFU of HSV2-gD27 i.n., 16,000 PFU s.c. or i.m., or uninfected B78H1-A10 cell lysate (no vacc) as a control. About 3 weeks after the second vaccination, mice were injected s.c. with 2 mg/mouse of medroxyprogesterone acetate. Five days later, mice were challenged intravaginally with 4 × 104 PFU of WT HSV-2 strain R519. (A) Percentages of mice (10 per group) that survived challenge. Mice were monitored for 30 days, but no deaths were observed after day 18. (B) Vaginal swabs were obtained 1, 2, 4, and 6 days after challenge, stored at −80°C, and titrated in Vero cells, and group mean swab titers were plotted. (C) gD antibody titers were determined by adding 10 μl of 1:10-diluted mouse serum to 1 × 106 light units (LU) of HSV-2 gD–Renilla luciferase fusion protein lysate, followed by adding protein A/G beads. After extensive washing, coelenterazine substrate was added, and the LU were measured in a luminometer.
Intramuscular and intranasal vaccinations induce comparable levels of serum gD antibody.
The ability of HSV2-gD27 to induce serum antibody was determined by LIPS assay (34). The i.m. and i.n. vaccinations induced comparable levels of serum gD antibody (P = 0.8933), while antibody levels of the mice vaccinated s.c. were lower (P < 0.0001 for s.c. versus i.m. or i.n. group) (Fig. 5C).
Intramuscular vaccination with HSV2-gD27 is more effective than vaccination with an HSV-2 subunit vaccine.
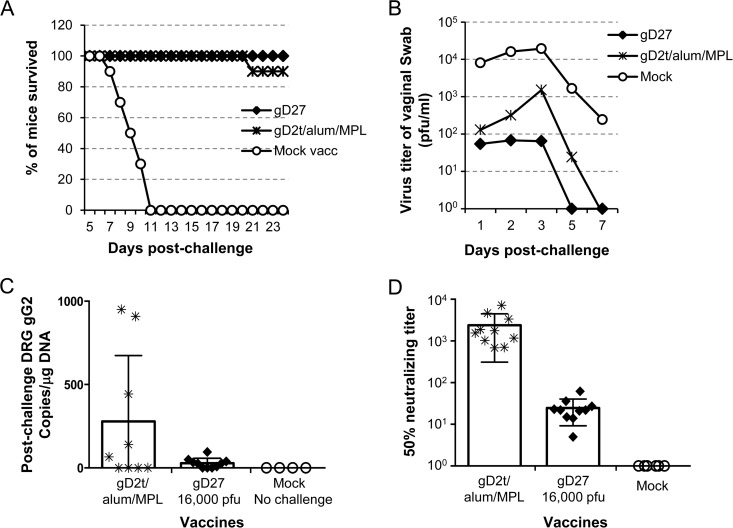
A soluble HSV-2 gD subunit vaccine in alum/MPL, which had been shown to be effective in guinea pigs (36), recently failed to reduce HSV-2 genital infection or disease in a phase 3 multicenter clinical trial (12). We postulated that a successful human vaccine will need to be more effective than the soluble HSV-2 gD subunit vaccine in the adjuvant used in the clinical trial. Therefore, we compared the abilities of HSV2-gD27 and the gD subunit vaccine gD2t/alum/MPL to protect mice from intravaginal challenge with HSV-2. Mice were vaccinated i.m. twice with 16,000 PFU of HSV2-gD27 or with gD2t/alum/MPL and challenged with HSV-2 R519 i.vag. Both vaccines protected mice from lethal challenge, and there was no significant difference between protection with HSV2-gD27 and that with gD2t/alum/MPL (P = 0.317; log rank test) (Fig. 6A). However, the ability of the vaccines to reduce challenge virus replication in the mouse vagina (monitored by virus titers of vaginal swabs) was different. Vaccination with gD2t/alum/MPL reduced the titer of challenge virus in the vagina on day 3 (the peak of virus replication) ∼13-fold compared with that observed with mock vaccination. In contrast, vaccination with HSV2-gD27 reduced the titer of challenge virus ∼300-fold compared with that observed with mock vaccination and 22-fold compared with that observed with gD2t/alum/MPL vaccination (Fig. 6B). In addition, mice vaccinated with HSV2-gD27 had no detectable shedding by day 5, while animals receiving gD2t/alum/MPL were still shedding virus at that time. Comparison of HSV-2 titers shed over days 1 to 5 showed that mice vaccinated with HSV2-gD27 and gD2t/alum/MPL shed less virus than mock-vaccinated animals (P < 0.0001 and P = 0.0002, respectively; t test) and that mice vaccinated with HSV2-gD27 shed less virus than those receiving gD2t/alum/MPL (P = 0.031; t test).
FIG 6.
HSV2-gD27 is superior to gD2t for preventing challenge virus replication in the vagina and latency in DRG, despite inducing lower titers of serum neutralizing antibody. Groups of 10 mice were vaccinated i.m. twice, 28 days apart, with 16,000 PFU of HSV2-gD27 (gD27), with gD2t/alum/MPL, or with B78H1-A10 cell lysate (mock vaccine control). Three weeks after the second vaccination, mice received 2 mg of medroxyprogesterone acetate, and 5 days later animals were challenged with 4 × 104 PFU of HSV-2 R519 intravaginally. (A) Percentage of mice in each group that survived challenge. (B) Vaginal swabs were obtained 1, 2, 3, 5, and 7 days after challenge and titrated in Vero cells. (C) DRG were harvested 33 days after challenge, DNAs were isolated, and real-time PCR using primers and a probe for the HSV-2 gG gene was performed with 150 ng of DRG DNA. Each symbol represents the value for one mouse. (D) Two weeks after the second dose of vaccine, sera were obtained and HSV-2 neutralizing antibody titers were determined by plaque assays with Vero cells. Plaque numbers in each well were counted, and 50% plaque inhibition titers were calculated with Prism software, using nonlinear regression. Each symbol represents the value for one mouse.
The ability of the vaccines to reduce latent infection in mouse ganglia was also assessed. Mice were euthanized 33 days after challenge, DRG were harvested, and DNAs were extracted from DRG for real-time PCR to determine the number of copies of HSV-2 DNA per μg of total DRG DNA. Mice vaccinated with HSV2-gD27 had a group mean of 28 copies of HSV-2 DNA per μg DRG DNA, while animals vaccinated with gD2t/alum/MPL had a group mean of 279 copies of HSV-2 DNA per μg of DRG DNA, i.e., there was a 10-fold difference; however, due to the large intragroup variation, the difference was not significant (P = 0.8513; t test) (Fig. 6C). While all of the mice vaccinated with HSV2-gD27 had <100 HSV-2 DNA copies per μg DRG DNA, 3 of 9 mice vaccinated with gD2t/alum/MPL had 442 to 950 HSV-2 copies per μg DRG DNA. Taken together, the data showed that live attenuated HSV2-gD27 was superior to the gD2t/alum/MPL subunit vaccine for reducing shedding and latent virus infection after i.vag. challenge with WT HSV-2.
The superior protection provided by HSV2-gD27 compared to gD2t/alum/MPL is not due to a higher titer of HSV-2 serum neutralizing antibody.
To compare serum neutralizing antibody titers of mice vaccinated i.m. with HSV2-gD27 and gD2t/alum/MPL, sera were collected 23 days after the second vaccination. Three experiments were conducted, and the group mean serum neutralizing antibody titers were 25, 42, and 34 for the HSV2-gD27 groups, while the titers were 2,390, 825, and 1,140 for the groups vaccinated with gD2t/alum/MPL. The results of one representative experiment, like the other two, showed a significantly higher level of neutralizing antibody with gD2t/alum/MPL than with the HSV2-gD27 vaccine (P < 0.0001; t test) (Fig. 6D). Since the mean serum neutralizing antibody titer in mice vaccinated with HSV2-gD27 was lower than expected, additional mice were vaccinated with a higher dose of HSV2-gD27 (160,000 PFU/mouse); the mean neutralizing titer in these animals was 139, which was still lower than that for mice receiving gD2t/alum/MPL. Therefore, while HSV2-gD27 provided better protection than gD2t/alum/MPL against i.vag. challenge with HSV-2, the level of serum neutralizing antibody was not responsible for the improved protection.
DISCUSSION
We have shown that HSV2-gD27, a candidate live attenuated virus vaccine, is stable when passaged in vitro and in vivo, is attenuated in mice even after intracranial inoculation, and protects mice from intravaginal challenge with HSV-2 better than a gD2 subunit vaccine does, even though HSV2-gD27 induces lower titers of neutralizing antibody than those with the subunit vaccine.
HSV2-gD27 has point mutations (D215G, R222N, and F223I) in the nectin-1 receptor binding domain of gD that result in impaired infection of neurons (33), which express nectin-1 (20, 21) but little to no HVEM (22–24). The genome stability of HSV2-gD27 is of concern when the virus is grown in cells that express both nectin-1 and HVEM, which could allow viruses with reversion of point mutations or with compensatory mutations to be selected. HSV-1 mutants impaired for using nectin-1 have been constructed but were found to be unstable (31, 32). Awasthi et al. (31) constructed HSV-1 KOS-gDA3C/Y38C, with mutations in HSV-1 gD codons 3 and 38. The cysteine mutation at codon 38 reverted to tyrosine during propagation of the virus in Vero cells, while the cysteine mutation at codon 3 was stable during passage in cell culture and in mice. Uchida et al. (32) constructed HSV-1 K26-gD:R222N/F223I, with mutations in HSV-1 gD codons 222 and 223; these two codons are homologous to two of the three codons that we mutated in HSV2-gD27. Uchida et al. observed phenotypic revertants when they passaged their mutant in cells expressing nectin-1. Some of the phenotypic revertants had one of the mutant codons changed, while others had new compensatory mutations at another codon in gD (32) or in gB (35). In contrast, we found that our HSV2-gD27 mutant virus was phenotypically and genotypically stable when passaged 19 times in human epithelial (ARPE-19) cells and after infection of mice. The increased genetic stability of the HSV-2 mutant compared to its HSV-1 counterpart may be due to differences between HSV-1 and HSV-2 for entry into cells. While HSV-1 K26-gD:R222N/F223I produced CPE in B78H1-C10 cells (32, 35), which express human nectin-1 but not HVEM, HSV2-gD27 did not yield CPE when inoculated onto these cells at MOIs of >10. This result is consistent with the observation made by Manoj et al. that gD-null HSV-1 pseudotyped with a gD protein bearing R222N/F223I mutations can efficiently enter CHO-nectin-1 cells, while gD-null HSV-2 333 pseudotyped with an HSV-2 gD protein bearing D215G/R222N/F223I mutations (identical to the mutations in HSV2-gD27) is impaired for entering CHO-nectin-1 cells (28). Since HSV-1 K26-gD:R222N/F223I produces CPE in B78H1 cells expressing nectin-1, replication of the virus in these cells allows it to mutate further, and any revertant virus that regains the ability to use nectin-1 will be selected over the K26-gD:R222N/F223I virus to grow in B78H1 cells. The inability of HSV2-gD27 to infect cells that express nectin-1 but not HVEM reduces the opportunity for the virus to mutate to use nectin-1 for entry. Thus, HSV2-gD27 is more stable than HSV-1 K26-gD:R222N/F223I. Nonetheless, if HSV2-gD27 is further developed as a vaccine, additional mutations would be needed to ensure its safety and stability.
HSV2-gD27 was markedly impaired for neurotropism. We previously showed that after i.m. injection of mice with HSV2-gD27, no infectious virus was detectable in DRG during acute infection, implying that the virus is impaired for neuroinvasiveness (33). Although 50% of mice died after i.c. injection with 625 PFU of HSV2-gD27 (titrated in B78H1-A10 cells; equivalent to 104 PFU in Vero cells), HSV-2 antigen could not be detected in the brain (Fig. 3B and C). However, HSV2-gD27 resulted in higher mortality in these experiments than in our previous report of a replication-defective mutant also given i.c. (37), and additional mutations would need to be engineered into HSV2-gD27 to improve its safety if it were to be used as a vaccine. While i.vag. vaccination with a live attenuated HSV-2 strain may not be feasible in humans, we inoculated mice i.vag. with HSV2-gD27 for additional safety testing of the virus. Infectious HSV2-gD27 was detected in DRG of 60% of the mice during acute infection, but titers were >1,000-fold lower than those in mice inoculated i.vag. with WT virus at 1/10 the dose of HSV2-gD27. However, low levels of HSV2-gD27 DNA were detected in DRG 36 days after inoculation. Latent infection with live attenuated virus vaccines may not necessarily be a problem if the virus remains highly attenuated and its genome is stable. For example, the live attenuated varicella-zoster Oka vaccine can establish latent infection (38).
HSV-2 infects mucosal surfaces, and it is possible that an HSV-2 vaccine given i.n. may induce better immune responses than a vaccine given i.m. Previously, i.n. immunization of mice with surfactant vesicles containing HSV-1 gB protected 90% of mice from lethal intravaginal challenge with HSV-2 (39). Vaccination of mice by the i.n. route with an adenovirus expressing HSV-1 gB was more effective than vaccination by the i.p. route to protect animals from genital challenge with HSV-2 (40). Vaccination of mice with a replication-defective HSV-2 ICP8-null mutant i.n. induced mucosal and systemic immunity and reduced challenge virus replication in the vagina (41). Vaccination of guinea pigs with vaccinia virus expressing HSV-2 gD i.n. reduced genital shedding after challenge with HSV-2 (42). Vaccination of mice by the i.n. route with an HSV-2 thymidine kinase mutant induced superior protection against intravaginal challenge with WT HSV-2 compared to intraperitoneal vaccination (43). We found that while i.n. vaccination with HSV2-gD27 protected mice from lethal challenge as effectively as i.m. immunization, i.n. vaccination was less effective than i.m. immunization to reduce virus replication in the vagina after i.vag. challenge.
While mice can be infected with HSV-2, the infection is much more likely to be lethal in mice than in humans. Most genital HSV-2 infections in humans are asymptomatic or result in mild disease, but most mice infected with HSV-2 intravaginally develop paralysis and urinary retention and die. In many prior studies, protection of mice from lethal genital challenge was used as an endpoint to imply that an HSV-2 vaccine candidate was effective. Here we showed that the gD2t subunit vaccine protected mice from lethal challenge almost as well as HSV2-gD27 did; however, HSV2-gD27 resulted in better protection from i.vag. challenge than that with gD2t when challenge virus replication in the vagina and the latent viral load in DRG were measured. Virus shedding and latent virus in DRG of mice are likely to be better predictors than survival for the efficacy of HSV-2 vaccines in humans, since humans shed virus in the genital tract and genital lesions are associated with higher genital viral loads in humans (44, 45) and mice (46).
Since HSV2-gD27 is expected to express all of the more than 80 HSV-2 proteins, while the gD2t subunit vaccine contains only one glycoprotein, and since HSV2-gD27 was superior to gD2t in reducing HSV-2 shedding and latent virus infection after i.vag. challenge with WT HSV-2, we expected that HSV2-gD27 would induce higher titers of neutralizing antibody than those obtained with the gD2t/alum/MPL vaccine. Surprisingly, the mean serum neutralizing antibody titer in mice that received the gD2t subunit vaccine was about 40-fold higher than that for animals that received HSV2-gD27. It is possible that the reduced level of HSV neutralizing antibody in mice vaccinated with HSV2-gD27 compared to that in mice vaccinated with gD2t/alum/MPL was partially due to the mutations in gD2 in HSV2-gD27. Connolly et al. reported that HSV-1 gD proteins with a D215A or R222A mutation, corresponding to the sites of two of the amino acids mutated in HSV2-gD27, D215G and R222N, lost reactivity with the monoclonal antibody DL11 (47). DL11 is a potent neutralizing antibody that blocks the interaction of gD with nectin-1 (48). If a gD mutant is not able to induce a DL11-like antibody, it may be less capable of inducing an HSV-2 neutralizing antibody that targets the nectin-1 binding site on gD. The observation that HSV2-gD27 induced better protection in mice than gD2t/alum/MPL did, but with a lower serum neutralizing antibody titer, clearly demonstrates that HSV2-gD27 induced a protective immune response that involved an activity other than neutralizing antibody activity. This immune response might include antibody-dependent cellular cytotoxicity (ADCC), complement-mediated cytotoxicity, or virus-specific CD4+ T and/or CD8+ T cells. Passive transfer of a panel of mouse monoclonal antibodies against HSV-2 glycoproteins with no HSV-2 neutralizing activity partially protected mice from HSV-2 footpad infection, and the effect correlated with ADCC activity of the monoclonal antibodies (49). An HSV-2 gG vaccine which induced low levels of serum neutralizing antibody but induced both ADCC and complement-mediated cytotoxicity partially protected mice from HSV-2 intravaginal challenge (50). More recently, vaccination of mice with a replication-defective HSV-2 gD deletion mutant protected animals from i.vag. challenge; while the vaccine induced very low titers of serum neutralizing antibody, it induced antibody with ADCC activity (15). In addition, live attenuated or replication-defective vaccines may induce protective immunity due to CD4+ or CD8+ T cells. Milligan et al. reported that depletion of T cells, especially CD4+ cells, from mice vaccinated with a live attenuated HSV-2 thymidine kinase-null mutant markedly reduced the protective efficiency of the vaccine (51). Similarly, Morrison found that depletion of CD4+ cells from B cell-deficient mice vaccinated with a replication-defective HSV-2 ICP8-null mutant resulted in severe genital and central nervous system disease after challenge with wild-type virus (52).
Taken together, our data show that HSV2-gD27 is stable during passage in vitro and acute infection in mice, has markedly reduced neurotropism, and is effective in a vaginal challenge model in mice. This suggests that viruses with alterations in receptor usage can be developed as live attenuated virus vaccine candidates. Live attenuated HSV-2 vaccines are likely to provide better and more durable protection than subunit vaccines and may induce a broader array of immune responses than solely neutralizing antibody to provide protection against HSV-2 infection and/or disease.
ACKNOWLEDGMENTS
We thank Gary H. Cohen and Roselyn J. Eisenberg for sharing reagents, Peter D. Burbelo for helping with the LIPS assay, and Jing Qin for helping with statistical analyses.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. 2015. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 10:e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. 2006. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS 20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein DI, Bellamy AR, Hook EW III, Levin MJ, Wald A, Ewell MG, Wolff PA, Deal CD, Heineman TC, Dubin G, Belshe RB. 2013. Epidemiology, clinical presentation, and antibody response to primary infection with herpes simplex virus type 1 and type 2 in young women. Clin Infect Dis 56:344–351. doi: 10.1093/cid/cis891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts CM, Pfister JR, Spear SJ. 2003. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis 30:797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- 5.Lafferty WE, Coombs RW, Benedetti J, Critchlow C, Corey L. 1987. Recurrences after oral and genital herpes simplex virus infection. Influence of site of infection and viral type. N Engl J Med 316:1444–1449. [DOI] [PubMed] [Google Scholar]
- 6.Berman PW, Gregory T, Crase D, Lasky LA. 1985. Protection from genital herpes simplex virus type 2 infection by vaccination with cloned type 1 glycoprotein D. Science 227:1490–1492. doi: 10.1126/science.2983428. [DOI] [PubMed] [Google Scholar]
- 7.Burke RL. 1991. Development of a herpes simplex virus subunit glycoprotein vaccine for prophylactic and therapeutic use. Rev Infect Dis 13(Suppl 11):11. [DOI] [PubMed] [Google Scholar]
- 8.Stanberry LR, Bernstein DI, Burke RL, Pachl C, Myers MG. 1987. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J Infect Dis 155:914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- 9.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM Jr, Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 282:331–340. [DOI] [PubMed] [Google Scholar]
- 10.Langenberg AG, Burke RL, Adair SF, Sekulovich R, Tigges M, Dekker CL, Corey L. 1995. A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity [corrected]. Ann Intern Med 122:889–898. doi: 10.7326/0003-4819-122-12-199506150-00001. [DOI] [PubMed] [Google Scholar]
- 11.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 12.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brans R, Akhrameyeva NV, Yao F. 2009. Prevention of genital herpes simplex virus type 1 and 2 disease in mice immunized with a gD-expressing dominant-negative recombinant HSV-1. J Invest Dermatol 129:2470–2479. doi: 10.1038/jid.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Costa XJ, Jones CA, Knipe DM. 1999. Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc Natl Acad Sci U S A 96:6994–6998. doi: 10.1073/pnas.96.12.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petro C, Gonzalez PA, Cheshenko N, Jandl T, Khajoueinejad N, Benard A, Sengupta M, Herold BC, Jacobs WR. 2015. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 4:e06054. doi: 10.7554/eLife.06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awasthi S, Zumbrun EE, Si H, Wang F, Shaw CE, Cai M, Lubinski JM, Barrett SM, Balliet JW, Flynn JA, Casimiro DR, Bryan JT, Friedman HM. 2012. Live attenuated herpes simplex virus 2 glycoprotein E deletion mutant as a vaccine candidate defective in neuronal spread. J Virol 86:4586–4598. doi: 10.1128/JVI.07203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halford WP, Puschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. 2011. A live-attenuated HSV-2 ICP0 virus elicits 10 to 100 times greater protection against genital herpes than a glycoprotein D subunit vaccine. PLoS One 6:e17748. doi: 10.1371/journal.pone.0017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wachsman M, Kulka M, Smith CC, Aurelian L. 2001. A growth and latency compromised herpes simplex virus type 2 mutant (ICP10DeltaPK) has prophylactic and therapeutic protective activity in guinea pigs. Vaccine 19:1879–1890. doi: 10.1016/S0264-410X(00)00446-1. [DOI] [PubMed] [Google Scholar]
- 19.Prichard MN, Kaiwar R, Jackman WT, Quenelle DC, Collins DJ, Kern ER, Kemble GM, Spaete RR. 2005. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine 23:5424–5431. doi: 10.1016/j.vaccine.2005.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haarr L, Shukla D, Rodahl E, Dal Canto MC, Spear PG. 2001. Transcription from the gene encoding the herpesvirus entry receptor nectin-1 (HveC) in nervous tissue of adult mouse. Virology 287:301–309. doi: 10.1006/viro.2001.1041. [DOI] [PubMed] [Google Scholar]
- 21.Shukla D, Dal Canto MC, Rowe CL, Spear PG. 2000. Striking similarity of murine nectin-1alpha to human nectin-1alpha (HveC) in sequence and activity as a glycoprotein D receptor for alphaherpesvirus entry. J Virol 74:11773–11781. doi: 10.1128/JVI.74.24.11773-11781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon BS, Tan KB, Ni J, Oh KO, Lee ZH, Kim KK, Kim YJ, Wang S, Gentz R, Yu GL, Harrop J, Lyn SD, Silverman C, Porter TG, Truneh A, Young PR. 1997. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem 272:14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery RI, Warner MS, Lum BJ, Spear PG. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427–436. doi: 10.1016/S0092-8674(00)81363-X. [DOI] [PubMed] [Google Scholar]
- 24.Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A. 1997. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem 272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 25.Spear PG. 2004. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol 6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- 26.Carfi A, Willis SH, Whitbeck JC, Krummenacher C, Cohen GH, Eisenberg RJ, Wiley DC. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol Cell 8:169–179. doi: 10.1016/S1097-2765(01)00298-2. [DOI] [PubMed] [Google Scholar]
- 27.Connolly SA, Landsburg DJ, Carfi A, Wiley DC, Cohen GH, Eisenberg RJ. 2003. Structure-based mutagenesis of herpes simplex virus glycoprotein D defines three critical regions at the gD-HveA/HVEM binding interface. J Virol 77:8127–8140. doi: 10.1128/JVI.77.14.8127-8140.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manoj S, Jogger CR, Myscofski D, Yoon M, Spear PG. 2004. Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism. Proc Natl Acad Sci U S A 101:12414–12421. doi: 10.1073/pnas.0404211101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Giovine P, Settembre EC, Bhargava AK, Luftig MA, Lou H, Cohen GH, Eisenberg RJ, Krummenacher C, Carfi A. 2011. Structure of herpes simplex virus glycoprotein D bound to the human receptor nectin-1. PLoS Pathog 7:e1002277. doi: 10.1371/journal.ppat.1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu G, Zhang N, Qi J, Li Y, Chen Z, Zheng C, Gao GF, Yan J. 2014. Crystal structure of herpes simplex virus 2 gD bound to nectin-1 reveals a conserved mode of receptor recognition. J Virol 88:13678–13688. doi: 10.1128/JVI.01906-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awasthi S, Lubinski JM, Eisenberg RJ, Cohen GH, Friedman HM. 2008. An HSV-1 gD mutant virus as an entry-impaired live virus vaccine. Vaccine 26:1195–1203. doi: 10.1016/j.vaccine.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uchida H, Shah WA, Ozuer A, Frampton AR Jr, Goins WF, Grandi P, Cohen JB, Glorioso JC. 2009. Generation of herpesvirus entry mediator (HVEM)-restricted herpes simplex virus type 1 mutant viruses: resistance of HVEM-expressing cells and identification of mutations that rescue nectin-1 recognition. J Virol 83:2951–2961. doi: 10.1128/JVI.01449-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, Kappel JD, Canders C, Davila WF, Sayre D, Chavez M, Pesnicak L, Cohen JI. 2012. A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM. J Virol 86:12891–12902. doi: 10.1128/JVI.01055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burbelo PD, Hoshino Y, Leahy H, Krogmann T, Hornung RL, Iadarola MJ, Cohen JI. 2009. Serological diagnosis of human herpes simplex virus type 1 and 2 infections by luciferase immunoprecipitation system assay. Clin Vaccine Immunol 16:366–371. doi: 10.1128/CVI.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchida H, Chan J, Goins WF, Grandi P, Kumagai I, Cohen JB, Glorioso JC. 2010. A double mutation in glycoprotein gB compensates for ineffective gD-dependent initiation of herpes simplex virus type 1 infection. J Virol 84:12200–12209. doi: 10.1128/JVI.01633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourne N, Bravo FJ, Francotte M, Bernstein DI, Myers MG, Slaoui M, Stanberry LR. 2003. Herpes simplex virus (HSV) type 2 glycoprotein D subunit vaccines and protection against genital HSV-1 or HSV-2 disease in guinea pigs. J Infect Dis 187:542–549. doi: 10.1086/374002. [DOI] [PubMed] [Google Scholar]
- 37.Hoshino Y, Pesnicak L, Dowdell KC, Lacayo J, Dudek T, Knipe DM, Straus SE, Cohen JI. 2008. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine 26:4034–4040. doi: 10.1016/j.vaccine.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gershon AA. 2013. Varicella zoster vaccines and their implications for development of HSV vaccines. Virology 435:29–36. doi: 10.1016/j.virol.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cortesi R, Ravani L, Rinaldi F, Marconi P, Drechsler M, Manservigi M, Argnani R, Menegatti E, Esposito E, Manservigi R. 2013. Intranasal immunization in mice with non-ionic surfactants vesicles containing HSV immunogens: a preliminary study as possible vaccine against genital herpes. Int J Pharm 440:229–237. doi: 10.1016/j.ijpharm.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 40.Gallichan WS, Johnson DC, Graham FL, Rosenthal KL. 1995. Mucosal immunization with a recombinant adenovirus vector induces local and systemic immunity and protection from herpes simplex virus. Adv Exp Med Biol 371B:1581–1585. [PubMed] [Google Scholar]
- 41.Morrison LA, Da Costa XJ, Knipe DM. 1998. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology 243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- 42.Bernstein DI. 2000. Effect of route of vaccination with vaccinia virus expressing HSV-2 glycoprotein D on protection from genital HSV-2 infection. Vaccine 18:1351–1358. doi: 10.1016/S0264-410X(99)00416-8. [DOI] [PubMed] [Google Scholar]
- 43.Sato A, Suwanto A, Okabe M, Sato S, Nochi T, Imai T, Koyanagi N, Kunisawa J, Kawaguchi Y, Kiyono H. 2014. Vaginal memory T cells induced by intranasal vaccination are critical for protective T cell recruitment and prevention of genital HSV-2 disease. J Virol 88:13699–13708. doi: 10.1128/JVI.02279-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schiffer JT, Corey L. 2013. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med 19:280–290. doi: 10.1038/nm.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryson YJ, Dillon M, Lovett M, Acuna G, Taylor S, Cherry JD, Johnson BL, Wiesmeier E, Growdon W, Creagh-Kirk T, Keeney R. 1983. Treatment of first episodes of genital herpes simplex virus infection with oral acyclovir. A randomized double-blind controlled trial in normal subjects. N Engl J Med 308:916–921. [DOI] [PubMed] [Google Scholar]
- 46.Overall JC Jr, Kern ER, Schlitzer RL, Friedman SB, Glasgow LA. 1975. Genital herpesvirus hominis infection in mice. I. Development of an experimental model. Infect Immun 11:476–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Connolly SA, Landsburg DJ, Carfi A, Whitbeck JC, Zuo Y, Wiley DC, Cohen GH, Eisenberg RJ. 2005. Potential nectin-1 binding site on herpes simplex virus glycoprotein D. J Virol 79:1282–1295. doi: 10.1128/JVI.79.2.1282-1295.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol 86:1563–1576. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balachandran N, Bacchetti S, Rawls WE. 1982. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun 37:1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorander S, Harandi AM, Lindqvist M, Bergstrom T, Liljeqvist JA. 2012. Glycoprotein G of herpes simplex virus 2 as a novel vaccine antigen for immunity to genital and neurological disease. J Virol 86:7544–7553. doi: 10.1128/JVI.00186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milligan GN, Bernstein DI, Bourne N. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol 160:6093–6100. [PubMed] [Google Scholar]
- 52.Morrison LA. 2008. Replication-defective virus vaccine-induced protection of mice from genital herpes simplex virus 2 requires CD4 T cells. Virology 376:205–210. doi: 10.1016/j.virol.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]