Abstract
UVC light is a widely used sterilization technology. However, UV lamps have several limitations, including low activity at refrigeration temperatures, a long warm-up time, and risk of mercury exposure. UV-type lamps only emit light at 254 nm, so as an alternative, UV light-emitting diodes (UV-LEDs) which can produce the desired wavelengths have been developed. In this study, we validated the inactivation efficacy of UV-LEDs by wavelength and compared the results to those of conventional UV lamps. Selective media inoculated with Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes were irradiated using UV-LEDs at 266, 270, 275, and 279 nm in the UVC spectrum at 0.1, 0.2, 0.5, and 0.7 mJ/cm2, respectively. The radiation intensity of the UV-LEDs was about 4 μW/cm2, and UV lamps were covered with polypropylene films to adjust the light intensity similar to those of UV-LEDs. In addition, we applied UV-LED to sliced cheese at doses of 1, 2, and 3 mJ/cm2. Our results showed that inactivation rates after UV-LED treatment were significantly different (P < 0.05) from those of UV lamps at a similar intensity. On microbiological media, UV-LED treatments at 266 and 270 nm showed significantly different (P < 0.05) inactivation effects than other wavelength modules. For sliced cheeses, 4- to 5-log reductions occurred after treatment at 3 mJ/cm2 for all three pathogens, with negligible generation of injured cells.
INTRODUCTION
UV light covers a wavelength spectrum from 100 to 380 nm and is subdivided into three regions by wavelength: UVA (320 to 400 nm), UVB (280 to 320 nm), and UVC (200 to 280 nm) (1). Among them, UVC has the strongest germicidal effect and is widely used in the form of mercury lamps to inactivate microorganisms. However, UV mercury lamps have several critical limitations. First, UV lamps are fragile and thus present a risk of mercury leakage through breakage when subjected to any shock. Also, the warm-up time is long and, moreover, cannot exhibit maximum efficacy at low temperatures according to an earlier study. Due to these critical weaknesses of mercury lamps, UV light-emitting diode (UV-LED) technology has been developed recently as an alternative. LED construction commonly consists of a junction between “n-type” and “p-type” semiconducting materials. Current is caused by mobile electrons in the “n-type” layer and carriers are positively charged holes in the “p-type” layer. To emit light, the electrons and holes reconnect at the junction (2). UV-LED lamps (UV-LEDs) are very small size compared to conventional lamps, so they can be easily incorporated into diverse designs of device (3). Also, UV-LEDs emit high-intensity light as soon as they are turned on; in other words, there is no warm-up time. Furthermore, Shin et al. (4) demonstrated that UV-LEDs contain no mercury and yield a consistent irradiation output regardless of temperature, which makes them effective even under refrigeration. Although UV mercury lamps emit only one wavelength (254 nm), UV-LEDs can be configured to emit certain target wavelengths. The most effective germicidal wavelength occurs at a peak of 260 to 265 nm at which DNA absorbs UV the most (5, 6), and LEDs can be designed to produce these specific wavelengths.
Listeria monocytogenes is the most important and critical pathogen of concern to the cheese industry. Every year, 1,600 people are hospitalized and 260 people die from listeriosis in the United States (7). Listeria outbreaks are commonly traced to soft cheese made from unpasteurized milk. Soft cheeses contain 45 to 50% moisture which are generally smooth and easy to ladle or spread. Soft cheeses made from unpasteurized milk are a very high-risk food and are 50 to 160 times more likely to be contaminated with Listeria than those made from pasteurized milk. Escherichia coli O157:H7 and Salmonella spp. are also important pathogens of concern to the dairy industry. In 2010, 38 persons were infected with E. coli O157:H7 in five states of the United States after consuming cheese. Due to this outbreak, 15 people were hospitalized and one person had hemolytic-uremic syndrome (8). In addition, several cases of salmonellosis have been reported from Canada and the United States that were traced to the consumption of cheese (9, 10).
Not only is it risky to use unpasteurized milk as an ingredient to make cheese, cheeses can also be contaminated with pathogens during cheese-making operations. Even if raw milk is pasteurized, it may become contaminated with pathogens when processed in an unsanitary environment (11). For these reasons, we chose sliced cheese as a target food in this study and their flat and even surfaces were suitable for applying UV light.
Recently, interest in UV-LED technology has been increasing, but the inactivating ability of UV-LED by wavelength has never been evaluated before. So in this study, we examined the efficacy of UV-LED to inactivate three major foodborne pathogens, E. coli O157:H7, Salmonella enterica serovar Typhimurium, and L. monocytogenes, on solid media and compared its germicidal ability relative to UVC wavelength. Also, the application of UV-LED to sliced cheese was implemented to assess its suitability as an antimicrobial control intervention.
MATERIALS AND METHODS
Experimental apparatus.
Four UV-LED modules (LG Innotek Co., Republic of Korea), each with the same peak wavelength, were connected onto electronic printed circuit boards (PCBs), and each set of PCBs had a different peak wavelength (266, 270, 275, or 279 nm). The specifications of UV-LED modules used in this experiment are indicated in Table 1. DC voltage from a power supply (TPM series; Toyotech, South Korea) was applied to all the PCBs in accordance with preset available current that provided 23 mA for 266-nm PCBs and 20 mA for 270-, 275-, and 279-nm PCBs. Based on Shin's study (4), we elected to use the four-corner arrangement of modules in this experiment with a 6-cm distance between modules and a 4-cm distance between LEDs and samples (90-mm-diameter petri dish, sliced cheese) for equally distributed irradiance and optimal LED configuration. The PCBs and inoculated media were placed in a treatment chamber (TH-TG-300; JEIO Tech, South Korea). A UVC lamp (G10T5/4P; 357 mm; Sankyo, Japan), which has a nominal output power of 16 W, was used in order to compare the two UV emitting sources for efficacy of pathogen inactivation. The peak wavelength of the UV-lamp was 254.31 nm.
TABLE 1.
Specifications of UV-LED modules used in the experiments
| Expt | Voltage (V) at various wavelengths/currentsa |
|||
|---|---|---|---|---|
| 266 nm/23 mA | 270 nm/20 mA | 275 nm/20 mA | 279 nm/20 mA | |
| 1 | 6.70 | 6.49 | 6.47 | 6.33 |
| 2 | 6.92 | 6.50 | 6.48 | 6.37 |
| 3 | 7.12 | 6.52 | 6.47 | 6.35 |
| 4 | 6.72 | 6.50 | 6.47 | 6.37 |
The voltage value was measured when the specified constant current was applied to each UV-LED module using a DC power supply.
Irradiance measurements.
Intensity of the UV-LED modules was measured with a spectrometer (AvaSpec-ULS2048-USB2-UA-50; Avantes, Netherlands) calibrated for a range of 200 to 400 nm to include the entire UV spectrum. For sample treatment, the distance between collimated LEDs and an optical probe was 4 cm, and the irradiance value of the spectrum at the peak wavelength was measured. The Petri factor, which indicates the evenness of UV irradiance reaching the petri dish, was calculated by scanning the surface of the petri dish every 5 mm with the probe (12). To calculate the corrected intensity, the maximum intensity value was multiplied by the obtained petri factor.
For the purpose of reducing the natural intensity of UV lamps in order to render comparable irradiance from UV-LEDs, which ranges from about 4 to 5 μW/cm2, the UV lamp was covered with 52 sheets of polypropylene (PP) film (thickness, 0.05 mm), and the distance between probe and lamp was set at 20 cm. The Petri factor and corrected intensity were calculated by method used for the UV-LEDs.
Bacterial strains.
Three strains each of E. coli O157:H7 (ATCC 35150, ATCC 43889, and ATCC 43890), S. Typhimurium (ATCC 19585, ATCC 43971, and DT104), and L. monocytogenes (ATCC 19111, ATCC 19115, and ATCC 15313) were obtained from the Food Science and Human Nutrition culture collection at Seoul National University (Seoul, South Korea). Stock cultures were kept frozen at −80°C in 0.7 ml of tryptic soy broth (TSB; MB Cell) and 0.3 ml of 50% glycerol. Working cultures were streaked onto tryptic soy agar (TSA; MB Cell), incubated at 37°C for 24 h, and stored at 4°C.
Culture preparation.
Each strain of E. coli O157:H7, S. Typhimurium, and L. monocytogenes was cultured in 5 ml of TSB at 37°C for 24 h and harvested by centrifugation at 4,000 × g for 20 min at 4°C. The obtained pelleted cells were resuspended in sterile 0.2% Bacto peptone (Becton-Dickinson, Sparks, MD) and centrifuged. This washing procedure was performed three times to purify the cells. The final pelleted cells were resuspended in 9 ml of peptone water (PW), corresponding to approximately 108 to 109 CFU/ml. Each strain of all three pathogen species was combined to make culture cocktails for use in experiments.
Sample preparation and inoculation.
Commercially processed sliced camembert cheese was purchased at a local grocery store (Seoul, South Korea). The sliced cheese was 85 by 85 by 2 mm. Samples were stored under refrigeration (4°C) and used within 2 days. For the medium surface experiments, the cocktail suspension was 10-fold serially diluted three times with 0.2% sterile PW so that the initial concentration of the inoculum was approximately 105 to 106 CFU/ml. Also, the culture suspension was subjected to an additional 10-fold serial dilution in 0.2% PW, and 0.1 ml of diluent was inoculated and spread onto selective media or nonselective agar, such as phenol red agar base (Difco) with 1% sorbitol (d-sorbitol; MB Cell) (SPRAB), and TSA, for injured-cell enumeration. Every medium was duplicate spread-plated with three sequential 10-fold dilutions. Sorbitol MacConkey agar (SMAC; Oxoid), xylose lysine desoxycholate agar (XLD; Oxoid), and Oxford agar base with antimicrobial supplement (OAB; MB Cell) were used as selective media to enumerate E. coli O157:H7, S. Typhimurium, and L. monocytogenes, respectively. For cheese inoculation, 0.1 ml of the same cocktail suspension used for medium experiments was applied to one piece of sliced cheese (ca. 25 g). The inoculum was spread by using a sterile glass spreader every 5 min for even distribution of pathogens, and the samples were dried inside of biosafety hood for 15 min without the fan running to avoid excessive surface aridity. The final cell concentration was approximately 106 to 107 CFU/25 g.
UV treatments.
Inoculated media were treated in the chamber at room temperature with UV-LED PCBs or PP-covered UV-lamp at five different peak wavelengths at dosages of 0.1, 0.2, 0.5, and 0.7 mJ/cm2. Treatment times for the doses were calculated by dividing UV doses by intensities with an appropriate conversion factor. After treatments, in order to minimize photoreactivation, all UV-treated petri dishes were covered with aluminum foil before incubating. Also, pieces of inoculated sliced cheese were treated with the same UV-LED PCBs at dosages of 1, 2, and 3 mJ/cm2 in the same environment and treatment chamber.
Bacterial enumeration.
After UV treatment in the medium surface experiment, treated media were immediately incubated at 37°C for 24 h. For food samples, treated sliced cheeses were transferred into sterile stomacher bags (Labplas, Inc., Canada), along with 225 ml of sterile 0.2% PW, and homogenized for 2 min using a Stomacher (EasyMix; AES Chemunex, France). Aliquots (1 ml) of sample were 10-fold serially diluted in 9-ml blanks of 0.2% PW, and 0.1 ml of diluent was spread plated onto each selective medium (described previously). All agar media from food sample treatments were incubated at 37°C for 24 to 48 h, and typical colonies were counted.
Enumeration of injured cells.
The overlay method was used to enumerate the injured cells of S. Typhimurium and L. monocytogenes (13). The nonselective medium TSA, which enables injured cells to resuscitate, was used so that not only uninjured cells but also sublethally injured cells could be enumerated. Portions (0.1 ml) of appropriate the aliquots were duplicate spread plated onto TSA medium, and the plates were incubated at 37°C for 2 h to permit the injured cells to recover. The plates were then overlaid with 7 to 8 ml of the selective medium XLD for S. Typhimurium or OAB for L. monocytogenes, respectively. Once the samples solidified, the plates were further incubated for an additional 22 h at 37°C. After incubation, typical black colonies of both S. Typhimurium and L. monocytogenes were enumerated. Enumeration of injured E. coli O157:H7 was accomplished with phenol red agar base with 1% sorbitol (SPRAB) (14). After 37°C, 24 h of incubation, typical white colonies were enumerated and, simultaneously, serological confirmation using a RIM E. coli O157:H7 latex agglutination test (Remel, Lenexa, KS) was performed on randomly selected presumptive colonies of E. coli O157:H7.
Color measurement.
A Minolta colorimeter (model CR400; Minolta Co., Japan) was used to quantify the color changes of treated samples to determine the effect of UV-LED treatment on the color of sliced cheese. CIE LAB measurement was implemented, and L* (lightness), a* (green-red), and b* (blue-yellow) of chromaticity were used for the test. Three randomly selected locations on sliced cheese surfaces were analyzed and averaged to compare changes in color during the UV-LED treatments.
Statistical analysis.
All experiments were duplicate-plated and replicated three times. All data were analyzed with ANOVA using a statistical analysis system (SAS Institute, Cary, NC) and Duncan's multiple-range test to determine whether there were significant differences (P < 0.05) in the mean values of log reduction of microorganism populations or color changes.
RESULTS
Emission spectrum of UV lamp and UV-LED.
The spectral intensity of the 254-nm UV lamp covered with PP films was measured with a spectrometer and the results are presented in Fig. 1b. The actual peak wavelength was 254.31 nm and, as the number of PP films increased, the irradiance of the UV lamp decreased. With 52 PP films, the intensity of the 254-nm lamp was determined to be 3.97 ± 0.02 μW/cm2, which was 0.47% of the intensity of the uncovered lamp. Also, the irradiance of UV-LED PCBs is shown in Fig. 1a. The actual peak wavelengths of LED PCBs were 266.25, 271.02, 275.80, and 279.37 nm, respectively, and the intensity values ranged from 4 to 5 μW/cm2.
FIG 1.
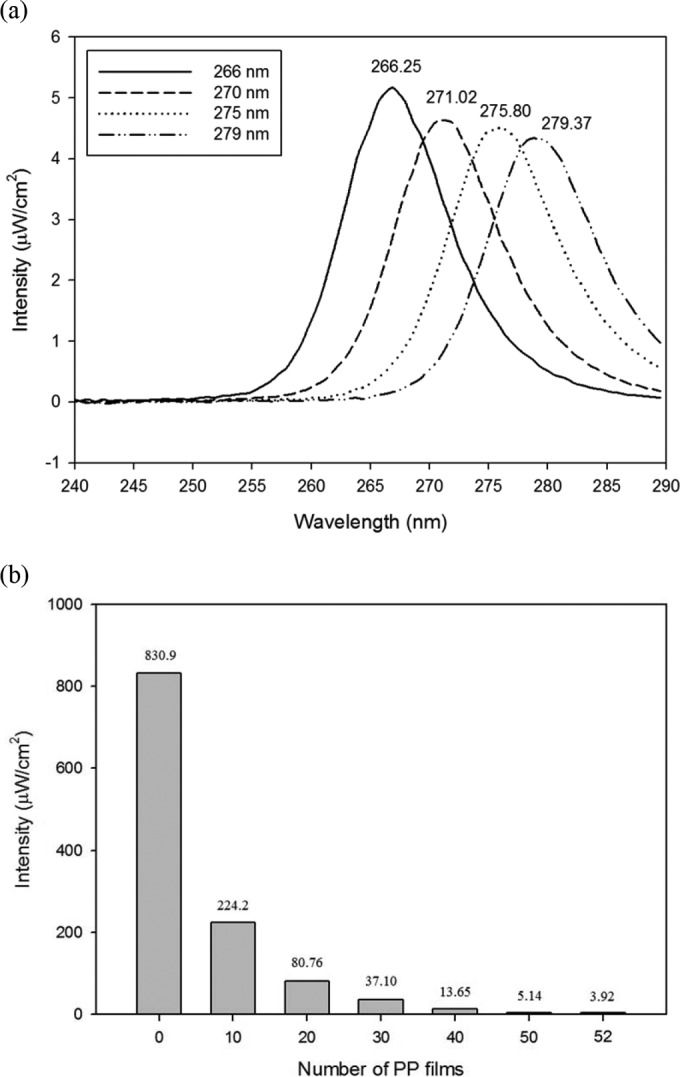
Emission spectra of four different peak wavelengths (266, 270, 275, and 279 nm) of UV-LED PCBs (a) and absolute intensity of a 254-nm UV lamp covered with various numbers of PP films at a 20-cm distance between UV sources and a spectrometer probe (b).
Comparison of microbial reductions between the 254-nm lamp and 266-nm UV-LED.
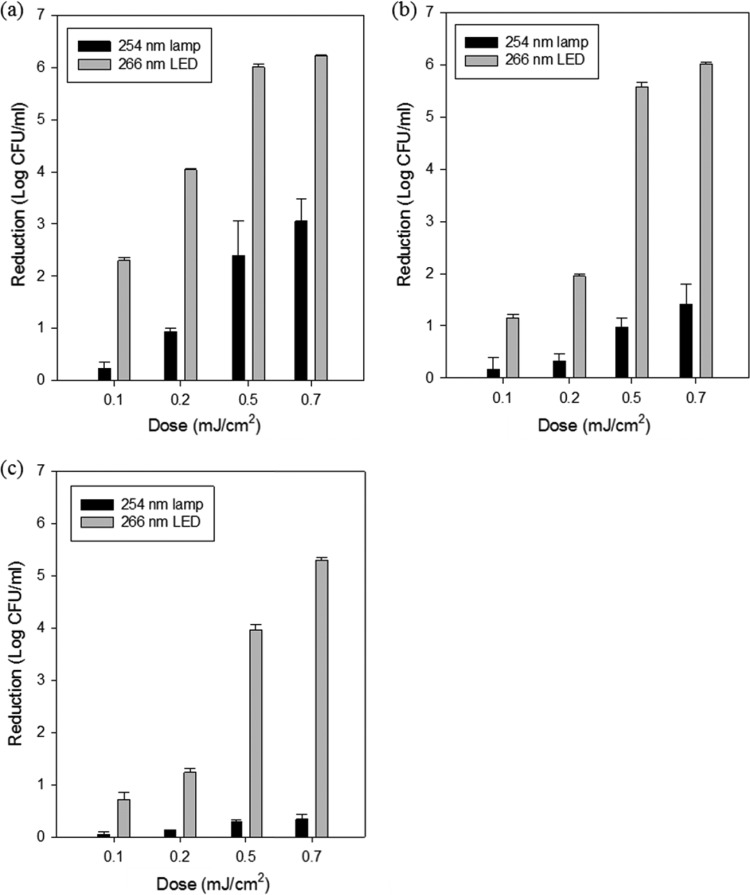
Fig. 2 shows the viable-count reduction levels of E. coli O157:H7, S. Typhimurium, and L. monocytogenes spread on selective media after treating with the 254-nm UV lamp or the 266-nm UV-LED. Both treatments presented the same pattern of foodborne pathogen reductions; that is, higher doses induced higher levels of inactivation. The 266-nm UV-LED treatment at a dose of 0.7 mJ/cm2 achieved ∼6-log reductions of E. coli O157:H7 and S. Typhimurium, respectively, and a 5.3-log reduction of L. monocytogenes. In other words, the 266-nm, 0.7-mJ/cm2 UV-LED treatment demonstrated that nearly all inoculated pathogens were inactivated at this dose. On the other hand, the reduction levels with UV lamp treatment were 3.06-, 1.42-, and 0.34-log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes, respectively, which were significantly less (P < 0.05) than the UV-LED inactivation levels at the same dose. The other doses (0.1, 0.2, and 0.5 mJ/cm2) also showed significant differences between reductions of the three foodborne pathogens treated with the UV lamp and UV-LED. For each dosage, the inactivation level of L. monocytogenes was least compared to E. coli O157:H7 and S. Typhimurium. Resuscitation of injured cells from either UV lamp or UV-LED treatment was observed in terms of numerical level (data not shown), but, statistically, there were no significant differences (P > 0.05).
FIG 2.
Reduction of E. coli O157:H7 (a), S. Typhimurium (b), and L. monocytogenes (c) cells on each selective medium (E. coli O157:H7; sorbitol MacConkey agar, S. Typhimurium; xylose lysine desoxycholate, L. monocytogenes; Oxford agar base with antimicrobial supplement) treated with a 254-nm UV-lamp and 266-nm UV-LED PCBs at 0.1, 0.2, 0.5, and 0.7 mJ/cm2.
Inactivation effect of UV-LED on various media caused by different wavelengths.
The log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes on media treated with four different wavelengths of UV-LED are shown in Table 2. Reduction levels showed an increasing tendency in accordance with treatment dose, achieving an ∼6-log reduction of E. coli O157:H7 and S. Typhimurium and a 5-log reduction of L. monocytogenes at a dose of 0.7 mJ/cm2. Comparison of the inactivation of foodborne pathogens with respect to wavelengths demonstrated that UV treatment with relatively short wavelengths (266 and 270 nm) had a pronounced bactericidal effect at low dosage levels. In the case of E. coli O157:H7, over 4-log reduction was demonstrated at 0.2 mJ/cm2 with 270-nm PCB treatment, and the other PCB treatments achieved 3- to 4-log reductions at the same dose, which were significantly lower (P < 0.05). At 0.5 mJ/cm2, >5-log reductions were achieved with the 266- and 270-nm PCBs on S. Typhimurium, values significantly greater than the reductions obtained with the longer wavelengths. Also, L. monocytogenes showed ∼4-log reductions only for 266- and 270-nm UV-LED treatments, which were 1.0 to 1.5 log greater than that seen with the 279-nm treatment.
TABLE 2.
Log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes on culture media after treatment with UV-LED PCBs at four different wavelengths
| Organism and wavelength (nm) | Mean log reductiona (log10 CFU/ml) ± SD at indicated dose |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 mJ/cm2 | 0.2 mJ/cm2 | 0.5 mJ/cm2 | 0.7 mJ/cm2 | |||||
| E. coli O157:H7 | SMAC |
SPRAB |
SMAC |
SPRAB |
SMAC |
SPRAB |
SMAC |
SPRAB |
| 266 | 2.30 ± 0.06 Ba | 2.86 ± 0.51 Aa | 4.04 ± 0.03 Ba | 4.05 ± 0.41 Aa | 6.01 ± 0.05 Aa | 5.83 ± 0.09 Ab | 6.23 ± 0.01 Aa | 5.82 ± 0.51 Aa |
| 270 | 2.93 ± 0.27 Aa | 2.75 ± 0.22 Aa | 4.49 ± 0.34 Aa | 4.27 ± 0.29 Aa | 5.85 ± 0.12 Aa | 5.92 ± 0.43 Aa | 6.17 ± 0.23 Aa | 5.88 ± 0.84 Aa |
| 275 | 2.10 ± 0.03 BCa | 2.72 ± 0.41 Aa | 3.79 ± 0.04 Ba | 4.17 ± 0.49 Aa | 6.02 ± 0.20 Aa | 5.83 ± 0.35 Aa | 6.27 ± 0.11 Aa | 6.31 ± 0.09 Aa |
| 279 | 1.89 ± 0.24 Cb | 2.65 ± 0.30 Aa | 3.16 ± 0.22 Cb | 3.95 ± 0.38 Aa | 5.86 ± 0.27 Aa | 5.21 ± 0.62 Aa | 6.17 ± 0.23 Aa | 6.05 ± 0.32 Aa |
| S. Typhimurium | XLD |
OV-XLD |
XLD |
OV-XLD |
XLD |
OV-XLDv |
XLD |
OV-XLD |
| 266 | 1.15 ± 0.07 ABa | 0.80 ± 0.39 Aa | 1.95 ± 0.04 ABa | 1.57 ± 0.46 Aa | 5.58 ± 0.09 Aa | 4.35 ± 0.44 Ab | 6.01 ± 0.03 Aa | 5.07 ± 0.15 ABb |
| 270 | 1.39 ± 0.27 Aa | 0.74 ± 0.30 ABb | 2.27 ± 0.31 Aa | 1.64 ± 0.41 Aa | 5.26 ± 0.47 Aa | 4.30 ± 0.33 Ab | 6.00 ± 0.10 Aa | 5.32 ± 0.22 Ab |
| 275 | 0.97 ± 0.02 Ba | 0.84 ± 0.30 Aa | 1.76 ± 0.07 Ba | 1.97 ± 0.89 Aa | 4.59 ± 0.05 Ba | 3.90 ± 0.41 ABb | 5.81 ± 0.33 Aa | 4.79 ± 0.38 Bb |
| 279 | 0.86 ± 0.21 Ba | 0.91 ± 0.50 Aa | 1.93 ± 0.26 ABa | 1.60 ± 0.38 Aa | 4.61 ± 0.23 Ba | 3.46 ± 0.12 Bb | 5.62 ± 0.37 Aa | 4.79 ± 0.38 Ba |
| L. monocytogenes | OAB |
OV-OAB |
OAB |
OV-OAB |
OAB |
OV-OAB |
OAB |
OV-OAB |
| 266 | 0.71 ± 0.15 Aa | 0.49 ± 0.05 Aa | 1.23 ± 0.08 Aa | 1.03 ± 0.05 Ab | 3.97 ± 0.09 Aa | 4.13 ± 0.48 Aa | 5.31 ± 0.05 Aa | 4.91 ± 0.34 Aa |
| 270 | 0.42 ± 0.11 Ba | 0.46 ± 0.07 ABa | 0.88 ± 0.18 Ba | 0.98 ± 0.18 ABa | 3.57 ± 0.05 Ba | 3.87 ± 0.44 Aa | 5.46 ± 0.26 Aa | 4.74 ± 0.57 Aa |
| 275 | 0.34 ± 0.18 Ba | 0.35 ± 0.08 BCa | 0.68 ± 0.10 Ba | 0.79 ± 0.09 BCa | 2.94 ± 0.29 Ca | 3.55 ± 0.32 ABa | 4.61 ± 0.34 Ba | 5.14 ± 0.19 Aa |
| 279 | 0.29 ± 0.10 Ba | 0.32 ± 0.04 CDa | 0.68 ± 0.10 Ba | 0.74 ± 0.13 Ca | 2.27 ± 0.20 Db | 3.08 ± 0.24 Ba | 4.20 ± 0.23 Ca | 4.54 ± 0.07 Aa |
Data represent means from three replications. Values followed by the same uppercase letters within columns and lowercase letters within rows for each dose are not significantly different. Media: SMAC, sorbitol MacConkey agar; SPRAB, phenol red agar base with 1% sorbitol; XLD, xylose lysine desoxycholate agar; OV-XLD, overlay XLD agar on TSA; OAB; Oxford agar base with antimicrobial supplement; OV-OAB, overlay OAB agar on TSA.
With regard to resuscitation of sublethally injured cells, only in the case of S. Typhimurium at 0.5 and 0.7 mJ/cm2 doses were there any significant differences (0.6 to 1 log unit) between inactivation of samples subjected to injured-cell recovery methods and those plated directly onto selective media. In numerical value, different level of reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes were observed for the overlay agar method (SPRAB in the case of E. coli O157:H7) than for selective agar. However, statistically significant differences between the inactivation levels obtained on each selective agar (SMAC, XLD, and OAB) versus the agar for recovering injured cells were not observed except for high dose treatments (0.5 and 0.7 mJ/cm2) on S. Typhimurium, as already mentioned.
Bactericidal effect by UV-LED treatment on sliced cheeses.
Log reductions of foodborne pathogens on sliced cheese samples following UV-LED treatments are presented in Table 3. A relationship between reduction levels and treatment doses was observed that was similar to that described previously for experiments involving selective media. Approximately 4- to 5-log reductions were accomplished at a 3 mJ/cm2 radiation intensity for E. coli O157:H7 and S. Typhimurium, and 3- to 4-log reductions for L. monocytogenes. Furthermore, UV-LED composed of 266-nm modules achieved 4.88-, 4.72-, and 3.52-log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes, respectively, while 279-nm modules achieved 4.04-, 3.91-, and 3.24-log reductions of each pathogen, respectively. Statistically significant differences (P < 0.05) in the numbers of surviving cells enumerated on selective media after exposure to relatively short peak wavelengths (266 and 270 nm) versus relatively long peak wavelengths (275 and 279 nm) were observed at 3 mJ/cm2, the highest treatment dose. The resuscitation of sublethally injured cells after UV-LED treatment was not demonstrated in the overall data.
TABLE 3.
Log reductions of E. coli O157:H7, S. Typhimurium, and L. monocytogenes on sliced cheese after treatment with UV-LED PCBs at four different wavelengths
| Organism and wavelength (nm) | Mean log reductiona (log10 CFU/g) ± SD at indicated dose |
|||||
|---|---|---|---|---|---|---|
| 1 mJ/cm2 | 2 mJ/cm2 | 3 mJ/cm2 | ||||
| E. coli O157:H7 | SMAC |
SPRAB |
SMAC |
SPRAB |
SMAC |
SPRAB |
| 266 | 3.50 ± 0.57 Aa | 3.21 ± 0.22 Aa | 4.09 ± 0.46 Aa | 3.43 ± 0.30 Aa | 4.88 ± 0.18 Aa | 4.49 ± 0.09 Ab |
| 270 | 2.83 ± 0.43 Aa | 3.09 ± 0.72 Aa | 3.99 ± 0.10 Aa | 3.73 ± 0.10 Ab | 4.81 ± 0.10 Aa | 4.14 ± 0.72 ABa |
| 275 | 2.78 ± 0.36 Aa | 2.74 ± 0.42 Aa | 3.79 ± 0.50 Aa | 3.39 ± 0.43 Aa | 4.31 ± 0.31 Ba | 4.13 ± 0.28 ABa |
| 279 | 2.80 ± 0.53 Aa | 2.86 ± 0.73 Aa | 3.46 ± 0.51 Aa | 3.38 ± 0.40 Aa | 4.04 ± 0.33 Ba | 3.64 ± 0.17 Ba |
| S. Typhimurium | XLD |
OV-XLD |
XLD |
OV-XLD |
XLD |
OV-XLD |
| 266 | 3.10 ± 0.24 Aa | 3.13 ± 0.25 Aa | 3.93 ± 0.68 Aa | 3.42 ± 0.46 Aa | 4.72 ± 0.02 Aa | 4.50 ± 0.37 Aa |
| 270 | 2.82 ± 0.33 Aa | 3.08 ± 0.47 Aa | 3.70 ± 0.12 Aa | 3.43 ± 0.41 Aa | 4.73 ± 0.05 Aa | 4.37 ± 0.39 Aa |
| 275 | 2.83 ± 0.31 Aa | 2.91 ± 0.20 Aa | 3.24 ± 0.36 Aa | 3.35 ± 0.28 Aa | 4.24 ± 0.26 Ba | 4.04 ± 0.22 Aa |
| 279 | 2.73 ± 0.38 Aa | 2.93 ± 0.37 Aa | 3.17 ± 0.39 Aa | 2.94 ± 0.61 Aa | 3.91 ± 0.05 Ca | 3.96 ± 0.28 Aa |
| L. monocytogenes | OAB |
OV-OAB |
OAB |
OV-OAB |
OAB |
OV-OAB |
| 266 | 3.09 ± 0.26 Aa | 2.55 ± 0.22 Ab | 3.10 ± 0.10 Aa | 3.03 ± 0.43 Aa | 3.52 ± 0.05 ABa | 3.32 ± 0.75 Aa |
| 270 | 2.89 ± 0.19 Aa | 2.66 ± 0.62 Aa | 2.97 ± 0.44 Aa | 2.73 ± 0.21 Aa | 3.94 ± 0.55 Aa | 3.06 ± 0.25 ABa |
| 275 | 2.54 ± 0.41 Aa | 2.04 ± 0.11 ABa | 2.72 ± 0.34 ABa | 2.43 ± 0.30 Aa | 3.31 ± 0.22 Ba | 2.57 ± 0.18 ABb |
| 279 | 2.33 ± 0.65 Aa | 1.72 ± 0.24 Ba | 2.37 ± 0.17 Ba | 2.07 ± 0.84 Aa | 3.24 ± 0.08 Ba | 2.27 ± 0.37 Bb |
Data represent means from three replications. Values followed by the same uppercase letters within columns and lowercase letters within rows for each dose are not significantly different. Media: SMAC, sorbitol MacConkey agar; SPRAB, phenol red agar base with 1% sorbitol; XLD, xylose lysine desoxycholate agar; OV-XLD, overlay XLD agar on TSA; OAB; Oxford agar base with antimicrobial supplement; OV-OAB, overlay OAB agar on TSA.
Effect of UV-LED treatment on product color values.
The CIE LAB color method was used to determine color changes in sliced cheese samples after a 3-mJ/cm2 UV-LED treatment. Numerical changes in the L*, a*, and b* values of UV-LED-treated sliced cheese were observed, but there were no significant differences (P > 0.05) between any of the treatments and the control (data not shown).
DISCUSSION
UVC is widely used for the surface sterilization of many foods, including fruits, vegetables, and processed foods, as well as equipment. UVC irradiation doses of 0.60 to 6.0 kJ/m2 achieved 2.3- to 3.5-log CFU/fruit reduction of E. coli O157:H7 and 2.15- to 3.1-log CFU/fruit reduction of Salmonella on the grape tomato surfaces (15). E. coli O157:H7, S. Typhimurium, and L. monocytogenes on fresh-cut lettuce were inactivated by >4 logs after a 10-min exposure to a UV lamp at 6.80 mW/cm2 (16). In a pulsed UV system in which 3,800 V of input was used to generate 1.27 J/cm2 per pulse for a lamp with a frequency of three pulses per s, L. monocytogenes inoculated onto unpackaged white American cheese slices (9 by 9 cm) was reduced by 1.1- to 3.08-log CFU/cm2 at distances of 13 and 8 cm at intervals from 5 to 40 s (17). Another promising disinfection method of cheese, photohydroionization technology, consisting of a combination effect of plasmas, 16.65-mJ/cm2 UV lamp irradiation, ozone, and hydrogen peroxide, decontaminated L. monocytogenes on sliced American cheese by slightly >2-log CFU/sample after 5 min of treatment (18). As shown by these earlier studies, UVC is obviously an effective sterilization technology available to the food industry and potentially useful for pasteurizing cheese using UV-LEDs as a highly competitive and promising novel intervention.
UVC emitted from LEDs is an emerging technology offering an alternative to mercury lamps to compensate for their limitations. There have been several studies involving UV-LEDs, but comparison of sterilization efficacy of UV-LEDs by wavelength in the UVC region has barely been studied before. One of the major strengths of UV-LED technology is that it can be configured to emit a specific wavelength. The inactivation ability of UV lamps has been evaluated only at a wavelength of 254 nm since it can only generate a peak wavelength of 254 nm. Therefore, an actual evaluation and comparison of disinfection efficacy of UVC by wavelength is needed at this time.
In this study, we investigated germicidal effects of UVC-LEDs at wavelengths of 266, 270, 275, and 279 nm, and a UV lamp at 254 nm was applied to the pathogens at an intensity similar to those of UV-LEDs. UV lamps emit a considerably high irradiation intensity of light in natural conditions, which leads to a high inactivation effect. However, according to our research, UV lamps showed significantly different (P < 0.05) sterilization capacity for all three pathogens than UV-LEDs when applied at similar intensity. It was assumed that this result was due to differences in irradiation characteristics between UV lamps and UV-LEDs. UV lamps radiate light from a point source that disperses in every direction, the intensity with distance following a classic inverse-square relationship. However, light from UV-LEDs converges at one point vertically. That is, UV lamps scatter light over a large area, and thus actual irradiation strength impinging on the target area may only be a small fraction of what was emitted. On the other hand, UV-LED light, rather than radiating in all directions, proceeds in a linear fashion without much loss of light intensity due to spreading. Thus, we postulate that LED light is concentrated onto the target area and is thus more efficacious than light from a UV lamp.
The UV-LED experiments were performed at an intensity of 4 μW/cm2; therefore, we covered the UV lamp with PP films to adjust its intensity to be almost equivalent to that of the UV-LED lamp. UV-LEDs are still under development, and the output power of erstwhile UV-LEDs are relatively low, so it was necessary to lower the UV lamp intensity for an exact comparison under the same conditions. Raising the radiation intensity of UV-LEDs to that of UV lamps is difficult with current technology, and this is a technical challenge that needs to be solved.
Among UV-LEDs of different wavelengths, 266- and 270-nm LEDs achieved more pathogen reductions than those of longer wavelengths, but these differences were not so critical. Other studies also demonstrated a similar tendency. Chevremont et al. (19) treated mesophilic bacteria, fecal enterococci, and coliforms in effluent with UVA and UVC-LED for 60 s. There was only <1-log reduction, and the inactivation efficacies of 254 and 280 nm were not significantly different. In our study, sterilization efficacy was more related to dose than to wavelength. UV-LEDs achieved >5-log reductions of E. coli O157:H7 after 0.5 mJ/cm2 and S. Typhimurium after 0.7 mJ/cm2, and in the case of L. monocytogenes they achieved >5-log reductions after 0.7 mJ/cm2 only at 266 and 270 nm. The inactivation level of L. monocytogenes was relatively less than those of E. coli O157:H7 or S. Typhimurium because L. monocytogenes is a Gram-positive bacterium and the other two pathogens are Gram-negative bacteria. UV light causes physical electron movements and destroys DNA bonds. UV light induces the formation of photoproducts due to the direct absorption of photons by pyrimidine and purine nucleic acid bases (20). Photoproducts lead to structural distortion in DNA and interrupt RNA transcription and DNA replication, finally causing cell mutagenesis or death. The major photoproducts caused by UV are cyclobutane pyrimidine dimers (CPDs) and pyrimidine 6-4 pyrimidone photoproducts (6-4pps) (21). Gram-positive bacteria are generally more resistant to UV light than are Gram-negative bacteria. This was demonstrated by the study of Beauchamp and Lacroix (22), who reported that L. monocytogenes produced 35% fewer CPDs and 10% fewer 6-4pps than E. coli during a UV lamp irradiation dose of >3 J/cm2. This low production of UV photoproducts indicates a greater resistance for Gram-positive bacteria. Also, after L. monocytogenes, Salmonella is more resistant to UV than E. coli (23).
The inactivation effect of UV-LEDs on pathogens itself is very meaningful, but every sterilization method may show very different results when applied to food. Through our experiments, we learned that UV lamps showed a significantly lowered (P < 0.05) germicidal effect than UV-LEDs at almost the same intensity through medium experiments, and actual application of UV-LEDs to a food matrix has never been implemented before. Therefore, we evaluated the application of UV-LEDs of 266, 270, 275, and 279 nm to inoculated sliced cheese. To inactivate pathogens on sliced cheese, much higher irradiation doses were needed compared to microbiological media. The reductions of pathogen populations on sliced cheese showed a tendency similar to what was observed in the medium experiments, including no significant differences (P > 0.05) within various wavelengths, and 3- to 4-log reductions were achieved after exposure at 3 mJ/cm2.
As for injured cells, nonselective TSA or SPRAB agar was used because stressed subpopulations are viable but not culturable in the presence of selective agents. They do have metabolic activity and can be resuscitated under the proper conditions but cannot be recovered or detected on typical selective media (24). E. coli O157:H7 and L. monocytogenes did not produce sublethally injured cells (Table 2), but S. Typhimurium after 0.5- and 0.7-mJ/cm2 exposures yielded about 1 log of injured cells at all of the wavelengths evaluated in our study. Choi et al. (25) investigated sublethally injured cells on cherry tomatoes inoculated with S. Typhimurium after a 2- to 10-kJ/m2 treatment with an UVC lamp, and injured cells increased from 60.73 to 93.14% as the irradiation dose increased. Also, there were no differences in L. monocytogenes population estimates in sterile-distilled water between samples enumerated on MOX and TSAYE (P > 0.05) after a 12.4-mJ/cm2 UV lamp exposure, a finding which indicates no sublethal injury occurred due to UV exposure (26). Although previous studies of UV-induced injured cells are not especially numerous, our results prove that UVC scarcely generates injured cells but that at high irradiation doses sublethally damaged cells can form in S. Typhimurium. However, the selective action of sodium desoxycholate in XLD is so powerful there is a tendency to underestimate actual live cell counts on this medium. Therefore, injured cells in XLD are not thought to be significant.
In conclusion, the use of UV-LEDs is an innovative and effective technology to decontaminate foodborne pathogens on agar media and sliced cheese. By irradiating sliced cheese only for approximately 10 min at a dosage of 3 mJ/cm2, ca. 99.99% of the pathogens were inactivated without affecting quality changes in color or generating significant numbers of injured cells.
ACKNOWLEDGMENTS
This research was supported by the Public Welfare and Safety Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (2012M3A2A1051679), and by the Agriculture, Food and Rural Affairs Research Center Support Program, Ministry of Agriculture, Food and Rural Affairs, Republic of Korea.
REFERENCES
- 1.Guerrero-Beltrán JA, Barbosa-Cánovas GV. 2004. Review: advantages and limitations on processing foods by UV light. Food Sci Technol Int 10:137–147. doi: 10.1177/1082013204044359. [DOI] [Google Scholar]
- 2.Dumé B. 2006. LEDs move into the ultraviolet. Physics World, Bristol, United Kingdom: http://physicsworld.com/cws/article/news/2006/may/17/leds-move-into-the-ultraviolet. [Google Scholar]
- 3.Mori M, Hamamoto A, Takahashi A, Nakano M, Wakikawa N, Tachibana S, Ikehara T, Nakaya Y, Akutagawa M, Kinouchi Y. 2007. Development of a new water sterilization device with a 365 nm UV-LED. Med Bio Eng Comput 45:1237–1241. doi: 10.1007/s11517-007-0263-1. [DOI] [PubMed] [Google Scholar]
- 4.Shin J-Y, Kim S-J, Kim D-K, Kang D-H. 2016. Fundamental characteristics of deep-UV light-emitting diodes and their application to control foodborne pathogens. Appl Environ Microbiol 82:2–10. doi: 10.1128/AEM.01186-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalisvaart BF. 2004. Re-use of wastewater: preventing the recovery of pathogens by using medium-pressure UV lamp technology. Water Sci Technol 50:337–344. [PubMed] [Google Scholar]
- 6.Sharma G. 2000. Ultraviolet light. Encyclopedia Food Microbiol 3:2208–2214. [Google Scholar]
- 7.CDC. 2015. Listeria outbreaks. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/listeria/outbreaks/index.html. [Google Scholar]
- 8.CDC. 2010. Multistate outbreak of Escherichia coli O157:H7 infections associated with cheese. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/ecoli/2010/cheese0157/index.html. [Google Scholar]
- 9.CDC. 1998. Salmonellosis associated with cheese consumption—Canada. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/mmwr/preview/mmwrhtml/00000370.htm. [Google Scholar]
- 10.CDC. 2008. Outbreak of multidrug-resistant Salmonella enterica serotype Newport infections associated with consumption of unpasteurized Mexican-style aged cheese—Illinois, March 2006–April 2007. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5716a4.htm. [PubMed] [Google Scholar]
- 11.CDC. 2013. Listeria and food. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/foodsafety/specific-foods/listeria-and-food.html. [Google Scholar]
- 12.Bolton JR, Linden KG. 2003. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J Environ Eng 129:209–215. doi: 10.1061/(ASCE)0733-9372(2003)129:3(209). [DOI] [Google Scholar]
- 13.Lee SY, Kang DH. 2001. Suitability of overlay method for recovery of heat-injured Listeria monocytogenes and Salmonella Typhimurium. Food Sci Biotechnol 10:323–326. [Google Scholar]
- 14.Rhee MS, Lee SY, Hiller VN, McCurdy SM, Kang DH. 2003. Evaluation of consumer-style cooking methods for reduction of Escherichia coli O157:H7 in ground beef. J Food Prot 66:1030–1034. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay S, Ukuku DO, Juneja V, Fan X. 2014. Effects of UV-C treatment on inactivation of Salmonella enterica and Escherichia coli O157:H7 on grape tomato surface and stem scars, microbial loads, and quality. Food Control 44:110–117. doi: 10.1016/j.foodcont.2014.03.027. [DOI] [Google Scholar]
- 16.Kim YH, Jeong SG, Back KH, Park KH, Chung MS, Kang DH. 2013. Effect of various conditions on inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in fresh-cut lettuce using ultraviolet radiation. Int J Food Microbiol 166:349–355. doi: 10.1016/j.ijfoodmicro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Can FO, Demirci A, Puri VM, Gourama H. 2014. Decontamination of hard cheeses by pulsed UV light. J Food Prot 77:1723–1731. doi: 10.4315/0362-028X.JFP-13-559. [DOI] [PubMed] [Google Scholar]
- 18.Saini JK, Marsden JL, Getty KJK, Fung DYC. 2014. Advanced oxidation technology with photohydroionization as a surface treatment for controlling Listeria monocytogenes on stainless steel surfaces and ready-to-eat cheese and turkey. Foodborne Pathogens Dis 11:295–300. doi: 10.1089/fpd.2013.1512. [DOI] [PubMed] [Google Scholar]
- 19.Chevremont AC, Farnet AM, Coulomb B, Boudenne JL. 2012. Effect of coupled UV-A and UV-C LEDs on both microbiological and chemical pollution of urban wastewaters. Sci Total Environment 426:304–310. doi: 10.1016/j.scitotenv.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Malo A, Palou E. 2005. Novel food processing technologies. CRC Press, Boca Raton, FL. [Google Scholar]
- 21.Friedberg EC, Walker GC, Siede W, Wood RD. 2006. DNA repair and mutagenesis, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 22.Beauchamp S, Lacroix M. 2012. Resistance of the genome of Escherichia coli and Listeria monocytogenes to irradiation evaluated by the induction of cyclobutane pyrimidine dimers and 6-4 photoproducts using gamma and UV-C radiations. Radiat Physics Chem 81:1193–1197. doi: 10.1016/j.radphyschem.2011.11.007. [DOI] [Google Scholar]
- 23.Rowan NJ, MacGregor SJ, Anderson JG, Fouracre RA, McIlvaney L, Farish O. 1999. Pulsed-light inactivation of food-related microorganisms. Appl Environ Microbiol 65:1312–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ueckert J, Breeuwer P, Abee T, Stephens P, Nebe von Caron G, ter Steeg PF. 1995. Flow cytometry applications in physiological study and detection of foodborne microorganisms. Int J Food Microbiol 28:317–326. doi: 10.1016/0168-1605(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 25.Choi DS, Park SH, Choi SR, Kim JS, Chun HH. 2015. The combined effects of ultraviolet-C irradiation and modified atmosphere packaging for inactivating Salmonella enterica serovar Typhimurium and extending the shelf life of cherry tomatoes during cold storage. Food Packaging Shelf Life 3:19–30. doi: 10.1016/j.fpsl.2014.10.005. [DOI] [Google Scholar]
- 26.McKinney J, Williams RC, Boardman GD, Eifert JD, Sumner SS. 2009. Dose of UV light required to inactivate Listeria monocytogenes in distilled water, fresh brine, and spent brine. J Food Prot 72:2144–2150. [DOI] [PubMed] [Google Scholar]