Abstract
Fusarium graminearum is the predominant component of the Fusarium head blight complex of wheat. F. graminearum ascospores, which initiate head infection, mature in perithecia on crop residues and become airborne. The effects of temperature (T) and moisture on perithecium production and maturation and on ascospore production on maize stalk residues were determined. In the laboratory, perithecia were produced at temperatures between 5 and 30°C (the optimum was 21.7°C) but matured only at 20 and 25°C. Perithecia were produced when relative humidity (RH) was ≥75% but matured only when RH was ≥85%; perithecium production and maturation increased with RH. Equations describing perithecium production and maturation over time as a function of T and RH (R2 > 0.96) were developed. Maize stalks were also placed outdoors on three substrates: a grass lawn exposed to rain; a constantly wet, spongelike foam exposed to rain; and a grass lawn protected from rain. No perithecia were produced on stalks protected from rain. Perithecium production and maturation were significantly higher on the constantly wet foam than on the intermittently wet lawn (both exposed to rain). Ascospore numbers but not their dispersal patterns were also affected by the substrate.
INTRODUCTION
Fusarium head blight (FHB) is one of the most widespread and dangerous diseases of wheat and other small-grain cereals (1, 2). Up to 17 species belonging to the genera Fusarium and Microdochium have been recognized as responsible for FHB (3). Fusarium graminearum has been considered the predominant species in most cereal-growing areas of the world (4, 5, 6). Symptoms of the disease include premature bleaching of spikes or spikelets, sterile florets, poorly filled grains, and grains that are shriveled and discolored (white to pale pink) (7). The disease reduces yield, grain quality, including contamination by mycotoxins, and seed quality (3).
Wheat spikes are receptive to infection from flowering to the soft-dough stage (8). Ascospores and macroconidia are the main inocula (3, 7, 9) and are typically produced on the residues of the previous crop (4, 7). The fungus can survive as a saprophyte on the previous crop's residue for 2 or more years (10, 11, 12). Although both ascospores and conidia can be detected in cereal crops, ascospores are more prevalent than conidia in many areas (3, 4, 7, 13). Macroconidia are produced in sporodochia and are dispersed for short distances by splashing rain (14, 15, 16). Ascospores mature in perithecia and are forcibly discharged into the air; they are then airborne and can travel meters to kilometers (9, 17, 18, 19).
The main factors influencing production of perithecia and ascospores are light, temperature, and moisture. Light with wavelengths between 300 and 320 nm is essential for the induction of perithecium (20). Paulitz (21) observed more perithecia on the side of debris exposed to direct light; perithecia were also found on grains buried at 5 to 10 cm, but they did not produce ascospores (10).
Perithecia and ascospores are formed under warm and moist conditions, but they require cooler conditions than conidia (20, 22, 23). Andries et al. (24) reported that, in the field, a daily average temperature of <9°C prevented the formation of perithecia. Under controlled conditions, perithecia developed at temperatures between 12 and 28°C (25) but not at 8 and 30°C (26), and they matured only at 16, 20, and 24°C. Tschanz et al. (20) observed mature perithecia (i.e., perithecia oozing ascospores and perithecia with similar size) in the range of 15 to 31°C but not at 32.5°C. The optimum temperature range for perithecium production was 20 to 24°C as reported by Dufault et al. (26) and 29°C as reported by Tschanz et al. (20). Sutton (7) documented that ascospores were produced at temperatures from 25 to 28°C. According to Xu (27), the minimal and optimal temperatures were 7 to 10°C and 15 to 20°C, respectively. Other studies have suggested that different strains of F. graminearum could produce fully developed perithecia and ascospores at 15°C or lower (24, 26).
Production of perithecia is favored by moisture (25, 28). Perithecium production was enhanced by a moisture level of −1.50 MPa at 20°C according to Sung and Cook (28) and by a level of −0.45 MPa at both 20 and 24°C according to Dufault et al. (25). The latter study, however, also reported a high production at −1.30 MPa, and few perithecia were formed at −5 to −6 MPa or below (28); Dufault et al. (25) reported an interaction between temperature and moisture. At −0.45 MPa, perithecia matured after 10 days at 20 and 24°C and after 15 days at 16°C; at −1.30 MPa, perithecia matured after 10 days at 20°C days and after 15 days at 24°C. However, the trend in perithecium production in response to temperature was similar among moisture levels.
The studies cited in the previous paragraphs focused on perithecium production and did not evaluate the effects of temperature and moisture on ascospore production. Dufault et al. (25) speculated that conditions favoring large numbers of perithecia of substantial size would result in high concentrations of ascospore inoculum, but this assumption needs to be verified. In addition, most of the previous studies were conducted in controlled environments, on agar media or maize stalk tissue, with constant temperature and moisture regimes. In the field, however, both temperature and moisture follow a diurnal pattern. Therefore, the fungus may exploit favorable conditions for further development before either temperature or moisture becomes limiting again, and it is possible that perithecia develop in a series of steps. It follows that further research is needed to investigate how perithecium and ascospore productions are affected by temperature and moisture under field or near-field conditions.
The objective of our study was to determine the effect of temperature and moisture conditions on perithecium production and maturation and on ascospore production by F. graminearum on maize stalk residues under both laboratory and outdoor conditions.
MATERIALS AND METHODS
Inoculation of maize stalks.
In February 2011 and 2012, maize stalk residues were collected from the surface of plots in which maize had been grown the previous season. Stalks were cut into pieces that were 5 cm long for laboratory experiments and 15 to 20 cm long for outdoor experiments. The pieces were immersed in distilled water for 12 h, removed from the water, and sterilized twice in an autoclave (120°C for 20 min each time). The sterilized pieces were then immersed for 2 min in a conidial suspension (3 × 104 conidia ml−1), which was obtained by mixing conidia produced by two strains of F. graminearum that had been previously tested for their ability to produce perithecia. One strain was isolated from wheat kernels in Italy (Emilia-Romagna) (strain MPVP074; culture collection of Università Cattolica del Sacro Cuore, Piacenza, Italy), and the other was isolated from oat grains in Germany (strain GERM001; Institut fur landwirtschaftliche Kulturen, Bundesanstalt fur Zuchtungsforschung an Kulturpflanzen [BAZ], OT Groß Lusewitz, Sanitz, Germany). Conidial suspensions used for stalk inoculation were obtained from 14-day-old colonies grown on potato dextrose agar (PDA) at 25°C with a 12-h photoperiod in white light. These colonies were washed twice with 20 ml of sterile water, and the surface was gently scraped. The resulting conidial suspensions were filtered through a double layer of sterile cheesecloth and adjusted to achieve a concentration of 3 × 104 conidia ml−1. After they were inoculated, the stalk pieces were incubated at room temperature (20 to 25°C) for 1 week in the dark to allow the fungus to colonize the residue.
Effect of constant temperatures on perithecium development.
Inoculated stalk pieces were placed in aluminum trays (20 by 15 cm), 10 stalk pieces per tray, with 500 ml of sterile water, and sealed in transparent plastic bags to maintain 100% relative humidity (RH). The stalk pieces were placed on a wire grid so that they did not touch the water. The trays were incubated for 8 weeks in incubators (Biolog-LUX, F.lli Galli G.&P., Milan, Italy) at 5, 10, 15, 20, 25, 30, 35, or 40°C (each temperature regime was represented by two replicate trays) with a 12-h dark/12-h light photoperiod, and a mixture of white and near-UV light was used to induce the production of perithecia (20). Five of the 10 stalk pieces per tray were examined once each week with a dissecting microscope (magnification, ×40); the number of perithecia was determined and expressed as numbers per square centimeter of stalk surface. At the same time (i.e., once a week), 10 perithecia were randomly collected from the remaining five stalk pieces with a needle. The collected perithecia were crushed on a microscope slide, examined with a light microscope (×200), and assigned to one of the following maturity classes (25): 1, initials (without pigmentation); 2, pigment just visible; 3, pigmented, no asci; 4, pigmented, asci beginning to differentiate; 5, asci well-differentiated, no ascospores; 6, asci fully formed, ascospores just beginning to differentiate; 7, with asci and partially formed ascospores; 8, with asci and fully formed ascospores, ascospores clearly septate. Starting from when the first perithecium of a treatment (i.e., temperature regime) was in stage 8, 10 additional perithecia were collected per tray, suspended in 25 μl of water in vials, and stirred twice with a vortex mixer at 2,400 rpm. The ascospores in the resulting suspension were counted using a Bürker hemocytometer, and the number of ascospores per perithecium was calculated. The experiment was performed twice. In each experiment, there were 8 temperature regimes, each of them with two replicate trays and 10 stalk pieces per tray.
Effect of constant relative humidity on perithecium development.
Inoculated stalk pieces were managed as described in the previous section, but the water on the bottom of the trays was replaced with saturated salt solutions to create different RH values: NH4NO3, NaCl, KCl, KNO3, and water were used to obtain RH values of 62.5, 75.5, 85.0, 92.5, and 100%, respectively (29). The actual level of RH in the trays incubated at 25°C was confirmed with a data logger (Tinytag Plus 2 TGP-4500; Gemini Data Loggers, Chichester, United Kingdom). Trays were incubated in incubators at 25°C for 8 weeks, and the numbers of perithecia, their maturation, and the numbers of ascospores were determined once each week as previously described. At the end of the experiment, the available water (aw) and the moisture content (M) of the stalk pieces were determined. The aw was determined with the instrument AquaLab lite (version 1.3; Decagon Devices Inc., Pullman, WA), and M was calculated as follows: M = [(fresh weight − dry weight)/fresh weight] ×100. For the determination of dry weight, stalks were dried at 120°C for 48 h. The experiment was performed twice. In each experiment, there were 5 RH levels, each of them with two replicate trays and 10 stalk pieces per tray.
Development of perithecia outdoors on different substrates.
In early March of 2011 and again in 2012, stalk pieces were closed in wire grid boxes (30 by 20 by 10 cm; the boxes prevented dispersal of the stalk pieces) that were placed outside at the University campus of Piacenza (North Italy). There were two kinds of stalk pieces: (i) stalk pieces artificially inoculated as previously described and (ii) stalk pieces naturally infested, collected in experimental plots of ARVALIS—Institut du Végétal (Boigneville, France). The boxes were placed on (i) a grass lawn in open air (exposed to rain); (ii) a piece of sponge-like florist foam in open air (the foam was exposed to rain and remained moist in the absence of rain because it sat in a pan of water); and (iii) a grass lawn under a transparent cover (not exposed to rain). The transparent cover, which was placed 20 cm above the soil, protected the stalk pieces from rainfall while permitting air circulation and light penetration. In a preliminary test, the cover did not alter air temperature or RH at 10 cm above the soil and did not prevent the formation of perithecia in moist stalks (data not shown). Boxes were arranged according to a split-plot design in which the exposure condition was the main factor and the stalk origin was the split factor, with five replicate boxes, 10 stalk pieces per box.
In each stalk piece, an area corresponding to the area visible under a dissecting microscope at a magnification of ×40 was randomly chosen and delimited with a permanent marker. These areas were examined twice each week for 20 weeks (from the beginning of March) with a dissecting microscope (magnification, ×40), and the perithecia were counted. The maturity stage of the perithecia was determined as previously described on 10 to 30 additional perithecia taken outside the areas used for the counts.
To determine ascospore discharge, spore traps were placed over the stalk pieces. Spore traps consisted of microscope slides (7.5 by 2.5 cm) coated with transparent double-sided adhesive tape (Tesa, Hamburg, Germany) (5.0 by l.2 cm). Slides were fixed 3 to 5 cm above the stalk pieces with the adhesive side facing down. There was one slide per wire box (i.e., 5 slides per each exposure condition and stalk origin). Slides were replaced once each week for 20 weeks. The slides were examined with a light microscope (magnification, ×200) to determine the numbers of F. graminearum ascospores and macroconidia; two length-wise stripes (0.213 mm wide) were examined on each slide. Ascospores and macroconidia were identified according to the methods of Headrick et al. (30) and Leslie and Summerell (31) and expressed as numbers per square millimeter of spore trap.
Weather data of air temperature, relative humidity, and rainfall during the outdoor experiments were recorded by a nearby automatic weather station (MeteoSense 2.0; Netsens s.r.l., Florence, Italy).
Data analysis.
Numbers of mature perithecia per square centimeter of stalk surface were estimated by multiplying the numbers of perithecia produced at any temperature or relative humidity condition tested by the proportion of mature perithecia assessed by microscopic observation of crushed perithecia under the same condition. Numbers of total and mature perithecia were then rescaled by dividing each value by the maximum obtained in the experiment (i.e., the number of perithecia at 20°C or at 100% RH after 56 days of incubation). Pooled data of the replicate experiments were regressed against temperature or relative humidity and incubation time.
Nonlinear regression models were used and compared based on the Akaike's information criterion (AIC); the following models provided the smallest AIC values and were therefore considered the most likely to be correct (32):
| 1 |
| 2 |
where Y is the rescaled production or maturation of perithecia, Teq values are equivalents of temperature calculated as (T − Tmin)/(Tmax − Tmin), where T is the temperature regime (in degrees Celsius) and Tmin and Tmax are the minimal and maximal temperature for production or maturation, RH is the relative humidity (in percentage), t is incubation day, and a to e are the equation parameters. Tmin and Tmax were considered equation parameters and estimated accordingly (33). The equation parameters were estimated using the nonlinear regression procedure of SPSS (version 21; IBM SPSS Statistics, IBM Corp., USA), which minimizes the residual sums of squares using the Marquardt algorithm.
The following goodness-of-fit indexes were calculated for equations 1 and 2 (34, 35, 36): concordance correlation coefficient (CCC), coefficient of residual mass (CRM), and modeling efficiency (EF). CCC measures the concordance between continuous, approximately normally distributed variables and ranges from −1 to 1, with a value of 1 indicating a perfect fit of predicted and observed data. CRM measures the tendency of the model to overestimate (CRM < 0) or underestimate (CRM > 0) the measurements, with a value of 0 indicating a perfect fit of predicted and observed data. EF compares the simulated values to the average value of measurements, with a value of 1 indicating a perfect fit.
Optimal temperatures (Topt) for perithecial production and maturation were estimated according to Analytis (37), based on parameter estimates of the following equation:
| 3 |
where Y is the rescaled production or maturation of perithecia after 56 days of incubation, and a, b, c, and Teq have the same meaning as in equation 1; Topt was then calculated as follows:
In the experiment conducted outdoors, the numbers of perithecia produced on maize stalks, the percentage of mature perithecia, and the numbers of ascospores trapped by spore traps under the three exposure conditions (lawn without rain, continuously wet foam, and lawn with rain) and stalk origin (artificially inoculated and naturally infested) were plotted against time (expressed as day of the year [DOY]). The cumulative numbers of total and mature perithecia produced over the season as well as the total numbers of ascospores trapped were used in an analysis of variance (ANOVA); before analysis, the values were transformed using the natural logarithm function to make the variances homogeneous. A factorial ANOVA was performed considering exposure conditions (fixed factor with 3 levels), stalk origin (fixed factor with 2 levels), and year (random factor with 2 levels), with 5 replicates.
RESULTS
Effects of constant temperatures on perithecium development.
Perithecia were formed at temperatures between 5 and 30°C; no perithecia were produced at 35 and 40°C, and only few were produced at 5°C. Perithecium production over time followed an S-shaped pattern, with a plateau after 42 days of incubation for most of the temperatures tested (Fig. 1A). Based on equation 3, with b equal to 17.2 ± 2.1 and c equal to 10.5 ± 1.2 and Tmin and Tmax estimated as 0 and 35°C, respectively (R2 = 0.99), the optimal temperature for perithecium production was 21.7°C, with a 95% confidence interval of 21.5 to 21.8°C.
FIG 1.
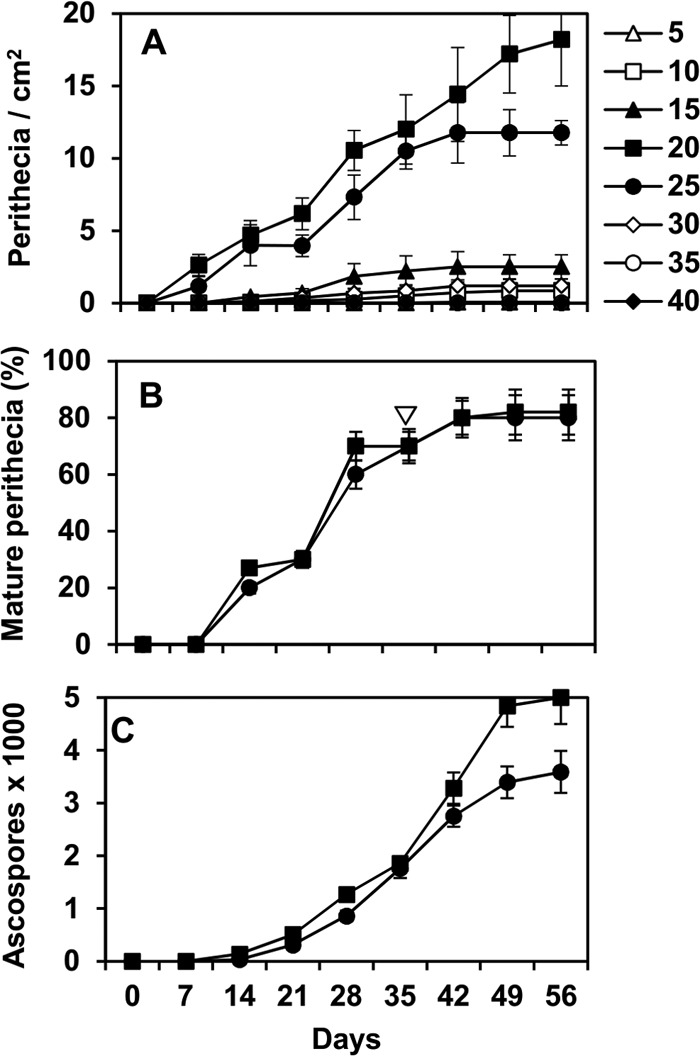
Numbers of Fusarium graminearum perithecia produced per square centimeter of maize stalk (A), percentage of mature perithecia (B), and cumulative number of mature ascospores in perithecia (C) as affected by temperature and days of incubation. Maize stalks were sterilized, inoculated with F. graminearum conidia, and incubated at different constant temperatures (shown on the right, in degrees Celsius). Maturity was evaluated by microscopic observation of crushed perithecia. In panel B, the white arrow indicates when empty perithecia were first observed. Numbers of mature ascospores were determined by suspending perithecia in water and counting the ascospores in the suspension with a hemocytometer; the average numbers of ascospores per perithecium were then calculated. Points are the averages and whiskers the standard errors for 40 values (2 experiments, 2 replicate trays with 10 stalk pieces each).
Perithecia began producing mature ascospores after 14 days of incubation, but only at 20 and 25°C (Fig. 1B). At either higher or lower temperatures, maturation did not advance beyond stage 3 (perithecia pigmented, no asci; data not presented). Based on equation 3, given b of 0.98 ± 0.06 and c of 1.41 ± 0.02, and with Tmin and Tmax estimated as 15 and 30°C, respectively (R2 = 0.99), the optimal temperature for perithecium maturation was 21.1°C, with a 95% confidence interval of 21.0 to 21.3°C. Empty perithecia (perithecia that had matured and discharged ascospores) were found at both 20 and 25°C. They were first observed after 35 days of incubation, and their numbers increased thereafter; 0 to 60% immature perithecia were detected at each sampling time (Fig. 1B). Mature ascospores were first found after 14 days of incubation at 20 and 25°C, and the number of mature ascospores increased with time (Fig. 1C).
Equation 1 provided a good fit of the rescaled data for the production and maturation of perithecia as a function of temperature and incubation time (Fig. 2A and B, respectively). Equation 1 is a logistic curve describing perithecial production and maturation over time of incubation having a temperature-dependent, Bete equation of Analytis (37) as the asymptote parameter. In the Bete equation, Tmin and Tmax were estimated as 0 and 35°C, respectively, for production and as 15 and 30°C for maturation (Table 1). The parameter a is a proportionality factor, such that the perithecial development at the optimum temperature is equal to 1, b is a rate parameter of production or maturation increase toward its optimum with increasing temperature, c is a rate parameter of production or maturation decrease from its optimum with increasing temperature. In the logistic equation, the parameter d defines the duration of the lag phase, while e defines the development rate at increasing time. Standard errors of equation parameters were low compared to the parameter estimates, and R2 values were 0.99 (Table 1). The goodness-of-fit of equation 1 to experimental data was confirmed by the following index values: CCC = 0.993, CRM = 0.028, and EF = 0.987 for perithecial production; CCC = 0.995, CRM = −0.004, and EF = 0.989 for maturation. The plot of predicted versus observed values did not show systematic deviations (Fig. 2), resulting in a regression line not different from the line of perfect agreement (y = x), with intercept a of 0.004 (P = 0.538 for a = 0), slope b of 1.001 (P = 0.340 for b = 1), and R2 of 0.99 for production (Fig. 2A); and a of 0.001 (P = 0.422 for a = 0), b of 0.991 (P = 0.478 for b = 1), and R2 of 0.99 for maturation (Fig. 2B).
FIG 2.
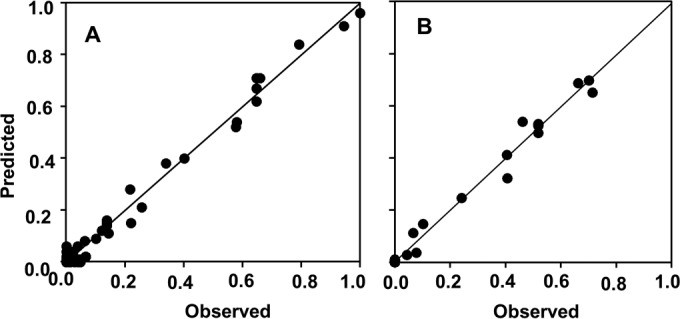
Observed and predicted production (A) and maturation (B) of Fusarium graminearum perithecia on maize stalks as affected by temperature and days of incubation. The observed values are those shown in Fig. 1, and the predicted values were generated by equation 1 (see Table 1 for equation parameters); the numbers of perithecia per square centimeter of stalk surface were rescaled by dividing each number by the maximum number obtained in the experiment. The line represents the linear fit of observed and predicted values; intercept a = 0.004, slope b = 1.001, R2 = 0.99 (A); a = 0.001, b = 0.991, R2 = 0.99 (B).
TABLE 1.
Parameters and statistics of the equations describing the relationship between constant temperatures (5 to 40°C), incubation time (0 to 56 days), and production and maturation of Fusarium graminearum perithecia on maize stalk residues in the laboratory
| Equation parametera | Perithecium production |
Perithecium maturation |
||||
|---|---|---|---|---|---|---|
| Parameter estimate | SE | R2 | Parameter estimate | SE | R2 | |
| Tmin | 0 | 0.1 | 0.99 | 15 | 0.3 | 0.99 |
| Tmax | 35 | 0.2 | 30 | 0.2 | ||
| a | 5.937 | 0.038 | 0.907 | 0.037 | ||
| b | 1.668 | 0.012 | 0.200 | 0.013 | ||
| c | 9.334 | 0.430 | 0.492 | 0.085 | ||
| d | 2.807 | 0.152 | 5.618 | 0.374 | ||
| e | 0.106 | 0.007 | 0.195 | 0.008 | ||
The regression equation was equation 1 (see Materials and Methods).
Effect of constant RH on perithecium development.
Perithecia were produced on stalks when RH was ≥75.5%, which corresponded to 15.8% stalk moisture, and matured when RH was ≥85% (16.0% stalk moisture). Numbers of perithecia increased as RH increased, and the maximum number was produced at 100% RH (76.4% stalk moisture) (Fig. 3A). Mature perithecia were first observed after 14 days of incubation at RH of ≥92.5% (corresponding to aw of ≥0.92 and 22.6% stalk moisture) and after 21 days at 85% RH (corresponding to 0.82 aw and 16.0% stalk moisture) (Fig. 3B). Empty perithecia were observed after 21 to 28 days of incubation, irrespective of stalk moisture (data not presented). The numbers of ascospores were higher in perithecia produced at 100% RH than at 92.5 and 85% RH (Fig. 3C).
FIG 3.
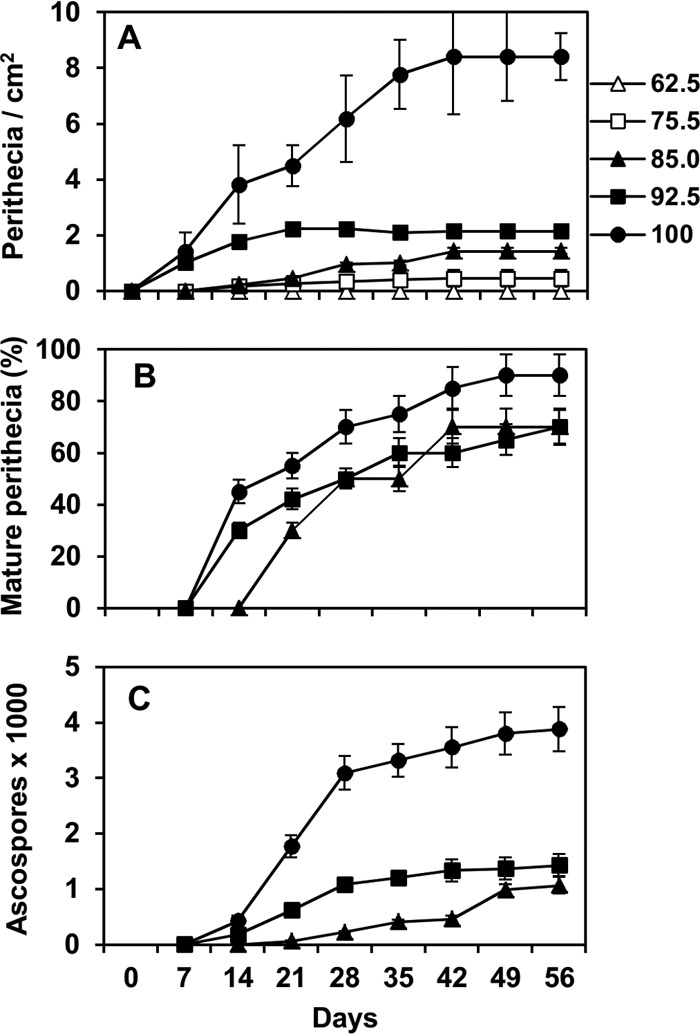
Numbers of Fusarium graminearum perithecia produced per square centimeter of maize stalk (A), frequency of mature perithecia (B), and cumulative numbers of mature ascospores in perithecia (C) as affected by constant relative humidity (RH, indicated on the right, in percentages) and days of incubation. Maize stalks were sterilized, inoculated with F. graminearum conidia, and incubated at constant RHs. Maturity was evaluated by microscopic observation of crushed perithecia. The numbers of mature ascospores were determined by suspending perithecia in water and counting the ascospores in the suspension with a hemocytometer; the average numbers of ascospores per perithecium were then calculated. Points are the averages and whiskers the standard errors of 40 values (2 experiments, 2 replicate trays with 10 stalk pieces each).
Equation 2 provided a good fit of the rescaled data for the production and maturation of perithecia as a function of relative humidity and incubation time (Fig. 4A and B, respectively). with R2 values of 0.96 and 0.99, respectively (Table 2). Equation 2 is a logistic curve describing perithecial production and maturation over time of incubation having an RH-dependent, asymptotic growth curve as the asymptote parameter. In this growth curve, the parameter c defines the developmental rate as a function of RH, with an asymptote of 1 at 100% RH. The parameter a defines the duration of the lag phase, while b defines the development rate at increasing time. Equation 2 had CCC of 0.980, CRM of 0.057, and EF of 0.959 for perithecial production, and CCC of 0.994, CRM of −0.019, and EF of 0.987 for perithecial maturation. There were no systematic deviations of the predicted versus the observed values (Fig. 4) resulting in a line not different from the line of perfect agreement (y = x), with intercept a of −0.008 (P = 0.460 for a = 0), slope b of 0.983 (P = 0.567 for b = 1), and R2 of 0.96 for production (Fig. 4A); and a of 0.0002 (P = 0.973 for a = 0), b of 1.017 (P = 0.310 for b = 1), and R2 of 0.99 for maturation (Fig. 4B).
FIG 4.

Observed and predicted production (A) and maturation (B) of Fusarium graminearum perithecia on maize stalks as affected by relative humidity (RH) and days of incubation. The observed values are those shown in Fig. 3, and predicted values were generated by equation 2 (see Table 2 for equation parameters). Numbers of perithecia per square centimeter of stalk surface were rescaled by dividing each number by the maximum number obtained in the experiment. The line represents the linear fit of observed and predicted values; intercept a = −0.008, slope b = 0.983, R2 = 0.96 (A); a = 0.0002, b = 1.017, R2 = 0.99 (B).
TABLE 2.
Parameters and statistics of the equations describing the relationship between constant relative humidity (RH = 62.5 to 100%), incubation time (0 to 56 days), and production and maturation of Fusarium graminearum perithecia on maize stalk residues in the laboratory
| Equation parametera | Perithecial production |
Perithecial maturation |
||||
|---|---|---|---|---|---|---|
| Parameter estimate | SE | R2 | Parameter estimate | SE | R2 | |
| a | 2.325 | 0.320 | 0.96 | 3.401 | 0.205 | 0.99 |
| b | 0.128 | 0.016 | 0.121 | 0.007 | ||
| c | 0.850 | 0.009 | 0.801 | 0.008 | ||
The regression equation was equation 2 (see Materials and Methods).
Development of perithecia outdoors under different conditions.
Based on the ANOVA, numbers of total and mature perithecia were significantly affected by exposure conditions. The number of mature perithecia was also affected by interaction exposure condition × stalk origin × year, even though this interaction accounted for only 0.04% of total variance (Table 3). In 2011, perithecia were first observed in mid-March in maize stalks on both wet florist foam and grass lawn exposed to rain. Thereafter, numbers of perithecia increased more rapidly on wet florist foam than on the grass lawn (Fig. 5A). By the end of the season, approximately 1,800 and 1,500 perithecia were cumulatively produced per square centimeter of stalk on wet florist foam and on the grass lawn, respectively (Fig. 5C). No perithecia were produced on stalks that were placed on a lawn and protected from rain. The percentage of mature perithecia increased over the season in stalks on wet foam, but perithecium maturation was delayed by approximately 2 months on the grass lawn (Fig. 5B). As a consequence, numbers of mature perithecia were lower in stalks on grass lawn than on wet foam (Fig. 5C).
TABLE 3.
Results of the ANOVA testsa
| Source of variationb | dfc | Total perithecia |
Mature perithecia |
Ascospores released |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % of varianced | F | P value | % of variance | F | P value | % of variance | F | P value | ||
| 1. Exposure condition | 2 | 97.65 | 793.3 | 0.001 | 93.53 | 193.3 | 0.005 | 25.14 | 1.9 | 0.348 |
| 2. Stalk type | 1 | 0.05 | 5.0 | 0.269 | 0.03 | 0.1 | 0.801 | 1.13 | 794.0 | 0.023 |
| 3. Year | 1 | 0.24 | 4.6 | 0.237 | 0.82 | 2.5 | 0.390 | 26.70 | 4.1 | 0.186 |
| 1 × 2 | 2 | 0.05 | 1.2 | 0.459 | 0.03 | 0.1 | 0.919 | 0.56 | 2.5 | 0.285 |
| 1 × 3 | 2 | 0.12 | 3.2 | 0.238 | 0.48 | 1.2 | 0.446 | 13.41 | 59.5 | 0.017 |
| 2 × 3 | 1 | 0.01 | 0.5 | 0.556 | 0.28 | 1.4 | 0.353 | 0.00 | 0.0 | 0.921 |
| 1 × 2 × 3 | 2 | 0.04 | 0.6 | 0.554 | 0.39 | 4.2 | 0.021 | 0.23 | 0.9 | 0.413 |
ANOVA tests were performed for total number of perithecia, numbers of mature perithecia, and numbers of ascospores produced on two types of maize stalk residues (artificially inoculated and naturally infested by Fusarium graminearum). Stalks were exposed outdoors in 2 years and on three exposure conditions: (i) on a grass lawn and exposed to rain; (ii) on a piece of wet florist foam and exposed to rain; (iii) on a grass lawn under a transparent cover.
A factorial ANOVA was performed considering exposure conditions (fixed factor with 3 levels), stalk origin (fixed factor with 2 levels), and year (random factor with 2 levels), with 5 replicates (n = 60).
df, degrees of freedom.
Percentage of total variance accounted for by the source of variation.
FIG 5.
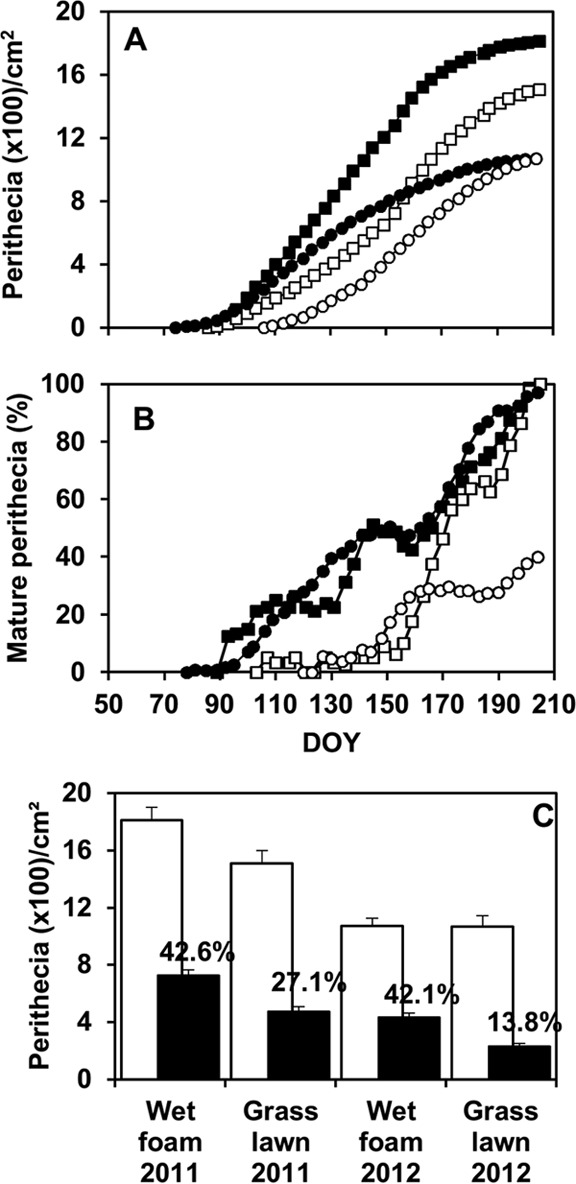
Production of Fusarium graminearum perithecia per square centimeter of maize stalk (A) and percentage of mature perithecia (B) in outdoor experiments in 2011 (□) and 2012 (○) (B) as affected by time (day of the year [DOY]) and exposure conditions. Maize stalks were arranged on a grass lawn (white) or on a wet florist foam (black); the stalks were exposed to rain and open air in both treatments, but the foam was always moist while the lawn was moist only during and shortly after rain events. (C) Cumulative numbers of total perithecia (white bars) and mature perithecia (black bars); values indicate the percentages of mature perithecia relative to the total number. Points in panels A and B and bars in panel C are averages of 10 values: two stalk types (artificially inoculated and naturally infested by F. graminearum) and 5 replicates per stalk type. Whiskers in panel C are standard errors of the 10 values.
In 2012, the first perithecia were produced in late March in stalks on wet foam and were produced 1 month later on grass lawn exposed to rain; at the end of the experiment, similar numbers of perithecia were produced on the two substrates that were exposed to rain (Fig. 5C). However, perithecium maturation was delayed and strongly reduced on the grass lawn compared to that on wet foam (Fig. 5B), so that the numbers of mature perithecia were about two times greater on the wet foam than on the grass lawn.
Patterns of ascospores trapping were similar in different years and with different substrates (Fig. 6A), while the number of ascospores trapped was 10 times greater in 2011 than in 2012 (Fig. 6B). The numbers of ascospores trapped above the maize stalks were significantly affected by the stalk origin and by the interaction exposure condition-year (Table 3), since the difference between ascospores trapped above wet foam and grass lawn was significant in 2011 but not in 2012 (Fig. 6B).
FIG 6.
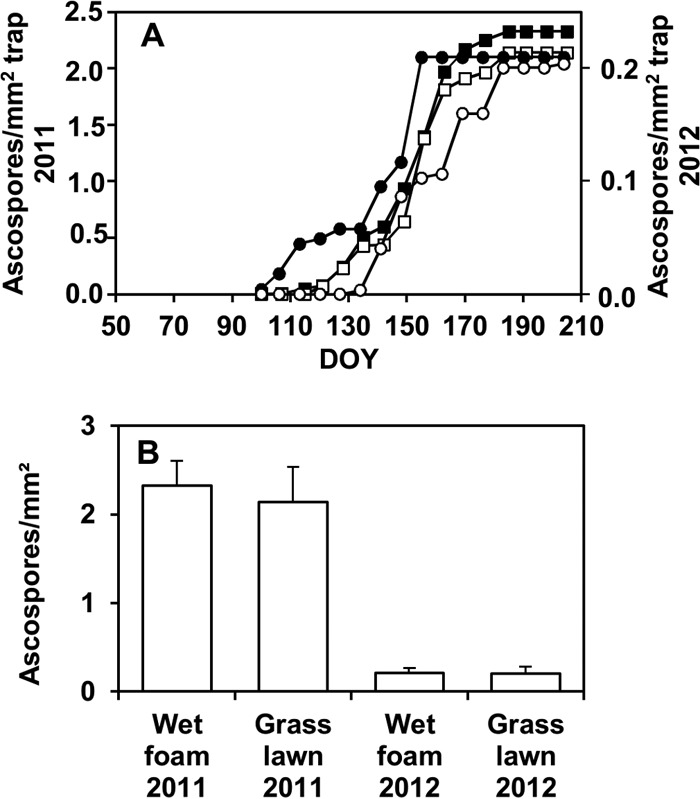
Cumulative numbers of Fusarium graminearum ascospores trapped over time (DOY) (A) and at the end of the trapping period (B) above maize stalks in outdoor experiments in 2011 (□) and 2012 (○) as affected by exposure conditions. Maize stalks were arranged on a grass lawn (white) or on a wet florist foam (black) as described in the legend to Fig. 5, and spore traps (microscope slides with adhesive tape facing down) were placed 3 to 5 cm above the stalk pieces. Points in panel A and bars in panel C are averages of 10 values: two stalk types (artificially inoculated and naturally infested by F. graminearum) and 5 replicates per stalk type. Whiskers in panel B are standard errors of the 10 values.
DISCUSSION
Our results have confirmed that temperatures between 20 and 25°C are good for perithecium production (20, 25); in our experiment, the optimum temperature was 21.7°C. Our results also agree with those of previous authors who reported that the upper temperature limit for perithecium production was ≥30°C. On the other hand, we observed that 5°C was the lower limit for perithecium production, while Dufault et al. (25) and Andries et al. (24) stated that temperatures of <9°C prevented perithecial production. The range of temperature allowing perithecium maturation was more restricted than that for production: perithecia matured only at 20 and 25°C after 14 days of incubation, with an optimum at 21.1°C; no maturation occurred at either higher or lower temperatures. These results agree with those of Tschanz et al. (20), who reported almost no maturation at 15°C, while Dufault et al. (25) observed mature perithecia at 16°C, although this maturation was reached later than at the optimal temperature.
Our experiments also confirmed the effect of moisture on perithecium production. Compared to previous studies, we tested a wider range of humidities (from 62.5 to 100% RH, corresponding to −66 MPa to 0 MPa). Dufault et al. (25) investigated perithecium production in the range of −4 to 0 MPa, and Sung and Cook (28) did the same in the range of −6 to 0 MPa. In our study, perithecia were not produced at RH of 62.5% (corresponding to 0.62 aw, 15.3% moisture, and −66 MPa water potential). Perithecia were produced at RH of 75.5% (0.75 aw, 15.8% moisture, and −38 MPa), and production increased with increasing RH. This is in contrast to the results from Sung and Cook (28), who reported no perithecium production at water potentials that were less than −5 MPa. Perithecium matured only at RH ≥85% (0.82 aw, 16.0% moisture, and −27 MPa water potential), and maturation was faster and more ascospores were produced as humidity increased above that threshold. The same trend was reported by Dufault et al. (25), i.e., the average maturity index increased as humidity increased.
Two main factors may explain the different results reported by the different authors in the previous paragraphs. One factor is the intrinsic variability among strains of the fungus. Genetic variability of strains belonging to F. graminearum has widely been reported (31). It is not clear, however, how this variability affects the fungal response to temperature. Brennan et al. (38), who analyzed the in vitro growth of several F. graminearum strains originating from three European countries, concluded that the country of origin affected the response to temperature. In contrast, other authors (23, 39, 40, 41) observed a consistent response to temperature among different strains. A second factor is the plant residue used for perithecium production because the plant tissue that is used as residue can differ in the C/N ratio and other properties depending on plant species, plant organ, and growth conditions (42). Khonga and Sutton (11) reported that the nutritional status of the residues supporting fungal development is the principal factor conditioning the temporal pattern of F. graminearum inoculum production, with perithecia being more rapidly formed on poor residues with a high C/N ratio. In the works previously mentioned, Tschanz et al. (20) used fresh carnation leaves and Sung and Cook (28) used PDA adjusted to different water potentials. Prussin et al. (43), who analyzed the differences between artificial media and plant residues, reported that maize stalks have an average C/N ratio of 60:1, while PDA has a C/N ratio of 10:1 (44). Dufault et al. (25) used maize stalks from mature plants. We used maize residues that had overwintered in the field, which is a substrate that would support perithecium production under natural conditions. Overwintering of the stalks probably resulted in the leaching of soluble compounds, which would increase the C/N ratio, and also involved cycles of wetting and drying and freezing and thawing (45). It therefore seems likely that overwintering affected residue characteristics, which in turn would have affected perithecium development.
In addition to determining the effect of temperature and humidity on perithecium production on maize stalks that had overwintered in the field, the current study provides new insights into the effects of temperature and humidity on perithecium maturation and ascospore production. Our results showed that perithecia matured only in a narrow range of temperatures and that low-moisture conditions inhibited or slowed maturation and ascospore production. Therefore, perithecium production may occur under a wider range of conditions (5°C ≤ T ≤ 30°C and 75.5% ≤ RH ≤ 100%) than perithecium maturation and ascospore production (20°C ≤ T ≤ 25°C and 85% ≤ RH ≤ 100%). Because of these differences in temperature and moisture requirements, perithecia were produced but did not mature in some treatments. It follows that using the number of perithecia observed on stalk residues may result in the overestimation of the quantity of inoculum. This inference is further supported by the observation that empty perithecia may remain in situ for some time after ascospores are discharged.
The outdoor experiments confirmed the results obtained under controlled conditions. The outdoor experiments also highlighted the substantial differences between the three moisture regimes (in which the residues were placed on grass with or without exposure to rain and on a sponge-like foam exposed to rain) and the 2 years. In both years, the numbers of perithecia produced were highest when the stalk residues were placed on the continuously moist foam; the numbers were intermediate on a grass lawn subjected to fluctuating moisture (exposed to rain), and no perithecia were detected on a grass lawn that was protected from rain. In addition, perithecia matured earlier on the moist foam exposed to rain than on the grass lawn exposed to rain, but ascospore production (evaluated by trapping discharged ascospores) was similar for the two treatments exposed to rain in the same year. The effect of moisture coming from the soil on inoculum production was previously mentioned by Xu (27), who reported that ascospore production is prevented when the soil moisture is <30% and is at its maximum when soil moisture is >80%. The dynamics of ascospore discharge were similar in the two conditions (stalks on a moist substrate versus stalks on a grass lawn exposed to rain), suggesting that ascospore discharge is not affected by stalk moisture but is mainly driven by rain and atmospheric humidity (18, 46, 47, 48).
Concerning differences between the 2 years of our outdoor experiment, fewer perithecia were produced, fewer of them matured, and fewer ascospores were discharged in the second year, when there was less rainfall, which again indicated the importance of rainfall for the production and dispersal of inoculum by F. graminearum on maize stalks. Temperature also appeared to play a role in the lower production and maturation of perithecia in the second year, because April and May were cooler in the second year than in the first. Dufault et al. (22), who also studied the development of perithecia in the field, reported that perithecia stopped developing when the temperature dropped below 7°C and that less inoculum was produced in the second year. Although laboratory experiments with constant conditions are useful, it is important to evaluate the effects of wetting and drying on perithecium development under natural conditions. This is because the response of the fungus to fluctuating conditions can differ from the response to constant conditions. In this regard, Fernando et al. (47) and Dufault et al. (25) postulated that, under fluctuating conditions, F. graminearum perithecia can develop in a series of steps, exploiting periods of favorable conditions for development.
In this paper, equations that describe the effects of temperature and stalk moisture on perithecium production and maturation were developed. These equations should enable researchers to estimate the relative amount of F. graminearum inoculum produced based on weather data. The equations could be integrated into, and thus improve, models that describe inoculum production, such as the Brazilian model GIBSIM (49) and the model elaborated in Italy (50), by providing an estimate of the ascosporic inoculum present.
ACKNOWLEDGMENTS
V.M. carried out this work within the Doctoral School in the Agro-Food System (Agrisystem) of the Università Cattolica del Sacro Cuore (Italy).
We thank Matthias Herrmann for providing the German fungal strain and Emmanuelle Gourdain for providing naturally infested maize stalks.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Gilbert J, Haber S. 2013. Overview of some recent research developments in Fusarium head blight of wheat. Can J Plant Pathol 35:149–174. doi: 10.1080/07060661.2013.772921. [DOI] [Google Scholar]
- 2.McMullen M, Bergstrom G, De Wolf E, Dill-Macky R, Hershman D, Shaner G, Van Sanford D. 2012. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis 96:1712–1728. doi: 10.1094/PDIS-03-12-0291-FE. [DOI] [PubMed] [Google Scholar]
- 3.Parry DW, Jenkinson P, Mcleod L. 1995. Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol 44:207–238. doi: 10.1111/j.1365-3059.1995.tb02773.x. [DOI] [Google Scholar]
- 4.Bai G, Shaner G. 1994. Scab of wheat: prospects for control. Plant Dis 78:760–766. doi: 10.1094/PD-78-0760. [DOI] [Google Scholar]
- 5.Goswami RS, Kistler HC. 2004. Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5:515–525. doi: 10.1111/j.1364-3703.2004.00252.x. [DOI] [PubMed] [Google Scholar]
- 6.Stepien L, Chelkowski J. 2009. Fusarium head blight of wheat: pathogenic species and their mycotoxins. World Mycotoxin J 3:107–119. [Google Scholar]
- 7.Sutton JC. 1982. Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum.. Can J Plant Pathol 4:195–209. doi: 10.1080/07060668209501326. [DOI] [Google Scholar]
- 8.Rossi V, Terzi V, Moggi F, Morcia C, Faccioli P, Haidukowski M, Pascale M. 2007. Assessment of Fusarium infection in wheat heads using a quantitative polymerase chain reaction (qPCR) assay. Food Addit Contam 24:1121–1230. doi: 10.1080/02652030701551818. [DOI] [PubMed] [Google Scholar]
- 9.Fernando WGD, Paulitz TC, Seaman WL, Dutilleul P, Miller JD. 1997. Head blight gradients caused by Gibberella zeae from area sources of inoculum in wheat field plots. Phytopathology 87:414–421. doi: 10.1094/PHYTO.1997.87.4.414. [DOI] [PubMed] [Google Scholar]
- 10.Inch SA, Gilbert J. 2003. Survival of Gibberella zeae in Fusarium-damaged wheat kernels. Plant Dis 87:282–287. doi: 10.1094/PDIS.2003.87.3.282. [DOI] [PubMed] [Google Scholar]
- 11.Khonga EB, Sutton JC. 1988. Inoculum production and survival of Gibberella zeae in maize and wheat residues. Can J Plant Pathol 10:232–239. doi: 10.1080/07060668809501730. [DOI] [Google Scholar]
- 12.Pereyra SA, Dill-Macky R, Sims AL. 2004. Survival and inoculum production of Gibberella zeae in wheat residue. Plant Dis 88:724–730. doi: 10.1094/PDIS.2004.88.7.724. [DOI] [PubMed] [Google Scholar]
- 13.Markell SG, Francl LJ. 2003. Fusarium head blight inoculum: species prevalence and Gibberella zeae spore type. Plant Dis 87:814–820. doi: 10.1094/PDIS.2003.87.7.814. [DOI] [PubMed] [Google Scholar]
- 14.Jenkinson P, Parry DW. 1994. Splash dispersal of conidia of Fusarium culmorum and Fusarium avenaceum.. Mycol Res 98:506–510. doi: 10.1016/S0953-7562(09)80468-1. [DOI] [Google Scholar]
- 15.Paul PA, El-Allaf SM, Lipps PE, Madden LV. 2004. Rain splash dispersal of Gibberella zeae within wheat canopies in Ohio. Phytopathology 94:1342–1349. doi: 10.1094/PHYTO.2004.94.12.1342. [DOI] [PubMed] [Google Scholar]
- 16.Rossi V, Languasco L, Pattori E, Giosuè S. 2002. Dynamics of airborne Fusarium macroconidia in wheat fields naturally affected by head blight. J Plant Pathol 84:53–64. [Google Scholar]
- 17.De Luna L, Bujold I, Carisse O, Paulitz TC. 2002. Ascospore gradients of Gibberella zeae from overwintered inoculum in wheat fields. Can J Plant Pathol 24:457–464. doi: 10.1080/07060660209507034. [DOI] [Google Scholar]
- 18.Francl L, Shaner G, Bergstrom GC, Gilbert J, Pedersen W, Dill-Macky R, Corwin B, Jin Y, Gallenberg D, Wiersma J. 1999. Daily inoculum levels of Gibberella zeae on wheat spikes. Plant Dis 83:662–666. doi: 10.1094/PDIS.1999.83.7.662. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado-Ramirez SL, Schmale DG, Shields EJ, Bergstrom GC. 2005. The relative abundance of viable spores of Gibberella zeae in the planetary boundary layer suggests the role of long-distance transport in regional epidemics of Fusarium head blight. Agric Forest Meteorol 132:20–27. doi: 10.1016/j.agrformet.2005.06.007. [DOI] [Google Scholar]
- 20.Tschanz AT, Horst RK, Nelson PE. 1976. The effect of environment on sexual reproduction of Gibberella zeae.. Mycologia 68:327–340. doi: 10.2307/3759003. [DOI] [Google Scholar]
- 21.Paulitz TC. 1996. Diurnal release of ascospores by Gibberella zeae in inoculated wheat plots. Plant Dis 80:674–678. doi: 10.1094/PD-80-0674. [DOI] [Google Scholar]
- 22.Dufault NS, De Wolf ED, Lipps PE, Madden LV. 2002. Identification of environmental variables that affect perithecial development of Gibberella zeae, p 141 Proc 2002 Natl Fusarium Head Blight Forum, Erlanger, KY. [Google Scholar]
- 23.Rossi V, Pattori E, Ravanetti A, Giosuè S. 2002. Effect of constant and fluctuating temperature regimes on sporulation of four fungi causing head blight of wheat. J Plant Pathol 84:95–105. [Google Scholar]
- 24.Andries C, Jarosz A, Trail F. 2000. Effects of rainfall and temperature on production of perithecia by Gibberella zeae in field debris in Michigan, p 118–112. Proc 2000 Natl Fusarium Head Blight Forum, Erlanger, KY. [Google Scholar]
- 25.Dufault NS, De Wolf ED, Lipps PE, Madden LV. 2006. Role of temperature and moisture in the production and maturation of Gibberella zeae perithecia. Plant Dis 90:637–644. doi: 10.1094/PD-90-0637. [DOI] [PubMed] [Google Scholar]
- 26.Dufault NS, De Wolf ED, Lipps PE, Madden LV. 2002. Relationship of temperature and moisture to Gibberella zeae perithecial development in a controlled environment, p 142–144. Proc 2002 Natl Fusarium Head Blight Forum, Erlanger, KY. [Google Scholar]
- 27.Xu X-M. 2003. Effects of environmental conditions on the development of Fusarium ear blight. Eur J Plant Pathol 109:683–689. doi: 10.1023/A:1026022223359. [DOI] [Google Scholar]
- 28.Sung J-M, Cook RJ. 1981. Effect of water potential on reproduction and spore germination by Fusarium roseum “Graminearum”, “Culmorum”, and “Avenaceum.” Phytopathology 71:499–504. [Google Scholar]
- 29.Dhingra OD, Sinclair JB. 1995. Basic plant pathology methods. CRC Press, Boca Raton, FL. [Google Scholar]
- 30.Headrick JM, Glawe DA, Pataky JK. 1988. Ascospore polymorphism in Gibberella zeae.. Mycologia 80:679–684. doi: 10.2307/3807718. [DOI] [Google Scholar]
- 31.Leslie JF, Summerell B. 2006. The Fusarium laboratory manual. Blackwell Publishing, Ames, IA. [Google Scholar]
- 32.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference. A practical information-theoretic approach. Springer-Verlag, New York, NY. [Google Scholar]
- 33.Xu X-M. 1996. On estimating non-linear response of fungal development under fluctuating temperatures. Plant Pathol 45:163–171. doi: 10.1046/j.1365-3059.1996.d01-134.x. [DOI] [Google Scholar]
- 34.Loague KM, Green RE. 1991. Statistical and graphical methods for evaluating solute transport models: overview and application. J Contam Hydrol 7:51–73. doi: 10.1016/0169-7722(91)90038-3. [DOI] [Google Scholar]
- 35.Madden LV, Hughes G, van der Bosch F. 2007. The study of plant disease epidemics. APS Press, St. Paul, MN. [Google Scholar]
- 36.Nash JE, Sutcliffe JV. 1970. River flow forecasting through conceptual models, part I—a discussion of principles. J Hydrol 10:282–290. doi: 10.1016/0022-1694(70)90255-6. [DOI] [Google Scholar]
- 37.Analytis S. 1980. Obtaining of sub-models for modeling the entire life cycle of a pathogen. Z Pflanzenk Pflanzen 87:371–382. [Google Scholar]
- 38.Brennan JM, Fagan B, Van Maanen A, Cooke BM, Doohan FM. 2003. Studies on in vitro growth and pathogenicity of European Fusarium fungi. Eur J Plant Pathol 109:577–587. doi: 10.1023/A:1024712415326. [DOI] [Google Scholar]
- 39.Cook RJ, Christen AA. 1976. Growth of cereal root-rot fungi as affected by temperature-water potential interactions. Phytopathology 66:193–197. doi: 10.1094/Phyto-66-193. [DOI] [Google Scholar]
- 40.Ramirez ML, Chulze S, Magan N. 2006. Temperature and water activity effects on growth and temporal deoxynivalenol production by two Argentinean strains of Fusarium graminearum on irradiated wheat grain. Int J Food Microbiol 106:291–296. doi: 10.1016/j.ijfoodmicro.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Rossi V, Ravanetti A, Pattori E, Giosuè S. 2001. Influence of temperature and humidity on the infection of wheat spikes by some fungi causing fusarium head blight. J Plant Pathol 83:189–198. [Google Scholar]
- 42.Kumar K, Goh KM. 1999. Crop residues and management practices: effects on soil quality, soil nitrogen dynamics, crop yield and nitrogen recovery. Adv Agron 68:197–319. doi: 10.1016/S0065-2113(08)60846-9. [DOI] [Google Scholar]
- 43.Prussin AJ, Szanyi NA, Welling PI, Ross SD, Schmale DG. 2014. Estimating the production and release of ascospores from a field-scale source of Fusarium graminearum inoculum. Plant Dis 98:497–503. doi: 10.1094/PDIS-04-13-0404-RE. [DOI] [PubMed] [Google Scholar]
- 44.Wyss GS, Charudattan R, Devalerio JT. 2001. Evaluation of agar and grain media for mass production of conidia of Dactylaria higginsii.. Plant Dis 85:1165–1170. doi: 10.1094/PDIS.2001.85.11.1165. [DOI] [PubMed] [Google Scholar]
- 45.Coûteaux MM, Bottner P, Berg B. 1995. Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66. doi: 10.1016/S0169-5347(00)88978-8. [DOI] [PubMed] [Google Scholar]
- 46.Del Ponte EM, Fernandes JMC, Pierobom CR. 2005. Factors affecting density of airborne Gibberella zeae inoculum. Fitopatol Bras 30:55–60. doi: 10.1590/S0100-41582005000100009. [DOI] [Google Scholar]
- 47.Fernando WGD, Miller JD, Seaman WL, Seifert K, Paulitz TC. 2000. Daily and seasonal dynamics of airborne spores of Fusarium graminearum and other Fusarium species sampled over wheat plots. Can J Bot 78:497–505. [Google Scholar]
- 48.Inch SA, Fernando WGD, Gilbert J. 2005. Seasonal and daily variation in the airborne concentration of Gibberella zeae (Schw.) Petch spores in Manitoba. Can J Plant Pathol 27:357–363. doi: 10.1080/07060660509507233. [DOI] [Google Scholar]
- 49.Del Ponte EM, Fernandes JMC, Pavan W. 2005. A risk infection simulation model for Fusarium head blight of wheat. Fitopatol Bras 30:634–642. doi: 10.1590/S0100-41582005000600011. [DOI] [Google Scholar]
- 50.Rossi V, Giosuè S, Pattori E, Spanna F, Del Vecchio A. 2003. A model estimating the risk of Fusarium head blight on wheat. EPPO Bull 33:421–425. doi: 10.1111/j.1365-2338.2003.00667.x. [DOI] [Google Scholar]


