Abstract
Low-pressure mercury UV (LP-UV) lamps have long been used for bacterial inactivation, but due to certain disadvantages, such as the possibility of mercury leakage, deep-UV-C light-emitting diodes (DUV-LEDs) for disinfection have recently been of great interest as an alternative. Therefore, in this study, we examined the basic spectral properties of DUV-LEDs and the effects of UV-C irradiation for inactivating foodborne pathogens, including Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes, on solid media, as well as in water. As the temperature increased, DUV-LED light intensity decreased slightly, whereas LP-UV lamps showed increasing intensity until they reached a peak at around 30°C. As the irradiation dosage and temperature increased, E. coli O157:H7 and S. Typhimurium experienced 5- to 6-log-unit reductions. L. monocytogenes was reduced by over 5 log units at a dose of 1.67 mJ/cm2. At 90% relative humidity (RH), only E. coli O157:H7 experienced inactivation significantly greater than at 30 and 60% RH. In a water treatment study involving a continuous system, 6.38-, 5.81-, and 3.47-log-unit reductions were achieved in E. coli O157:H7, S. Typhimurium, and L. monocytogenes, respectively, at 0.5 liter per minute (LPM) and 200 mW output power. The results of this study suggest that the use of DUV-LEDs may compensate for the drawbacks of using LP-UV lamps to inactivate foodborne pathogens.
INTRODUCTION
Food safety is an important component of public health. Each year, more than 16% of the U.S. population acquire a foodborne illness, and 3,000 people are killed by consuming contaminated foods (http://www.cdc.gov/foodborneburden/index.html). Also, the United States is burdened by more than $50 billion in economic costs related to foodborne illnesses each year (1). The major bacterial causes of foodborne illness outbreaks are generally recognized to be Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes. E. coli O157:H7 has become a prominent foodborne pathogen, causing severe hemorrhagic colitis and hemolytic-uremic syndrome (2). The symptoms of Salmonella sp. infection include diarrhea, abdominal pain, mild fever, blood-tinged stools, and vomiting (3). L. monocytogenes can survive and grow at refrigeration temperatures and adversely affects pregnant women and immunocompromised individuals, such as the elderly (4).
UV light (UV), which covers the region of the electromagnetic spectrum from 100 to 400 nm, is classified as UV-A (320 to 400 nm), UV-B (280 to 320 nm), and UV-C (200 to 280 nm) (5). UV-C light is considered to be the most effective germicidal region of the UV spectrum for inactivating microorganisms, such as bacteria, viruses, protozoa, fungi, yeasts, and algae, by the formation of photoproducts in the DNA (6, 7). The pyrimidine dimer, which forms between adjacent pyrimidine molecules in the same DNA strand, is the most prominent product. These dimers can interrupt both proper transcription and replication of DNA, eventually leading to cell death (5, 8, 9).
The use of UV irradiation as a pathogen control method to treat foods has been approved by the U.S. Food and Drug Administration (10). So far, the majority of UV treatment is performed with low-pressure mercury UV (LP-UV) lamps at 253.7 nm in academic, as well as industrial, fields. However, these lamps have some potential drawbacks, such as the possibility of mercury leakage, a short lifetime, and significant energy requirements (11). As an alternative to UV mercury lamps, the application of deep-UV-C light-emitting diodes (DUV-LEDs) has been under development. DUV-LEDs have numerous advantages over conventional mercury lamps. While the emission wavelength of low-pressure mercury lamps is fixed at 253.7 nm, the emission wavelength of DUV-LEDs can be tuned to various individual wavelengths across the UV spectrum. Adjustment to match the most effective wavelength for disinfection under a wide range of environmental conditions can enhance the efficiency of inactivation (12). Also, DUV-LEDs have fracture resistance to external shock, flexible spatial application due to compact size, and reduced heat generation.
Several investigative studies have been performed to assay the bactericidal efficacy of DUV-LEDs in water (13, 14). Würtele et al. (15) and Oguma et al. (16) developed both a static test and a flowthrough test system to examine the inactivation efficiency. Although they developed unique types of water decontamination systems, both continuous systems still have some limitations for practical use. To be economically feasible, such treatment systems must be able to process water at a flow rate of more than 1 liter per minute. Also, to be effective in a high-flow-rate system, DUV-LED modules should be applied at high power for short UV exposure times, and radiation loss during treatment must be low. For these reasons, a new type of flowthrough water disinfection system was designed for the present study in order to prevent the loss of UV treatment radiation. The system can be applied to sterilize municipal water.
The objectives of this study were to examine the basic properties of DUV-LEDs, such as the spectrum and intensity relative to the distance between the LED and the subject and he arrangement of LEDs. Also, the efficacy of UV-C irradiation for inactivating E. coli O157:H7, Salmonella enterica serovar Typhimurium, and L. monocytogenes on solid media at various temperatures and levels of relative humidity (RH), as well as the comparison of batch and continuous water treatment systems, were investigated.
MATERIALS AND METHODS
Collimated UV radiation design.
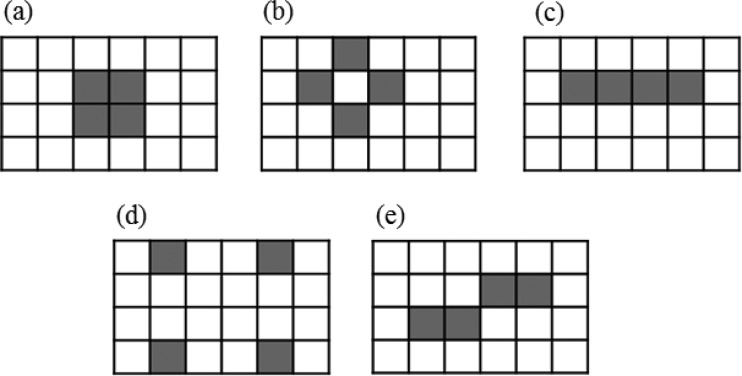
Four DUV-LED modules (LG Innotek Co., Seoul, Republic of Korea) were connected to an electronic printed circuit board (PCB) to get a constant electric current of 20 mA from a DC power supply (TPM series; Toyotech, Incheon, Republic of Korea). All of these LEDs emitted a single wavelength, 275 ± 3 nm. Several spatial arrangements of four DUV-LEDs were devised and analyzed to clearly fix the optimal LED configuration that produced collimated radiation. Figure 1 shows the five kinds of LED arrangements that were tested in this study (11).
FIG 1.
Various arrangements for LEDs strengthen the overall effect. Shown are original (a), evenly spaced (b), straight-line (c), 4-corners (d), and staggered-line (e) configurations.
Experimental setup.
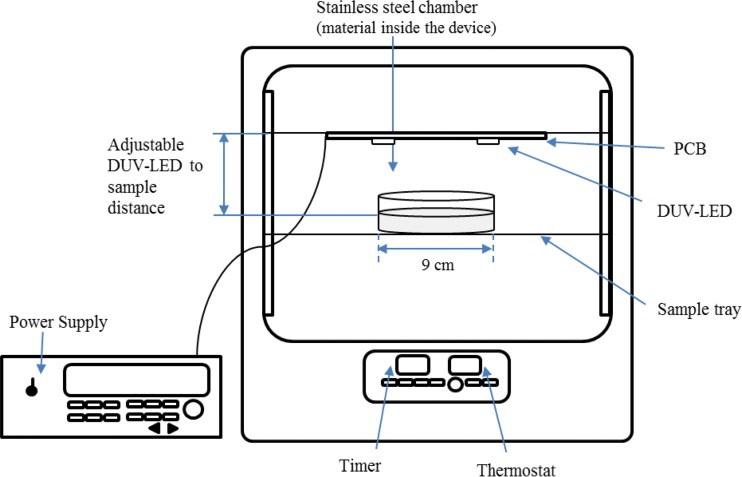
Four LED modules were arranged at each corner at a distance of 6 cm from each other and with a 4-cm distance between the LEDs and a petri dish. This design showed generally equal intensity across the whole petri dish (90-mm diameter) at a level 4 cm above the sample. More concisely, the Petri factor, which indicates the area of even distribution of irradiated light on a petri dish, was higher than 0.9, which provides a nearly ideal and uniform exposure of the whole petri dish to UV irradiation (17). The PCB with LEDs and inoculated media was placed in a constant-temperature chamber (IL-11; Lab Companion, Daejeon, Republic of Korea) to optimize the treatment conditions. The petri dish was located directly below the LEDs to receive maximum UV exposure (Fig. 2).
FIG 2.
Schematic diagram of the DUV-LED irradiation system.
Irradiance measurements.
Radiation intensities were compared between DUV-LEDs and an 8-W LP-UV lamp (G6T5; Sankyo Denki, Kanagawa, Japan) by increasing the time and temperature. The irradiance levels of the DUV-LEDs and the LP lamp were measured with a spectrometer (AvaSpec-ULS2048-USB2-UA-50; Avantes, Apeldoorn, Netherlands) calibrated for a 200- to 400-nm range within the UV spectrum. To reveal the best arrangement of LEDs in the PCB and to decide the most effective distance between the PCB and the material accepting light, an optical probe was placed 2, 4, 6, and 8 cm above the LED and the peak irradiance value of the spectrum was read. In order to calculate the Petri factor over a petri dish, the optical probe scanned and took measurements of 7.95 cm2, which corresponds to the area of one-eighth of the petri dish surface. The area that could be representative of the whole petri dish was measured for every 5 mm (17). The intensity of each point was divided by the maximum intensity of the relevant configuration, and the Petri factor was calculated as the average ratio of the intensities. The maximum intensity value was multiplied by the Petri factor to obtain the corrected irradiance, which indicates the average fluence (UV dose) rate.
Bacterial strains and inoculum conditions.
Three strains of E. coli O157:H7 (ATCC 35150, ATCC 43889, and ATCC 43890), S. Typhimurium (ATCC 19585, ATCC 43971, and DT 104), and L. monocytogenes (ATCC 7644, ATCC 19114, and ATCC 19115) were obtained from the Bacterial Culture Collection at Seoul National University (Seoul, Republic of Korea). Stock cultures were grown in tryptic soy broth (TSB) (Difco, Becton Dickinson and Company, Sparks, MD, USA) at 37°C for 24 h and stored at −80°C (0.7 ml of TSB culture plus 0.3 ml of sterile 50% glycerol solution). To obtain working cultures, bacteria were streaked onto tryptic soy agar (TSA) (Difco), incubated at 37°C for 24 h, stored at 4°C, and used within 3 days.
Culture preparation and inoculation.
Each strain of E. coli O157:H7, S. Typhimurium, and L. monocytogenes was cultured in 5 ml TSB at 37°C for 24 h and harvested by centrifugation at 4,000 × g for 20 min at 4°C, and the supernatant was discarded. The cell pellets obtained were resuspended in sterile 0.2% Bacto peptone (Becton, Dickinson and Company, Sparks, MD, USA) and centrifuged. This washing procedure was performed three times. The final pellets were resuspended in 9 ml sterile 0.2% Bacto peptone water (PW), corresponding to approximately 108 to 109 CFU/ml.The resuspended pellets of each strain of all the pathogen species were combined to constitute a 3-pathogen mixed-culture cocktail.
For experiments performed on medium surfaces, in order to set a control solution, the mixed-culture cocktail was 10-fold serially diluted three times (10−3 dilution) with 0.2% sterile PW, resulting in a final concentration of approximately 5- to 6-log CFU/ml. For inoculation, the culture suspension was further 10-fold serially diluted with 0.2% sterile PW to obtain countable colonies. One-tenth milliliter of selected diluents was spread plated onto selective or nonselective medium. Sorbitol MacConkey agar (SMAC) (Difco), xylose lysine desoxycholate agar (XLD) (Difco), and Oxford agar base with antimicrobial supplement (MOX) (MB Cell, Seoul, Republic of Korea) were used as selective media to enumerate E. coli O157:H7, S. Typhimurium, and L. monocytogenes bacteria, respectively. Phenol red agar base (Difco) with 1% sorbitol (MB Cell) (SPRAB) was used to enumerate injured E. coli O157:H7 cells, and the overlay (OV) method was used to enumerate injured S. Typhimurium and L. monocytogenes cells. To obtain countable numbers of colonies on the tested media, two levels of sequential 10-fold serial dilutions were spread plated. After inoculation, the media were dried for approximately 30 min prior to UV treatment.
For water treatment, sterile distilled water (DW) was used. In the case of the small-batch system for water decontamination, 0.1 ml of mixed-culture cocktail was inoculated into 25 ml of DW at room temperature. For the continuous water decontamination system, 8 ml of culture cocktail was inoculated into 2 liters of DW.
UV treatment.
In order to minimize photoreactivation, all DUV-LED-treated petri dishes were covered with aluminum foil. Inoculated samples were treated with 275-nm DUV-LEDs at 5.57-μW/cm2 intensity for 0, 0.5, 1, 3, and 5 min at room temperature. DUV-LED doses were calculated by multiplying DUV-LED intensities by the irradiation times. For testing at different temperatures, DUV-LED irradiation was applied to samples for 1 min at 0, 4, 15, 25, and 37°C. For all temperature tests, samples were kept at a controlled temperature inside the chamber for 5 min prior to treatment to allow equilibration. For examining the impact of RH on DUV-LED irradiation, a temperature and humidity chamber (TH-TG-300; JEIO Tech, Daejeon, Republic of Korea) was used to adjust the humidity to 30, 60, and 90% RH, with the temperature maintained at 25°C.
For the small-scale water decontamination system, inoculated samples were treated with a 278-nm DUV-LED at a 4.5-cm distance between the sample and the DUV-LED PCBs. Ten milliliters of inoculated water sample was placed in a petri dish (50 by 15 mm [internal dimensions]). The sample was mixed continuously with a magnetic stirrer (HY-HS11; Hanyang Science, Seoul, Republic of Korea) to allow even irradiation.
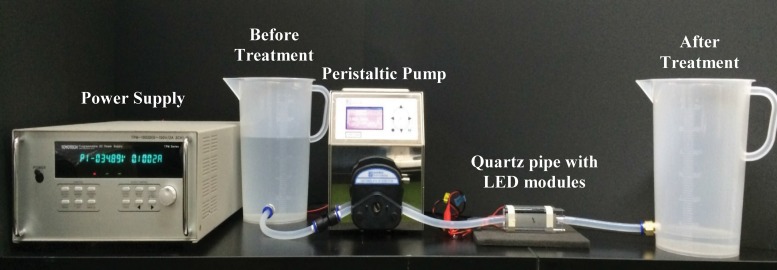
The continuous water treatment system (Fig. 3) consisted of a power supply, a peristaltic pump (JWS600; JenieWell, Seoul, Republic of Korea), and a manufactured quartz pipe (Kum-Kang Quartz, Gyeonggi, Republic of Korea), which was attached with LED modules. The inoculated water sample was transported by the pump through the silicon tubing and treated while flowing. Two to four LED modules that had an output power of 50 mW were attached to the quartz pipe, and the flow rates were adjusted to 0.5, 1, 1.5, or 2 liters per minute (LPM).
FIG 3.
Continuous water decontamination system.
Bacterial enumeration.
After UV treatment of water, 1-ml sample aliquots were 10-fold serially diluted in 9-ml blanks of 0.2% PW, and 0.1 ml of sample or diluent was spread plated onto the selective media described previously to enumerate the three pathogens. All selective agar media were incubated at 37°C for 24 to 48 h, and typical colonies were counted.
Enumeration of injured cells.
Assessing the generation of injured cells was performed only for media subjected to dose and temperature treatments and for the batch water treatment. The OV method was used to count injured cells of S. Typhimurium and L. monocytogenes (18). TSA, a nonselective medium, was used to resuscitate injured cells. One-tenth-milliliter aliquots of appropriate dilutions were spread plated in duplicate, and the plates were incubated at 37°C for 2 h to enable injured cells to recover. The resuscitated media plates were then overlaid with 7 ml of the selective medium XLD for S. Typhimurium or MOX for L. monocytogenes. The solidified plates were incubated at 37°C for an additional 22 h. After incubation, typical black colonies characteristic of either S. Typhimurium or L. monocytogenes were counted. Enumeration of injured E. coli O157:H7 cells was accomplished with SPRAB (19). After incubation at 37°C for 24 h, white colonies characteristic of E. coli O157:H7 were enumerated, and simultaneously, serological confirmation (RIM; E. coli O157:H7 latex agglutination test; Remel, Lenexa, KS, USA) was performed on randomly selected presumptive E. coli O157:H7 colonies.
Statistical analysis.
All experiments were replicated three times. All data were analyzed by analysis of variance (ANOVA) using the Statistical Analysis System (SAS Institute, Cary, NC, USA) and Tukey's multiple-range test to determine if there were significant differences (P < 0.05) in the mean values of microorganism populations.
RESULTS
Emission spectrum of DUV-LEDs.
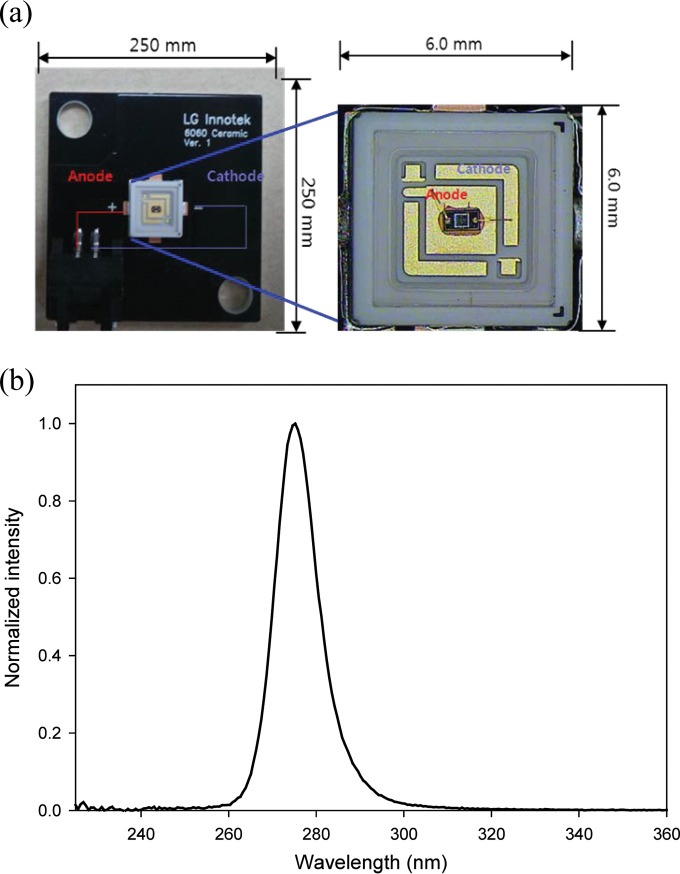
An external view of the LED module is presented in Fig. 4a. The typical spectral irradiance of 275-nm DUV-LEDs was measured with a spectrometer as shown in Fig. 4b. The full width at half maximum (FWHM), defined as the wavelength gap between the output half-peak-intensity values, was 11.3 nm for 275-nm LEDs.
FIG 4.
External view (a) and emission spectrum (b) of the DUV-LED module.
Comparison of properties between DUV-LED and LP-UV lamps.
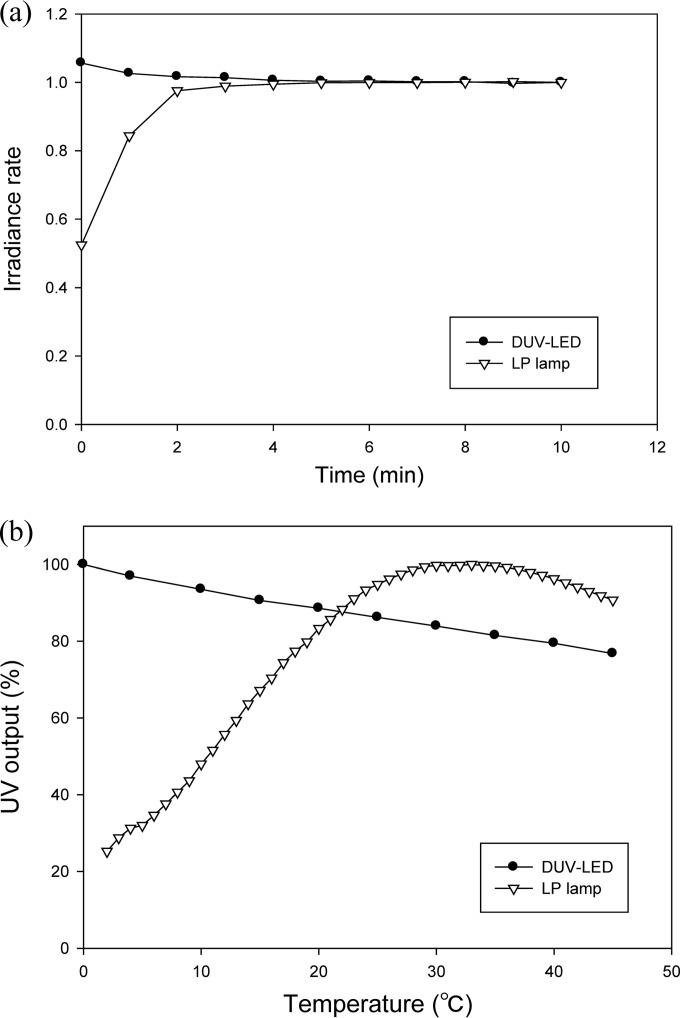
The warm-up time for both DUV-LED and LP-UV lamps was determined by measuring the irradiance intensity over time (0 to 10 min). After 5 min, the intensity of DUV-LEDs decreased by about 5.45%, whereas the intensity of LP-UV lamps increased by about 90.43% (Fig. 5a). Intensity changes relative to temperature presented different patterns between DUV-LED and LP-UV lamps. As the temperature increased, the intensity of the LEDs slightly decreased, while LP-UV lamps showed increasing intensity until they reached peak intensity at around 30°C and then decreased (Fig. 5b).
FIG 5.
Comparison between DUV-LED and LP-UV lamps for warm-up time (a) and variation of intensity according to temperature (b).
Influence of the LED arrangement on the effective area.
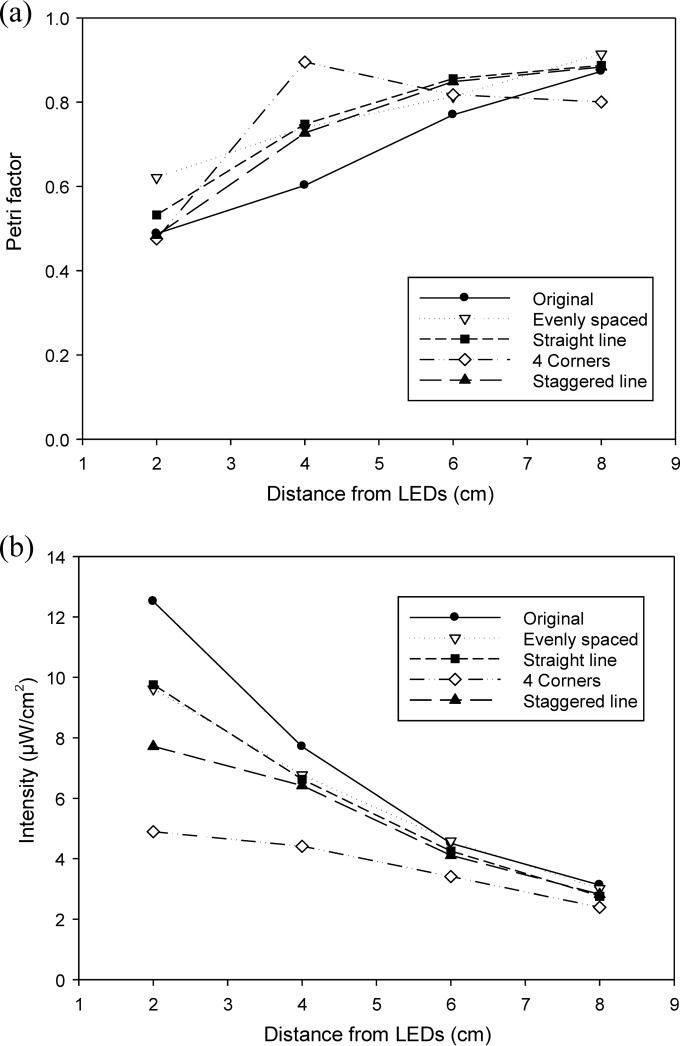
The intensities at specified distances from DUV-LEDs were measured, and the Petri factor of each point was calculated (Fig. 6). In order to get a collimated beam of UV irradiation, the Petri factor should exceed 0.90. For the 4-corners arrangement, the Petri factor was calculated as 0.48, 0.90, 0.82, and 0.80 at distances of 2, 4, 6, and 8 cm, respectively (Fig. 6a). For other configurations, the Petri factor steadily increased from around 0.5 to 0.9 relative to distance, but all the configurations showed decreasing intensity with increasing distance between the centers of the LEDs and the probe (Fig. 6b). The 4-corners configuration at 4-cm distance had an intensity of 4.41 μW/cm2, while the other configurations at 8-cm distance had an intensity of 3.2 μW/cm2, yielding the same Petri factor of 0.90.
FIG 6.
Petri factors (a) and intensities (b) of four LEDs according to spatial arrangements and distance from the LEDs.
Bactericidal effect of UV treatment on media.
The inactivation of foodborne pathogens following UV radiation is presented in Table 1. As the UV radiation dose increased from 0 to 1.67 mJ/cm2, E. coli O157:H7 and S. Typhimurium experienced over 6-log-unit reductions and L. monocytogenes underwent a >5-log-unit reduction. Only after a dose of 0.34 mJ/cm2 did E. coli O157:H7 experience a >5-log-unit reduction, and log reductions increased with increasing UV dose. For S. Typhimurium, the overall reduction patterns were similar to those of E. coli O157:H7. Populations of S. Typhimurium were reduced by 2.11 and 6.05 log CFU/ml after UV-C irradiation of 0.17 and 1.67 mJ/cm2, respectively. With regard to L. monocytogenes, reductions ranged from 0.83 to 5.10 log CFU/ml after UV dosages of 0.17 to 1.67 mJ/cm2. For all UV treatments, low numbers of injured cells occurred, but none differed significantly (P > 0.05) from the numbers of noninjured cells (Table 1).
TABLE 1.
Log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes on selective media following UV-C irradiation for various times at an intensity of 5.57 μW/cm2
| Treatment time (min) | Dose (mJ/cm2) | Log reduction (CFU/ml)a |
|||||
|---|---|---|---|---|---|---|---|
|
E. coli O157:H7 |
S. Typhimurium |
L. monocytogenes |
|||||
| SMAC | SPRAB | XLD | OV-XLD | MOX | OV-MOX | ||
| 0.5 | 0.17 | 3.51 ± 0.58 Ba | 3.27 ± 0.16 Ca | 2.11 ± 0.60 Ba | 1.69 ± 0.07 Ca | 0.83 ± 0.21 Ba | 0.90 ± 0.04 Da |
| 1 | 0.34 | 5.87 ± 0.41 Aa | 4.62 ± 0.15 Bb | 3.14 ± 0.61 Ba | 3.26 ± 0.23 Ba | 1.41 ± 0.68 Ba | 2.44 ± 0.16 Ca |
| 3 | 1.00 | 6.32 ± 0.05 Aa | 5.89 ± 0.51 Aa | 6.05 ± 0.51 Aa | 5.96 ± 0.39 Aa | 4.91 ± 0.57 Aa | 4.46 ± 0.40 Ba |
| 5 | 1.67 | 6.32 ± 0.05 Aa | 6.23 ± 0.13 Aa | 6.05 ± 0.51 Aa | 5.36 ± 0.32 Aa | 5.10 ± 0.29 Aa | 5.39 ± 0.39 Aa |
The data represent means ± standard deviations from three replications. Means followed by the same uppercase letter in the same column are not significantly different (P > 0.05). Means followed by the same lowercase letter in the same row for the same bacterium are not significantly different (P > 0.05).
Effect of temperature on UV irradiation.
Table 2 shows the bactericidal effect of 1-min treatment with UV irradiation against the three foodborne bacteria at 0 to 37°C. Log reductions of all three foodborne pathogens increased with increasing temperature. For E. coli O157:H7, significant inactivation (P < 0.05) was observed at 0°C, which achieved a 5.07-log-unit reduction. As the treatment temperature increased from 0 to 37°C, reduction levels of E. coli O157:H7 gradually increased. Reductions of 2.25 to 5.44 log CFU/ml were observed in S. Typhimurium after 1 min of UV treatment at 0 to 37°C. Also, treatment at 37°C significantly reduced (P < 0.05) levels of S. Typhimurium by >5 log units compared to the control. Log reductions of UV-treated L. monocytogenes showed significant reduction (P < 0.05) compared to the control, but at differing treatment temperatures, irradiated samples were not significantly different (P > 0.05).
TABLE 2.
Log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes on selective media following 1 min of UV-C irradiation at different treatment temperatures
| Treatment temp (°C) | Log reduction (CFU/ml)a |
|||||
|---|---|---|---|---|---|---|
|
E. coli O157:H7 |
S. Typhimurium |
L. monocytogenes |
||||
| SMAC | SPRAB | XLD | OV-XLD | MOX | OV-MOX | |
| 0 | 5.07 ± 0.25 Aa | 4.85 ± 0.25 Ba | 2.25 ± 0.19 Ba | 1.86 ± 0.53 Aa | 1.02 ± 0.12 Aa | 1.19 ± 0.45 Aa |
| 4 | 5.44 ± 0.53 Aa | 5.29 ± 0.48 ABa | 2.83 ± 0.40 Ba | 1.71 ± 0.31 Ab | 1.01 ± 0.18 Aa | 1.08 ± 0.55 Aa |
| 15 | 5.82 ± 0.63 Aa | 6.07 ± 0.42 Aa | 3.32 ± 0.31 Ba | 1.74 ± 0.48 Ab | 1.15 ± 0.13 Aa | 1.08 ± 0.39 Aa |
| 25 | 6.14 ± 0.33 Aa | 6.05 ± 0.29 Aa | 4.94 ± 0.72 Aa | 2.15 ± 0.35 Ab | 1.37 ± 0.14 Aa | 1.30 ± 0.55 Aa |
| 37 | 6.03 ± 0.38 Aa | 5.74 ± 0.23 ABa | 5.44 ± 0.22 Aa | 2.00 ± 0.17 Ab | 1.76 ± 0.65 Aa | 1.19 ± 0.23 Aa |
The data represent means ± standard deviations from three replications. Means followed by the same uppercase letter in the same column are not significantly different (P > 0.05). Means followed by the same lowercase letter in the same row for the same bacterium are not significantly different (P > 0.05).
Effect of relative humidity on UV irradiation.
Log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes on media following UV treatment at 30, 60, and 90% RH are presented in Table 3. Only E. coli O157:H7 showed a significant reduction (P < 0.05) in relation to RH; approximately 0.5-log-unit greater reduction occurred at 90% than at 30 and 60% RH. In the cases of S. Typhimurium and L. monocytogenes, there were no significant (P > 0.05) differences relative to RH.
TABLE 3.
Log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes on selective media following 1 min of UV-C irradiation at 25°C and various levels of RH
| Treatment RH (%) | Log reduction (CFU/ml)a |
||
|---|---|---|---|
| E. coli O157:H7 | S. Typhimurium | L. monocytogenes | |
| 30 | 4.04 ± 0.20 B | 2.18 ± 0.11 A | 1.59 ± 0.07 A |
| 60 | 3.97 ± 0.19 B | 2.27 ± 0.12 A | 1.58 ± 0.12 A |
| 90 | 4.48 ± 0.05 A | 2.24 ± 0.11 A | 1.59 ± 0.15 A |
The data represent means ± standard deviations from three replications. Values followed by the same letters within the same column are not significantly different (P > 0.05).
Bactericidal effect of UV treatment on a batch water system.
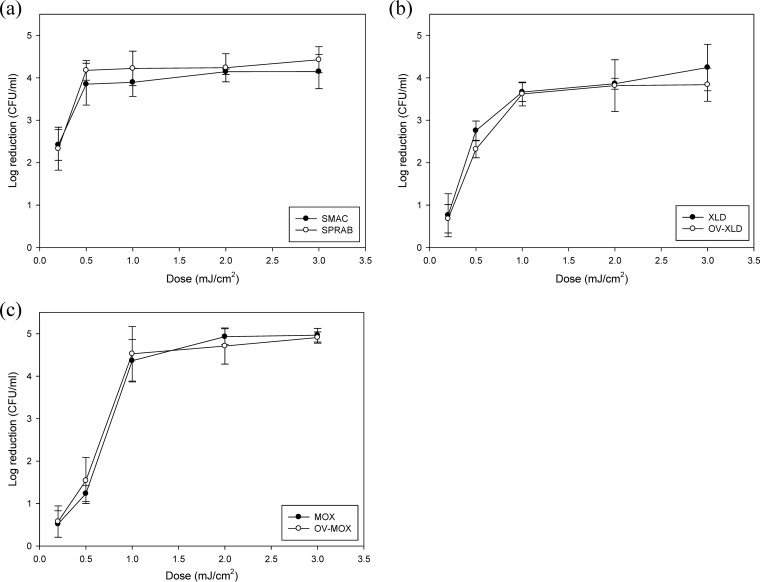
Log reductions (CFU/ml) of E. coli O157:H7, S. Typhimurium, and L. monocytogenes in 10 ml of water during UV treatment are depicted in Fig. 7. Inactivation levels for all three pathogens were more than 4 log units. Reductions of E. coli O157:H7 were 2.42 to 4.85 log units after irradiation with 0.2 to 3 mJ/cm2. For S. Typhimurium, the overall inactivation pattern was similar to that of E. coli O157:H7. Reductions of 0.76 to 4.24 log units were observed after UV doses of 0.2 to 3 mJ/cm2. In the case of L. monocytogenes, the levels of reduction increased from 0.52 to 4.97 log units as the irradiation dose increased from 0.2 to 2 mJ/cm2. The number of injured cells was not significantly different from the number of noninjured cells (P > 0.05).
FIG 7.
Viable-count reductions of 3 foodborne pathogens in a batch water system following UV-C irradiation. (a) E. coli O157:H7. (b) S. Typhimurium. (c) L. monocytogenes. The error bars indicate standard deviations.
Bactericidal effect of UV treatment on a continuous water system.
The reduction of E. coli O157:H7, S. Typhimurium, and L. monocytogenes in a continuous water system during UV irradiation is presented in Table 4. In general, the reduction levels of E. coli O157:H7, S. Typhimurium, and L. monocytogenes increased with decreasing flow rate and increasing UV light power output. For E. coli O157:H7, UV treatment at 100 mW with a flow rate of less than 1.0 LPM, 150 mW with a flow rate under 1.5 LPM, and 200 mW with less than a 2.0-LPM flow rate accomplished greater than 3-log-unit reductions. For S. Typhimurium, intensities of 150 mW at a flow rate of 0.5 LPM and 200 mW at less than 1.0 LPM accomplished more than 3-log-unit reductions. For L. monocytogenes, the trend of reduction was similar to those of E. coli O157:H7 and S. Typhimurium. However, only at an intensity of 200 mW with a flow rate of 0.5 LPM did a 3-log-unit reduction of L. monocytogenes occur.
TABLE 4.
Reductions in levels of E. coli O157:H7, S. Typhimurium, and L. monocytogenes in a continuous water system following UV-C irradiation at various flow rates and intensities
| Bacterium | Flow rate (LPM) | Log [log10 (n0/n)] (%) reduction by treatment ata: |
||
|---|---|---|---|---|
| 100 mW | 150 mW | 200 mW | ||
| E. coli O157:H7 | 2.0 | 1.97 ± 0.32 (98.93) Cb | 2.82 ± 0.28 (99.85) Ca | 3.36 ± 0.30 (99.97) Da |
| 1.5 | 2.36 ± 0.12 (99.56) Cb | 3.81 ± 0.31 (99.98) Ba | 4.06 ± 0.19 (>99.99) Ca | |
| 1.0 | 3.24 ± 0.32 (99.94) Bc | 4.20 ± 0.11 (>99.99) ABb | 4.90 ± 0.10 (>99.99) Ba | |
| 0.5 | 4.69 ± 0.40 (>99.99) Ab | 5.02 ± 0.57 (>99.99) Ab | 6.38 ± 0.06 (>99.99) Aa | |
| S. Typhimurium | 2.0 | 1.20 ± 0.17 (93.74) Ca | 1.38 ± 0.39 (95.83) Ba | 1.85 ± 0.39 (98.59) Ca |
| 1.5 | 1.40 ± 0.19 (96.05) BCb | 1.81 ± 0.37 (98.46) Bab | 2.27 ± 0.38 (99.46) BCa | |
| 1.0 | 1.87 ± 0.29 (98.66) Bb | 2.11 ± 0.35 (99.22) Bab | 3.06 ± 0.53 (99.91) Ba | |
| 0.5 | 2.87 ± 0.25 (99.86) Ab | 3.67 ± 0.26 (99.98) Ab | 5.81 ± 0.43 (>99.99) Aa | |
| L. monocytogenes | 2.0 | 0.42 ± 0.14 (62.27) Ba | 0.44 ± 0.28 (63.97) Ba | 0.93 ± 0.27 (88.16) Ba |
| 1.5 | 0.58 ± 0.23 (73.56) Ba | 0.60 ± 0.27 (75.07) Ba | 1.19 ± 0.38 (93.49) Ba | |
| 1.0 | 0.63 ± 0.15 (76.56) Bb | 0.89 ± 0.09 (87.02) Bab | 1.49 ± 0.41 (96.79) Ba | |
| 0.5 | 1.48 ± 0.17 (96.69) Ab | 2.31 ± 0.42 (99.51) Ab | 3.47 ± 0.41 (99.97) Aa | |
The data represent means ± standard deviations from three replications. Means followed by the same uppercase letter in the same column are not significantly different (P > 0.05). Means followed by the same lowercase letter in the same row are not significantly different (P > 0.05). n0, number of bacteria before irradiation (time zero); n, number of bacteria after irradiation.
DISCUSSION
UV irradiation has been used for disinfecting surfaces, air, and water for many years. Also, much research has verified the decontamination efficacy of UV. Caminiti et al. (20) investigated the inactivation efficacy of UV lamps on apple juice. Over 5-log-unit reductions of both Listeria innocua and E. coli K-12 were achieved at a dosage of 2,660 mJ/cm2. Reduction of foodborne pathogens in fresh-cut lettuce by using UV lamps was conducted by Kim et al. (21). Double-sided irradiation for a 10-min treatment with an intensity of 6.80 mW/cm2 effected 3- to 4-log-unit reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes.
As one of the nonthermal methods for reducing a broad range of microorganisms, including some pathogens, UV is effective, but safety hazards are associated with its use. LP-UV lamps, which are in common use, have some disadvantages, such as low energy efficiency and potential for mercury leakage, so it would be highly advantageous to replace the technology. Therefore, in this study, we validated the basic spectral characteristics of DUV-LEDs and demonstrated their bactericidal efficacy as an alternative to LP-UV lamps. Also, the real pasteurization effect of UV irradiation by DUV-LED technology in water systems was investigated.
For this research, DUV-LEDs that had a wavelength of 275 ± 3 nm were selected because of their cost and germicidal range. Worldwide, DUV-LED technology is still in the development stage, and only a few companies, such as Sensor Electronic Technology (SET) and LG Innotek, are now producing LED prototypes with a UV-C range of less than 280 nm. That is the reason for the current high cost of DUV-LED production. UV-C radiation in the 200- to 280-nm range has a pronounced germicidal effect, depending on the wavelength. The maximum DNA absorption of UV-C is at the wavelength peak of 260 to 265 nm (22, 23). After considering both cost and germicidal effectiveness, LEDs with a wavelength of 275 nm were chosen for the medium treatment study and LEDs with a wavelength of 278 nm were chosen for the water treatment study.
It is important to minimize the amount of light loss by providing optimal LED alignment and distance adjustment while lowering overall cost. Bowker et al. (11) used the Comsol Multiphysics method to develop an optimal collimated irradiation design. They reported that the simulated 4-corners configuration showed the highest Petri factor over other types of arrays, but it had limitations regarding predictability. Therefore, actual evaluation of the Petri factor and intensity for DUV-LEDs was required. By comparing the arrangements, a Petri factor of >0.9 was obtained at a 4-cm distance for the 4-corners array and at an 8-cm distance for the others. When considered along with the irradiance factor, the 4-corners arrangement at a distance of 4 cm showed higher intensity than an 8-cm distance with the other arrangements, so it was selected as the most effective irradiation design.
As shown in Fig. 5a, the intensities of LP-UV lamps reached their maximum range after 5 min or more, so there was a possibility of a delayed disinfecting effect, but in the case of DUV-LEDs, high intensity was measured from the outset, and it was consistently maintained. For these reasons, LP lamp warm-up time could be eliminated by switching to DUV-LED technology. Figure 5b shows that another benefit of DUV-LED technology is the wide range of working temperatures, especially at low temperatures around 0 to 4°C. By comparing the DUV-LED intensities at 4°C and room temperature, 4.5% higher irradiance power was measured at the lower temperature. Peak intensity of LP lamps was observed at room temperature and decreased to about 62.5% at 4°C. Similar results have been reported by Crawford et al. (24). Numerous psychrophilic microorganisms are capable of growing at low temperatures, particularly L. monocytogenes, which is one of the major foodborne pathogens known to grow under refrigeration (25). For inactivating these bacteria, DUV-LEDs may therefore be more appropriate than LP lamps.
Log reductions of all three pathogens showed a tendency to increase as the applied UV energy increased. L. monocytogenes, a Gram-positive bacterium, had more resistance to UV radiation than Gram-negative bacteria, such as E. coli O157:H7 and S. Typhimurium. This added resistance may be due to the thick peptidoglycan wall that surrounds the cytoplasmic membrane in Gram-positive bacteria, whereas Gram-negative bacteria possess only an external membrane (26). Many research studies investigating the sanitization of foodborne pathogens in various foods report that L. monocytogenes is considered to be one of the most UV-resistant bacteria (27, 28, 29).
Most of the UV-related studies have focused on the storage temperature following UV treatment (30, 31). Along with storage temperature, treatment temperature is one of the key factors. In the present study, the treatment temperature had a profound effect on inactivation of E. coli O157:H7 and S. Typhimurium. When the temperature increased from 0 to 37°C, log reductions of both pathogens increased. However, S. Typhimurium produced significant numbers of injured cells when the treatment temperatures exceeded 4°C, whereas E. coli O157:H7 and L. monocytogenes experienced no formation of injured cells regardless of the treatment temperature. A related study showed that about 60.73 to 93.14% of S. Typhimurium cells on cherry tomatoes became injured following UV lamp irradiation at 2 to 10 kJ/m2 (32). However, the selective action of sodium desoxycholate in XLD for Salmonella is so powerful that actual live (noninjured) cell populations have a tendency to be underestimated. Therefore, injured cells of S. Typhimurium are assumed to be less prevalent and thus less important.
Although DUV-LEDs emit a higher intensity of radiation at lower temperatures, the inactivation effect showed the opposite results. The enhanced inactivation at higher temperatures might be explained by phase transitioning of the phospholipid molecules that are found in the cell membrane (33). Thayer and Boyd (34) stated that the level of inactivation of bacteria by gamma radiation was directly related to chemical reactions at treatment temperatures, Rather than a direct effect of irradiation, cellular inactivation is due to interactions with radiolytic products of water. This phenomenon can be understood in the context of temperature-dependent UV radiation because of photochemical reactions that can occur as a direct result of UV radiation energy (35). Also, the fluidity of the cell membrane increases with heating, making the affected cells more sensitive to UV exposure (36). On the other hand, the UV treatment temperature did not affect the reduction rate of L. monocytogenes because of its characteristics as a Gram-positive bacterium mentioned above.
DUV-LEDs applied to a batch water system inactivated foodborne pathogens effectively without generating injured cells. In 2009, Lakretz et al. (37) investigated the inactivation efficacy of medium-pressure (MP) UV lamps in a static solution system. The MP lamps generating polychromatic light were filtered with band-pass filters to select the desired peak wavelength. Pseudomonas aeruginosa in phosphate-buffered saline (PBS) was inactivated by 2.5 to 3.5 log units at various wavelengths, which induced a lower level of biofilm formation. Kim et al. (38) observed around 6-log-unit reductions of E. coli, L. monocytogenes, and S. Typhimurium suspended in water with 60-s exposure to UV-TiO2 photocatalytic lamps.
To adopt LED technology commercially, a continuous water treatment system is more practical than a batch system for sterilizing large amounts of water on an ongoing basis, but a continuous-type decontamination system deserves careful examination. In Table 4, reductions of pathogen populations are shown for each flow rate and irradiation intensity. As the flow rate increased, reduction levels of each pathogen decreased because of the shorter UV treatment time. By combining the two factors flow rate and intensity, the inactivation effect in a continuous system can be explained as a function of the UV dosage factor (intensity times treatment time). As a result, the effectiveness of the UV treatment system for inactivating pathogens is ultimately determined by the UV dose.
Conclusions.
Our overall results suggest that conventional LP-UV lamps for the inactivation of foodborne pathogens can be completely replaced with DUV-LED technology. The spectral characteristics of DUV-LEDs, such as fast-stabilizing intensity and insensitivity to temperature, should be regarded as great advantages over LP-UV lamps. DUV-LED use led to effective inactivation of E. coli O157:H7, S. Typhimurium, and L. monocytogenes both on medium surfaces and in water systems under various conditions with minimal generation of injured cells. Furthermore, to date, this is the first report of the direct application of DUV-LED technology for the inactivation of foodborne pathogens. In addition, water treatment by DUV-LEDs in large capacities and at high flow rates has not been previously reported. DUV-LEDs could be a very promising alternative technology for UV irradiation in the field of controlling foodborne pathogens. Moreover, application of this technology to actual food samples and comparison of disinfection effectiveness between types of UV lamps must be studied in the future.
ACKNOWLEDGMENTS
This research was supported by the Public Welfare and Safety Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (grant 2012M3A2A1051679). The research was also supported by the Agriculture, Food and Rural Affairs Research Center Support Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
REFERENCES
- 1.Scharff RL. 2012. Economic burden from health losses due to foodborne illness in the United States. J Food Prot 75:123–131. doi: 10.4315/0362-028X.JFP-11-058. [DOI] [PubMed] [Google Scholar]
- 2.Sodha SV, Heiman K, Gould LH, Bishop R, Iwamoto M, Swerdlow DL, Griffin PM. 2015. National patterns of Escherichia coli O157 infections, USA, 1996-2011. Epidemiol Infect 143:267–273. doi: 10.1017/S0950268814000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zansky S, Wallace B, Schoonmaker-Bopp D, Smith P, Ramsey F, Painter J. 2002. Outbreak of multidrug-resistant Salmonella Newport—United States, January–April 2002. JAMA 288:951–953. [PubMed] [Google Scholar]
- 4.Datta AR. 2003. International handbook of foodborne pathogens. Marcel Dekker, New York, NY. [Google Scholar]
- 5.Guerrero-Beltrán JA, Barbosa-Cánovas GV. 2004. Advantages and limitations on processing foods by UV light. Food Sci Technol Int 10:137–148. doi: 10.1177/1082013204044359. [DOI] [Google Scholar]
- 6.Bintsis T, Tzanetaki EL, Robinson RK. 2000. Existing and potential applications of ultraviolet light in the food industry—a critical review. J Sci Food Agric 80:637–645. doi:. [DOI] [PubMed] [Google Scholar]
- 7.Yaun BR, Sumner SS, Eifert JD, Marcy JE. 2004. Inhibition of pathogens on fresh produce by ultraviolet energy. Int J Food Microbiol 90:1–8. doi: 10.1016/S0168-1605(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 8.Franz CMAP, Specht I, Cho G, Graef V, Stah MR. 2009. UV-C inactivation of microorganisms in naturally cloudy apple juice using novel inactivation equipment based on Dean vortex technology. Food Control 20:1103–1107. doi: 10.1016/j.foodcont.2009.02.010. [DOI] [Google Scholar]
- 9.Lopez-Malo A, Palou E. 2005. Novel food processing technologies. CRC Press, Boca Raton, FL. [Google Scholar]
- 10.US Food and Drug Administration. 2000. Ultraviolet radiation for the processing and treatment of food. Code of Federal Regulations 21 (Part 179.39). FDA, Washington, DC. [Google Scholar]
- 11.Bowker C, Sain A, Shatalov M, Ducoste J. 2011. Microbial UV fluence-response assessment using a novel UV-LED collimated beam system. Water Res 45:2011–2019. doi: 10.1016/j.watres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Bettles T, Schujman S, Smart JA, Liu W, Schowalter L. 2007. UV light emitting diodes; their applications and benefits. Int Ultraviolet Assoc News 9:11–15. [Google Scholar]
- 13.Hamamoto A, Mori M, Takahashi A, Nakano M, Wakikawa N, Akutagawa M, Kinouchi Y. 2007. New water disinfection system using UVA light-emitting diodes. J Appl Microbiol 103:2291–2298. doi: 10.1111/j.1365-2672.2007.03464.x. [DOI] [PubMed] [Google Scholar]
- 14.Chatterley C, Linden K. 2010. Demonstration and evaluation of germicidal UV-LEDs for point-of-use water disinfection. J Water Health 8:479–486. doi: 10.2166/wh.2010.124. [DOI] [PubMed] [Google Scholar]
- 15.Würtele MA, Kolbe T, Lipsz M, Külberg A, Weyers M, Kneissl M, Jekel M. 2011. Application of GaN-based ultraviolet-C light emitting diodes—UV LEDs—for water disinfection. Water Res 45:1481–1489. doi: 10.1016/j.watres.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Oguma K, Kita R, Sakai H, Murakami M, Takizawa S. 2013. Application of UV light emitting diodes to batch and flow-through water disinfection systems. Desalination 328:24–30. doi: 10.1016/j.desal.2013.08.014. [DOI] [Google Scholar]
- 17.Bolton JR, Linden KG. 2003. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J Environ Eng 129:2009–2215. [Google Scholar]
- 18.Lee SY, Kang DH. 2001. Suitability of overlay method for recovery of heat-injured Listeria monocytogenes and Salmonella Typhimurium. Food Sci Biotechnol 10:323–326. [Google Scholar]
- 19.Rhee MS, Lee SY, Hiller VN, McCurdy SM, Kang DH. 2003. Evaluation of consumer-style cooking methods for reduction of Escherichia coli O157:H7 in ground beef. J Food Prot 66:1030–1034. [DOI] [PubMed] [Google Scholar]
- 20.Caminiti IM, Palgan I, Muñoz A, Noci F, Whyte P, Morgan DJ, Cronin DA, Lyng JG. 2012. The effect of ultraviolet light on microbial inactivation and quality attributes of apple juice. Food Bioprocess Technol 5:680–686. doi: 10.1007/s11947-010-0365-x. [DOI] [Google Scholar]
- 21.Kim YH, Jeong SG, Back KH, Park KH, Chung MS, Kang DH. 2013. Effect of various conditions on inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in fresh-cut lettuce using ultraviolet radiation. Int J Food Microbiol 166:349–355. doi: 10.1016/j.ijfoodmicro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Kowalski W. 2009. Ultraviolet germicidal irradiation handbook: UVGI for air and surface disinfection. Springer, New York, NY. [Google Scholar]
- 23.US Environmental Protection Agency. 2006. Ultraviolet disinfection guidance manual. EPA 815-R-06-007. Office of Water, US Environmental Protection Agency, Washington, DC. [Google Scholar]
- 24.Crawford MH, Banas MA, Ross MP, Ruby DS, Nelson JS, Boucher A, Allerman AA. 2005. Final LDRD report: ultraviolet water purification systems for rural environments and mobile applications. Sandia report. US Department of Energy, Washington, DC. [Google Scholar]
- 25.Azevedo I, Regalo M, Mena C, Almeida G, Carneiro L, Teixeira P, Hogg T, Gibbs PA. 2005. Incidence of Listeria spp. in domestic refrigerators in Portugal. Food Control 16:121–124. doi: 10.1016/j.foodcont.2003.12.006. [DOI] [Google Scholar]
- 26.Virto R, Manas P, Alvarez I, Condon S, Raso J. 2005. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl Environ Microbiol 71:5022–5028. doi: 10.1128/AEM.71.9.5022-5028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu G, Li C, Liu P. 2011. UV inactivation of milk-related microorganisms with a novel electrodeless lamp apparatus. Eur Food Res Technol 233:79–87. doi: 10.1007/s00217-011-1498-5. [DOI] [Google Scholar]
- 28.Guerrero-Beltran JA, Barbosa-Canovas GV. 2005. Reduction of Saccharomyces cerevisiae, Escherichia coli and Listeria innocua in apple juice by ultraviolet light. J Food Process Eng 28:437–452. doi: 10.1111/j.1745-4530.2005.00040.x. [DOI] [Google Scholar]
- 29.Gabriel AA, Nakano H. 2009. Inactivation of Salmonella, E. coli and Listeria monocytogenes in phosphate-buffered saline and apple juice by ultraviolet and heat treatments. Food Control 20:443–446. doi: 10.1016/j.foodcont.2008.08.008. [DOI] [Google Scholar]
- 30.Lemoine ML, Civello PM, Martinez GA, Chaves AR. 2007. Influence of postharvest UV-C treatment on refrigerated storage of minimally processed broccoli (Brassica oleracea var. Italica). J Sci Food Agric 87:1132–1139. doi: 10.1002/jsfa.2826. [DOI] [Google Scholar]
- 31.Gonzalez-Aguilar G, Wang CY, Buta GJ. 2004. UV-C irradiation reduces breakdown and chilling injury of peaches during cold storage. J Sci Food Agric 84:415–422. doi: 10.1002/jsfa.1675. [DOI] [Google Scholar]
- 32.Choi DS, Park SH, Choi SR, Kim JS, Chun HH. 2015. The combined effects of ultraviolet-C irradiation and modified atmosphere packaging for inactivating Salmonella enterica serovar Typhimurium and extending the shelf life of cherry tomatoes during cold storage. Food Packaging Shelf Life 3:19–30. doi: 10.1016/j.fpsl.2014.10.005. [DOI] [Google Scholar]
- 33.Jayaram S, Castle GSP, Margaritis A. 1992. Kinetics of sterilization of Lactobacillus brevis cells by the application of high voltage pulses. Biotechnol Bioeng 40:1412–1420. doi: 10.1002/bit.260401116. [DOI] [PubMed] [Google Scholar]
- 34.Thayer DW, Boyd G. 1995. Radiation sensitivity of Listeria monocytogenes on beef as affected by temperature. J Food Sci 60:237–240. doi: 10.1111/j.1365-2621.1995.tb05645.x. [DOI] [Google Scholar]
- 35.Gayán E, Mañas P, Álvarez I, Condón S. 2013. Mechanism of the synergistic inactivation of Escherichia coli by UV-C light at mild temperatures. Appl Environ Microbiol 79:4465–4473. doi: 10.1128/AEM.00623-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gayán E, Condón S, Álvarez I. 2014. Biological aspects in food preservation by ultraviolet light: a review. Food Bioprocess Technol 7:1–20. doi: 10.1007/s11947-013-1168-7. [DOI] [Google Scholar]
- 37.Lakretz A, Ronb EZ, Mamane H. 2010. Biofouling control in water by various UVC wavelengths and doses. Biofouling 26:257–267. doi: 10.1080/08927010903484154. [DOI] [PubMed] [Google Scholar]
- 38.Kim S, Ghafoor K, Lee J, Feng M, Hong J, Lee DU, Park J. 2013. Bacterial inactivation in water, DNA strand breaking, and membrane damage induced by ultraviolet-assisted titanium dioxide photocatalysis. Water Res 47:4403–4411. doi: 10.1016/j.watres.2013.05.009. [DOI] [PubMed] [Google Scholar]