Abstract
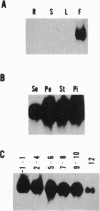
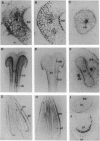
Flowering is known to be associated with the induction of many cell wall proteins. We report here five members of a tobacco gene family (CELP, Cys-rich extensin-like protein) whose mRNAs are found predominantly in flowers and encode extensin-like Pro-rich proteins. CELP mRNAs accumulate most abundantly in vascular and epidermal tissues of floral organs. In the pistil, CELP mRNAs also accumulate in a thin layer of cells between the transmitting tissue and the cortex of the style and in a surface layer of cells of the placenta in the ovary. This unique accumulation pattern of CELP mRNAs in the pistil suggests a possible role in pollination and fertilization processes. CELP genes encode a class of plant extracellular matrix proteins that have several distinct structural features: a Pro-rich extensin-like domain with Xaa-Pro3-7 motifs and Xaa-Pro doublets, a Cys-rich region, and a highly charged C terminus. The extensin-like domains in these proteins differ significantly in their length and these differences appear to be results of both long and short deletions within the coding regions of their genes. Furthermore, the number of charged amino acid residues in the C-terminal region varies among the CELPs. These structural differences may contribute to functional versatility in the CELPs. On the other hand, the Cys-rich domain is highly conserved among CELPs and the positions of the Cys residues are conserved, suggesting that this region may have a common functional role. The presence of a Pro-rich domain and a Cys-rich domain in these CELPs is reminiscent of a class of hydroxyproline-rich glycoproteins, solanaceous lectins, that are believed to be important in cell-cell recognition. The structure of these CELPs indicates that they may be multifunctional and that their genes may have arisen from recombinational events.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. C., Coen E. S., Dickinson H. G. The ptl1 gene expressed in the transmitting tissue of Antirrhinum encodes an extensin-like protein. Plant J. 1992 Sep;2(5):733–739. doi: 10.1046/j.1365-313x.1992.t01-14-00999.x. [DOI] [PubMed] [Google Scholar]
- Chen C. G., Cornish E. C., Clarke A. E. Specific expression of an extensin-like gene in the style of Nicotiana alata. Plant Cell. 1992 Sep;4(9):1053–1062. doi: 10.1105/tpc.4.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A. Y., May B., Kawata E. E., Gu Q., Wu H. M. Characterization of cDNAs for stylar transmitting tissue-specific proline-rich proteins in tobacco. Plant J. 1993 Jan;3(1):151–160. [PubMed] [Google Scholar]
- Ertl H., Hallmann A., Wenzl S., Sumper M. A novel extensin that may organize extracellular matrix biogenesis in Volvox carteri. EMBO J. 1992 Jun;11(6):2055–2062. doi: 10.1002/j.1460-2075.1992.tb05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard J. L., Jako C., Saint-Guily A., Weil J. H., Kuntz M. Anther-specific, developmentally regulated expression of genes encoding a new class of proline-rich proteins in sunflower. Plant Mol Biol. 1991 Feb;16(2):271–281. doi: 10.1007/BF00020558. [DOI] [PubMed] [Google Scholar]
- Gilbert W. The exon theory of genes. Cold Spring Harb Symp Quant Biol. 1987;52:901–905. doi: 10.1101/sqb.1987.052.01.098. [DOI] [PubMed] [Google Scholar]
- Goldman M. H., Pezzotti M., Seurinck J., Mariani C. Developmental expression of tobacco pistil-specific genes encoding novel extensin-like proteins. Plant Cell. 1992 Sep;4(9):1041–1051. doi: 10.1105/tpc.4.9.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q., Kawata E. E., Morse M. J., Wu H. M., Cheung A. Y. A flower-specific cDNA encoding a novel thionin in tobacco. Mol Gen Genet. 1992 Jul;234(1):89–96. doi: 10.1007/BF00272349. [DOI] [PubMed] [Google Scholar]
- Hong J. C., Nagao R. T., Key J. L. Developmentally regulated expression of soybean proline-rich cell wall protein genes. Plant Cell. 1989 Sep;1(9):937–943. doi: 10.1105/tpc.1.9.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltunow A. M., Truettner J., Cox K. H., Wallroth M., Goldberg R. B. Different Temporal and Spatial Gene Expression Patterns Occur during Anther Development. Plant Cell. 1990 Dec;2(12):1201–1224. doi: 10.1105/tpc.2.12.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Neale A. D., Wahleithner J. A., Lund M., Bonnett H. T., Kelly A., Meeks-Wagner D. R., Peacock W. J., Dennis E. S. Chitinase, beta-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell. 1990 Jul;2(7):673–684. doi: 10.1105/tpc.2.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ori N., Sessa G., Lotan T., Himmelhoch S., Fluhr R. A major stylar matrix polypeptide (sp41) is a member of the pathogenesis-related proteins superclass. EMBO J. 1990 Nov;9(11):3429–3436. doi: 10.1002/j.1460-2075.1990.tb07550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts K. Structures at the plant cell surface. Curr Opin Cell Biol. 1990 Oct;2(5):920–928. doi: 10.1016/0955-0674(90)90093-t. [DOI] [PubMed] [Google Scholar]
- Roberts K. The plant extracellular matrix. Curr Opin Cell Biol. 1989 Oct;1(5):1020–1027. doi: 10.1016/0955-0674(89)90074-4. [DOI] [PubMed] [Google Scholar]
- Varner J. E., Lin L. S. Plant cell wall architecture. Cell. 1989 Jan 27;56(2):231–239. doi: 10.1016/0092-8674(89)90896-9. [DOI] [PubMed] [Google Scholar]
- Wyatt R. E., Nagao R. T., Key J. L. Patterns of soybean proline-rich protein gene expression. Plant Cell. 1992 Jan;4(1):99–110. doi: 10.1105/tpc.4.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. H., Varner J. E. Tissue-Specific Expression of Cell Wall Proteins in Developing Soybean Tissues. Plant Cell. 1991 Jan;3(1):23–37. doi: 10.1105/tpc.3.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst G. J., Martin S. R., Allen A. K., Ashford D., Desai N. N., Neuberger A. Protein conformation of potato (Solanum tuberosum) lectin determined by circular dichroism. Biochem J. 1986 Feb 1;233(3):731–736. doi: 10.1042/bj2330731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]