Abstract
Approximately 30 years ago, it was discovered that free-living bacteria isolated from cold ocean depths could produce polyunsaturated fatty acids (PUFA) such as eicosapentaenoic acid (EPA) (20:5n-3) or docosahexaenoic acid (DHA) (22:6n-3), two PUFA essential for human health. Numerous laboratories have also discovered that EPA- and/or DHA-producing bacteria, many of them members of the Shewanella genus, could be isolated from the intestinal tracts of omega-3 fatty acid-rich marine fish. If bacteria contribute omega-3 fatty acids to the host fish in general or if they assist some bacterial species in adaptation to cold, then cold freshwater fish or habitats should also harbor these producers. Thus, we undertook a study to see if these niches also contained omega-3 fatty acid producers. We were successful in isolating and characterizing unique EPA-producing strains of Shewanella from three strictly freshwater native fish species, i.e., lake whitefish (Coregonus clupeaformis), lean lake trout (Salvelinus namaycush), and walleye (Sander vitreus), and from two other freshwater nonnative fish, i.e., coho salmon (Oncorhynchus kisutch) and seeforellen brown trout (Salmo trutta). We were also able to isolate four unique free-living strains of EPA-producing Shewanella from freshwater habitats. Phylogenetic and phenotypic analyses suggest that one producer is clearly a member of the Shewanella morhuae species and another is sister to members of the marine PUFA-producing Shewanella baltica species. However, the remaining isolates have more ambiguous relationships, sharing a common ancestor with non-PUFA-producing Shewanella putrefaciens isolates rather than marine S. baltica isolates despite having a phenotype more consistent with S. baltica strains.
INTRODUCTION
Omega-3 fatty acids such as eicosapentaenoic acid (EPA) (20:5n-3), docosapentaenoic acid (DPA) (22:5n-3), and docosahexaenoic acid (DHA) (22:6n-3) and omega-6 fatty acids such as linoleic acid (18:2n-6) are essential nutrients in vertebrate species. Currently, a need exists to find abundant, inexpensive, and safe ways to supplement our diets with omega-3 fatty acids from sources other than fish or shellfish (1). A safe and potentially inexpensive solution to this demand was triggered by the discovery in 1986 by DeLong and Yayanos (2) that certain deep-sea marine bacteria, and not just eukaryotic microbes (3), could produce omega-3 fatty acids such as EPA and/or DHA. This discovery suggested that marine bacteria could be a source of omega-3 fatty acids either by direct isolation from the bacteria or through genetic engineering allowing overproduction in numerous hosts (1, 4, 5). Soon after the initial discovery, many laboratories also isolated omega-3 fatty acid-producing bacteria from ocean depths, from colder regions of the ocean, and from gastrointestinal tracts of omega-3 fatty acid-containing marine fish, such as mackerel, or shellfish (6–9). Many of the producers identified were members of the gammaproteobacterial clade, such as the genera Moritella, Photobacteria, Shewanella, and Vibrio, which are reviewed in reference 10. Members of the Cyanobacteria phylum (11, 12) and Flavobacteriaceae family, such as Psychroflexus torquis (13), have also been shown to be able to produce omega-3 fatty acids. Recently, in a study of marine bacteria from Antarctica (14), members of the genera Cellulophaga, Pibocella, and Polaribacter were identified as EPA and/or DHA producers.
In 1996, Yazawa (15) demonstrated that five genes (and their products) were responsible for the ability of Shewanella to produce omega-3 fatty acids such as EPA. Metz et al. (16) explored the pathway of omega-3 fatty acid synthesis for both Shewanella and a marine protist (Schizochytrium) and showed in both organisms that it progressed via a novel type II fatty acid synthesis (FAS II) pathway related to polyketide synthesis pathways for antibiotics. Allen and Bartlett (17) characterized the EPA biosynthetic genes pfaA to pfaD from the deep-sea bacterium Photobacterium profundum and demonstrated an analogous pathway of synthesis in this organism. The pathway also appears to exist in other proteobacteria but not in cyanobacteria, which predominantly make C18 PUFA such as alpha-linolenic acid (18:3n-3) (reviewed in reference 18). A comparison of Shewanella isolates that make omega-3 fatty acids with those that do not shows that some nonproducers contain the cluster. However, in isolates such as Shewanella putrefaciens 200, there is a transposon inserted in the pfaA gene (19). In contrast, Shewanella oneidensis strain MR-1 also contains the cluster but produces only small amounts of omega-3 fatty acids (20). Thus, possessing the cluster is necessary but not sufficient for omega-3 fatty acid synthesis. A number of functions have been proposed for the omega-3 fatty acids in these bacteria, including adaptation to cold and high pressure. Not surprisingly, it has been shown in Shewanella (21) and Photobacteria (8) that the synthesis of omega-3 fatty acids is regulated by cold and pressure. Furthermore, strains of Shewanella livingstonensis that are defective in EPA synthesis as a result of mutations are defective for growth at lower temperatures (22). It is believed that the unsaturated fatty acids increase membrane fluidity after being incorporated into phospholipids, thereby improving membrane function at lower temperatures (23). The effect of increasing membrane fluidity appears to facilitate folding of outer membrane proteins (24, 25). In contrast, the absence of omega-3 fatty acids in mutants of Photobacterium profundum (8) has been shown to have only minor effects during growth under conditions of low temperature or high pressure even though the organism was isolated from the deep sea. EPA production has also been shown to protect Shewanella marinintestina from hydrogen peroxide (26), suggesting a role in protection against reactive oxygen species, possibly against those generated as part of the host's defense against microbes. In toxic marine microalgae, omega-3 fatty acids are believed to play a role in toxicity to fish or shellfish, perhaps after oxidation by reactive oxygen intermediates (27). Recently, EPA production by S. marinintestina was shown to protect the bacteria from water-soluble antibiotics but made them more susceptible to hydrophilic compounds such as membrane uncouplers (28).
In 1992, Ringo et al. (29) demonstrated that EPA-producing Vibrio species could also be isolated from freshwater Arctic charr (Salvelinus alpinus), a species which is also found in marine habitats (30). Bowman et al. (31) have shown that novel omega-3-producing bacteria could be isolated from Antarctic coastal waters and Antarctic meromictic lakes. Skerratt et al. (32) were even able to isolate omega-3 fatty acid-producing bacteria from a temperate river estuary. A recent study (9) has shown that omega-3 fatty acid-producing bacteria also exist in temperate marine waters at shallow depths. Furthermore, Minicystis rosea (33), a relative of Myxobacterium, has been isolated from soil and shown to produce omega-3 fatty acids in addition to steroids. Thus, the habitats and ecological niches of omega-3 fatty acid-producing bacteria are more diverse than previously thought. Although most of the marine omega-3 producers are halophiles (10), no evidence suggests that the production is part of an adaptation to the increased salinity of marine environments. However, if enteric microbiota are major contributors to omega-3 concentrations in fish tissue or if production of omega-3 fatty acids protects the bacteria from the host immune system, then freshwater fish with high omega-3 tissue concentrations could harbor bacterial producers. Cold freshwater habitats may also contain bacteria that produce omega-3 fatty acids if synthesis is required for cold adaptation for some species. Although numerous marine Shewanella spp. have been identified as omega-3 fatty acid producers, previous studies showed that freshwater Shewanella isolates either fail to produce omega-3 fatty acids or produce very low levels (20, 21). Thus, it is possible that producers are not going to be found in freshwater habitats.
In this study, using recently developed selection techniques (34), we isolated EPA-producing Shewanella spp. from the gastrointestinal tracts of five freshwater fish species that live in environments that seasonally approach a temperature of 0°C and from four cold freshwater habitats. Thus, these niches contain EPA producers too.
MATERIALS AND METHODS
Bacterial strains.
Escherichia coli K-12 strain W3110 was obtained from the E. coli Genetic Stock Center (Yale University). All other strains listed in Table 1 were isolated during this study from the gastrointestinal tracts of freshwater fish supplied by either Douglas Borys (Concordia University Wisconsin), the Inter-tribal Fisheries and Assessment Program, or the Wisconsin Department of Natural Resources. Samples from water came from Lake Michigan (WI), Horicon Marsh (WI), or Wild Rose Fish Hatchery (WI). Sediment samples came from Lake Michigan (WI).
TABLE 1.
Description of bacterial strains used in this study and identification of omega-3 fatty acid producers by PCR, GC, and MS
| Strain | Taxon (collection date) (mo/day/yr) | Location (map coordinates, latitude and longitude) | Source | % total fatty acids in cells grown at 10°C determined by GC in indicated growth medium |
Omega-3 fatty acid content determined by MS in TSB-2% NaCl | pfaA KS+c | |||
|---|---|---|---|---|---|---|---|---|---|
| TSB-2% NaCl |
M9 minimal mediuma |
||||||||
| EPA | DHA | EPA | DHA | ||||||
| CW2 | Shewanella baltica (11/5/2011) | Lake Superior, MI (46°31′N, 84°38′W) | Salvelinus namaycush (lean lake trout) | 7.2 | NDb | 6.5 | ND | EPA | + |
| CW6 | Pseudomonas sp. (11/5/2011) | Lake Superior, MI (46°31′N, 84°38′W) | Coregonus clupeaformis (whitefish) | ND | ND | ND | |||
| CW7 | Shewanella morhuae (11/5/2011) | Lake Superior, MI (46°31′N, 84°38′W) | Coregonus clupeaformis (whitefish) | 4.5 | ND | 3.5 | ND | EPA | + |
| CW10 | Psychrobacter sp. (11/5/2011) | Lake Superior, MI (46°31′N, 84°38′W) | Coregonus clupeaformis (whitefish) | ND | ND | ND | − | ||
| CW15 | Psychrobacter sp. (11/5/2011) | Lake Superior, MI (46°31′N, 84°38′W) | Coregonus clupeaformis (whitefish) | ND | ND | ND | − | ||
| WE21 | Shewanella sp. (8/28/2012) | Lac des Mille, Western Ontario, Canada (48°84′N, 90°51′W) | Sander vitreus (walleye) | 7.3 | ND | 5.9 | ND | EPA | + |
| LMW1 | Shewanella sp. (8/28/2014) | Lake Michigan, WI (43°25′N, 87°54′W) | Freshwater | 7.3 | ND | EPA | + | ||
| LMS4 | Shewanella sp. (8/28/2014) | Lake Michigan, WI (43°25′N, 87°54′W) | Freshwater sediment | 7.2 | ND | EPA | + | ||
| BT2 | Shewanella sp. (11/19/2014) | C. D. Besadny Anadromous Fish Facility, Kewaunee, WI (44°45′N, 87°50′W) | Salmo trutta (seeforellen brown trout) | EPA | + | ||||
| BT11 | Shewanella sp. (11/19/2014) | C. D. Besadny Anadromous Fish Facility, Kewaunee, WI (44°45′N, 87°50′W) | Salmo trutta (seeforellen brown trout) | EPA | + | ||||
| CHO4 | Shewanella sp. (11/19/2014) | C. D. Besadny Anadromous Fish Facility, Kewaunee, WI (44°45′N, 87°50′W) | Oncorhynchus kisutch (coho salmon) | EPA | + | ||||
| CHO5 | Shewanella sp. (11/19/2014) | C. D. Besadny Anadromous Fish Facility, Kewaunee, WI (44°45′N, 87°50′W) | Oncorhynchus kisutch (coho salmon) | EPA | + | ||||
| HMS18 | Shewanella sp. (11/10/2014) | Horicon Marsh, WI (43°45′N, 88°68′W) | Freshwater | EPA | + | ||||
| WRW4 | Shewanella sp. (4/9/2015) | Wild Rose Fish Hatchery, WI (44°19′N, 89°25′W) | Freshwater | EPA | + | ||||
| W3110 | E. coli K-12 | Stock culture | ND | ND | ND | − | |||
Succinate as sole carbon and energy source.
ND, not detectable.
By PCR analysis for ketosynthase (KS) domains found in EPA- or DHA-producing bacteria.
Isolation of putative omega-3 fatty acid-producing bacteria.
Gastrointestinal tracts from lean lake trout (Salvelinus namaycush), lake whitefish (Coregonus clupeaformis), both from Lake Superior (MI), and walleye (Sander vitreus) caught in Lac des Mille (Ontario, Canada) were harvested for this study. Gastrointestinal tracts from coho salmon (Oncorhynchus kisutch) and seeforellen brown trout (Salmo trutta) were provided by the Wisconsin Department of Natural Resources. Bacteria were isolated from the gastrointestinal tracts as described previously (34). After 1 or 2 days of growth in BD Bacto tryptic soy broth (TSB) at 15°C, bacteria were diluted and plated on 50% marine agar (Difco marine broth 2216) plus 0.1% (wt/vol) tetrazolium chloride (TTC) (Sigma-Aldrich), essentially as described previously (34), for 3 to 7 days at 15°C. Red colonies were picked as putative omega-3-producing bacteria and streaked onto the same medium at least twice for purification. For fresh-living isolates, we collected water samples and sediment samples and stored them at 4°C until use. One milliliter of each water sample was then inoculated into 9 ml of LB (35) or directly plated onto selective medium. Ten grams of sediment was inoculated into 10 ml of LB. Samples grown in LB were kept at 4°C for 1 day and then grown at 10°C until the cultures became dense (ca. 3 days). Cultures were then plated on the same TTC medium as described above but incubated at 10°C. Red colonies were picked after 4 days of growth to 50% marine agar and purified twice.
Initial characterization of isolates.
Gram staining was done on putative omega-3 producers using a Gram staining kit (BD) using cells grown in TSB at 15°C. Oxidase tests were performed using discs (Oxoid) and swabs (Gibson Bioscience). Catalase tests were performed using 3% hydrogen peroxide. Hydrogen sulfide production was tested on triple sugar iron (TSI) slants (BBL-Difco). Motility was observed by phase-contrast microscopy, and chemotaxis was tested on tryptone broth swarm plates (36) at 25°C.
Growth of cells at various temperatures and sodium chloride concentrations.
Growth at various temperatures was tested on both LB agar (37) and 50% marine agar (MA). We measured growth rates at 25°C in liquid medium containing 1% (wt/vol) Bacto tryptone and 0.5% (wt/vol) yeast extract, with NaCl added to achieve the desired concentration of 0 to 3% (wt/vol). We also tested the ability of the isolates to grow in 0%, 1%, and 6% (wt/vol) NaCl on 1% (wt/vol) Bacto tryptone agar. M9 minimal agar medium was used to test for carbon utilization (35). The carbon sources were used at the following concentrations: citrate, dl-lactate, malate, and succinate, 40 mM; glucose, maltose, and sucrose, 0.5% (wt/vol); l-arabinose and d-gluconate, 0.4% (wt/vol). The agar concentration in all plate media was 1.5% (wt/vol).
Phenotypic analysis.
API 20 NE strips (bioMérieux, Inc.) were used to perform additional phenotypic testing. Cells were grown in Trypticase soy agar (TSA) (BD BBL) at 25°C, resuspended in 0.85% NaCl, and inoculated into the strips per the manufacturer's instruction. The strips were incubated at 25°C, and results were recorded at 24 and 48 h. Results shown in Table 2 are after 24 h unless otherwise noted.
TABLE 2.
Phenotypic characteristics of omega-3 fatty acid producersa
| Characteristic | Medium or ID kit | CW2 | CW7 | WE21 | LMW1 | LMS4 | CHO4 | CHO5 | BT2 | BT11 | HMS18 | WRW4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth in: | ||||||||||||
| 0% NaCl | 1% tryptone | + | − | + | + | + | + | + | + | + | + | + |
| 6% NaCl | 1% tryptone | + | − | − | − | − | − | − | − | − | − | − |
| Assimilation ofb: | ||||||||||||
| Lactate | M9 | + | − | + | + | + | + | + | + | + | + | + |
| Sucrose | M9 | + | − | + | − | − | − | − | − | + | − | + |
| d-Glucose | API 20 NE | + | − | − | − | − | − | − | − | − | − | − |
| Arabinose | API 20 NE | − | − | − | − | +c | − | +c | − | − | − | − |
| N-Acetylglucosamine | API 20 NE | + | + | + | + | + | + | + | + | + | + | + |
| Gluconate | API 20 NE | + | + | + | −c | + | + | + | + | + | + | + |
| Maltose | API 20 NE | +c | +c | +c | + | + | + | + | + | + | + | + |
| Malate | API 20 NE | +c | +c | +c | + | + | + | + | + | + | + | + |
| Citrate | API 20 NE | +c | −c | −c | − | − | − | − | − | − | − | −c |
All strains produced NO2 from NO3, failed to produce nitrogen gas from NO3, were urease and indole negative, did not ferment glucose, failed to assimilate capric acid, mannose, mannitol, phenylacetate, or adipic acid, and lacked arginine dihydrolase activity (API 20 NE). All were hydrogen sulfide positive, gelatinase positive (API 20 NE), oxidase positive, catalase positive, motile, and chemotactic. All assimilated succinate on M9 minimal medium. Morphology was bacillus for all.
Grown at 25°C.
Same result when tested on minimal medium.
Extraction and testing of isolates for omega-3 fatty acid production.
Samples were grown at 10°C on TSB medium with NaCl at 2% (wt/vol) for initial screening and analysis. E. coli strain W3110 was grown on LB at 25°C as a negative control. Late log-phase to early stationary-phase cells were spun down and washed twice in 50% TSB and then briefly dried in an Eppendorf Vacufuge or air dried. Samples were also grown on succinate M9 minimal agar plates at the same temperature until medium-size colonies formed at 10°C. Colonies were scraped off the plates, washed once in M9 salts, and briefly dried. Strain WE21 was also grown on modified LB medium at various NaCl concentrations and temperatures to an optical density at 600 nm (OD600) of ∼1 and harvested as described above. Fatty acid methyl esters (FAME) were generated by a methanolic acid methylation protocol similar to that described previously (29, 36).
Bacterial pellets were suspended in 1 ml of an 8% HCl solution in methanol and resuspended to a final volume of 4 ml of an 8% HCl-methanol solution. Suspensions were heated at 80°C for 60 min in sealed glass vials. After the suspensions were cooled and 4 ml hexane (Fluka) was added, the samples were vortexed vigorously in 15-ml glass conical tubes. Samples were then spun at 1,000 × g for 10 min using a swinging-bucket rotor (Eppendorf A.4.62) in an Eppendorf 5810 centrifuge. The hexane layer was removed, respun as described above, and evaporated with nitrogen gas. Dry extracts were resuspended in 300 μl hexane. Three micrograms of a pentacosanoic acid methyl ester (Cayman Chemical) was added as an internal standard to some samples, and for other samples, 3 μg of a pentacosanoic acid (Cayman Chemical) was added before the methylation step to assess methylation efficiency.
Solvents were analytical grade and purchased from Thermo Fisher Scientific (Waltham, MA). Fatty acid methyl esters were quantitated via external standard curves of Nu-Chek Prep (Elysian, MN) no. 462 FAME mixtures. Eight-point standard curve points ranged from 0.83 to 106.3 μg/ml.
FAME instrumentation and analysis.
Screening analyses for possible omega-3 fatty acid producers were performed on a Thermo Ultra Trace gas chromatograph (GC) with a programmed temperature vaporization (PTV) inlet, AS3000 autosampler, and flame ionization detector (FID). Quantification of omega-3 fatty acids was later performed on a Thermo Trace 1310 GC with a PTV inlet, AS3000 autosampler, and FID detector (data collection rate, 10 Hz). An Agilent DB23 column (60 m by 0.25 mm, 0.25 μm) was used for both screening analyses and quantification, with hydrogen as a carrier gas, and run at 3.3 and 1.8 ml/min, respectively. Screening data were processed using ChromQuest 5.0 software. After initial screenings, select positive cultures and select negative cultures were rerun for quantitation on the Trace 1310 GC (Thermo Scientific) and analyzed using Chromeleon 7 software.
MS methods.
Mass spectrometry (MS) was performed by the following protocol to confirm the identity of the fatty acids after cells were grown and harvested under the same conditions as those used for the GC analysis. Dried pellet lipids were saponified in 4 ml of 1% NaOH heated to 80°C for 60 min. Cooled lipids were extracted with 5 ml hexane, centrifuged for 25 min at 2,500 × g, removed, and then dried with nitrogen gas. Fatty acids were resuspended in 500 μl of methanol and then diluted 10-fold in methanol.
Chromatographic conditions for fatty acid determination by MS.
Mass spectrometry confirmation was based on that described previously (38) and performed using an ABSciex 4000 Qtrap equipped with a Dionex RSLC 3000 ultrahigh-performance liquid chromatograph (UHPLC). A Waters Acquity BEH C18 column (2.1 mm by 100 mm) with a 1.7-μm particle size was used. The injection volume was maintained at 5 μl for all samples. The column temperature was set at 50°C, and samples were left at room temperature. Mobile phase A consisted of 2 mM ammonium acetate in 0.1% formic acid, and mobile phase B consisted of acetonitrile. The UHPLC system started with 85% phase B at −0.5 min from injection and then went from 55% phase B from time zero to 1.0 min from injection. The system then followed a linear gradient from 55% phase B at 1.0 min to 95% phase B at 2.0 min and then down to 85% phase B at 3.0 min. The system held the column at 85% phase B for the remainder of the run. The total run time was 4.6 min, and the flow rate was set to 0.7 ml/min throughout. The PTV inlet was programmed in a splitless mode at an initial temperature of 58°C, an injection time of 0.1 min, a transfer rate of 5°C/s to 260°C, a splitless time of 1.35 min, and a split flow of 20 ml/min. The FID was set to a base temperature of 260°C, with gas flows of air, hydrogen, and nitrogen (makeup) of 350, 35, and 30 ml/min, respectively. The screening oven settings were similar to those of the quantitation method. The quantitation oven ramp settings were as follows: (i) initially 50°C and held for 1 min, (ii) ramped to 175°C at 25°C/min, (iii) ramped to 190°C at 2.4°C/min and held for 1 min, (iv) ramped to 198°C at 0.5°C/min, and (v) finally ramped to 260°C at 30°C/min and held for 1 min. The temperature ramp for screening runs differed after 175°C due to variation in resolution among different DB23 columns.
MS conditions.
An AB Sciex 4000 Q TRAP with a turbospray ion source was operated in the negative-ion mode. The instrument voltages were optimized by direct infusion of free fatty acid standard solutions. MS/UHPLC data were collected and processed using Analyst (version 1.5.1) software and Dionex Chromatography MS Link (DCMS Link version 2.8.0.263). Multiple reaction monitoring (MRM) transitions of m/z 301.2 to 257.0 and m/z 302.5 to 258.5 were chosen for EPA. Transitions of m/z 303.2 to 259.0 and m/z 304.5 to 260.5 were chosen for arachidonic acid. Transitions of m/z 327.2 to 283.0, m/z 328.5 to 284.5, m/z 330.500 to 286.500 and m/z 332.500 to 288.500 were chosen for DHA. The optimized conditions for the MS were as follows: source temperature, 650°C; ion spray voltage, −4,500 V; gas 1, 60 lb/in2; gas 2, 80 lb/in2; curtain gas, 60 lb/in2; collisionally activated dissociation gas, Hi; entrance potential, 10 eV; collision energy, −18 eV; declustering potential, −70 eV; and exit potential, −12 eV.
Polymerase chain amplification and cloning of 16S rRNA and gyrB genes.
All PCRs were performed using a Master Cycler Pro S thermocycler (Eppendorf Scientific). All oligonucleotide primers were purchased from Eurofins MWG/operon via Fisher Scientific. GoTaq master mixes (Promega) were used for all PCRs, and DNA was then purified using a Wizard SV gel and a PCR Clean-Up system (Promega) per the manufacturers' instructions. Chromosomal DNA for PCRs was prepared using ArchivePure DNA yeast and Gram-positive kits (5 Prime). Partial 16S rRNA gene sequences were obtaining by using the following primers, which are based on universal primers E8F and U1115R, as described in reference 39. Sequences specifically used were rRNAFR1N1, 5′-GCTCAGATTGAAC-3′ (bp 22 to 34 of Escherichia coli sequence), and rRNAR1, 5′-TCGTTGCGGGACTTAACCCAAC-3′ (bp 1104 to 1083 of E. coli sequence). Parameters for this amplification were, after an initial denaturation at 94°C for 2 min, 30 cycles with the denaturing temperature at 94°C for 30 s, followed by an annealing step at 53°C for 30 s, an elongation step at 72°C for 75 s, and an extension step after the last elongation step for an additional 8 min. Isolated PCR fragments of the predicted size were ligated into Classic pCR2.1 (Life Technologies) per the manufacturer's protocol and transformed into chemically prepared E. coli TOP10 competent cells (Life Sciences Technologies). The gyrB gene was amplified using the universal primers and method described previously (40), except that the annealing temperature was 56°C. PCR-amplified fragments were isolated as described above and were ligated into pGEMT vector or pGEMT easy vector (Promega) and transformed into E. coli strain HB101 or JM109 per the manufacturer's protocol with selection for ampicillin-resistant colonies. Promega SV plus miniprep kits were used to isolate plasmids from white colonies (Lac−). Plasmids containing correctly sized inserts were sequenced by the Yale Sequencing Center (New Haven, CT, USA). Sequences obtained were compiled using MacVector software (Cary, NC, USA). DNA sequence similarity, protein identities and similarities, and putative domains in the protein were predicted using programs and databases made available by NCBI. PCR analysis for the presence of the ketosynthase domain in the pfaA gene present in EPA and DHA producers was done as described previously (9).
Genomic sequencing.
Cells were grown in succinate M9 liquid medium at 25°C and harvested at an OD600 of ∼0.6. Chromosomal DNA was extracted using MagAttract kits (Qiagen). PacBio sequencing was performed by the Institute of Genome Sciences (IGS), University of Maryland, Baltimore, MD. Long-insert libraries were made, and each genomic library DNA was run on two SMART cells. The IGS Annotation Engine Manatee was used for structural and functional annotation of the sequences (41).
Construction of phylogenetic trees.
Nucleotide sequences used in the phylogenetic analyses that were not produced by the authors were gathered from NCBI. Taxon sampling followed the phylogeny of Satomi et al. (42), in which a clade consisting of S. japonica, S. olleyana, and S. pacifica was sister to a clade consisting of Shewanella baltica, S. decolorationis, S. denitrificans, S. frigidimarina, S. gaetbuli, S. glacialipiscicola, S. hafniensis, S. livingstonensis, S. morhuae, S. oneidensis, S. profunda, and S. putrefaciens. Sequences were aligned with MAFFT v7 (43, 44) using the following algorithms: for 16S rRNA, FFT-NS-i; for gyrB, L-INS-i; and for recA, L-INS-i. The default scoring matrix (200PAM/K = 2), gap opening penalty (1.53), and offset values (0) were used in all three alignments. Additionally, regions with gaps were left unaligned. Phylogenetic analyses were conducted using RAxML v8 (45), employing 1,000 rapid bootstrap replicate searches followed by a search for the best tree using gamma rate variation and 10 randomized parsimony starting trees. Trees were visualized with Dendroscope v3.2.10 (46). Bootstrap support with 70% or higher support is indicated on the branches. Trees were rooted with Alteromonas macleodii strain “Deep ecotype,” Aeromonas hydrophila subsp. hydrophila ATCC 7966, and Colwellia psychrerythraea 34H as described by Dikow (47).
Nucleotide sequence accession numbers.
Sequences were submitted to GenBank under the following accession numbers: KM347974, KM347975, KM357913, KM357914, KM363230, KM363231, KM386672 to KM386674, KM396206 to KM396214, KM407144 to KM407146, KP164550, KP164551, KP890205 to KP890209, KT183385, KT633940 to KT633942, and KT724967.
RESULTS
Isolation and initial characterization of putative omega-3 fatty acid-producing bacteria from the gastrointestinal tracts from three native freshwater fish.
Broth cultures of fish intestinal contents from lake whitefish, lean lake trout, and walleye yielded numerous red bacterial colonies on TTC plates. Over 30 (minimum of four from each fish) were saved for further analysis. Gram staining results of the possible omega-3 producers showed that all the bacteria isolated were Gram-negative bacilli or coccobacilli. All were oxidase and catalase positive. However, some were motile, and others were not. Based upon Gram staining results, colony morphologies and pigmentation patterns on TSA and 50% MA plates, and motility phenotypes, we identified at least three different types of bacteria.
Identification of omega-3 fatty acid producers from fish intestines by gas chromatography and mass spectrometry.
We tested 12 independent isolates representing the different classes all together for the ability to produce omega-3 fatty acids when grown in TSB plus 2% NaCl at 10°C by the first GC method for analysis of lipids. After determining which isolates were unique (see below), we reanalyzed the samples by the second GC method (Table 1). Results of that analysis are shown for the unique representative isolates CW2, CW6, CW7, CW10, CW15, and WE21 (Table 1). E. coli strain W3110 was included as a negative control. Isolates CW2, CW7, and WE21 produced the omega-3 fatty acid EPA when grown on TSB-2% NaCl (Table 1). Isolates CW6, CW10, and CW15 did not produce either EPA or DHA despite their red phenotype on TTC plates. Isolates CW2 and WE21 produced roughly the same amounts (∼7% total fatty acids) of EPA, while isolate CW7 produced less (ca. 4.5% total fatty acids). All three isolates produced almost the same amounts of EPA when grown on succinate minimal medium as when grown in TSB-2% NaCl. Isolates CW2 and WE21 also produced roughly the same amounts of EPA when grown on sucrose minimal medium as on succinate minimal medium (data not shown). Production of EPA was also confirmed for isolates CW2, CW7, and WE21 by mass spectrometry analysis (Table 1) on TSB-2% NaCl grown cells. E. coli strain W3110 was again included as a negative control. All producers turned out to be motile, chemotactic, and both oxidase and catalase positive (Table 2). They also all produced hydrogen sulfide, formed salmon-colored colonies, and were Gram-negative rods.
Species determination based on 16S rRNA gene, gyrB gene, and recA gene sequencing.
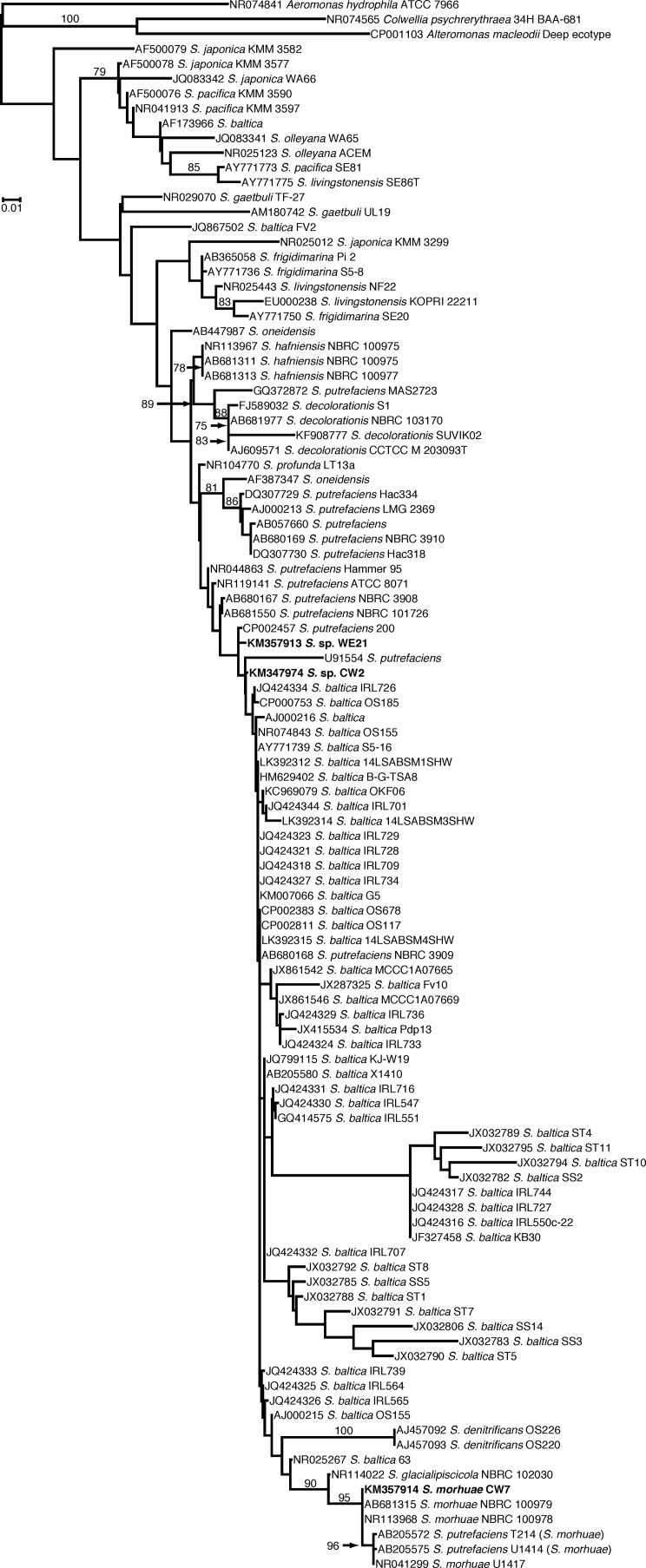
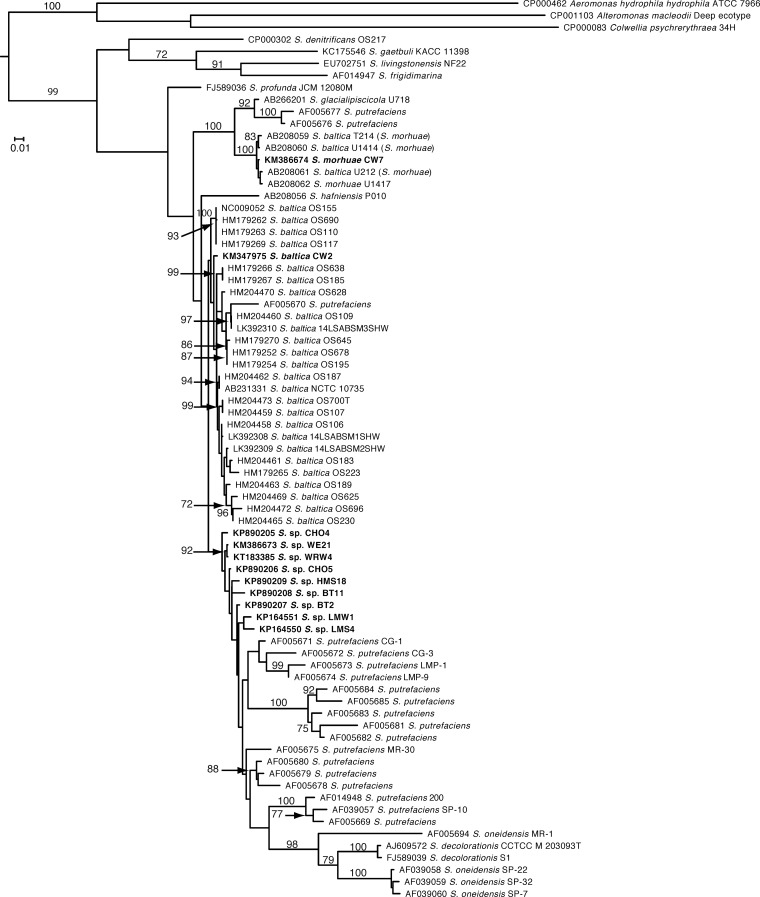
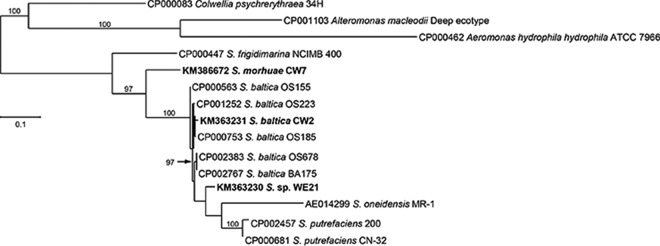
We sequenced the 16S rRNA gene from many of the producers and some of the nonproducers and determined that all of the producers were members of the Shewanella genus and the nonproducers were members of the Psychrobacter genus or Pseudomonas genus (data not shown). Sequencing also suggested that we most likely isolated only three unique isolates (one from each fish species), since the partial 16S rRNA gene sequences were identical (data not shown). We chose to further investigate three strains representing one of the unique isolates. Since the genomic sequences became available for the three strains (CW2, CW7, and WE21), we included the complete sequence of them in the 16S rRNA analysis rather than the partial sequences. Sequences were then compared to the sequences available in the NCBI database. The results of our 16S rRNA phylogenetic analysis are shown in Fig. 1. Strain CW2 was found to be sister to a large grade (i.e., paraphyletic assemblage) consisting mainly of S. baltica strains such as OS185. Strain CW7 was included in a well-supported (95% bootstrap support [BS]) monophyletic clade consisting of S. morhuae isolates. The 16S rRNA phylogeny suggests that strain WE21 shares a common ancestor with S. putrefaciens 200, S. baltica, S. denitrificans, S. glacialipiscicola, and S. morhuae. We sequenced the gyrB gene from several of the isolates to determine which previously isolated Shewanella strains our strains were most closely related to and to further determine if some of our isolates were in fact the same strain. Results confirmed that we likely have only one unique isolate from each of the fish. However, we used the gyrB sequence data from the genomic sequencing for the representative unique isolates, CW2, CW7, and WE21, for phylogenetic analysis of gyrB. Our results, shown in Fig. 2, confirm that strain CW2 is likely a member of the Shewanella baltica species based on the phylogenetic tree; however, there was no deep-level support for this clade. The gyrB phylogeny showed a strong affinity for inclusion of strain CW7 with S. morhuae, as it was included in a monophyletic clade with 100% BS support. Isolate WE21 was sister to a grade of S. baltica strains. This grade was sister to a paraphyletic assemblage of S. putrefaciens plus S. oneidensis plus S. decolorationis. A strong sister relationship (92% BS support) was found between Shewanella sp. strain WE21 and Shewanella sp. strain WRW4, a novel isolate of ours (see below). We also constructed a recA phylogenetic tree from the available genomic sequences. Results (Fig. 3) suggested that isolate CW2 is more closely related to S. baltica isolates, although there is no support for this clade and S. baltica was not recovered as monophyletic. We could not compare the recA sequence of strain CW7 to that of S. morhuae or S. glacialipiscicola in the tree because there were no deposited sequences for these species. However, the placement of strain CW7 on the tree is consistent with it being distinct from S. baltica. A comparison of the recA sequence of isolate WE21 suggests that the isolate shares a most recent common ancestor with S. oneidensis MR-1, S. putrefaciens 200, and S. putrefaciens CN-32 and not with the S. baltica strains included in the phylogenetic analysis.
FIG 1.
Phylogenetic tree based on 16S rRNA gene sequences. The accession number is given before the species name. The species name is that designated in GenBank, but in several cases the up-to-date classification is shown in parentheses. Only bootstrap values greater than 70% are shown. S. = Shewanella.
FIG 2.
Phylogenetic tree based on gyrB sequences. The accession number is given before the species name. The species name is that designated in GenBank, but in several cases the up-to-date classification is shown in parentheses. Only bootstrap values greater than 70% are shown. S. = Shewanella.
FIG 3.
Phylogenetic tree based on recA sequences. The accession number is given before the species name. The species name is that designated in GenBank, but in several cases the up-to-date classification is shown in parentheses. Only bootstrap values greater than 70% are shown. S. = Shewanella.
Production of EPA by strain WE21 at various temperatures and salt concentrations.
Our temperature studies showed that EPA levels were highest when cells were grown at 10°C and lowest when they were grown at 25°C (Table 3). Since strain WE21 grows well in the absence of NaCl (Table 2), we tested the effect of NaCl on EPA levels in this strain. Results (Table 3) show that the NaCl concentration affects the EPA levels. Significant amounts of EPA were present when NaCl concentrations were 0, 0.4, or 1%. At concentrations of 2% or 3% NaCl, EPA concentrations were lower.
TABLE 3.
Salt and temperature regulation of the EPA production in isolate WE21a
| Temp (°C) | EPA production (% of total lipid production) at NaCl concn (%) of: |
||||
|---|---|---|---|---|---|
| 0 | 0.4 | 1 | 2 | 3 | |
| 10 | 6.3 | ||||
| 15 | 4.7 | 5.2 | 4.4 | 3.1 | 2.1 |
| 25 | 1.5 | <1 | <1 | ||
EPA production determined by gas chromatography. Cells were grown in 1% tryptone–0.5% yeast extract.
Genomic sequencing analysis.
PacBio genomic sequencing results are shown in Table 4. Strain CW2 has a chromosomal GC content of 46.4%, similar to the published contents of other S. baltica isolates (48). However, the genome size of 4.93 Mb is slightly smaller than that reported for other S. baltica isolates. It also lacks a type III secretion system, in contrast to other S. baltica strains (48). The genome of strain CW7 is approximately 44.2% GC, similar to that previously reported for S. morhuae (49), and has a size of 4.3 Mb, including a 120-kb megaplasmid. Strain WE21 has a genome size of 5.3 Mb and a GC content of 45.2%, less than the 46% GC content reported for S. baltica strains (48) or our S. baltica isolate CW2 but higher than the GC content of 44.5% reported for S. putrefaciens strain 200 (50). Isolate WE21 also carries genes for a type III secretion system. Figure 4 shows the organization of the EPA biosynthetic cluster in strain CW2. Included are not only the pfaA, -B, -C, -D, and -E genes but also a hypothetical regulatory gene (epaR). The genetic organization in strains CW7 and WE21 is identical (results not shown). The genetic organization of the putative omega-3 fatty acid synthesis cluster is identical to that of the characterized EPA producers S. baltica MAC (4) and Shewanella sp. BR-2 (51), as well as to the EPA clusters of other S. baltica isolates deposited in GenBank, such as S. baltica OS185. However, there are differences in the genes in the cluster from those found in Shewanella species that do not make omega-3 fatty acids, such as S. putrefaciens 200, or to those that make small amounts of omega-3 fatty acids, such as S. oneidensis MR-1 (21). Significantly, the pfaA gene product would be predicted to have five acyl carrier protein (ACP) binding sites for isolates CW2, CW7, and WE21 (Fig. 4), compared to four for S. putrefaciens strain 200 and S. oneidensis strain MR-1. Also, the ketosynthase domains present in the pfaA genes of our isolates are more similar to those of EPA-producing bacteria. The analyses also show that all three have the critical gene pfaE linked to the EPA synthesis cluster (Fig. 4). The sequence of isolate CW2 (including the epaR gene and intervening regions) is almost 100% identical to the sequence of the cluster encoded by Shewanella sp. BR-2 (51). There is a difference of only a few base pairs between their pfaA genes, which does not result in any changes in the putative protein products (data not shown). The genes in isolate CW7 show highest identity to S. baltica strains, such as BA175, and also to Shewanella sp. BR-2. There is 91% identity to the pfaA, -C, and -D genes, 83% to the pfaB gene, and 84% to the pfaE gene of Shewanella sp. BR-2. Isolate WE21 shows identity in the ranges of 88 to 92% of the genes in the cluster to S. baltica isolates and to Shewanella sp. BR-2. The pfaA, -B, -C, and -D genes also show high identity to the cluster in S. putrefaciens 200 and CN-32, with the pfaC and pfaD genes with values of 94 to 95% and slightly less for the pfaA (90% and 87%) and pfaB (ca. 88%) genes. Although the pfaE gene of WE21 shows 90 to 91% identity to the entire gene sequence found in S. baltica or Shewanella sp. BR-2, there is only about 76% identity to a limited region of the gene (ca. 55%) found in S. putrefaciens strains 200 and CN-32 (data not shown).
TABLE 4.
Data derived from genomic sequencing
| Isolate | GC% | Genome size (Mb) | Megaplasmid (size in kb) | Type III secretion system |
|---|---|---|---|---|
| WE21 | 45.3 | 5.3 | Absent | Present |
| CW2 | 46.4 | 4.9 | Absent | Absent |
| CW7 | 44.0 | 4.3 | Present (120) | Absent |
FIG 4.
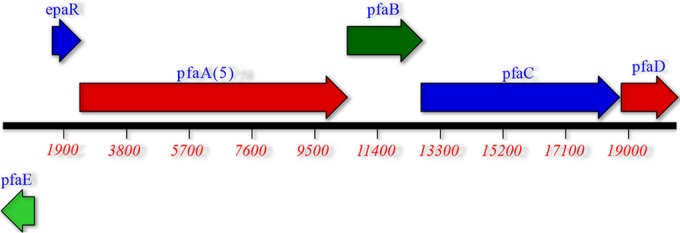
Genetic organization of the EPA biosynthetic cluster in strain CW2. Derived from genomic sequence data. pfaA(5) indicates that there were five ACP binding domains based upon the predicted protein product for pfaA. The numbers in red indicate numbers of base pairs.
Isolation and characterization of EPA producers from the gastrointestinal tracts of coho salmon and seeforellen brown trout.
We also attempted to isolate omega-3 fatty acid-producing bacteria from two nonnative but omega-3 fatty acid-rich fish, coho salmon and seeforellen brown trout. We thought they may be exceptions to the finding that omega-3 fatty acid-rich fish from the Great Lakes region contain producers. However, if they exist, we wished to characterize them. We were able to isolate numerous possible omega-3 fatty acid producers based upon the ability to form red colonies on TTC plates. Approximately 20 red colonies from each fish were saved for further study. Next we performed PCR analysis for the specific domain present in the pfaA gene in omega-3-producing bacteria but not in nonproducers (9). Many of the isolates were determined to be positive for this region (Table 1). We tested several isolates for omega-3 fatty acid production by mass spectrometry (Table 1). We confirmed that isolates such as CHO4, CHO5, BT2, and BT11 (isolates that possess the key domain in the pfaA gene) were EPA producers and also showed that several isolates that lacked the domain (data not shown) were nonproducers. Since we were able to isolate producers, we characterized them further to see if they were more similar to strain CW2, CW7, or WE21. We sequenced the gyrB gene of several of these isolates and confirmed four novel isolates. The partial gyrB sequences of the novel isolates (CHO4, CHO5, BT2, and BT11) were included in the construction of the phylogenetic tree (Fig. 2). Strains CHO4, CHO5, BT2, and BT11 were found to be distinct from our original three isolates but do share an ancestor with Shewanella sp. WE21 rather than S. baltica CW2 or S. morhuae CW7. Results of the gyrB phylogeny also suggest that these isolates share an ancestor with S. putrefaciens, S. oneidensis, and S. decolorationis rather than the included S. baltica isolates (Fig. 2).
Isolation and characterization of free-living omega-3 fatty acid producers from freshwater and sediment.
We attempted to isolate free-living producers of omega-3 fatty acids as described in Materials and Methods since our isolates from the gastrointestinal tracts of the fish could grow on minimal medium and low salt (Table 2) and because several isolates, such as strain WE21, appear to be related to free-living freshwater isolates of Shewanella (52) based upon their gyrB sequences (Fig. 2). We tested sediment samples and water samples from the same location in Lake Michigan as well as water samples from a marsh and a fish hatchery as described in Materials and Methods. Several red colonies from TTC plates were isolated and tested from the Lake Michigan samples, six in all (three from water samples and three from sediment samples). Two of the six, strains LMW1 and LMS4, were shown to be producers of EPA both by GC analysis and by mass spectrometry (Table 1). Under the conditions tested, both produce intermediate levels of EPA (∼7% total fatty acids). Preliminary characterization of these isolates is shown in Tables 1 and 2. Both appear to be Shewanella species based upon their colony appearance and Gram stain results and on their oxidase-positive, catalase-positive, and H2S-positive phenotype (Table 2). Sequencing of part of the gyrB gene and phylogenetic tree construction (Fig. 2) confirm that they are two novel but closely related isolates that share a most recent common ancestor with S. putrefaciens, S. oneidensis, and S. decolorationis, rather than S. baltica. We were also able to isolate several EPA producers from a marsh and fish hatchery water samples. However, in this case, the putative producers identified by their red appearance on TTC plates were first screened for the presence of the critical omega-3-producing domain in the pfaA gene, as described for the isolates from brown trout and coho salmon before lipid analysis. Two positive isolates, HMS18 and WRW4, were shown to produce EPA by mass spectrometry (Table 1). We sequenced part of the gyrB gene from these two producers and used those sequences in the construction of the phylogenetic tree (Fig. 2). The results showed that they are unique isolates but closely related to our other freshwater isolates LMW1 and LMS4. However, the free-living isolate HMS18 appears more related to isolates from the gastrointestinal tracts of coho salmon and brown trout than the two free-living strains LMW1 and LMS4. Free-living isolate WRW4 is sister to isolate WE21, with 92% support (Fig. 2), whose phenotype it also matches (Table 2).
Phenotypic testing.
We did further phenotypic testing in an attempt to clarify what species or isolates were most closely related to our strains (Table 2). All isolates grew well at 10°, 15°, 25°, and 30°C, with 1 day to form medium-size colonies. All isolates were unable to grow at 37°C, in agreement with previously isolated S. baltica or S. morhuae strains but in contrast to what is expected of members of the S. putrefaciens species (49, 53) (results not shown). Growth at 4°C was very slow for all isolates, taking 5 to 7 days to form medium-size colonies. We tested the ability of some of the isolates to grow at different NaCl concentrations. Isolates CW2 and WE21 grew in the absence of NaCl on 1% tryptone agar, while strain CW7 did not grow (Table 2). Isolate CW7 did grow in LB liquid medium without NaCl, but it grew half as fast as it did in medium containing ≥1% NaCl (data not shown). Isolates CW2 and WE21 showed no impairment in growth rate (data not shown) in LB medium with NaCl concentrations of 0 to 1%. All isolates grew in the presence of 3% NaCl, but strain WE21's generation time at 25°C was now ca. 70 min compared to 45 min in the presence of 1% NaCl (data not shown). Isolate CW2 was citrate positive and sucrose positive, in agreement with the phenotype for previously described S. baltica isolates (52, 53). Results of testing the strains on API 20 NE strips are shown in Table 2. Strain CW2 results were similar to those reported for the S. baltica isolate OS195 as well as other isolates. Isolate CW2 differs from isolate OS195 (an isolate with which it shares a common ancestor based on Fig. 2) only in that strain CW2 was capric acid negative (53). It also grew in the presence of 6% NaCl, as do other S. baltica strains. Thus, the phenotype is consistent with the phylogenetic trees constructed for strain CW2. The results for strain CW7 match those of S. morhuae strains (49), with the exceptions being that strain CW7 was maltose positive on API 20 NE strips and also on minimal medium. It also possessed the ability to grow on several minimal media without supplements, in contrast to what was reported for the related S. morhuae isolates (49). It was also lactate negative, a trait of S. morhuae that is in contrast to the lactate-positive phenotype of the very closely related species S. glacialipiscicola (42). Strain CW7 failed to grow at all in 6% NaCl (Table 2), consistent with it being a member of the S. morhuae species. However, the phenotypes of the other isolates (strains BT2, BT11, CHO4, CHO5, HMS18, LMW1, LMS4, WE21, and WRW4) do not match the phenotypes of S. baltica or S. putrefaciens strains (Table 2). In contrast to strain CW2 and other S. baltica isolates, these isolates were weak assimilators or nonassimilators of glucose, did not grow on citrate, and grew slowly in the presence of 6% NaCl. They were also capric acid negative. However, they all utilized N-acetylglucosamine and malate and were gelatinase positive, traits of S. baltica but not S. putrefaciens strains (53). Of the ambiguous isolates, only strain LMW1 was gluconate negative on API strips (Table 2) and on minimal medium (results not shown), a phenotype associated with S. putrefaciens but not S. baltica (52–54). Strains CHO5 and LMS4 were the only arabinose utilizers, a trait associated with S. putrefaciens strains but not S. baltica (49, 53). Strains BT11, CW2, WE21, and WRW4 were sucrose utilizers, a phenotype found in S. baltica strains but not S. putrefaciens isolates (49, 52, 53).
DISCUSSION
We have isolated several strains of bacteria that produce omega-3 fatty acids from five different species of omega-3-rich fish caught in freshwater by first looking for red colonies on TTC plates. Many of the red colonies, but not all, were confirmed to be EPA producers by GC and/or MS analyses. Our success rate using this selection technique was not 100%, in agreement with the study done by Bianchi et al. (14). However, when we used the PCR screening technique for the presence of the critical pfaA beta-ketosynthase domains in addition to the first screen, we found that 100% of the red colonies that contained the domain were EPA producers and none of the negative isolates tested were producers (Table 1). This ease of isolation suggests that omega-3 fatty acid-rich freshwater fish and freshwater niches commonly harbor omega-3 fatty acid-producing bacteria. The association with omega-3 fatty acid-rich fish suggests that they may contribute to the omega-3 content of the fish. In addition, they may protect the bacteria from host defense, perhaps by conferring protection from oxygen radicals and antimicrobial compounds or by damaging the host's immune system after being converted to a toxic compound by reactive oxygen intermediates. They also may confer the ability to grow at lower temperatures. We characterized three of the bacterial strains in depth. Strain CW2 is sister to S. baltica isolates based upon 16S rRNA gene, gyrB, and recA sequences, and its phenotype is consistent with its being a member of the S. baltica species (48, 49, 52, 53). Analysis of CW2's genome also supports its designation as a member of the S. baltica species (results not shown). The marine S. baltica MAC1 isolate (4) and a strain closely related to S. baltica isolates, Shewanella sp. BR-2 (51), have been shown to possess the ability to produce EPA. Therefore, it is not surprising that this closely related freshwater isolate also produces EPA. Isolate CW2 is also closely related to S. baltica strains that were isolated as free living and not from fish. However, it is possible that the closely related marine isolates (48) can also inhabit the gastrointestinal tracts of marine fish, since it has been previously shown that S. baltica can be isolated from the gastrointestinal tracts of shellfish (55). Based on 16S rRNA gene sequence, gyrB sequence, and phenotype, strain CW7 is related to S. morhuae strains such as U1414, U1417, and T214, which were not analyzed for omega-3 fatty acid production (49). With the third isolate, strain WE21, the species designation is not as clear. The analysis of the phylogenetic trees based on 16S rRNA, gyrB, and recA gene sequences does not clearly indicate the isolate as a member of either S. baltica or S. putrefaciens. Clearly, strain WE21 is not a member of the S. baltica clade sensu stricto; rather, it shares a most recent common ancestor with Shewanella sp. WRW4, S. putrefaciens, S. oneidensis, and S. decolorationis in the gyrB analysis and S. baltica, S. denitrificans, and S. morhuae in the 16S rRNA gene analysis. However, there is no support for this designation based on Fig. 1 and 2. Isolate WE21's position on the gyrB phylogenetic tree also shows its relatedness to several free-living Shewanella isolates previously isolated from North American freshwaters (52), such as S. putrefaciens MR-30 and CG-1. It would be valuable to know if these closely related S. putrefaciens isolates also produce omega-3 fatty acids. The genome of isolate WE21 (and related isolates) is being analyzed further. Data will be used to construct additional phylogenetic trees, which will give us additional support to assign the ambiguous isolates to the S. baltica or S. putrefaciens species or perhaps to a new species. The phenotypic data for strain WE21 (and related isolates) do not match the expected phenotypes of either S. baltica or S. putrefaciens. For instance, isolate WE21 is gelatinase positive, as are S. baltica strains. This trait has been used to differentiate between S. baltica and S. putrefaciens (52). However, WE21 is citrate negative, in contrast to all S. baltica isolates but not S. putrefaciens strains. Thus, it has a unique phenotype, which perhaps reflects the ecological niches it inhabits. Additional phenotypic data will also be used for species determination.
We analyzed the sequence of the EPA biosynthetic cluster in strains CW2, CW7, and WE21. The cluster contains the FAS II pathway genes (pfaA through pfaE) (18, 56). The pfaE gene product, 4′-phosphopantetheinyl transferase, is crucial to the synthesis of the long-chain (>C20) unsaturated fatty acids in bacteria such as Shewanella or Moritella (57, 58). Also crucial is the pfaA gene product, which comprises multiple ACP binding domains and malonyl-coenzyme A, i.e., ACP transacylase, ketoreductase, and beta-ketosynthase domains. This gene product plays a crucial role in synthesis, in part, because the number of ACP binding domains in the predicted protein correlates with levels of omega-3 fatty acids produced by the organism. When more domains are present, higher levels of omega-3 fatty acids are produced (59). In addition, the beta-ketosynthase domains present in the pfaA-encoded products also play a role in determining the nature of the omega-3 fatty acids generated (9). The pfaB gene product has been shown (60) to be crucial in determining whether the omega-3 fatty acid produced is EPA or DHA and contains a crucial acyltransferase domain (16, 17). The PfaC protein contains a ketosynthase domain as well as two dehydratase/isomerase domains and a domain for chain length determination. The pfaD gene encodes an enoyl reductase homologous to that seen in shorter-chain fatty acid synthesis. Comparison of the biosynthetic cluster to the sequence found in previously identified EPA-producing strains shows they are also homologous to the cluster in S. baltica isolates. All three of these isolates contain a pfaA gene, which could putatively encode a protein with five acyl carrier protein (ACP) binding domains. pfaA genes encoding proteins with five putative ACP binding domains produce intermediate amounts of omega-3 fatty acids, consistent with the concentrations that isolates CW2 and WE21 were found to produce (maximum of 7.2 and 7.3%, respectively). These levels are similar to levels reported for Shewanella sp. BR-2 of up to 7.7% total fatty acid (59) and for a Shewanella marinintestina strain (7.0% total fatty acid) isolated by the selection methods we used (34). However, the levels these isolates produce is much lower than that of S. olleyana strains ACEM 6 and ACEM 9 (almost 24% of total fatty acid at 4°C) (32). In contrast, isolate CW7 is a lower-level producer, but it is possible that we grew it under less than optimal conditions. Analyses also show that the genes in the three clusters are similar to the genes found in both S. putrefaciens 200 and S. putrefaciens CN-32. However, the predicted products of the pfaE gene are less similar. Isolate WE21's predicted pfaE gene product, 4′-phosphopantetheinyl transferase, for example, shows only 57 and 56% identity to the predicted products of the S. putrefaciens strains, compared to 82% identity to the predicted products from the omega-3 fatty acid-producing strain Shewanella sp. BR-2 and other S. baltica strains. This difference may be one of the reasons strain WE21 produces omega-3 fatty acids and the related Shewanella putrefaciens strains do not (31). However, the predicted pfaA gene product from these nonproducers may be an additional reason for the inability of the organisms to make EPA. The predicted product encoded by S. putrefaciens CN-32 is expected to have four ACP binding domains based on the GenBank sequence, and the predicted product encoded by S. putrefaciens 200 would be very different due to an insertion (21). Shewanella oneidensis MR-1 (a freshwater isolate) produces very low levels of EPA (<1% of the total fatty acids) (20), despite its having the cluster (61). However, the predicted pfaA gene product would only have only four ACP binding domains (59). Furthermore, its predicted pfaE product shows only 47% identity with strain WE21's putative product (data not shown).
Previously (10), a survey of marine omega-3 fatty acid producers showed that most were halophiles, with S. frigidimarina being an exception. In contrast to marine isolates, most of our isolates are nonhalophiles. Strain CW7 is the only isolate that does not grow on tryptone medium without NaCl. Unlike marine EPA producers, strains CW2, CW7, and WE21 grew well when the salt concentration was 1% and 2% but more slowly when it was 3%. Since it has been suggested that marine bacteria produce omega-3 fatty acids in response to their environment, such as the temperature, we investigated whether isolate WE21's production of EPA omega-3 fatty acids varied with temperature and salinity. Temperature did affect the levels of EPA present in the organism, as it does in marine species, including Shewanella sp. BR-2 (51). Thus, production of omega-3 fatty acids in our isolates may be due in part due to cold adaptation in Shewanella species (62). Our results showed that the concentration of NaCl in which isolate WE21 was grown did affect the levels of EPA present. When the concentration was elevated to 2% or 3%, the level of EPA was reduced, not increased. However, this could be related to a lower growth rate, which has previously been shown to affect lipid composition in S. geldimarina (62).
We were able to isolate two different isolates of EPA producers from the same coho salmon and two different isolates from the same brown trout despite the fact that both fish species were introduced into the Great Lakes only 50 years ago (63). Partial sequencing of their gyrB genes was used in the construction of the gyrB phylogenetic tree (Fig. 2). This tree suggests that these isolates, which share a most recent common ancestor, are closely related to each other and our freshwater fish strain WE21, rather than the S. baltica (sensu stricto) isolates. Isolates BT2, CHO4, and CHO5 are sucrose negative, a trait of some S. putrefaciens isolates (31), but all isolates are gelatinase positive, unlike S. putrefaciens strains. These four new isolates also failed to grow on 6% NaCl and grew slower on 3% NaCl than on 0% or 1% NaCl (data not shown), in agreement with them likely being freshwater bacteria even though strains CHO4 and CHO5 came from coho salmon, a transplanted anadromous marine fish. Since both brown trout and coho salmon caught in the Great Lakes are rich in omega-3 fatty acids (63), it is possible that these fish must harbor omega-3 fatty acid-producing bacteria to be healthy. Thus, acquiring omega-3 fatty acid bacteria may be important in their adaptation to a new freshwater habitat. Recently, a detailed study on the autochthonous gut microbiota of laboratory-raised brown trout was published (64). 16S rRNA gene sequencing showed that Shewanella spp. were present but were not identified in the cultured isolates, presumably the result of their not being major members of the gut microbiota in those brown trout. None of our bacterial isolates from fish produced DHA, even though the fish are reported to contain DHA (65, 66). This is probably due to the predicted pfaA and pfaB gene products present in our bacteria being more similar to those expected of an EPA producer and not to a DHA producer. There are several possible reasons why we identified only EPA-producing bacterial isolates. The fish may harbor DHA-producing bacteria that we were not successful in isolating, the fish may convert EPA to DHA endogenously, or other food sources may be their primary source of DHA. Further characterization of more bacteria from the species of fish studied here or from other DHA-rich freshwater fish may yield such producers. It is possible that omega-3 fatty acid-producing bacteria present in the gastrointestinal tracts contribute to the fitness of the fishes and thus could be of benefit as probiotic additives to freshwater fish in aquaculture. In support of this hypothesis, recent work has shown that a Shewanella putrefaciens isolate, when used as a dietary probiotic in Senegalese sole larviculture, improved the growth and fitness of the fish (67). This could be the result of the omega-3 fatty acid production by the Shewanella species; however, this ability was not reported.
We tested for the possibility that cold freshwater habitats contain free-living omega-3 producers since our isolates are related to free-living Shewanella spp. and can grow and produce EPA when grown on minimal media. We were successful in isolating two unique producers from Lake Michigan, WI. Both strains (LMW1 and LMS4) produce EPA but not DHA. Our results suggest that they are members of the Shewanella genus but are different from the fish isolates. In fact, like strain WE21, they share a more recent common ancestor with S. putrefaciens, S. oneidensis, and S. decolorationis rather than the S. baltica isolates included in our analysis (Fig. 2), despite the fact that some of their closest relatives do not produce omega-3 fatty acids (21). We were also able to isolate two other unique free-living EPA producers (isolates HMS18 and WRW4). Based on phenotype, they are very similar to the two other freshwater EPA producers we isolated. However, their phenotype is more similar to that of the bacteria isolated from walleye, coho salmon, and brown trout. Partial sequencing of the gyrB gene from strain HMS18 suggests that it shares a more recent common ancestor with the isolates from coho salmon and brown trout than the two free-living isolates LMW1 and LMS4. Our gyrB phylogeny, which demonstrates the greatest number of supported branches of the three genes analyzed, presents the strongest evidence that these isolates of freshwater omega-3 fatty acid-producing Shewanella species are not associated with the marine omega-3 producing S. baltica isolates; rather, they share a most recent common ancestor with a clade that includes the freshwater non-omega-3 fatty acid-producing isolates of S. putrefaciens. Strain WRW4's gyrB sequence, strong bootstrap support, and phenotype data suggest that it is a sister to strain WE21, despite the fact that they were isolated from different niches. Although strain WRW4 was collected as a water sample, it was collected in the region of a hatchery where fish fecal material collected, suggesting it may also be a colonizer of fish. It has been previously published (68) that omega-3 fatty acids could be found in freshwater and levels correlate with fitness. It is very possible that bacteria such as Shewanella spp., and not just plankton, play a role in increasing omega-3 fatty acids in certain freshwater habitats and thus contribute to the fitness too.
In conclusion, Shewanella has been described as a metabolically diverse genus with a major impact on the environment (69). The study presented here adds further examples of the diversity of its isolates. Although most of our isolates appear closely related, the fact that every fish and habitat we examined contained different isolates suggests that there are many diverse and unique strains of Shewanella that are adapted to specific ecological niches. In support of this hypothesis, it was recently reported that the intestinal microbiota of fish can be very specific to the fish species they colonize (70).
ACKNOWLEDGMENTS
We acknowledge the contribution of Jacob Bognar (Concordia University Wisconsin) for the initial characterization of isolate WE21. We also acknowledge the angling skills of Doug Borys (Concordia University Wisconsin) and the Inter-tribal Fisheries Assessment Program and the Wisconsin Department of Natural Resources, specifically Bridget Baker, Meghan Finley, and Steven Fajfer, for the fish gastrointestinal tracts and hatchery samples used in this study. We also thank the Institute for Genome Sciences Annotation Engine Service at the University of Maryland, School of Medicine, for providing structural and functional annotation of the sequences in addition to the genomic sequencing.
Funding Statement
This project was funded in part by the School of Pharmacy (Concordia University Wisconsin) and by a CIRG grant (Concordia University Wisconsin) to F.E.D. and J.E.M. In addition, Dudley and Lois Johnson supported J.P.L. as the Dudley and Lois Johnson Lipid Fellow. This work was also funded by the National Institute of Environmental Health Children’s Environmental Health Sciences Core Center through the University of Wisconsin—Milwaukee pilot research grant program (National Institute of Environmental Health Sciences [NIEHS] grant P30ES004184) to J.E.M.
REFERENCES
- 1.Adarme-Vega TC, Thomas-Hall SR, Schenk PM. 2014. Towards sustainable sources for omega-3 fatty acids production. Curr Opin Biotechnol 26:14–18. doi: 10.1016/j.copbio.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 2.DeLong EF, Yayanos AA. 1986. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl Environ Microbiol 51:730–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajpai P, Bajpai PK. 1993. Eicosapentaenoic acid (EPA) production from microorganisms: a review. J Biotechnol 30:161–183. doi: 10.1016/0168-1656(93)90111-Y. [DOI] [PubMed] [Google Scholar]
- 4.Amiri-Jami M, Griffiths MW. 2010. Recombinant production of omega-3 fatty acids in Escherichia coli using a gene cluster isolated from Shewanella baltica MAC1. J Appl Microbiol 109:1897–1905. doi: 10.1111/j.1365-2672.2010.04817.x. [DOI] [PubMed] [Google Scholar]
- 5.Amiri-Jami M, LaPointe G, Griffiths MW. 2014. Engineering of EPA/DHA omega-3 fatty acid production by Lactococcus lactis subsp. cremoris MG1363. Appl Microbiol Biotechnol 98:3071–3080. doi: 10.1007/s00253-013-5381-0. [DOI] [PubMed] [Google Scholar]
- 6.Russell NJ, Nichols DS. 1999. Polyunsaturated fatty acids in marine bacteria—a dogma rewritten. Microbiology 145:767–779. doi: 10.1099/13500872-145-4-767. [DOI] [PubMed] [Google Scholar]
- 7.Satomi M, Oikawa H, Yano Y. 2003. Shewanella marinintestina sp. nov., Shewanella schlegeliana sp. nov. and Shewanella sairae sp. nov., novel eicosapentaenoic-acid-producing marine bacteria isolated from sea-animal intestines. Int J Syst Evol Microbiol 53:491–499. doi: 10.1099/ijs.0.02392-0. [DOI] [PubMed] [Google Scholar]
- 8.Allen EE, Facciotti D, Bartlett DH. 1999. Monounsaturated but not polyunsaturated fatty acids are required for growth of the deep-sea bacterium Photobacterium profundum SS9 at high pressure and low temperature. Appl Environ Microbiol 65:1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shulse CN, Allen EE. 2011. Diversity and distribution of microbial long-chain fatty acid biosynthetic genes in the marine environment. Environ Microbiol 13:684–695. doi: 10.1111/j.1462-2920.2010.02373.x. [DOI] [PubMed] [Google Scholar]
- 10.Nichols DS, McMeekin TA. 2002. Biomarker techniques to screen for bacteria that produce polyunsaturated fatty acids. J Microbiol Methods 48:161–170. doi: 10.1016/S0167-7012(01)00320-7. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto T, Wada H, Nishida I, Ohmori M, Murata N. 1994. Identification of conserved domains in the Δ12 desaturases of cyanobacteria. Plant Mol Biol 24:643–650. doi: 10.1007/BF00023560. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto T, Higashi S, Wada H, Murata N, Bryant DA. 1997. Low-temperature-induced desaturation of fatty acids and expression of desaturase genes in the cyanobacterium Synechococcus sp. PCC 7002. FEMS Microbiol Lett 152:313–320. [DOI] [PubMed] [Google Scholar]
- 13.Bowman JP, McCammon SA, Lewis T, Skerratt JH, Brown JL, Nichols DS, McMeekin TA. 1998. Psychroflexus torquis gen. nov., sp. nov., a psychrophilic species from Antarctic sea ice, and reclassification of Flavobacterium gondwanense as Psychroflexus gondwanense gen. nov., comb. nov. Microbiology 144:1601–1609. doi: 10.1099/00221287-144-6-1601. [DOI] [PubMed] [Google Scholar]
- 14.Bianchi AC, Olazabal L, Torre A, Loperena L. 2014. Antarctic microorganisms as source of the omega-3 polyunsaturated fatty acids. World J Microbiol Biotechnol 30:1869–1878. doi: 10.1007/s11274-014-1607-2. [DOI] [PubMed] [Google Scholar]
- 15.Yazawa K. 1996. Production of eicosapentaenoic acid from marine bacteria. Lipids 31(Suppl):S297–S300. doi: 10.1007/BF02637095. [DOI] [PubMed] [Google Scholar]
- 16.Metz JG, Roessler P, Facciotti D, Levering C, Dittrich F, Lassner M, Valentine R, Lardizabal K, Domergue F, Yamada A, Yazawa K, Knauf V, Browse J. 2001. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290–293. doi: 10.1126/science.1059593. [DOI] [PubMed] [Google Scholar]
- 17.Allen EE, Bartlett DH. 2002. Structure and regulation of the omega-3 polyunsaturated fatty acid synthase genes from the deep-sea bacterium Photobacterium profundum strain SS9. Microbiology 148:1903–1913. doi: 10.1099/00221287-148-6-1903. [DOI] [PubMed] [Google Scholar]
- 18.Schweizer E, Hofmann J. 2004. Microbial type I fatty acid synthases (FAS): major players in a network of cellular FAS systems. Microbiol Mol Biol Rev 68:501–517. doi: 10.1128/MMBR.68.3.501-517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Wang J, Jian H, Zhang B, Li S, Wang F, Zeng X, Gao L, Bartlett DH, Yu J, Hu S, Xiao X. 2008. Environmental adaptation: genomic analysis of the piezotolerant and psychrotolerant deep-sea iron reducing bacterium Shewanella piezotolerans WP3. PLoS One 3:e1937. doi: 10.1371/journal.pone.0001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abboud R, Popa R, Souza-Egipsy V, Giometti CS, Tollaksen S, Mosher JJ, Findlay RH, Nealson KH. 2005. Low-temperature growth of Shewanella oneidensis MR-1. Appl Environ Microbiol 71:811–816. doi: 10.1128/AEM.71.2.811-816.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang F, Xiao X, Ou HY, Gai Y, Wang F. 2009. Role and regulation of fatty acid biosynthesis in the response of Shewanella piezotolerans WP3 to different temperatures and pressures. J Bacteriol 191:2574–2584. doi: 10.1128/JB.00498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato S, Kurihara T, Kawamoto J, Hosokawa M, Sato SB, Esaki N. 2008. Cold adaptation of eicosapentaenoic acid-less mutant of Shewanella livingstonensis Ac10 involving uptake and remodeling of synthetic phospholipids containing various polyunsaturated fatty acids. Extremophiles 12:753–761. doi: 10.1007/s00792-008-0182-6. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto J, Kurihara T, Yamamoto K, Nagayasu M, Tani Y, Mihara H, Hosokawa M, Baba T, Sato SB, Esaki N. 2009. Eicosapentaenoic acid plays a beneficial role in membrane organization and cell division of a cold-adapted bacterium, Shewanella livingstonensis Ac10. J Bacteriol 191:632–640. doi: 10.1128/JB.00881-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kurihara T, Kawamoto J. 2014. Chemical approach to analyze the physiological function of phospholipids with polyunsaturated fatty acyl chain. Yakugaku Zasshi 134:507–513. (In Japanese.) doi: 10.1248/yakushi.13-00251-2. [DOI] [PubMed] [Google Scholar]
- 25.Dai XZ, Kawamoto J, Sato SB, Esaki N, Kurihara T. 2012. Eicosapentaenoic acid facilitates the folding of an outer membrane protein of the psychrotrophic bacterium, Shewanella livingstonensis Ac10. Biochem Biophys Res Commun 425:363–367. doi: 10.1016/j.bbrc.2012.07.097. [DOI] [PubMed] [Google Scholar]
- 26.Nishida T, Morita N, Yano Y, Orikasa Y, Okuyama H. 2007. The antioxidative function of eicosapentaenoic acid in a marine bacterium, Shewanella marinintestina IK-1. FEBS Lett 581:4212–4216. doi: 10.1016/j.febslet.2007.07.065. [DOI] [PubMed] [Google Scholar]
- 27.Dorantes-Aranda JJ, Seger A, Mardones JI, Nichols PD, Hallegraeff GM. 2015. Progress in understanding algal bloom-mediated fish kills: the role of superoxide radicals, phycotoxins and fatty acids. PLoS One 10:e0133549. doi: 10.1371/journal.pone.0133549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishida T, Hori R, Morita N, Okuyama H. 2010. Membrane eicosapentaenoic acid is involved in the hydrophobicity of bacterial cells and affects the entry of hydrophilic and hydrophobic compounds. FEMS Microbiol Lett 306:91–96. doi: 10.1111/j.1574-6968.2010.01943.x. [DOI] [PubMed] [Google Scholar]
- 29.Ringo E, Jostensen JP, Olsen RE. 1992. Production of eicosapentaenoic acid by freshwater Vibrio.. Lipids 27:564–566. doi: 10.1007/BF02536141. [DOI] [Google Scholar]
- 30.Jensen JL, Rikardsen AH, Thorstad EB, Suhr AH, Davidsen JG, Primicerio R. 2014. Water temperatures influence the marine area use of Salvelinus alpinus and Salmo trutta.. J Fish Biol 84:1640–1653. doi: 10.1111/jfb.12366. [DOI] [PubMed] [Google Scholar]
- 31.Bowman JP, McCammon SA, Nichols DS, Skerratt JH, Rea SM, Nichols PD, McMeekin TA. 1997. Shewanella gelidimarina sp. nov. and Shewanella frigidimarina sp. nov., novel Antarctic species with the ability to produce eicosapentaenoic acid (20:5ω3) and grow anaerobically by dissimilatory Fe(III) reduction. Int J Syst Bacteriol 47:1040–1047. doi: 10.1099/00207713-47-4-1040. [DOI] [PubMed] [Google Scholar]
- 32.Skerratt JH, Bowman JP, Nichols PD. 2002. Shewanella olleyana sp. nov., a marine species isolated from a temperate estuary which produces high levels of polyunsaturated fatty acids. Int J Syst Evol Microbiol 52:2101–2106. doi: 10.1099/00207713-52-6-2101. [DOI] [PubMed] [Google Scholar]
- 33.Garcia R, Gemperlein K, Muller R. 2014. Minicystis rosea gen. nov., sp. nov., a polyunsaturated fatty acid-rich- and steroid-producing soil myxobacterium. Int J Syst Evol Microbiol 64:3733–3742. doi: 10.1099/ijs.0.068270-0. [DOI] [PubMed] [Google Scholar]
- 34.Ryan J, Farr H, Visnovsky S, Vyssotski M, Visnovsky G. 2010. A rapid method for the isolation of eicosapentaenoic acid-producing marine bacteria. J Microbiol Methods 82:49–53. doi: 10.1016/j.mimet.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed, vol 3 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 36.Parkinson JS. 1976. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. J Bacteriol 126:758–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bertani G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol 62:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowen CL, Kehler J, Evans CA. 2010. Development and validation of a sensitive and selective UHPLC–MS/MS method for simultaneous determination of both free and total eicosapentaeonic acid and docosahexenoic acid in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 878:3125–3133. doi: 10.1016/j.jchromb.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Baker GC, Smith JJ, Cowan DA. 2003. Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55:541–555. doi: 10.1016/j.mimet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto S, Harayama S. 1995. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol 61:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galens K, Orvis J, Daugherty S, Creasy HH, Angiuoli S, White O, Wortman J, Mahurkar A, Giglio MG. 2011. The IGS standard operating procedure for automated prokaryotic annotation. Stand Genomic Sci 4:244–251. doi: 10.4056/sigs.1223234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satomi M, Vogel BF, Venkateswaran K, Gram L. 2007. Description of Shewanella glacialipiscicola sp. nov. and Shewanella algidipiscicola sp. nov., isolated from marine fish of the Danish Baltic Sea, and proposal that Shewanella affinis is a later heterotypic synonym of Shewanella colwelliana.. Int J Syst Evol Microbiol 57:347–352. doi: 10.1099/ijs.0.64708-0. [DOI] [PubMed] [Google Scholar]
- 43.Katoh K, Kuma K, Toh H, Miyata T. 2005. MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katoh K, Misawa K, Kuma K, Miyata T. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- 46.Huson DH, Richter DC, Rausch C, Dezulian T, Franz M, Rupp R. 2007. Dendroscope: an interactive viewer for large phylogenetic trees. BMC Bioinformatics 8:460. doi: 10.1186/1471-2105-8-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dikow RB. 2011. Genome-level homology and phylogeny of Shewanella (Gammaproteobacteria: lteromonadales: Shewanellaceae). BMC Genomics 12:237. doi: 10.1186/1471-2164-12-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caro-Quintero A, Auchtung J, Deng J, Brettar I, Hofle M, Tiedje JM, Konstantinidis KT. 2012. Genome sequencing of five Shewanella baltica strains recovered from the oxic-anoxic interface of the Baltic Sea. J Bacteriol 194:1236. doi: 10.1128/JB.06468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Satomi M, Vogel BF, Gram L, Venkateswaran K. 2006. Shewanella hafniensis sp. nov. and Shewanella morhuae sp. nov., isolated from marine fish of the Baltic Sea. Int J Syst Evol Microbiol 56:243–249. doi: 10.1099/ijs.0.63931-0. [DOI] [PubMed] [Google Scholar]
- 50.Nakhamchik A, Wilde C, Chong H, Rowe-Magnus DA. 2010. Evidence for the horizontal transfer of an unusual capsular polysaccharide biosynthesis locus in marine bacteria. Infect Immun 78:5214–5222. doi: 10.1128/IAI.00653-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee SJ, Seo PS, Kim CH, Kwon O, Hur BK, Seo JW. 2009. Isolation and characterization of the eicosapentaenoic acid biosynthesis gene cluster from Shewanella sp. BR-2. J Microbiol Biotechnol 19:881–887. doi: 10.4014/jmb.0902.090. [DOI] [PubMed] [Google Scholar]
- 52.Venkateswaran K, Moser DP, Dollhopf ME, Lies DP, Saffarini DA, MacGregor BJ, Ringelberg DB, White DC, Nishijima M, Sano H, Burghardt J, Stackebrandt E, Nealson KH. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol 49:705–724. doi: 10.1099/00207713-49-2-705. [DOI] [PubMed] [Google Scholar]
- 53.Ziemke F, Hofle MG, Lalucat J, Rossello-Mora R. 1998. Reclassification of Shewanella putrefaciens Owen's genomic group II as Shewanella baltica sp. nov. Int J Syst Bacteriol 48:179–186. doi: 10.1099/00207713-48-1-179. [DOI] [PubMed] [Google Scholar]
- 54.Brettar I, Christen R, Hofle MG. 2002. Shewanella denitrificans sp. nov., a vigorously denitrifying bacterium isolated from the oxic-anoxic interface of the Gotland Deep in the central Baltic Sea. Int J Syst Evol Microbiol 52:2211–2217. doi: 10.1099/00207713-52-6-2211. [DOI] [PubMed] [Google Scholar]
- 55.Richards GP, Watson MA, Crane EJ III, Burt IG, Bushek D. 2008. Shewanella and Photobacterium spp. in oysters and seawater from the Delaware Bay. Appl Environ Microbiol 74:3323–3327. doi: 10.1128/AEM.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shulse CN, Allen EE. 2011. Widespread occurrence of secondary lipid biosynthesis potential in microbial lineages. PLoS One 6:e20146. doi: 10.1371/journal.pone.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugihara S, Orikasa Y, Okuyama H. 2008. An EntD-like phosphopantetheinyl transferase gene from Photobacterium profundum SS9 complements pfa genes of Moritella marina strain MP-1 involved in biosynthesis of docosahexaenoic acid. Biotechnol Lett 30:411–414. doi: 10.1007/s10529-007-9579-z. [DOI] [PubMed] [Google Scholar]
- 58.Orikasa Y, Nishida T, Hase A, Watanabe K, Morita N, Okuyama H. 2006. A phosphopantetheinyl transferase gene essential for biosynthesis of n-3 polyunsaturated fatty acids from Moritella marina strain MP-1. FEBS Lett 580:4423–4429. doi: 10.1016/j.febslet.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 59.Jiang H, Zirkle R, Metz JG, Braun L, Richter L, Van Lanen SG, Shen B. 2008. The role of tandem acyl carrier protein domains in polyunsaturated fatty acid biosynthesis. J Am Chem Soc 130:6336–6337. doi: 10.1021/ja801911t. [DOI] [PubMed] [Google Scholar]
- 60.Orikasa Y, Tanaka M, Sugihara S, Hori R, Nishida T, Ueno A, Morita N, Yano Y, Yamamoto K, Shibahara A, Hayashi H, Yamada Y, Yamada A, Yu R, Watanabe K, Okuyama H. 2009. pfaB products determine the molecular species produced in bacterial polyunsaturated fatty acid biosynthesis. FEMS Microbiol Lett 295:170–176. doi: 10.1111/j.1574-6968.2009.01582.x. [DOI] [PubMed] [Google Scholar]
- 61.Wu WF, Wang FP, Li JH, Yang XW, Xiao X, Pan YX. 2013. Iron reduction and mineralization of deep-sea iron reducing bacterium Shewanella piezotolerans WP3 at elevated hydrostatic pressures. Geobiology 11:593–601. doi: 10.1111/gbi.12061. [DOI] [PubMed] [Google Scholar]
- 62.Nichols DS, Olley J, Garda H, Brenner RR, McMeekin TA. 2000. Effect of temperature and salinity stress on growth and lipid composition of Shewanella gelidimarina.. Appl Environ Microbiol 66:2422–2429. doi: 10.1128/AEM.66.6.2422-2429.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams MCW, Schrank C, Anderson HA. 2014. Fatty acids in thirteen Wisconsin sport fish species. J Great Lakes Res 40:771–777. doi: 10.1016/j.jglr.2014.05.002. [DOI] [Google Scholar]
- 64.Al-Hisnawi A, Ringø E, Davies SJ, Waines P, Bradley G, Merrifield DL. 2014. First report on the autochthonous gut microbiota of brown trout (Salmo trutta Linnaeus). Aquaculture Res doi: 10.1111/are.12451. [DOI] [Google Scholar]
- 65.Pantazopoulos P, Sawyer JM, Turyk ME, Diamond M, Bhavsar SP, Mergler D, Schantz S, Ratnayake N, Carpenter DO. 2013. Fatty acids in Great Lakes lake trout and whitefish. J Great Lakes Res 39:120–127. doi: 10.1016/j.jglr.2012.12.012. [DOI] [Google Scholar]
- 66.Williams M, Schrank C. April 2014. A healthy dose of flavor. Wisconsin Natural Resources Magazine. Wisconsin Department of Natural Resources, Madison, WI. [Google Scholar]
- 67.Lobo C, Moreno-Ventas X, Tapia-Paniagua S, Rodriguez C, Morinigo MA, de La Banda IG. 2014. Dietary probiotic supplementation (Shewanella putrefaciens Pdp11) modulates gut microbiota and promotes growth and condition in Senegalese sole larviculture. Fish Physiol Biochem 40:295–309. doi: 10.1007/s10695-013-9844-0. [DOI] [PubMed] [Google Scholar]
- 68.Muller-Navarra DC, Brett MT, Park S, Chandra S, Ballantyne AP, Zorita E, Goldman CR. 2004. Unsaturated fatty acid content in seston and tropho-dynamic coupling in lakes. Nature 427:69–72. doi: 10.1038/nature02210. [DOI] [PubMed] [Google Scholar]
- 69.Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JL, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM. 2008. Towards environmental systems biology of Shewanella.. Nat Rev Microbiol 6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- 70.Li XM, Zhu YJ, Yan QY, Ringø E, Yang DG. 2014. Do the intestinal microbiotas differ between paddlefish (Polyodon spathala) and bighead carp (Aristichthys nobilis) reared in the same pond? J Appl Microbiol 117:1245–1252. doi: 10.1111/jam.12626. [DOI] [PubMed] [Google Scholar]