Abstract
Solar disinfection (SODIS) of drinking water in polyethylene terephthalate (PET) bottles is a simple, efficient point-of-use technique for the inactivation of many bacterial pathogens. In contrast, the efficiency of SODIS against viruses is not well known. In this work, we studied the inactivation of bacteriophages (MS2 and ϕX174) and human viruses (echovirus 11 and adenovirus type 2) by SODIS. We conducted experiments in PET bottles exposed to (simulated) sunlight at different temperatures (15, 22, 26, and 40°C) and in water sources of diverse compositions and origins (India and Switzerland). Good inactivation of MS2 (>6-log inactivation after exposure to a total fluence of 1.34 kJ/cm2) was achieved in Swiss tap water at 22°C, while less-efficient inactivation was observed in Indian waters and for echovirus (1.5-log inactivation at the same fluence). The DNA viruses studied, ϕX174 and adenovirus, were resistant to SODIS, and the inactivation observed was equivalent to that occurring in the dark. High temperatures enhanced MS2 inactivation substantially; at 40°C, 3-log inactivation was achieved in Swiss tap water after exposure to a fluence of only 0.18 kJ/cm2. Overall, our findings demonstrate that SODIS may reduce the load of single-stranded RNA (ssRNA) viruses, such as echoviruses, particularly at high temperatures and in photoreactive matrices. In contrast, complementary measures may be needed to ensure efficient inactivation during SODIS of DNA viruses resistant to oxidation.
INTRODUCTION
Access to an improved source of drinking water is compromised for about 11% of the world's population (approximately 800 million people), while more than 1.8 million people use water that is unsafe (1). This dramatic situation may worsen globally in the future, partly due to climatic change and human demographic growth (2, 3). Inevitably, consumption of contaminated water leads to many fecal-oral infections, such as gastroenteritis caused by bacteria, viruses, or parasites. According to the United Nations, more than 20% of diarrheal cases could be prevented by introducing effective interventions to increase water quality at the distribution sources or point-of-use interventions within households (4). It is desirable that point-of-use treatments be low cost, easy to apply, and sustainable. Among other strategies, such as boiling, chlorination, and filtration, solar disinfection (SODIS) has been used in recent decades as a very cheap, clean, and simple method for improving the microbial quality of drinking water in many developing countries (5, 6).
SODIS is a simple treatment strategy that harnesses the antimicrobial effects of sunlight. Specifically, transparent containers, typically bottles made of polyethylene terephthalate (PET), are filled with water and are then exposed to sunlight for one full day (6 h of sunshine, including midday hours), or for two consecutive days under cloudy conditions (5). Additional interventions may be implemented to enhance the efficiency of the overall process. For instance, turbid water may be filtered prior to solar exposure, or bottles may be placed on a refracting surface (such as aluminum, or a roof made with corrugated iron sheets) (5, 7, 8). In addition to disinfecting the water, SODIS has the benefit of eliminating the need for a separate, potentially contaminated storage container.
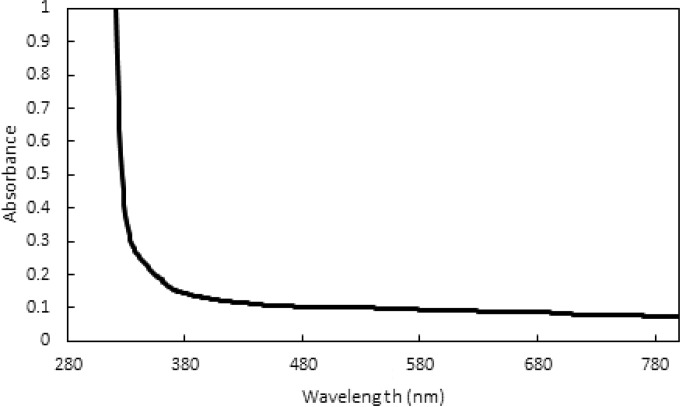
Generally, microorganisms can be inactivated by sunlight through two processes: direct and indirect photoinactivation. In direct inactivation, light in the solar UVB range (290 to 320 nm) is absorbed by DNA or RNA genomes, causing the formation of various photoproducts that may block the replication of the damaged microorganisms (9, 10). However, transparent PET containers do not transmit light in the UVB region (Fig. 1), eliminating the possibility of a contribution of direct inactivation to SODIS. In contrast, indirect photoinactivation can also be triggered by light in the UVA and visible ranges (>320 nm), which can penetrate PET bottles to a greater extent. Indirect inactivation is initiated by the light excitation of chromophores. Excited chromophores can then react with solution constituents, mainly dissolved oxygen, to form reactive (oxygen) species (RS). RS, as well as the excited chromophores themselves, can inactivate microorganisms by oxidizing vital constituents necessary for microbial infectivity. Generally, bacteria contain endogenous chromophores which, upon irradiance by light, act as sensitizers for the formation of RS. RS are therefore produced in the immediate vicinity of vital bacterial constituents, and thus, indirect endogenous inactivation is often very efficient against bacteria. In contrast, enteric viruses simply consist of a genome and a protein capsid and rarely contain endogenous chromophores capable of absorbing light in the UVA/visible range. Instead, indirect photoinactivation of viruses can depend entirely on the presence of an exogenous sensitizer in the water (11). For example, organic matter (OM), nitrate, nitrite, and iron-containing solution constituents can absorb light and produce RS such as superoxide (O2−), hydrogen peroxide (H2O2), singlet oxygen (1O2), and the hydroxyl radical (OH·).
FIG 1.
Absorbance of the 0.5-liter PET bottles used in this study over a wavelength range from 280 to 800 nm.
SODIS has proven to be efficient at inactivating a wide range of bacteria under laboratory and field conditions and, more importantly, at reducing the number of diarrhea cases in field surveys (5). However, studies on the inactivation of enteric viruses by SODIS are scarce, and even fewer studies have been conducted in PET bottles, simulating actual SODIS conditions (7, 12). The available data are further complicated by the facts that (i) standardized experimental procedures for the study of SODIS are lacking and (ii) the solution conditions promoting virus inactivation by SODIS are mostly unknown and hence are not reported. Reports published by different laboratories are therefore only poorly comparable. Nevertheless, previous publications support the notion that SODIS is less efficient for viruses than for bacteria (8, 12–14).
The aims of this study were to characterize the efficacy of SODIS for the inactivation of viruses in drinking water under different simulated field conditions by using PET bottles and to evaluate the influence of viral and environmental factors on the success of the treatment. For this purpose, we conducted our experiments in drinking water of diverse compositions collected from Chennai, India, and Lausanne, Switzerland, and at increasing temperatures (15, 22, 26, and 40°C) under simulated sunlight. Specifically, we studied the efficacy of SODIS against two commonly used surrogates of human viruses (phages MS2 and ϕX174) and two human viruses (human adenoviruses and echoviruses) with different structural and genetic characteristics. Overall, the outcomes of this work enhance our understanding of the efficacy of SODIS for virally contaminated water.
MATERIALS AND METHODS
Virus preparation and enumeration.
Phage MS2 (DSMZ 3767) and ϕX174 (ATCC 13706-B1) stocks were propagated and purified as described previously (15). For both phages, the concentration of the stocks obtained was around 1 × 1013 PFU/ml, and the suspensions were kept in phosphate-buffered saline (PBS) (5 mM phosphate, 10 mM NaCl [pH 7.5]) at 4°C until use. The phages in the suspensions were enumerated during the experiments using the double-layer agar method, as described previously (15). Human adenovirus type 2 (HAdV) stocks (NCPV 00213; kindly provided by Rosina Girones, University of Barcelona) were produced by infecting A549 cells. The cells were grown at 37°C under 5% CO2 with high-glucose Dulbecco's modified Eagle medium (DMEM) (Gibco, Frederick, MD) supplemented with 1% penicillin-streptomycin (Gibco, Frederick, MD) per ml and 10% (growth medium) or 2% (maintenance medium) heat-inactivated fetal bovine serum (FBS) (Gibco, Frederick, MD). Human echovirus 11 (EV) stocks (Gregory strain; ATCC VR737) were produced by infecting BGM cells (kindly provided by Rosina Girones, University of Barcelona). BGM cells were also cultivated at 37°C under 5% CO2 with modified Eagle medium (MEM) (Gibco, Frederick, MD) supplemented with 1% penicillin-streptomycin per ml and 10% (growth medium) or 2% (maintenance medium) heat-inactivated FBS. HAdV and EV were released from infected cells by freezing and thawing the culturing flasks 3 times. A centrifugation step at 3,500 × g for 5 min was applied to eliminate cell debris. The supernatant obtained was membrane filtered (pore size, 0.22 μm; Millipore), and the viruses were washed three times with PBS and were concentrated with 15-ml Amicon Ultra centrifugal filter units (nominal molecular weight limit, 100,000 Da). To quantify these viruses, a most probable number (MPN) assay was used. Briefly, A549 or BGM cells were grown to 95% confluence in flat-bottom 96-well plates (Greiner Cellstar; Sigma-Aldrich). Then HAdV or EV samples were diluted over a 10-fold dilution series in DMEM or MEM, respectively, supplemented with 2% FBS. The medium in each well was discarded and was replaced with 150 μl of the diluted sample. Infected plates were then incubated for 7 or 5 days, respectively, and the presence of a cytopathic effect was observed by inverted microscopy in order to differentiate between infected (positive) and noninfected (negative) wells. HAdV and EV concentrations were then determined as the most probable number of cytopathic units (MPNCU) per milliliter by using an MPN table. The stocks obtained (~108 MPNCU/ml) were stored at 4°C until use.
Water collection, storage, and analysis.
In order to characterize the influence of water composition on virus inactivation by SODIS, three different water matrices were tested. One was collected from a tap at the EPFL (Lausanne, Switzerland), stirred overnight in an open beaker to volatilize any remaining chlorine, and kept at 4°C over the course of the experiments. Swiss tap water (STW) obtained on different days led to slight differences in the experimental results. Each self-contained set of experiments was therefore conducted in a single batch of water. The other two water matrices were collected in India and were kindly provided by the Sandec team from EAWAG (Dübendorf, Switzerland). One was groundwater, and the other was tap water, collected in the city of Chennai. All Indian water samples were frozen at −20°C until use, although 0.5 liter of each sample was kept at 4°C for immediate experiments.
For each type of water, the total organic carbon (TOC) and inorganic carbon (IC) contents were determined using a Shimadzu TOC-VCPH analyzer. Iron and copper cations were analyzed with a PerkinElmer inductively coupled plasma mass spectrometry (ICP-MS) Elan DRC II device. Anion (chloride, nitrite, bromide, nitrate, phosphate, and sulfate) concentrations were determined using a Dionex ICS 3000 ion chromatograph. In addition, all water types were checked for free chlorine residuals using the N,N-diethyl-p-phenylenediamine (DPD) colorimetric method (16). In all water samples, the concentration of free chlorine was below the detection limit (<0.5 mg/liter). The absorption spectra of the three types of water were measured with a UV-visible (UV-Vis) spectrophotometer (UV-2550; Shimadzu). All types of water absorbed little light above 320 nm (see Fig. S1 in the supplemental material), such that light shielding by the water matrix in our experimental setup was negligible (<5%).
SODIS inactivation experiments.
SODIS experiments were conducted using a solar simulator (Sun 2000; Abet Technologies) equipped with a 1,000-W xenon lamp, an Air Mass (AM) 1.5 filter, and a 2-mm-thick atmospheric edge (AE) filter to mimic the solar radiation spectrum. The fluence rate below the simulator was measured with a spectroradiometer (ILT 900-R; International Light Technologies, Peabody, MA). The average fluence rate, computed for the wavelength range from 280 to 800 nm, was 249 ± 14 W/m2, resulting in fluences of 0.53 kJ/cm2 and 2.1 kJ/cm2 after 6 and 24 h of irradiation, respectively. Two different reactor setups were tested and compared (see Fig. S2 in the supplemental material): specifically, SODIS experiments were conducted either in a PET bottle (0.5 liter) filled with 500 ml of a water sample or in an open glass beaker (30 ml) filled with 20 ml of a water sample and placed inside an empty PET bottle (0.5 liter). The absorbance of the PET bottle was measured with a UV-Vis spectrophotometer (UV-2550; Shimadzu). The UVB radiation (<320 nm) emitted by the solar simulator was mostly removed due to the PET bottle absorbance (Fig. 1). The rest of the sunlight spectrum was only slightly affected.
The reactors were spiked with the viruses studied in order to establish initial virus concentrations of 104 to 109 PFU/ml for MS2, 105 to 107 PFU/ml for ϕX174, 106 MPNCU/ml for HAdV, or 105 MPNCU/ml for EV. MS2 was present in all experiments as a process control. Its inactivation kinetics were used to confirm that the presence of other viruses did not significantly alter the light spectrum and hence the SODIS inactivation potential. The containers were placed under the solar simulator and were stirred continuously with a magnetic stirrer at 150 rpm to ensure good mixing. The containers were kept at a constant temperature (22°C, unless specified otherwise) by using a water cooler (Julabo). In each experiment, 150-μl samples were taken approximately every 2 h during light exposure, diluted in 450 μl of PBS, and then stored at 4°C before enumeration on the same day.
Experiments were also performed in PBS to quantify the extent of direct photoinactivation of MS2 during SODIS in PET bottles. Furthermore, experiments without stirring were also conducted in order to study the need for, or interference of, stirring. Finally, dark controls were exposed to the same conditions as the irradiated samples but were shielded from light by wrapping aluminum foil around the glass beakers.
To confirm that the experiments conducted under our solar simulator adequately represented natural conditions, control experiments were conducted under natural sunlight. PET bottles (0.5 liter) were filled with Swiss tap water, and the water was spiked with MS2 to an initial virus concentration of 6 × 106 PFU/ml. The bottle was placed at a 30° angle and was always oriented toward the sun for 6 h (from 10:30 a.m. to 4:30 p.m.) on a clear day in May 2013 in Lausanne, Switzerland. The fluence rate at the PET bottle surface and the temperature inside the bottle were measured every hour. The total fluence after 6 h of irradiation, computed for the wavelength range from 280 to 800 nm, corresponded to 1.34 kJ/cm2 (Fig. 2). The temperature fluctuated between 18 and 32°C throughout the experiment. Samples (150 μl diluted in 450 μl PBS) were withdrawn every hour and were stored at 4°C before enumeration on the same day. A dark control was conducted in an open beaker wrapped in aluminum foil over the same period. The number of experimental replicates conducted for each assay is specified in the figure legends.
FIG 2.
Fluence rates between 200 and 800 nm (A and C) or between 280 and 400 nm (B and D) outside (black lines) or inside (red lines) the PET bottles used in this study under natural sunlight (A and B) as measured on 27 March 2013 at 2:30 p.m. in Lausanne, Switzerland (46°52′N, 6°57′E), or under the solar simulator (C and D). The internal fluence rate (red lines) was determined by correcting the outside fluence rate (black lines) by the absorbance of the PET bottle (Fig. 1).
Data analysis.
Virus inactivation was analyzed by fitting the data obtained to a first-order decay model as follows:
| (1) |
where C0 is the initial infective virus concentration, C is the remaining infective virus concentration after irradiation time t (in hours), and kvirus is the time-based first-order inactivation rate constant (per hour). The remaining virus concentration C can also be expressed as function of the fluence F (in kilojoules per square centimeter) received at the surface of the PET bottle; here, κvirus (in square centimeters per kilojoule) is the fluence-based inactivation rate constant. Note that in equation 1 and throughout this article, “virus” refers to MS2, ϕX174, HAdV, or EV.
Furthermore, to account for SODIS-independent inactivation, we quantified the first-order inactivation rate constants in the dark controls (kdark, virus). The light-specific contribution to SODIS (klight, virus or κlight, virus) could then be determined by subtracting the dark contribution from the total inactivation rate constant (kvirus or κvirus).
The inactivation rate constants and the corresponding 95% confidence intervals were determined using Prism (version 5.0, 2007; GraphPad Software). The observed inactivation rate constants were compared by analysis of covariance (ANCOVA) in Prism.
RESULTS AND DISCUSSION
Development of an experimental setup for the study of SODIS. (i) Comparison of MS2 inactivation under natural and simulated sunlight.
In order to ensure that our simulated sunlight was representative of the inactivation occurring outdoors, simultaneous experiments were conducted under natural sunlight and under the solar simulator. Specifically, a 0.5-liter PET bottle was filled with Swiss tap water spiked with MS2 and was exposed either to natural sunlight or to the solar simulator. During equivalent exposure times, the extent of MS2 inactivation was higher in the PET bottle exposed to natural sunlight (5-log inactivation in 6 h) than in the bottles exposed to simulated sunlight (2-log inactivation in 6 h). This could be attributed to the greater fluence rate of natural sunlight in Lausanne at the time of the experiment (on average, 650 W/m2) than of the solar simulator (249 ± 14 W/m2) (Fig. 2). Accordingly, when determined as a function of fluence rather than exposure time, the MS2 inactivation rate constants were statistically equivalent (κMS2 = 5.95 ± 1.24 cm2/kJ under the simulator and 6.57 ± 2.84 cm2/kJ under natural sunlight [P = 0.33]). These results suggest that the solar simulator is a good tool with which to represent inactivation under natural sunlight, despite the differences in the solar spectrum (Fig. 2) and the temperature regimen (a constant 22°C under the simulator; fluctuation between 18 and 32°C under natural conditions).
Such differences between time-based and fluence-based data analysis in SODIS experiments highlight the need to report inactivation rate constants in SODIS experiments in terms of the fluence received by the samples rather than in terms of the duration of sunlight exposure. A fluence-based analysis also facilitates the comparison of SODIS results obtained in different laboratories under different experimental or field conditions. Finally, since the fluence of sunlight varies with latitude, the geographic position where SODIS is applied will influence the exposure time needed to reach a certain extent of inactivation but not the inherent sensitivity of the virus to inactivation.
(ii) Comparison of MS2 inactivation by sunlight in PET bottles and in glass beakers.
The use of an actual PET bottle during SODIS experiments is key for adequate representation of SODIS in the field, since PET absorbs light in the UVB range (Fig. 1) and hence blocks the most efficient wavelength contributing to the photoinactivation of pathogens. However, the use of PET bottles involves conducting laboratory experiments in large volumes of water (at least 500 ml for each experimental condition) and thus requires the preparation and handling of large quantities of viruses as well. To date, therefore, only a few investigators have conducted SODIS experiments using PET bottles such as those typically used in the field (7, 12). To circumvent the need for large volumes, we compared the inactivation of MS2 in 0.5-liter PET bottles with that in 20-ml glass beakers placed inside 0.5-liter PET bottles exposed to our solar simulator. During equivalent exposure times, MS2 inactivation in PET bottles (κMS2 = 6.40 ± 2.13 cm2/kJ) was equivalent to that in glass beakers placed inside PET bottles (κMS2 = 6.45 ± 1.24 cm2/kJ) (P = 0.94). Thus, further assays were conducted using 20-ml glass beakers placed inside 0.5-liter PET bottles.
(iii) Influence of stirring on MS2 inactivation by SODIS.
We conducted additional experiments under the solar simulator in order to further identify potential deviations of our setup from field conditions. While in SODIS applications, water in PET bottles is rarely stirred or mixed when the bottles are exposed to sunlight, in the experimental setup used here, the water was continuously stirred by means of a magnetic stir bar. Therefore, we conducted an experiment to compare the inactivation of MS2 in a stirred and a nonstirred PET bottle. The inactivation rate constants obtained were statistically equivalent in this experiment (κMS2 = 9.23 ± 2.23 cm2/kJ in the stirred bottle and 8.91 ± 1.47 cm2/kJ in the unstirred bottle [P = 0.63]). These results indicate that stirring had no influence on MS2 inactivation in our SODIS experiments. Hence, to ensure good homogeneity of the virus concentration in the beakers and PET bottles, our experiments were always conducted under magnetic stirring.
(iv) Influence of initial MS2 concentrations on SODIS.
The virus concentrations used in this study were higher than those typically encountered in the field, in order to allow the monitoring of virus inactivation over several orders of magnitude. To ensure that the high concentrations applied did not cause any experimental artifacts, we investigated the effects of different initial virus concentrations on virus inactivation kinetics. Specifically, a high initial virus concentration may be accompanied by high concentrations of cell culture impurities, which may influence the steady-state concentration of RS and hence the extent of inactivation. This issue became particularly apparent when a nonpurified MS2 virus stock was used for preliminary SODIS experiments conducted in our laboratory. This virus stock led to 3-fold-slower inactivation of MS2 than that in a purified stock with the same virus concentration (P < 0.001) (see Fig. S3 in the supplemental material). However, the inactivation rate constants obtained in experiments with different MS2 starting concentrations (5 × 105, 5 × 107, and 5 × 109 PFU/ml) were statistically equivalent (P = 0.86). These results indicate that within the range of conditions tested, the initial virus concentration did not influence the inactivation rate constants observed during SODIS (see Fig. S3). Thus, all subsequent assays were conducted using initial virus concentrations of 5 × 107 PFU/ml or MPNCU/ml or less.
Efficacy of SODIS against RNA and DNA viruses.
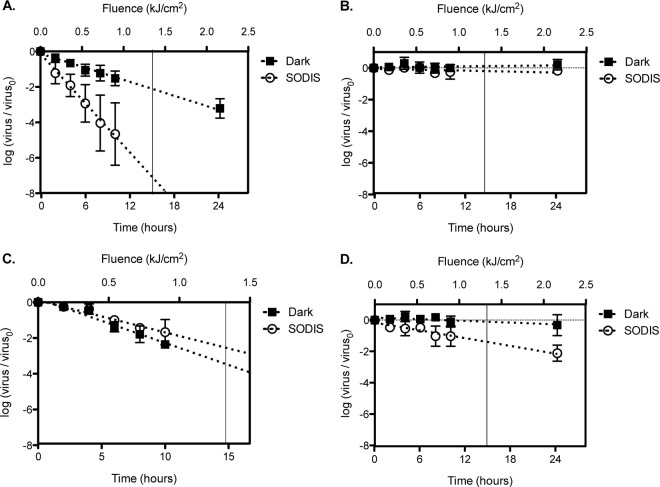
In order to characterize the efficacies of SODIS against different viruses, experiments with either an RNA virus (MS2 and EV) or a DNA virus (bacteriophage ϕX174 and HAdV) were conducted in Swiss tap water at 22°C. The inactivation curves obtained, along with the results for dark controls, are shown in Fig. 3. The corresponding rate constants are compiled in Table S1 in the supplemental material. In addition to discussing the observed inactivation trends, we specifically report the viral inactivation results obtained at a fluence of 1.34 kJ/cm2. This fluence corresponds to the SODIS-recommended 6 h of sunlight exposure on a sunny day in May in Lausanne, Switzerland (Fig. 2A).
FIG 3.
Inactivation kinetics of bacteriophages MS2 (A) and ϕX174 (B) and human viruses HAdV (C) and EV (D). The experiments were conducted in Swiss tap water at 22°C in 0.5-liter PET bottles that were either kept in the dark or exposed to SODIS. Exposure time is shown along the lower x axis, whereas the equivalent fluence for light-exposed bottles is shown along the upper x axis. MS2 results were obtained from six different experimental replicates. The results for the other viruses were obtained from two different experimental replicates and in the presence of MS2 as a process control. The vertical line in each panel marks the fluence of 1.34 kJ/cm2.
(i) SODIS against bacteriophage MS2.
We extensively characterized MS2 inactivation by SODIS in the absence of other viruses. Based on the data shown in Fig. 3A, a total decay of >6 log units can be extrapolated for a fluence of 1.34 kJ/cm2 (κMS2 = 11.91 ± 0.32 cm2/kJ). In the dark controls, only 2-log inactivation was observed after an equivalent exposure time. Previous work has shown that MS2 bacteriophages are readily inactivated by reactive species (17, 18). Here, since PET bottles block most UVB radiation, we expect that MS2 inactivation is due strictly to indirect photoinactivation mediated by sensitizers present in the water. In a previous study conducted with tap water in PET bottles, Harding and Schwab reported MS2 inactivation of 1.4 log units after 2.5 h of exposure to natural sunlight (7). Unfortunately, the actual fluence rate in these experiments was not recorded, and thus, these results are not directly comparable to ours. However, the solar simulator used here produces a lower fluence rate than natural sunlight (Fig. 2). We can therefore conclude that the Swiss tap water used in our experiments was more photoreactive than the water matrix used by Harding and Schwab, resulting in similar inactivation rates despite the lower fluence rate.
(ii) SODIS against bacteriophage ϕX174.
In contrast to the MS2 results, no ϕX174 inactivation was observed after exposure to a fluence of 1.34 kJ/cm2 (Fig. 3B). The inactivation rate constant for ϕX174 (κϕX174 = 0.25 ± 0.11 cm2/kJ) did not differ statistically from zero (P = 0.32) and was equivalent to the inactivation rate constant observed in dark experiments (P = 0.26). These results are consistent with previous work demonstrating that ϕX174 is more resistant to oxidation by RS than MS2 (18). The unfortunate implication of this result is that solar disinfection in containers that cut off UVB, such as PET bottles, may not be sufficient to inactivate viruses that are relatively resistant against oxidants, as is ϕX174.
(iii) SODIS against human adenoviruses.
HAdVs are large double-stranded DNA pathogens (Table 1) that cause a wide variety of infections and are widely prevalent in the environment (19–21). They have been suggested to be indicators of human fecal contamination and have been included in many disinfection studies as a process control (22–27). From our data, ∼3-log inactivation would occur at a fluence of 1.34 kJ/cm2 (κHAdV = 4.58 ± 0.26 cm2/kJ) (Fig. 3C). Interestingly, the inactivation rate constant for HAdV observed in the dark controls was similar to that observed under simulated sunlight (P = 0.064). This indicates that exposure to light did not contribute significantly to the observed inactivation of HAdV during SODIS. A similar result was obtained by Silverman et al., who observed equivalent inactivation rate constants for HAdV in water from the Tijuana River estuary exposed to simulated sunlight with a cutoff in the UVB region and the same water kept in the dark (kHAdV = 0.11 ± 0.03 h−1; kdark, HAdV = 0.087 ± 0.075 h−1) (28). Remarkably, HAdVs and MS2 have been shown to be similarly susceptible to RS (17). The negligible importance of photoinactivation to the overall HAdV inactivation was therefore unexpected. A possible explanation lies in the magnitude of light-independent processes of HAdV inactivation in this study, as well as in the study by Silverman et al. In the tap water used here, HAdV exhibited the most rapid inactivation in the dark of all the viruses studied (Fig. 3A to D). It thus appears that the light-independent inactivation of HAdV masks the effects of direct or indirect photoinactivation in our experimental system. In contrast, in previous work by our group using a different batch of Swiss tap water than the one tested herein, we observed a very low rate of dark inactivation and consequently a measurable effect of simulated sunlight on HAdV inactivation in a PET bottle (25). Similarly, Carratalà et al. observed a 2-log decay of HAdV in mineral water exposed to UVA and visible light for 24 h, while inactivation in the dark was negligible (23). Combined, these data suggest that HAdV inactivation can be strongly influenced by the experimental system used, and the resulting data should be interpreted accordingly.
TABLE 1.
Main properties of the viruses studied
| Virus | Host | Virion size (nm) | Genome type | Genome size (nt) | Capsid thickness (Å)a |
|---|---|---|---|---|---|
| MS2 | E. coli | 27 | Single-stranded RNA | 3,569 | 39 |
| ϕX174 | E. coli | 27 | Single-stranded DNA | 5,386 | 75 |
| Echovirus 11 | Human | 24–30 | Single-stranded RNA | 7,438 | 63 |
| Human adenovirus 2 | Human | 90–100, with spikes | Double-stranded DNA | 35,937 | 102 |
The capsid thickness of each virus was calculated using outer and inner measures of particle radius available at VIPERdb (http://viperdb.scripps.edu/).
(iv) SODIS against human echoviruses.
Echoviruses are single-stranded RNA enteroviruses belonging to the family Picornaviridae (Table 1). Enteroviruses are widely prevalent in the environment and cause a wide range of infections, from mild diarrhea to meningitis (29, 30). As shown in Fig. 3D, EV was sensitive to SODIS. In contrast to dark controls, where no significant inactivation was observed (P = 0.098), ∼1.5-log inactivation was achieved under SODIS at a fluence of 1.34 kJ/cm2 (κEV = 2.11 ± 0.15 cm2/kJ). Silverman et al. investigated the inactivation of poliovirus (PV), a structurally similar enterovirus, in various coastal waters containing natural photosensitizers (28). Those authors observed different inactivation rate constants for each water matrix investigated; the highest inactivation observed was 0.4 log unit for 6 h of exposure to a solar simulator with a UVB-blocking filter and a fluence rate of 187 W/m2 (kPV = 0.16 ± 0.01 h−1; κPV = 2.38 ± 0.15 cm2/kJ). This fluence-based inactivation rate constant is similar to that observed in our experiments with EV. It should also be noted, however, that the fluences in the two studies were computed for slightly different wavelength ranges, which may in part explain the differing inactivation rates for these two enteroviruses.
(v) Virus properties governing SODIS susceptibility.
Minor differences in structure and genetic composition are known to influence virus inactivation by different disinfectants (17, 31). Here the observed susceptibility to RS was greatest for MS2, intermediate for EV, and lowest for HAdV and ϕX174 (with approximately similar susceptibilities). While further research is required to identify the specific structural determinants of virus inactivation and resistance to RS, our results indicate that the single-stranded RNA viruses studied are more susceptible to oxidants than the DNA viruses. Notably, genome type is related to virus capsid thickness and genome package density, and these physical parameters have been reported previously to account for differences in virus mortality (32, 33). Due to their electrostatic interactions, DNA genomes are contained within virus capsids at higher pressures than single-stranded RNA genomes; therefore, DNA viruses generally have thicker capsids. It is conceivable that thicker capsids could protect virus genomes against oxidants by reducing the access and penetration of exogenous sensitizers and RS into the virion. As shown in Table 1, the capsids of MS2 and EV (39 and 63 Å, respectively) are thinner than those of ϕX174 and HAdV (75 and 102 Å, respectively) (34). Thus, our results are consistent with the hypothesis that viruses with thicker capsids may be less susceptible to oxidation by RS during SODIS. This hypothesis is supported by other work on the solar disinfection of rotaviruses (35). Rotaviruses are important enteric pathogens with a double-stranded RNA genome and a structure consisting of three complex concentric capsids that confers a capsid thickness of 390 Å (34). Only minor inactivation (a <1-log reduction over 12 h) of rotavirus was observed upon exposure to UVA and visible light, in agreement with the notion that thick capsids protect viruses from RS.
An additional explanation for the higher susceptibility of single-stranded RNA viruses than DNA viruses to RS is the fact that double-stranded DNA viruses, such as human adenoviruses, may employ their host replication machinery to repair genome damage (10). However, repair of DNA viruses within their hosts does not protect against potential protein damage caused by the RS generated during SODIS.
Effect of the water matrix on the efficacy of SODIS against MS2.
To demonstrate the drastic effects of the water matrix on SODIS efficiency, virus inactivation in three different types of water matrices, and in PBS, were compared. PBS does not contain sensitizers, so no indirect exogenous inactivation could occur. Furthermore, because the experiments were conducted in UVB-blocking PET bottles, direct inactivation of MS2 was also prevented. The only possible inactivation mechanism was therefore indirect endogenous inactivation initiated by light in the UVA/visible range. However, no significant MS2 inactivation was observed in PBS (Fig. 4), confirming the assumption stated in the introduction that indirect endogenous inactivation does not contribute to MS2 inactivation in PET bottles. From this PBS experiment, we can thus deduce that neither direct nor indirect endogenous inactivation of MS2 occurs in PET bottles. If MS2 inactivation by SODIS is nevertheless observed, it must be due to exogenous inactivation induced by sensitizers present in the matrix.
FIG 4.
Effect of water composition on MS2 inactivation rate constants in SODIS experiments. Triplicate experiments were conducted in beakers filled with Swiss tap water (STW), Indian groundwater (IGW), or Indian tap water (ITW). PBS results were obtained in a single experimental replicate. All experiments were conducted by placing the beakers inside 0.5-liter PET bottles and exposing them to the solar simulator at 22°C. Error bars represent standard deviations.
Waters from different sources contain different amounts and types of exogenous sensitizers. As a result, different photoreactivities and hence different SODIS efficiencies can be expected for different water matrices. Among the three different water matrices tested, MS2 inactivation was most efficient in Swiss tap water. The inactivation rate constant in STW (κMS2 = 11.45 ± 2.45 cm2/kJ) was significantly greater (P < 0.0001) than those in the two water types from India: 3.01 ± 0.26 cm2/kJ in Indian groundwater (IGW) and 0.98 ± 0.72 cm2/kJ in Indian tap water (ITW) (Fig. 4). At a fluence of 1.34 kJ/cm2, 6.7-log, 1.8-log, and 0.6-log reductions in infective virus concentrations were observed in STW, IGW, and ITW, respectively. In comparison, SODIS data obtained in PET bottles by Fisher and colleagues showed 3-log inactivation of MS2 in 33 h, which corresponded to a fluence of 8 kJ/cm2 in their experimental setup (12). They thus observed an inactivation rate constant of 1.35 cm2/kJ for MS2 in wastewater diluted in PBS (40 ml wastewater to 800 ml PBS), which is similar to the rate constants observed in the two Indian waters tested here.
To identify the solution components that determine the efficiency of a water matrix for SODIS of viruses, the compositions of the three water matrices used in this study were characterized (Table 2). In both waters from India, the total organic carbon (TOC) content was higher than that in the water from Switzerland. TOC is a measure of the organic matter (OM) present in the water. OM is an important material that leads to the formation of RS, but it can simultaneously consume such species. Therefore, the lower inactivation rate constants observed for Indian waters may be due to their higher content of OM, which may consume a large proportion of RS in the water. Nitrate can also be a source of hydroxyl radicals when excited by UVB radiation. However, since PET bottles block short-wavelength radiation, this process does not occur, or occurs only minimally, during SODIS. Therefore, the higher nitrate concentration in the ITW did not translate into faster inactivation of MS2 in our experiments.
TABLE 2.
Anion concentrations, total organic carbon, inorganic carbon, and total iron and copper contents of the different waters used in this study
| Compound (unit of measurement)a | Concn in: |
||
|---|---|---|---|
| Swiss tap water | Indian groundwater | Indian tap water | |
| Fe (μg/liter) | 99.6 | 77.8 | Below detection limit |
| Cu (μg/liter) | 7.7 | 4.8 | 7.3 |
| NO3− (mg/liter) | 3.3 | 4.6 | 52.1 |
| SO42− (mg/liter) | 44.0 | 53.3 | 91.3 |
| Cl− (mg/liter) | 10.7 | 92.9 | 81.1 |
| Br− (mg/liter) | Below detection limit | 0.1 | 0.1 |
| NO2− (mg/liter) | Below detection limit | Below detection limit | Below detection limit |
| PO43− (mg/liter) | Below detection limit | Below detection limit | Below detection limit |
| TOC (mg/liter) | 1.8 | 8.4 | 4.7 |
| IC (mg/liter) | 33.3 | 56.4 | |
TOC, total organic carbon; IC, inorganic carbon. The detection limit was 1 μg/liter for iron and copper, 0.4 mg/liter for nitrate, 0.8 mg/liter for sulfate and chloride, 0.1 mg/liter for bromide, and 0.2 mg/liter for nitrite and phosphate.
Both STW and IGW had high levels of iron, whereas the iron content in ITW was below the detection limit (<1 μg/liter). Iron can produce hydroxyl radicals by the decomposition of hydrogen peroxide (formed during the irradiation of OM) and thereby contribute to virus inactivation (36). Hence, in STW and IGW, iron could be a good source of hydroxyl radicals. Since hydroxyl radicals are quickly consumed by OM, the higher content of OM in IGW than in STW could result in lower steady-state concentrations of hydroxyl radicals in IGW. This could explain the faster inactivation in STW than in IGW. This hypothesis, however, is based only on chemical analysis of the waters and needs further investigation.
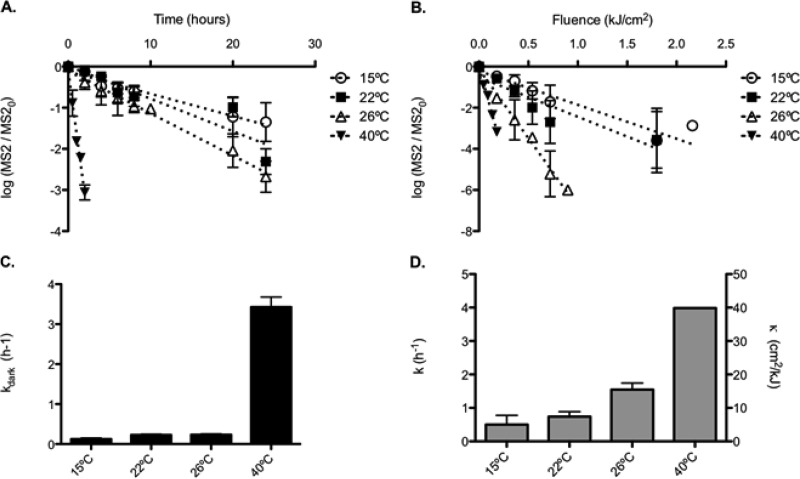
Effect of temperature on the efficacy of SODIS against MS2.
During SODIS, the water temperature in the PET bottle can vary as a function of air temperature and solar irradiance. Temperature changes have been shown previously to affect the exogenous photoinactivation of viruses (35). We therefore evaluated the inactivation of MS2 in Swiss tap water at four different temperatures (15°C, 22°C, 26°C, and 40°C), which span the range of air temperatures most commonly encountered in field applications of SODIS worldwide. As anticipated, inactivation increased with temperature, resulting in inactivation rate constants of 5.00 ± 2.75 cm2/kJ at 15°C, 7.41 ± 1.44 cm2/kJ at 22°C, 15.46 ± 1.99 cm2/kJ at 27°C, and 39.87 ± 0.007 cm2/kJ at 40°C (Fig. 5). After an exposure time of 15 h under the solar simulator, equivalent to a fluence of 1.34 kJ/cm2, this would lead to ∼3-, 4-, 9-, and 23-log inactivation at 15, 22, 26, and 40°C, respectively. These results indicate that temperature plays an important synergistic role during SODIS and may be a key factor to consider for efficient treatment of drinking water to inactivate viral pathogens.
FIG 5.
Effects of temperature on MS2 inactivation curves (A and B) and rate constants (C and D) in experiments conducted in beakers filled with Swiss tap water, placed inside 0.5-liter PET bottles, in the dark (A and C) or under our solar simulator (B and D). In plot D, the left vertical axis represents the observed inactivation rate constant in time units (per hour), and the right vertical axis represents the rate constant in fluence units (see equation 1). Results were obtained in two experimental replicates. Error bars represent standard deviations.
In any water matrix, the temperature dependence of the inactivation of a virus can be described by the Arrhenius relationship:
| (2) |
where κvirus is the virus inactivation rate constant (in square centimeters per kilojoule), A is a preexponential factor (in square centimeters per kilojoule), Ea is the activation energy (in joules per mole), R is the universal gas constant (in joules per kelvin per mole), and T is the absolute temperature (in kelvins). Once such a relationship is established for a given virus and matrix, it allows one to determine the expected level of inactivation at any temperature within the tested range. From the Arrhenius plot established for MS2 and Swiss tap water (see Fig. S4 in the supplemental material), the activation energy (Ea) of the overall processes leading to inactivation in our experiments was 64,313 J/mol, and the preexponential factor A was 2.18 × 1012 cm2/kJ. These values do not reflect actual physical-chemical parameters associated with specific reactions, since the underlying mechanisms are not fully understood at a molecular level. Nevertheless, this information can be used to estimate the inactivation rate constant of MS2 in the water matrix used at a given temperature below 40°C. At higher temperatures, thermal inactivation becomes dominant over inactivation by RS. This observation becomes evident in this study from the results obtained at 40°C, where similar levels of MS2 inactivation were observed in the dark and under the solar simulator. Since our Arrhenius plot is based on data measured at temperatures of <40°C, at which indirect exogenous inactivation exceeds the effect of thermal inactivation, at temperatures above 40°C such predictions may represent a “worst-case” scenario, with inactivation rate constants obtained by considering only oxidation and not thermal inactivation of pathogens. Overall, we expect that SODIS treatments in PET bottles are most efficient in geographical locations where both the temperatures and the fluence rates are high.
Implications for the use of SODIS to inactivate viruses.
Despite the significant contribution of viruses to the global burden of enteric infections in both developing and industrialized countries, efforts to ensure their inactivation by drinking water treatment are generally scarce. Previous work has shown that at a household level, SODIS is an effective, low-cost method for inactivating bacteria in drinking water. However, its effectiveness against viruses has not been investigated extensively. In this work, ∼6-log inactivation was observed for MS2 at 22°C in photoreactive Swiss drinking water receiving a sunlight fluence of 1.34 kJ/cm2 (corresponding to 6 h of sunlight exposure in Switzerland). In comparison, Berney et al. (37) observed 6-log inactivation of Escherichia coli at a fluence of only 0.24 kJ/cm2. Hence, as expected, MS2 is much more resistant to SODIS than E. coli. This comparison highlights the challenge associated with virus inactivation during SODIS, particularly since MS2 is relatively labile compared to the other viruses investigated here, due to its high sensitivity to RS (17). Given that virus decay during SODIS in PET bottles is caused by indirect exogenous photoinactivation, it can be expected that virus susceptibility to oxidation by RS is an important determinant for the success of SODIS against viral pathogens.
Recent WHO guidelines for evaluating the efficacy of household water treatments are based on the levels of inactivation achieved for certain reference pathogens, particularly Campylobacter jejuni, Cryptosporidium spp., and rotavirus, or nonpathogenic indicators, such as MS2 or ϕX174 (38). For virus inactivation, the treatment is classified as “highly protective” if ≥5-log inactivation is achieved for MS2 and ϕX174, or as “protective” if ≥3-log inactivation is achieved for both viruses under the conditions specified in the testing protocol (39). At a fluence of 1.34 kJ/cm2 in Swiss tap water at 22°C, we observed >6-log decay of MS2 and negligible inactivation of ϕX174. In the other water matrices tested, lower levels of inactivation were achieved. While these results were not obtained according to the WHO test protocol and thus cannot be strictly rated according to the WHO classification, the resistance of ϕX174 to SODIS makes it unlikely that SODIS can be classified as “protective” or even “highly protective.” The use of UVB-transmitting containers instead of PET bottles may provide great benefits for virus inactivation by SODIS, since this would allow direct photoinactivation of viral pathogens that are resistant to RS (12). For RS-susceptible viruses (such as enteroviruses) and for photoreactive matrices, however, SODIS could be an effective treatment technology, particularly given the synergistic action between water temperature and exogenous photoinactivation. In most developing countries, where SODIS is generally implemented, temperatures are much higher than in Switzerland, and the fluence rates in the exposed water bottles are often higher as well. We therefore expect that the effectiveness of SODIS against RS-susceptible viruses in these countries will be considerably higher than that observed in the present study.
Finally, an important factor that should be taken into consideration in implementing SODIS in the field is the chemical composition of the water used. From our results, the efficiency of SODIS inactivation of viruses can vary greatly as a function of water characteristics. Iron, an element often found in water, has been suggested as a potentially important water constituent that could be responsible for the formation of reactive oxygen species, such as hydroxyl radicals, that cause virus inactivation (40, 41). In contrast, organic matter may act as both a source and a quencher of reactive oxygen species. Hence, these two water constituents should always be analyzed in SODIS efficacy studies. However, more studies are needed in order to accurately link the presence and concentration of iron and OM in water, the formation of reactive oxygen species, and the efficiency of SODIS against viruses.
To conclude, this study contributes to elucidating the effectiveness of SODIS in PET bottles against enteric viruses. In conjunction with previous studies, our results show that while SODIS efficiently inactivates many bacteria, it is less efficient in the treatment of viruses. Thus, SODIS application procedures recommended on the basis of bacterial inactivation do not apply to enteric viruses. Nevertheless, removal during SODIS may be substantial for viruses susceptible to RS, especially if the water temperature is high and the organic matter content of the source water is low. It should be noted that even if certain viruses are resistant to oxidation by RS, their concentrations may be reduced by the effect of temperature during SODIS.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by EPFL.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02897-15.
REFERENCES
- 1.Onda K, LoBuglio J, Bartram J. 2012. Global access to safe water: accounting for water quality and the resulting impact on MDG progress. Int J Environ Res Public Health 9:880–894. doi: 10.3390/ijerph9030880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United Nations. 2014. The millennium development goals report. United Nations, New York, NY. [Google Scholar]
- 3.McMichael AJ, Woodruff RE, Hales S. 2006. Climate change and human health: present and future risks. Lancet 367:859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- 4.WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. 2012. Progress on drinking water and sanitation. WHO, Geneva, Switzerland. [Google Scholar]
- 5.McGuigan KG, Conroy RM, Mosler H-J, du Preez M, Ubomba-Jaswa E, Fernandez-Ibañez P. 2012. Solar water disinfection (SODIS): a review from bench-top to roof-top. J Hazard Mater 235–236:29–46. doi: 10.1016/j.jhazmat.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 6.Reed RH. 2004. The inactivation of microbes by sunlight: solar disinfection as a water treatment process. Adv Appl Microbiol 54:333–365. doi: 10.1016/S0065-2164(04)54012-1. [DOI] [PubMed] [Google Scholar]
- 7.Harding AS, Schwab KJ. 2012. Using limes and synthetic psoralens to enhance solar disinfection of water (SODIS): a laboratory evaluation with norovirus, Escherichia coli, and MS2. Am J Trop Med Hyg 86:566–572. doi: 10.4269/ajtmh.2012.11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heaselgrave W, Kilvington S. 2012. The efficacy of simulated solar disinfection (SODIS) against coxsackievirus, poliovirus and hepatitis A virus. J Water Health 10:531–538. doi: 10.2166/wh.2012.128. [DOI] [PubMed] [Google Scholar]
- 9.Moeller R, Douki T, Rettberg P, Reitz G, Cadet J, Nicholson WL, Horneck G. 2010. Genomic bipyrimidine nucleotide frequency and microbial reactions to germicidal UV radiation. Arch Microbiol 192:521–529. doi: 10.1007/s00203-010-0579-3. [DOI] [PubMed] [Google Scholar]
- 10.Eischeid AC, Meyer JN, Linden KG. 2009. UV disinfection of adenoviruses: molecular indications of DNA damage efficiency. Appl Environ Microbiol 75:23–28. doi: 10.1128/AEM.02199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohn T, Nelson KL. 2007. Sunlight-mediated inactivation of MS2 coliphage via exogenous singlet oxygen produced by sensitizers in natural waters. Environ Sci Technol 41:192–197. doi: 10.1021/es061716i. [DOI] [PubMed] [Google Scholar]
- 12.Fisher MB, Iriarte M, Nelson KL. 2012. Solar water disinfection (SODIS) of Escherichia coli, Enterococcus spp., and MS2 coliphage: effects of additives and alternative container materials. Water Res 46:1745–1754. doi: 10.1016/j.watres.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Love DC, Silverman A, Nelson KL. 2010. Human virus and bacteriophage inactivation in clear water by simulated sunlight compared to bacteriophage inactivation at a Southern California beach. Environ Sci Technol 44:6965–6970. doi: 10.1021/es1001924. [DOI] [PubMed] [Google Scholar]
- 14.Walker DC, Len S-V, Sheehan B. 2004. Development and evaluation of a reflective solar disinfection pouch for treatment of drinking water. Appl Environ Microbiol 70:2545–2550. doi: 10.1128/AEM.70.4.2545-2550.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pecson BM, Martin LV, Kohn T. 2009. Quantitative PCR for determining the infectivity of bacteriophage MS2 upon inactivation by heat, UV-B radiation, and singlet oxygen: advantages and limitations of an enzymatic treatment to reduce false-positive results. Appl Environ Microbiol 75:5544–5554. doi: 10.1128/AEM.00425-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clesceri LS, Greenberg AE, Eaton AD (ed). 1999. Standard methods for the examination of water and wastewater, 20th ed American Public Health Association, Washington, DC. [Google Scholar]
- 17.Mattle MJ, Vione D, Kohn T. 2015. Conceptual model and experimental framework to determine the contributions of direct and indirect photoreactions to the solar disinfection of MS2, ϕX174, and adenovirus. Environ Sci Technol 49:334–342. doi: 10.1021/es504764u. [DOI] [PubMed] [Google Scholar]
- 18.Sommer R, Pribil W, Appelt S, Gehringer P, Eschweiler H, Leth H, Cabaj A, Haider T. 2001. Inactivation of bacteriophages in water by means of non-ionizing (UV-253.7 nm) and ionizing (gamma) radiation: a comparative approach. Water Res 35:3109–3116. doi: 10.1016/S0043-1354(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 19.Calgua B, Barardi CRM, Bofill-Mas S, Rodriguez-Manzano J, Girones R. 2011. Detection and quantitation of infectious human adenoviruses and JC polyomaviruses in water by immunofluorescence assay. J Virol Methods 171:1–7. doi: 10.1016/j.jviromet.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Manzano J, Alonso JL, Ferrús MA, Moreno Y, Amorós I, Calgua B, Hundesa A, Guerrero-Latorre L, Carratalà A, Rusiñol M, Girones R. 2012. Standard and new fecal indicators and pathogens in sewage treatment plants, microbiological parameters for improving the control of reclaimed water. Water Sci Technol 66:2517–2522. doi: 10.2166/wst.2012.233. [DOI] [PubMed] [Google Scholar]
- 21.Bofill-Mas S, Rusiñol M, Fernandez-Cassi X, Carratalà A, Hundesa A, Girones R. 2013. Quantification of human and animal viruses to differentiate the origin of the fecal contamination present in environmental samples. Biomed Res Int 2013:192089. doi: 10.1155/2013/192089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girones R, Carratalà A, Calgua B, Calvo M, Rodriguez-Manzano J, Emerson S. 2014. Chlorine inactivation of hepatitis E virus and human adenovirus 2 in water. J Water Health 12:436–442. doi: 10.2166/wh.2014.027. [DOI] [PubMed] [Google Scholar]
- 23.Carratalà A, Rusiñol M, Rodriguez-Manzano J, Guerrero-Latorre L, Sommer R, Girones R. 2013. Environmental effectors on the inactivation of human adenoviruses in water. Food Environ Virol 5:203–214. doi: 10.1007/s12560-013-9123-3. [DOI] [PubMed] [Google Scholar]
- 24.de Abreu Corrêa A, Carratalà A, Barardi CR, Calvo M, Girones R, Monte R, Bofill-Mas S. 2012. Comparative inactivation of murine norovirus, human adenovirus, and human JC polyomavirus by chlorine in seawater. Appl Environ Microbiol 78:6450–6457. doi: 10.1128/AEM.01059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosshard F, Armand F, Hamelin R, Kohn T. 2013. Mechanisms of human adenovirus inactivation by sunlight and UVC light as examined by quantitative PCR and quantitative proteomics. Appl Environ Microbiol 79:1325–1332. doi: 10.1128/AEM.03457-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page MA, Shisler JL, Mariñas BJ. 2010. Mechanistic aspects of adenovirus serotype 2 inactivation with free chlorine. Appl Environ Microbiol 76:2946–2954. doi: 10.1128/AEM.02267-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hijnen WAM, Beerendonk EF, Medema GJ. 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res 40:3–22. doi: 10.1016/j.watres.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 28.Silverman AI, Peterson BM, Boehm AB, McNeill K, Nelson KL. 2013. Sunlight inactivation of human viruses and bacteriophages in coastal waters containing natural photosensitizers. Environ Sci Technol 47:1870–1878. doi: 10.1021/es3036913. [DOI] [PubMed] [Google Scholar]
- 29.Tapparel C, Siegrist F, Petty TJ, Kaiser L. 2013. Picornavirus and enterovirus diversity with associated human diseases. Infect Genet Evol 14:282–293. doi: 10.1016/j.meegid.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Lodder WJ, de Roda Husman AM. 2005. Presence of noroviruses and other enteric viruses in sewage and surface waters in The Netherlands. Appl Environ Microbiol 71:1453–1461. doi: 10.1128/AEM.71.3.1453-1461.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigstam T, Gannon G, Cascella M, Pecson BM, Wigginton KR, Kohn T. 2013. Subtle differences in virus composition affect disinfection kinetics and mechanisms. Appl Environ Microbiol 79:3455–3467. doi: 10.1128/AEM.00663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gelbart WM, Knobler CM. 2009. Pressurized viruses. Science 323:1682–1683. doi: 10.1126/science.1170645. [DOI] [PubMed] [Google Scholar]
- 33.De Paepe M, Taddei F. 2006. Viruses' life history: towards a mechanistic basis of a trade-off between survival and reproduction among phages. PLoS Biol 4(7):e193. doi: 10.1371/journal.pbio.0040193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carrillo-Tripp M, Shepherd CM, Borelli IA, Venkataraman S, Lander G, Natarajan P, Johnson JE, Brooks CL III, Reddy VS. 2009. VIPERdb2: an enhanced and web API enabled relational database for structural virology. Nucleic Acids Res 37(Database issue):D436–D442. doi: 10.1093/nar/gkn840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero OC, Straub AP, Kohn T, Nguyen TH. 2011. Role of temperature and Suwannee River natural organic matter on inactivation kinetics of rotavirus and bacteriophage MS2 by solar irradiation. Environ Sci Technol 45:10385–10393. doi: 10.1021/es202067f. [DOI] [PubMed] [Google Scholar]
- 36.Ortega-Gómez E, Ballesteros Martín MM, Carratalà A, Fernández Ibañez P, Sánchez Pérez JA, Pulgarín C. 2015. Principal parameters affecting virus inactivation by the solar photo-Fenton process at neutral pH and μM concentrations of H2O2 and Fe2+/3+. Appl Catal B Environ 174–175:395–402. doi: 10.1016/j.apcatb.2015.03.016. [DOI] [Google Scholar]
- 37.Berney M, Weilenmann H-U, Simonetti A, Egli T. 2006. Efficacy of solar disinfection of Escherichia coli, Shigella flexneri, Salmonella Typhimurium and Vibrio cholerae.. J Appl Microbiol 101:828–836. doi: 10.1111/j.1365-2672.2006.02983.x. [DOI] [PubMed] [Google Scholar]
- 38.WHO. July 2011. Evaluating household water treatment options. WHO, Geneva, Switzerland. [Google Scholar]
- 39.WHO. February 2014. Procedure for evaluation. WHO international scheme to evaluate household water treatment (HWT) technologies. WHO, Geneva, Switzerland. [Google Scholar]
- 40.Nieto-Juarez JI, Pierzchla K, Sienkiewicz A, Kohn T. 2010. Inactivation of MS2 coliphage in Fenton and Fenton-like systems: role of transition metals, hydrogen peroxide and sunlight. Environ Sci Technol 44:3351–3356. doi: 10.1021/es903739f. [DOI] [PubMed] [Google Scholar]
- 41.Kim JY, Lee C, Sedlak DL, Yoon J, Nelson KL. 2010. Inactivation of MS2 coliphage by Fenton's reagent. Water Res 44:2647–2653. doi: 10.1016/j.watres.2010.01.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.