ABSTRACT
Pneumonia virus of mice (PVM) is a natural rodent pathogen that replicates in bronchial epithelial cells and reproduces many clinical and pathological features of the more severe forms of disease associated with human respiratory syncytial virus. In order to track virus-target cell interactions during acute infection in vivo, we developed rK2-PVM, bacterial artificial chromosome-based recombinant PVM strain J3666 that incorporates the fluorescent tag monomeric Katushka 2 (mKATE2). The rK2-PVM pathogen promotes lethal infection in BALB/c mice and elicits characteristic cytokine production and leukocyte recruitment to the lung parenchyma. Using recombinant virus, we demonstrate for the first time PVM infection of both dendritic cells (DCs; CD11c+ major histocompatibility complex class II+) and alveolar macrophages (AMs; CD11c+ sialic acid-binding immunoglobulin-like lectin F+) in vivo and likewise detect mKATE2+ DCs in mediastinal lymph nodes from infected mice. AMs support both active virus replication and production of infectious virions. Furthermore, we report that priming of the respiratory tract with immunobiotic Lactobacillus plantarum, a regimen that results in protection against the lethal inflammatory sequelae of acute respiratory virus infection, resulted in differential recruitment of neutrophils, DCs, and lymphocytes to the lungs in response to rK2-PVM and a reduction from ∼40% to <10% mKATE2+ AMs in association with a 2-log drop in the release of infectious virions. In contrast, AMs from L. plantarum-primed mice challenged with virus ex vivo exhibited no differential susceptibility to rK2-PVM. Although the mechanisms underlying Lactobacillus-mediated viral suppression remain to be fully elucidated, this study provides insight into the cellular basis of this response.
IMPORTANCE Pneumonia virus of mice (PVM) is a natural mouse pathogen that serves as a model for severe human respiratory syncytial virus disease. We have developed a fully functional recombinant PVM strain with a fluorescent reporter protein (rK2-PVM) that permits us to track infection of target cells in vivo. With rK2-PVM, we demonstrate infection of leukocytes in the lung, notably, dendritic cells and alveolar macrophages. Alveolar macrophages undergo productive infection and release infectious virions. We have shown previously that administration of immunobiotic Lactobacillus directly to the respiratory mucosa protects mice from the lethal sequelae of PVM infection in association with profound suppression of the virus-induced inflammatory response. We show here that Lactobacillus administration also limits infection of leukocytes in vivo and results in diminished release of infectious virions from alveolar macrophages. This is the first study to provide insight into the cellular basis of the antiviral impact of immunobiotic L. plantarum.
INTRODUCTION
Human respiratory syncytial virus (hRSV, family Paramyxoviridae, genus Pneumovirus) is a major cause of morbidity and death among infants and children worldwide and is recognized as a significant health concern among the elderly (1). The anti-hRSV antibody paluvizumab is highly effective when used as prophylaxis in infants identified as high risk (2), although there are many infants hospitalized with severe hRSV disease who are not identified in any of the high-risk cohorts (3). Treatment options for hRSV disease are primarily supportive. The antiviral agent ribavirin is currently recommended only for severely ill and immunocompromised children (4, 5). New antiviral agents that focus specifically on hRSV are in development (6–8), and the inflammatory responses characteristic of severe hRSV disease are also recognized as targets for therapeutic intervention (9, 10).
Pneumonia virus of mice (PVM) is a rodent pathogen of the same family and genus as hRSV. PVM infection in inbred strains of mice reproduces many of the clinical and pathological features of the more severe forms of hRSV disease, which has facilitated the exploration of new therapeutic strategies in vivo (11, 12). Similar to hRSV (13), PVM infects bronchial epithelial cells and promotes influx of granulocytes to the lung in association with the production of proinflammatory cytokines and chemokines. Blockade of proinflammatory signaling pathways, including those involving the chemokine receptor CCR1 and also chemerin R23, cysteinyl-leukotrienes, and sphingosine-1-phosphate (14–17), promotes improved outcomes by targeting the lethal inflammatory sequelae of PVM infection.
As part of our ongoing interest in the host antiviral inflammatory response, we have explored the immunomodulatory potential of various Lactobacillus species. While the impact of oral administration of probiotics, including Lactobacillus, in improving outcomes secondary to respiratory virus infection remains uncertain (18, 19), we have shown that direct priming of the respiratory tract with live or heat-inactivated Lactobacillus plantarum results in robust and sustained protection against a subsequent lethal PVM infection in association with profound suppression of virus-induced proinflammatory cytokines (20–22). This is a unique example of heterologous immunity, a response of the innate immune system that offers cross-protection from unrelated pathogens after a primary inflammatory or infectious event; this is known in other contexts as trained immunity, innate imprinting, or innate memory (23–25). Among several relevant examples of this concept, Wiley and colleagues (26) found that inhalation of Methanococcus-derived nanoparticles protected mice against several different acute respiratory virus infections. Likewise, Easton and colleagues (27) found that intranasal administration of defective interfering influenza virus particles is protective against a subsequent lethal PVM infection challenge, and Schnoeller and colleagues (28) recently reported that an attenuated preparation of Bordetella pertussis protects mice against symptoms related to a subsequent challenge with hRSV. Remaining unclear in all of these examples and likewise in response to priming with L. plantarum is the fate of the respiratory virus, specifically, whether the priming agent alters not only virus clearance but also the way in which the virus interacts with innate immune target cells in the respiratory tract.
In order to address these questions, we have generated a recombinant virus featuring PVM strain J3666 that incorporates the far-red fluorescent protein monomeric Katushka 2 (mKATE2) (29) by using a bacterial artificial chromosome (BAC)-based methodology developed by Hotard and colleagues (30). Using mKATE2 fluorescence to detect PVM-infected cells, we focused on interactions of the virus with resident leukocytes (e.g., alveolar macrophages [AMs]), as well as with cells that are recruited to the respiratory tract in response to acute infection.
MATERIALS AND METHODS
Mice.
BALB/c mice (6- to 8-week-old females) were from the Charles River Laboratories, Frederick, MD, facility. All mouse studies were approved by NIAID and carried out in accordance with Animal Care and Use Committee guidelines.
Lactobacillus.
L. plantarum BAA-793 was grown in Mann-Rogosa-Sharpe medium; the ratio of the optical density at 600 nm (OD600) to the CFU count was determined experimentally (20). Bacterial cells were washed, inactivated by serial freezing-thawing (20), and stored at −80°C at 1011/ml.
Generation of PVM minigenome.
The PVM minigenome reporter pGEM-PVM-Luc was constructed by replacing the RSV leader and trailer in a similar plasmid, pRSVlucM5 (31), with the leader and trailer sequences derived from PVM strain J3666. The PVM leader sequences (PVM 5′ untranslated region [UTR], GenBank accession no. NC_006579 bp 1 to 42), the PVM N gene start (bp 1036 to 1044 [32]), the PVM N noncoding region (bp 1045 to 1066) with flanking NotI and BamHI sites, the PVM trailer (PVM L noncoding region, GenBank accession no. NC_006579 bp 14653 to 14657), the PVM L gene end (bp 14781 to 14794 [32]), and the PVM 3′ UTR (bp 14795 to 14885) with flanking XhoI and HindIII sites were inserted to replace the RSV sequences. All DNA fragments were synthesized by GeneArt (Invitrogen); sequences were confirmed prior to transfer in order to generate pGEM-PVM-Luc. The pGEM-PVM-Luc plasmid was transfected into BSR T7/5 cells (33) as described below by using 0.8 μg of pGEM-PVM-Luc. Luciferase activity was monitored at 24 h by using the dual-luciferase reagent in accordance with the manufacturer's protocol (Promega).
Generation of the recombinant pSynK-PVMJ3666 antigenome in a BAC.
The antigenome of PVM strain J3666 (GenBank accession no. NC_006579.1) was synthesized in three fragments that were provided to us in the pMA-T cloning vector (GeneArt; Invitrogen). To facilitate sequential cloning of the three fragments, ZraI and SphI restriction sites were introduced between the BstB1 and MluI sites already present in BAC vector pKBS3 via a 47-bp linker, generating pKBS3-LINKER. Among the unique features of this construct, the gene encoding the red fluorescent protein mKATE2 (29) was introduced between the PVM N and L noncoding regions prior to the NS1 gene to allow tracking of PVM-infected cells by fluorescence as described previously for recombinant RSV line 19F (30). These and other features of the antigenome construct, to which we refer as pSynK-PVMJ3666, are provided in Table 1.
TABLE 1.
Features of the pSynK-PVMJ3666 constructa
| Feature | Location(s) | Size (nt)b | Description |
|---|---|---|---|
| BstBI | 11–16 | 6 | BstBI restriction site |
| phi 10 T7 promoter | 18–43 | 26 | T7 promoter |
| GGG | 41–43 | 3 | Miscellaneous |
| AAA to AAG | 44–46 | 3 | Elimination of MluI site |
| HamRz | 47–94 | 48 | Hammerhead ribozyme |
| Leader | 95–136 | 42 | 5′ UTR |
| PVM N GSc | 137–145 | 9 | N gene start |
| PVM N NC sequenced | 146–167 | 22 | N noncoding region |
| mKate2 | 168–866 | 699 | CDS,e red fluorescent protein |
| 5-nt PVM L NC sequence | 867–871 | 5 | L noncoding region |
| PVM L GEf | 872–885 | 14 | L gene end |
| PVM NS1/NS2 IGg | 886–897 | 12 | NS1-NS2 intergenic region |
| NS1 gene | 898–1303 | 306 | NS1 gene |
| NS1 CDS | 936–1277 | 342 | NS1 CDS, 113 aah |
| NS2 gene | 1316–1881 | 566 | NS2 gene |
| NS2 CDS | 1329–1799 | 471 | NS2 CDS, 156 aa |
| N gene | 1891–3105 | 1,215 | N gene |
| N gene start | 1891–1899 | 9 | N gene start |
| N CDS | 1922–3103 | 1,182 | N CDS, 393 aa |
| P-1 gene | 3121–4023 | 903 | P gene |
| P-1 gene start | 3121–3129 | 9 | P gene start |
| P-1 CDS | 3130–4017 | 888 | P CDS, 295 aa |
| P-2 CDS | 3251–3664 | 414 | P2 CDS, 156 aa |
| M gene | 4035–4961 | 927 | M gene |
| M CDS | 4045–4818 | 774 | M CDS, 257 aa |
| 4-bp addition | 4966–4969 | 4 | GACG added to make ZraI site |
| ZraI | 4966–4971 | 6 | ZraI restriction site |
| SH gene | 4972–5361 | 390 | SH gene |
| SH GS | 4972–4980 | 9 | SH gene start |
| SH CDS | 4982–5326 | 345 | SH CDS, 114 aa |
| 3-bp addition | 5369–5371 | 3 | CGA added to make ClaI site |
| G gene | 5373–6701 | 1,329 | G gene |
| G CDS | 5455–6645 | 1,191 | G CDS, 396 aa |
| 3-bp deletion, 4-bp insertion | 6707–6710 | 4 | Removal of native TAA and insertion of CGCG to make SacII site |
| F gene | 6724–8380 | 1,657 | F gene |
| F CDS | 6733–8346 | 1,614 | F CDS, 537 aa |
| G, GA insertions | 8403, 8406–8407 | 5 | Insertion of G and GA to make SalI site |
| M2-1 gene | 8444–9312 | 869 | M2-1 gene |
| M2-1 CDS | 8448–8978 | 531 | M2-1 CDS, 176 aa |
| M2-2 CDS | 8912–9208 | 297 | M2-2 CDS, 98 aa |
| L gene | 9329–15654 | 6,326 | L gene |
| L CDS | 9338–15460 | 6,123 | L CDS, 2,020 aa |
| SphI | 11,419–11424 | 6 | SphI restriction site |
| L GE | 15,647–15,654 | 8 | L gene end |
| Trailer | 15,655–15,756 | 102 | 3′ UTR |
| Insertion | 15663–15667 | 5 | Insertion to make BsiWI site |
| Hepatitis delta virus ribozyme | 15757–15840 | 84 | Hepatitis delta virus ribozyme |
| T7 terminator | 15841–15963 | 123 | T7 terminator |
| MluI | 15964–15969 | 6 | MluI restriction site |
| Chloramphenicol resistance | 16096–16755 | 660 | Chloramphenicol resistance CDS |
| OriS | 17782–18047 | 266 | Replication origin |
| repE | 18092–18847 | 756 | CDS for plasmid replication and regulation of copy no. |
| parA | 19426–20601 | 1,176 | CDS for plasmid partitioning to daughter cells during division and stable BAC maintenance |
| parB | 20601–21572 | 972 | CDS for plasmid partitioning to daughter cells during division and stable BAC maintenance |
| parC | 21645–22351 | 707 | CDS for plasmid partitioning to daughter cells during division and stable BAC maintenance |
The PVMJ3666 genome sequence (GenBank accession no. NC_006579) was used to construct this plasmid.
nt, nucleotides.
GS, gene start.
NC, noncoding.
CDS, coding sequence.
GE, gene end.
IG, intergenic.
aa, amino acids.
Isolation of pSynK-PVMJ3666 BAC DNA.
NEB10β competent Escherichia coli (New England BioLabs) was transformed with isolated DNA in accordance with the manufacturer's directions with the following modifications. Bacteria were permitted to recover from heat shock for 2 h at 32°C with aeration at 250 rpm. After 2 h, the transformed bacteria were collected by centrifugation and resuspended in 250 μl of super optimal broth with glucose; 20 or 200 μl was then plated on Luria-Bertani agar plates containing 20 ng/ml chloramphenicol (Sigma) and grown overnight at 32°C. Colonies from these plates were grown at 32°C at 250 rpm in medium containing 20 ng/ml chloramphenicol. BAC DNA was isolated with a Qiagen midi kit and a user-optimized protocol (QP01; Qiagen, Hilden, Germany). Approximately 2 μg of DNA was recovered from a 100-ml culture.
Generation and isolation of helper plasmids expressing PVMJ3666 L, M2-1, N, and P proteins.
The cDNA sequences for these plasmids were also based on the sequence of PVM strain J3666. The gene sequences encoding the PVM nucleoprotein (N), phosphoprotein (P), transcription factor M2-1, and polymerase (L) were converted to human codon preference, provided with a Kozak consensus sequence (34) at the ATG initiation codon, and inserted within the HindIII and EcoRI cloning sites of the pcDNA3.1 expression vector (GeneArt; Invitrogen). The nucleotide sequences of all support plasmids were confirmed directly. Bacteria transformed with these plasmids were grown at 32°C; plasmids were isolated with the Marligen maxiprep kit in accordance with the manufacturer's instructions.
Generation of recombinant mKATE2-PVM (rK2-PVM).
BSR T7/5 cells (33) were maintained in 10% fetal bovine serum (FBS) in Glasgow's minimum essential medium (GMEM; Invitrogen) and passed into six-well plates so that the cells would be confluent on the day of transfection. With a 3:1 ratio of Lipofectamine 2000 (microliters) to DNA (micrograms), the plasmids were transfected into these cells in the following quantities: 0.8 μg of pSynK-PVM J3666; 0.8 μg of pKBS3-LINKER (negative control); 0.4 μg each of pcDNA3.1 helper plasmids encoding the PVM J3666 M2-1, N, and P proteins; and 0.2 μg of plasmid pcDNA3.1 encoding the PVM J3666 L protein in a total volume of 500 μl of Opti-MEM (Invitrogen). After a 2-h incubation at room temperature on a plate shaker rotating at 300 rpm, an additional 500 μl of Opti-MEM was added to each well and the cells were incubated at 37°C in a 5% CO2 incubator for 24 h. Opti-MEM was then replaced with 2 ml of 3% FBS in GMEM, and the cultures were moved to 32°C. The cells were monitored daily by fluorescence microscopy for the expression of mKATE2. On day 3 posttransfection, the supernatant was collected and stored at −80°C prior to use in exploring the infectivity of rK2-PVM produced by these cells in naive cell monolayers in vitro. The remaining cells from the monolayer were then treated with trypsin and transferred into a T75 flask containing 106 parental BHK21 cells (CCL-10; ATCC). These cells were maintained until 50% were mKATE2 positive by fluorescence microscopy, and then the cells were passaged two more times onto fresh monolayers of BHK-21 cells as described by Krempl and colleagues (35). After three passages, the infected cells were scraped into the medium, disrupted by vortex mixing, and stored in aliquots at −80°C for use in the generation of high-titer virus stocks in mice.
Infection of RAW 264.7 cells.
Two milliliters of supernatant from BSR T7/5 cells transfected with either pSynK-PVMJ3666 or pKBS3-LINKER (negative control) were used to infect a subconfluent monolayer of RAW 264.7 cells (ATCC TIB-71) in an 80-cm2 flask. Cells inoculated as described above were incubated for 2 h at room temperature on a plate shaker at 300 rpm and then moved to 37°C at 5% CO2 for 24 h. After 24 h, the supernatant was replaced with 10 ml of RPMI 1640 medium with 2% FBS and the cells were moved to 32°C. The cells were monitored daily by fluorescence microscopy for the expression of mKATE2.
TCFD50 analysis.
Virus stocks were quantified by a variation of the standard 50% tissue culture infective dose analysis performed essentially as described previously (36) by detection of mKATE2+ fluorescent cells as the endpoint. Serial 10-fold dilutions of dialyzed virus were prepared in 2% FBS in RPMI medium with glutamine, penicillin, and streptomycin and evaluated on RAW 264.7 or A72 (ATCC CRL1542) cells in 96-well plates incubated at 32°C. The cells were monitored daily for mKATE2+ cells, and the 50% endpoint based on fluorescence (TCFD50/ml) was calculated at 5 to 6 days postinfection (37). By this method, the calculated TCFD50s of the mouse-passaged virus stocks ranged from 2.1 to 4.6 × 106 U/ml.
Amplification and evaluation of high-titer virus stocks.
rK2-PVM generated by the transfection of BSR T7/5 cells was amplified in mice. BALB/c mice received 100-μl inocula of the supernatant from the third-passage supernatant collected from the parental BHK21 cells. Lung tissue was collected 7 days after inoculation, and virus stocks were prepared as previously described (38). Briefly, lungs were collected under aseptic conditions and placed in 1 ml of ice-cold 10% FBS in Dulbecco's modified Eagle medium. The lung tissue was blade homogenized, and cellular debris was removed by two rounds of centrifugation. The clarified supernatants were stored at −80°C or liquid nitrogen and used for subsequent passages. Mouse-passaged rK2-PVM was used for all of the in vivo studies described in this report, unless otherwise noted.
Infection of BALB/c mice with native and recombinant PVM and priming with L. plantarum.
BALB/c mice under isoflurane anesthesia were inoculated intranasally with 50 μl of PVM strain J3666 (0.2 TCFD50) or rK2-PVM (2.1 to 210 TCFD50) diluted in Iscove's modified Dulbecco's medium. In the experiments indicated, BALB/c mice were first inoculated intranasally with heat-inactivated L. plantarum (50 μl, 2 × 1010 cells/ml) on day −14 and again on day −7 prior to a virus challenge on day 0, as previously described (20). Anti-PVM IgG1 in mouse serum was detected by enzyme-linked immunosorbent assay (ELISA; catalog no. SMART-M12; Biotech Trading Partners, El Cerrito, CA).
Isolation of airway macrophages from L. plantarum-primed, rK2-PVM-infected mice.
Bronchoalveolar lavage (BAL) fluid was collected from control and L. plantarum-primed, PVM-infected mice in a total of 4 ml of sterile phosphate-buffered saline with 0.1% bovine serum albumin (PBS-BSA). Cells were centrifuged at 2,000 × g for 10 min at 4°C and resuspended in RPMI medium containing 10% FBS, 2 mM glutamine, 100 IU of penicillin, and 10 μg/ml streptomycin (complete RPMI). Equal numbers of cells from all of the BAL fluid samples were plated. After 2 h, the nonadherent cells were removed and the adherent macrophages remaining were cultured for an additional 48 h. After 48 h, the culture supernatant was collected and dialyzed against 106 volumes of complete RPMI with 2% instead of 10% FBS across a 50-kDa membrane to remove low-molecular-weight cytokines prior to evaluation in a TCFD50 assay as described in the next section. In some experiments, airway macrophages were isolated from mice that were control or L. plantarum primed as described above but not virus infected.
Detection of rK2-PVM-infected cells by flow cytometry.
Single-cell suspensions from mouse lung tissue were prepared as previously described (20). Mediastinal lymph nodes from either side of the thymus dorsal to the heart (39) were dissected, and single-cell suspensions were prepared in RPMI 1640 by passing tissue through a 40-μm cell strainer. Cells were incubated with Live-Dead Fixable Aqua Dead Cell stain (Life Technologies) for 30 min prior to washing with PBS-BSA and then stained with fluorochrome-conjugated antibodies against CD16/CD32, CD8a, CD19, sialic acid-binding immunoglobulin-like lectin F (Siglec-F), and Gr1 (BD Biosciences); CD45, CD3, CD4, CD11c, and major histocompatibility complex class II (MHC-II)-A/I-K (eBioscience); and CD11b, CD24, and CD64 (BioLegend). After incubation with antibodies for 30 min at 4°C, the cells were washed with 3 ml of PBS-BSA and then fixed in 4% paraformaldehyde. The samples were stored at 4°C in the dark until they were analyzed. A minimum of 100,000 events was collected on an LSRII flow cytometer (BD Biosciences), and data were analyzed in FlowJo (Tree Star, Inc.).
Isolation of RNA and determination of virus titers.
RNA was isolated from cultured cells and supernatants of virus stocks with the RNeasy minikit in accordance with the manufacturer's instructions (Qiagen). Virus titers in lung tissue were determined as described previously (20, 40), except that the sequence 5′-AAG CAT TGC TAC ATC AGG C-3′, targeting the PVM SH gene, replaced the reverse primer.
Cytokine analysis.
ELISAs (R&D Systems) of clarified homogenates of lung tissue were performed and corrected for total protein by BCA assay (Pierce) as previously described (20).
Statistical analysis.
Findings presented as the mean ± the standard error of the mean were analyzed for statistical significance by two-way analysis of variance (ANOVA), Mann-Whitney U test, or log rank test, as indicated, in GraphPad PRISM 6.
RESULTS
Recombinant mKATE2-PVM (rK2-PVM): production and recovery of infectious recombinant virions.
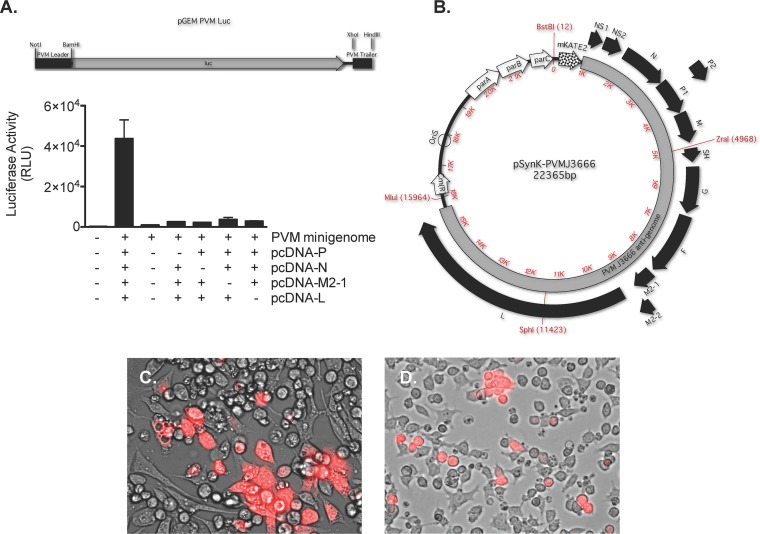
We first evaluated the effectiveness and specificity of the four individual helper plasmids, each with human codon-optimized sequences of the virus genes for P, N, M2-1, and L, at promoting the activity of a PVM minigenome reporter (pGEM-PVM-Luc) in BSR T7/5 cells (33) (Fig. 1A). As shown, significant luciferase activity from the pGEM-PVM-Luc reporter was detected only with the cotransfection of all four helper plasmids.
FIG 1.
Generation of a BAC-based reverse genetics system for PVM strain J3666. (A) Luciferase activity (in relative light units [RLU]) detected in lysates of BSR T7/5 cells transfected with the pGEM-PVM-Luc minigenome reporter together with individual helper plasmids encoding the PVM N, P, M2-1, and L proteins. The results shown are representative of an experiment performed in triplicate (n = 2). (B) The antigenome of PVM strain J3666 in BAC vector pKBS3. Red fluorescent protein mKATE2 (29) was introduced at the 3′ end of the viral genome to facilitate the detection of infected cells by fluorescence; all other features are as described in Table S1 in the supplemental material. (C) Detection of mKATE2 following the transfection of BSR T7/5 cells with pSynK-PVMJ3666 and the helper plasmids listed in panel A. Original magnification, ×40. mKATE2, pseudocolored red, is shown at 13 days posttransfection. The image shown is representative of two transfections. (D) Detection of mKATE2 following infection of RAW264.7 mouse macrophages with supernatant from the culture shown in panel C. Original magnification, ×40. mKATE2 pseudocolored red, is shown at 6 days postinfection. The image shown is representative of two experiments.
The antigenome of PVM strain J3666 preceded by the sequence encoding the far-red fluorescent protein mKATE2 (29) and flanked by PVM leader and trailer sequences was cloned into the BAC vector pKBS-LINKER, a modified version of vector pKBS3 that was altered to include ZraI, SphI, and MluI restriction enzyme sites in the multicloning site (pSynK-PVMJ3666; Fig. 1B). BSR T7/5 cells were transfected with pSynK-PVMJ3666 and the aforementioned four helper plasmids. Expression of mKATE2 in BSR T7/5 cells was detected from day 3 posttransfection (Fig. 1C). Supernatant from the transfected BSR T7/5 cells was used as a source of recombinant virus (rK2-PVM) to infect naive target cells of the RAW 264.7 mouse macrophage line. Expression of mKATE2 in the naive target macrophage cells was detected as early as 24 h after infection (Fig. 1D). No fluorescence was detected in cells infected with supernatants from BSR T7/5 cells transfected with helper plasmids and control plasmid pKBS3-LINKER (data not shown).
Infection with rK2-PVM is lethal in mice and elicits the characteristic inflammatory response.
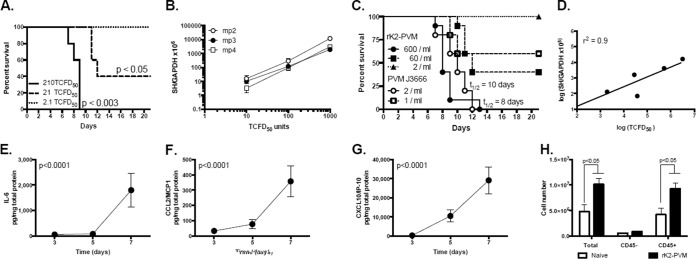
BALB/c mice were inoculated intranasally with mouse-passaged rK2-PVM. As shown in Fig. 2A, 210 TCFD50 in a 50-μl inoculation volume resulted in a fully lethal infection with a median survival time (t1/2) of 9 days. In contrast, a 60% mortality rate was observed in response to 21 TCFD50/50 μl. No deaths were observed in response to 2.1 TCFD50 in 50 μl, although seroconversion was observed (40% of mice at day 21). As shown in Fig. 2B, the recombinant virus is stable, with minimal variation (virion copy number versus TFCD50) observed over three passages in vivo. Although rK2-PVM is significantly (∼300 times) less virulent than parent PVM strain J3666 (Fig. 2C), fluorescence units (TCFD50) correlated directly with the standard measure of absolute virus copy number (absolute number of copies of virus SH per absolute copy of virus glyceraldehyde 3-phosphate dehydrogenase [GAPDH]) over a 104 range of virus concentrations (replication and clearance, days 3 to 11; Fig. 2D). As observed previously in response to infection with PVM strain J3666, proinflammatory cytokines interleukin-6 (IL-6) (Fig. 2E), CCL2 (Fig. 2F), and CXCL10 (Fig. 2G) were detected in lung tissue in response to infection with rK2-PVM. Furthermore, infection with rK2-PVM results in significant leukocyte (CD45+ cell) recruitment to the lung (Fig. 2H).
FIG 2.
Recombinant K2-PVM elicits symptomatic infection in mice. (A) Percent survival of BALB/c mice inoculated intranasally at t = 0 with increasing doses of rK2-PVM. Each mouse was inoculated with 2.1, 21, or 210 TCFD50 in a 50-μl inoculum. The P values shown reflect the log rank versus the lowest dose (n = 5 mice per group). (B) Evaluation of virus titer by both qRT-PCR (number of virion copies [SH gene] per copy of cellular GAPDH) and mKATE2 fluorescence in target RAW 264.7 cells (TCFD50) on dilutions of serial mouse-passaged stocks of rK2-PVM. mp, mouse passage number. (C) Percent survival of BALB/c mice inoculated at t = 0 with increasing doses of rK2-PVM (mp2) or parent PVM strain J3666. The virus titer in the inoculum was determined by qRT-PCR (1 to 600 virion copies/50-μl inoculation volume). Combined data from three experiments are shown (n = 5 to 10 mice per group). (D) Correlation of virus titer (number of virion copies [SH] per copy of GAPDH) with TCFD50 on target RAW 264.7 cells. Shown are titers of rK2-PVM evaluated on days 3, 6, 7, 9, and 11 of virus infection (r2 = 0.9). IL-6 (E), CCL2 (F), and CXCL10 (G) were measured in lung homogenates (in picograms per milligram of total protein) in response to 210 TCFD50 of rK2-PVM in a 50-μl inoculum. The P values shown are from one-way ANOVA. (H) Total cells, CD45− cells (nonleukocytes), and CD45+ cells (leukocytes) detected in single-cell suspensions of naive and rK2-PVM-infected mice at day 7 of infection (n = 5 to 10 mice per group combined from two experiments). P values were determined by Mann-Whitney U test.
Differential detection of mKATE2 among myeloid cells in rK2-PVM-infected mice.
Both mKATE2+ leukocytes (CD45+) and mKATE2+ nonleukocytes (CD45−) were detected at day 7 after inoculation. Of the leukocyte subsets examined, we detected significant fractions of mKATE2+ AMs, dendritic cells (DCs), and neutrophils. The remaining leukocyte subsets, including eosinophils, NK cells, B cells, CD4+ T cells, and CD8+ T cells, had no substantial mKATE2+ populations at that time point (<1%).
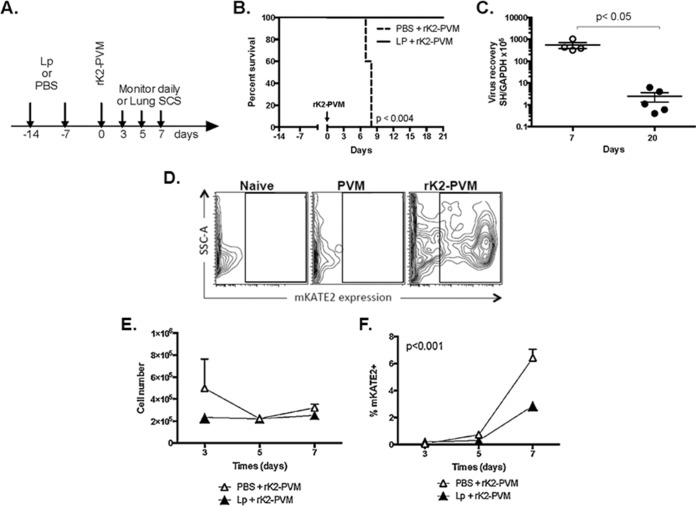
Priming with L. plantarum averts the lethal sequelae of rK2-PVM infection and limits PVM infection of CD45− cells.
We have shown previously that intranasal inoculation or priming of the respiratory tract with live or heat-inactivated preparations of L. plantarum results in robust and sustained protection from the lethal sequelae of severe pneumovirus infection in association with suppression of virus-induced inflammation (20–22). Our standard L. plantarum priming protocol is shown in Fig. 3A. Mice received an intranasal inoculation of heat-inactivated L. plantarum (109 cells in 50 μl) or diluent alone (PBS-BSA) on day −14 and again on day −7, followed by rK2-PVM (210 TCFD50 in 50 μl) on day 0. Similar to our previous findings with the parent strain, PVM J3666 (20, 21), mice that received diluent did not survive (t1/2 = 8 days) while those that were primed with L. plantarum were fully protected (100% survival) (Fig. 3B), with all undergoing seroconversion by day 21. As shown in Fig. 3C, L. plantarum-primed, rK2-PVM-infected wild-type mice cleared the virus from their lung tissue; minimal residual virus was detected at day 20 after inoculation.
FIG 3.
Priming of the respiratory tract with L. plantarum results in survival in response to lethal PVM infection and limits virus infection of CD45− cells in the respiratory tract. (A) The experimental protocol was intranasal inoculation of 109 CFU heat-inactivated L. plantarum (Lp) or diluent (PBS-BSA) in 50 μl on days −14 and −7, followed by 210 TCFD50/50 μl of rK2-PVM on day 0. Single-cell suspensions (SCS) were prepared from lung tissue on days 3, 5, and 7 as indicated. (B) Survival of mice inoculated as described for panel A. n = 5 mice per group; P < 0.04 (log rank test). (C) Virus recovery from L. plantarum-primed mice at 7 and 20 days postinoculation. P < 0.05 (Mann-Whitney U test); n = 4 or 5 mice per group. (D) Cells from lungs of uninfected mice (naive), mice infected with PVM J3666, and mice infected with rK2-PVM, demonstrating gating for mKATE2. (E) Total CD45− cells (SSC, side scatter) and (F) percentages of CD45− cells identified as mKATE2+ are shown. n = 4 to 10 mice per time point in two experiments. The P value is from two-way ANOVA comparing treatments over time.
An example of gating, comparing lung cell suspensions prepared from naive, PVM strain J3666-infected, and rK2-PVM-infected mice is shown in Fig. 3D. As shown in Fig. 3E, L. plantarum priming had no impact on the number of CD45− cells (nonleukocytes, predominantly type I pneumocytes [41]) detected in lung tissue. However, L. plantarum priming did suppress the infection of these cells, reducing the mKATE2+ population by approximately one-third (Fig. 3F, P < 0.001).
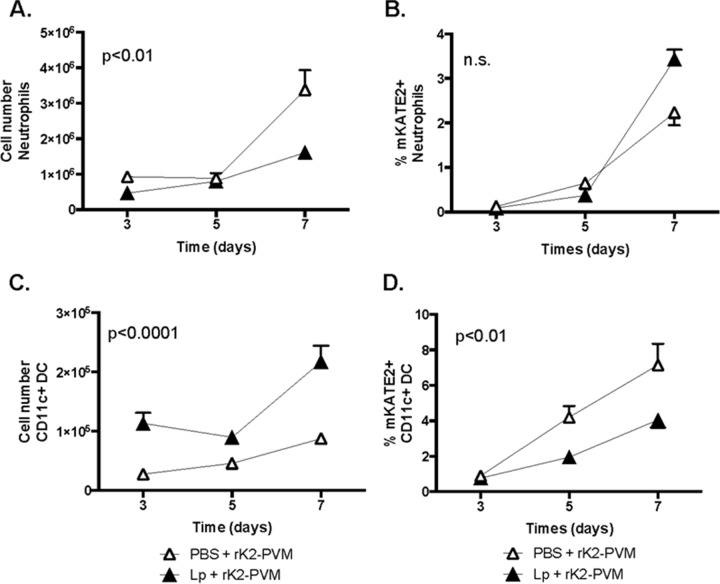
Priming with L. plantarum augments recruitment while suppressing infection of CD11c+ DCs.
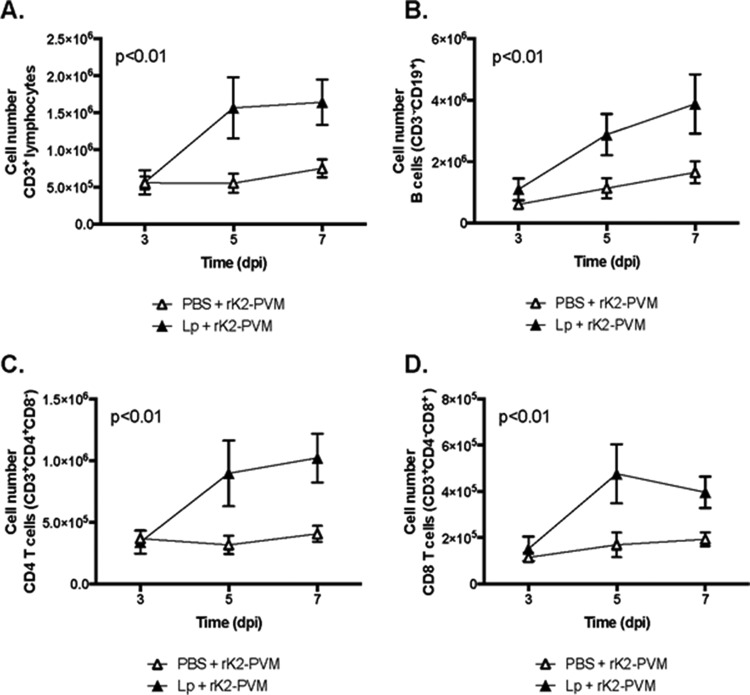
Given our earlier findings, we focused on AMs (CD11c+ Siglec-F+), DCs (CD11c+ MHCII+), and neutrophils (CD11c− Gr1+) as the significant leukocyte targets of L. plantarum priming. Consistent with our previous work (20), L. plantarum priming prior to virus infection resulted in diminished neutrophil recruitment (Fig. 4A; P < 0.01), although it had no impact on the relatively small fraction of neutrophils (>5%) that were mKATE2+ (Fig. 4B). In contrast, while PVM infection has been associated previously with the recruitment of CD11clo plasmacytoid DCs (42), we show here that L. plantarum priming resulted in augmented recruitment of CD11c+ MHC-II+ DCs (Fig. 4C; P < 0.0001) while at the same time reducing the fraction of infected mKATE2+ DCs by half (Fig. 4D; P < 0.01). Interestingly, although CD4+ T, CD8+ T, and B lymphocytes are not major targets of rK2-PVM infection and B cells are not critical for virus clearance or L. plantarum-mediated protection (22), priming with L. plantarum resulted in recruitment of these cells to the lungs at levels that exceed those observed in response to rK2-PVM infection alone (Fig. 5A to D).
FIG 4.
L. plantarum (Lp) priming targets DCs. The experimental protocol is the same as that described in the legend to Fig. 3A. (A) Total neutrophils (CD45+ CD11c− Gr1+). (B) Percentage of neutrophils identified as mKATE2+. (C) Total DCs (CD45+ CD11c+ MHC-II+). (D) Percentage of DCs identified as mKATE2+. For all panels, n = 5 to 10 mice per time point in two experiments. The P values shown are from two-way ANOVA comparing treatments over time. n.s., not significant.
FIG 5.
L. plantarum (Lp) priming augments the recruitment of T and B lymphocytes. The experimental protocol is the same as that described in the legend to Fig. 3A. Shown are total T cells (CD45+ CD3+) (A), CD8+ T cells (CD45+ CD3+ CD4− CD8+) (B), CD4+ T cells (CD45+ CD3+ CD4+ CD8−) (C), and B cells (CD3− CD19+) (D) recruited to the lung. n = 5 to 10 mice per time point in two experiments. The P values shown are from two-way ANOVA comparing treatments over time.
Priming with L. plantarum targets AMs.
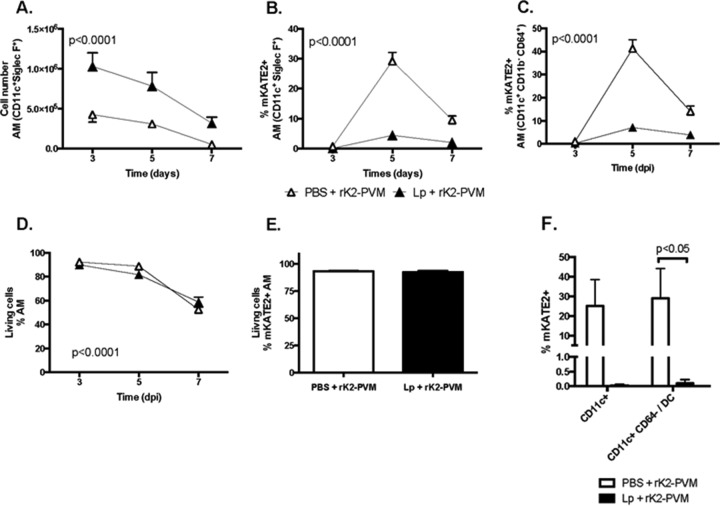
Priming resulted in a significant difference in the number of CD11c+ Siglec-F+ AMs detected, which also diminished over the course of infection (Fig. 6A; P < 0.001). AMs are long-lived cells that are capable of self-renewal and are not typically replenished from circulating progenitors under homeostatic conditions; however, exogenous perturbation, such as virus infection (and perhaps L. plantarum priming) may result in recruitment of monocytes from the periphery that will differentiate into AMs (reviewed in reference 43). L. plantarum priming also had a substantial impact on the fraction of mKATE2+ AMs (Fig. 6B and C; P < 0.0001). Specifically, at the peak (day 5), 30 to 40% of the AMs from control-primed mice were mKATE2+, while only 5 to 7% of the AMs from L. plantarum-primed mice were mKATE2+. These results are consistent whether we use the immunophenotyping protocol described by Guilliams and colleagues (44), in which AMs are reported as CD45+ CD11c+ Gr1− MHC-II− Siglec-F+ (utilizing Siglec-F, the only unique marker for AMs, when comparing them to other lung monocyte/macrophage and DC populations) (45), or the protocol of Misharin and colleagues (46), in which AMs are identified as CD45+ CD11c+ CD11b− CD64+. Exploring this observation further, we note that L. plantarum priming did not result in diminished viability of total AMs preferentially in rK2-PVM-infected mice; however, we did observe significant loss of AMs in both control and L. plantarum-primed, rK2-PVM-infected mice over time (P < 0.001, Fig. 6D), a finding that may account for some (or nearly all) of the decline in total AMs shown in Fig. 5A. Likewise, L. plantarum priming promotes no differential viability of the infected (mKATE2+) subpopulation of these cells (Fig. 6E). We also performed experiments to determine whether L. plantarum priming might promote the migration of rK2-PVM-infected cells to draining lymph nodes. Total and mKATE2+ cells were isolated from the mediastinal lymph nodes of control and L. plantarum-primed mice at 5 days after rK2-PVM inoculation. As shown in Fig. 6F, 25% ± 13% of the total CD11c+ cells, including 29% ± 15% of the CD45+ CD11c+ CD64− DCs (45) identified in mediastinal lymph nodes from control-primed, rK2-PVM-infected mice, were mKATE2+. In contrast, few (<0.1%) to no mKATE2+CD11c+ cells were detected in single-cell suspensions from mediastinal lymph nodes isolated from mice that were L. plantarum primed prior to rK2-PVM infection. Overall, our findings suggest that L. plantarum priming limits rather than promotes the transit of rK2-PVM and/or rK2-PVM-infected leukocytes to draining lymph nodes.
FIG 6.
L. plantarum (Lp) priming targets AMs. The experimental protocol is the same as that described in the legend to Fig. 3A. The total AMs (CD45+ CD11c+ Siglec-F+) (A), the percentage of AMs (CD45+ CD11c+ Siglec-F+) identified as mKATE2+ (B), the percentage of AMs (CD11c+ CD11b− CD64+) identified as mKATE2+ (C), and live AMs as a percentage of the total AMs (D) are shown. In panels A to D, n = 5 to 10 mice per time point in two experiments. Statistical significance was determined via two-way ANOVA comparing treatments over time. (E) Live mKATE2+ AMs as a percentage of total mKATE2+ AMs. (F) mKATE2+ cells as a percentage of the total CD11c+ cells and of total CD11c+ CD64+ cells in the mediastinal lymph nodes. n = 5 mice per group. The P value shown is from a Mann-Whitney U test.
Airway macrophages support active replication of rK2-PVM and produce replication-competent virions.
In order to evaluate the possibility of productive infection and the impact of L. plantarum priming on this process, we isolated macrophages from the airways of rK2-PVM-infected mice primed with L. plantarum or diluent alone (Fig. 7A). There was no difference in the AMs (defined as CD45+ CD11c+ Gr1− MHC-II− Siglec-F+) as a percentage of the total leukocytes (%CD45+ cells) isolated from the airways of L. plantarum-primed versus control-primed, rK2-PVM-infected mice (Fig. 7B). However, as might be anticipated from the results in Fig. 5A, we recovered significantly more CD11c+ Siglec-F+ AMs from the airways of L. plantarum-primed mice than from those of control mice (58 × 103 ± 15 × 103 versus 8.5 × 103 ± 1.8 × 103 per mouse; P < 0.01; Fig. 7C). As shown in Fig. 5B, although we isolated more CD45+ CD11c+ Gr1− MHC-II− Siglec-F+ AMs from the airways of L. plantarum-primed mice, the fraction of these AMs that were also mKATE2+ was substantially smaller (0.5% ± 0.1% mKATE2+ cells from L. plantarum-primed, rK2-PVM-infected mice versus 38% ± 7% mKATE2+ cells from control-primed, rK2-PVM-infected mice P < 0.01; Fig. 7D). To evaluate productive infection, equivalent numbers of airway macrophages from each group were evaluated ex vivo (100,000/well); no differential survival was observed after 48 h in culture (Fig. 7E), although there were significantly fewer mKATE2+ fluorescent foci among the airway macrophages from L. plantarum-primed, rK2-PVM-infected mice (34% ± 10% versus 2% ± 1%, P < 0.05; Fig. 7F). Supernatants from both cultures were evaluated in TCFD50 assays; those from control mice demonstrated a 100-fold (2-log) higher titer (TCFD50/ml) than those from L. plantarum-primed mice (Fig. 7G; P < 0.01). These results indicate that airway macrophages undergo productive infection when challenged with rK2-PVM and that L. plantarum priming of the respiratory tract limits not only virus replication but productive infection from these cells. It is most intriguing that when airway macrophages are isolated from L. plantarum-primed mice, introduced into tissue culture as described above, and challenged with rK2-PVM ex vivo, virus recovery is indistinguishable from that from control-primed counterparts (Fig. 7H). The implications of this finding with respect to L. plantarum priming and its mechanism of antiviral action are discussed below.
FIG 7.
L. plantarum (Lp) priming results in diminished recovery of infectious virus from airway macrophages. (A) Freshly isolated cells from the airways of rK2-PVM-infected mice. Original magnification, ×63; mKATE2+ pseudocolored red. (B) AMs (CD11c+ Siglec-F+) as a percentage of the CD45+ cells from the airways of control or L. plantarum-primed, rK2-PVM-infected mice on day 5 after inoculation. n = 5 mice per group. (C) Total AMs (CD11c+ Siglec-F+) isolated from the airways of control or L. plantarum-primed, rK2-PVM-infected mice (day 5). n = 5 mice per group; P < 0.01. (D) mKATE2+ AMs (CD11c+ Siglec-F+) as a fraction of the total AMs isolated from the airways as in panel C. n = 5 mice per group; P < 0.01. (E) Number of adherent cells per 10× microscopic field after 48 h in culture. n = 5 mice per group. (F) Number of fluorescent foci among adherent cells per 10× microscopic field after 48 h in culture. n = 5 mice per group; P < 0.05. (G) TCFD50/ml identified from supernatants of cultures in panels E and F. n = 3 to 5 cultures from individual mice; P < 0.01. All P values were determined by Mann-Whitney U test. (H) Virus titers in AMs isolated from PBS or L. plantarum-primed mice that were infected with rK2-PVM (2,000 TCFD50) ex vivo on day 5 after inoculation. n = 10 mice per group in two experiments.
DISCUSSION
In this study, we explored the interactions of PVM with leukocytes in the respiratory tract, identified both DCs and AMs as significant targets of infection in vivo, and explored the antiviral actions of immunobiotic L. plantarum administered to the respiratory tract.
As a crucial component of this study, we present a novel a reverse genetics system for the production of mKATE2-tagged recombinant PVM strain J3666 (rK2-PVM). This method is based on a reverse genetics system developed by Hotard and colleagues (30) for the production of recombinant RSV A-line 19F. There are several features that distinguish our system and that of Hotard and colleagues from others that have generated recombinant pneumoviruses (35, 47). Among the most prominent differences is the use of a BAC to support the virus antigenome. Similar methodologies have been used to generate recombinant Coronaviridae and Flaviviridae (reviewed in reference 48); among the advantages of this approach is the ease with which mutations can be introduced into the virus backbone via methods developed for the genetic engineering of E. coli. Another distinguishing feature of our systems is the conversion of the sequences encoding the helper plasmids to human codon preference in an effort to optimize protein expression. Lastly, the sequence encoding the fluorescent protein mKATE2 was added at the 3′ end of the PVM genome to facilitate virus detection both in vitro and in vivo. This 3′-end insertion may be responsible for (all or part of) the lower virulence of rK2-PVM than the parent PVM J3666 strain (Fig. 2B); as genes at the 3′ end of the virus genome are transcribed with greater frequency than those at the 5′ end (49), this insertion may lead to an overall reduction in the transcription of more distal PVM genes. However, we found that addition of mKATE2 had no impact on virus stability; there was no significant change in virus recovery, as determined by fluorescence intensity or by virion copy number, over several passages in mouse lung tissue (Fig. 2C).
As PVM is a natural rodent pathogen that promotes acute symptomatic respiratory disease in mice, our initial goal was to utilize rK2-PVM to identify and to characterize interactions of the virus with target cells in the respiratory tract. In previous studies, we demonstrated that PVM replicates in mouse macrophage cell lines and also in primary peritoneal macrophages isolated from mice and challenged ex vivo (50, 51). However, we demonstrate here for the first time that PVM infects both CD11c+ MHC-II+ DCs and CD11c+ Siglec-F+ AMs in vivo and that AMs support both active replication and production of infectious virions.
DCs have been characterized extensively as targets of respiratory virus infection, although infection is typically not productive of infectious virions (reviewed in references 43 and 52 to 55). hRSV specifically infects culture-derived monocyte-derived DCs (56, 57), as well as primary myeloid (CD11c+) and plasmacytoid (CD11clo/−) DCs, the former with greater efficiency (58). Human and mouse myeloid DCs are also susceptible to infection with influenza virus; infection is likewise not productive of infectious virions (59, 60) but can result in impaired antigen presentation (61).
Our findings, that recombinant PVM infects AMs in vivo and that AMs are a primary target of infection, are consistent with results of influenza virus infection studies (53, 54). In studies featuring pneumoviruses, isolated AMs from neonatal lambs support the replication of bovine RSV (62), as do AMs from hRSV-infected children (63). Adult human AMs can also be infected with hRSV ex vivo (64); this infection is productive and can generate virions in culture, although virion productivity has been described as short-lived (65).
These reports lead directly to our final observations. As shown in our earlier studies (20–22), priming of the respiratory tract with L. plantarum promotes heterologous immunity and results in full protection from the lethal sequelae of a subsequent acute respiratory virus infection in association with both profound suppression of the antiviral inflammatory response and diminished virus recovery from lung tissue. It is not immediately clear how priming with L. plantarum might alter virus kinetics (e.g., reduce the susceptibility of target cells and/or augment clearance via exogenous or endogenous means). We showed clearly that L. plantarum priming does not promote the trafficking of infected CD11c+ leukocytes to draining lymph nodes (Fig. 6F) or promote the differential survival of AMs in vivo (Fig. 6D). Considering first the case for endogenous mechanisms, several groups have shown that AMs are a major source of type I interferons (IFNs) in virus-challenged mice (66, 67). In our microarray analysis of whole lung tissue, we noted that L. plantarum versus control priming, followed by PVM infection, resulted in the differential regulation of numerous IFN-regulated genes, notably, IFN-induced GTP binding proteins Mx1 and Mx2, Stat3, and IFN regulatory proteins Irf1 and Irf7 (see Gene Expression Omnibus file GSE66721 and additional details in reference 68). Furthermore, L. plantarum priming resulted in the differential expression of transcripts encoding IFN-abR1, IFN-b1, Oas1b, and Isg15, among others, in AMs from rK2-PVM-infected mice (K. D. Dyer and H. F. Rosenberg, unpublished results). The role of type I IFNs in the limitation of virus replication in response to L. plantarum priming is currently under study. Interestingly, and despite our understanding of IFNs as potent antiviral regulators (69), L. plantarum-primed mice with deletions of genes for type I (or II) IFN receptors remain fully protected from the lethal sequelae of PVM infection (21, 69), which may be due to the substantial redundancy in pathways promoting protection (T. A. Rice et al., unpublished data).
Lactobacillus-mediated priming of the respiratory tract also results in the profound suppression of a specific subset of proinflammatory cytokines (20, 68), which may alter the responses of AMs in vivo (and perhaps not ex vivo, as discussed further below). We have also detected prominent upregulation of transcripts characteristic of alternatively activated (M2) macrophages, including resistin-like alpha and Ym1 (GSE66721; Rice and Rosenberg, unpublished findings). The impact of alternative activation and the role of M2 macrophages in the mitigation of negative sequelae of respiratory virus infection remain controversial points (70, 71); indeed, the entire issue of distinct M1/M2 states of macrophage activation has recently undergone substantial reconsideration (72). Interestingly, human cytomegalovirus and dengue virus replicate more (as opposed to less) efficiently in alternatively activated macrophages (73, 74).
A final point to consider is the impact of L. plantarum priming on lymphocyte recruitment. We have shown previously that L. plantarum priming augments the recruitment of lymphocytes to the lungs in response to PVM infection (20); we have added to these findings, and we show here that L. plantarum priming prior to rK2-PVM infection results specifically in greater recruitment of B cells, CD4+ T cells, and CD8+ T cells than that observed in response to rK2-PVM infection alone (Fig. 5). An examination of this finding is particularly important, as augmented levels of T cell-mediated virus clearance might explain the differences observed between virus replication in macrophages in vivo (in the presence of T cells) and that observed when primed AMs were challenged in tissue culture (i.e., Fig. 7G and H). We have already ruled out any substantial contributions from B cells alone; specifically, we observed diminished virus recovery and protection from lethal sequelae of PVM infection, both indistinguishable in both L. plantarum-primed wild-type and L. plantarum-primed B cell-deficient μMT mice (22). With respect to T cells and their role in acute PVM infection, Frey and colleagues (75) found that simultaneous depletion of both CD4+ and CD8+ T cells (and not single-lineage depletions) was necessary for any measurable impact on virus recovery. Thus, we have explored L. plantarum-mediated heterologous immunity in an initial series of experiments with wild-type and Rag1−/− (T and B cell-deficient) mice; both wild-type and Rag1−/− mice were protected acutely from the lethal sequelae of PVM in response to L. plantarum administration to the respiratory tract, and furthermore, virus recoveries at day 7 after inoculation with PVM strain J3666 are indistinguishable from one another (C. M. Percopo and H. F. Rosenberg, unpublished findings). Although these results suggest that T-cell mediated virus clearance may not play a prominent role in the L. plantarum antiviral mechanism overall, future experiments with rK2-PVM in Rag1−/− mice that focus on virus replication in AMs will permit a more comprehensive evaluation of this potential interaction.
In summary, in order to build on our understanding of virus-target cell interactions and the cellular basis of heterologous immunity in our system, we have generated a fluorescent-protein (mKATE2)-tagged recombinant PVM J3666 strain (rK2-PVM) that we have used to examine interactions with endogenous leukocytes during acute infection in vivo. In association with protection from the lethal sequelae of respiratory virus infection, we found that L. plantarum priming is significantly antiviral, notably targeting AMs, with a reduction from a peak of 40% to <10% mKATE2+ cells in association with a 2-log drop in the release of infectious virions. While the mechanisms underlying the observed antiviral response remain to be fully elucidated, they may involve Lactobacillus-mediated induction of antiviral cytokines in target AMs, notably, type I IFNs, which may have an impact on target cell susceptibility and/or virus clearance.
ACKNOWLEDGMENTS
We thank Klaus Conzelmann for his generous gift of the BSR T7/5 cell line. We also thank Jesse D. Keicher (Symmune Therapeutics, Raleigh, NC), Kirk M. Druey, and the members of the MSTS/LAD/NIAID for their many thoughtful comments and suggestions relating to this work.
This work was funded by NIH grants 1R01AI087798 and 1U19AI095227 to M.L.M. and Division of Intramural Research funding AI000943 to H.F.R.
REFERENCES
- 1.Tregoning JS, Schwarze J. 2010. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev 23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andabaka T, Nickerson JW, Rojas-Reyes MX, Rueda JD, Bacic Vrca V, Barsic B. 2013. Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children. Cochrane Database Syst Rev 4:CD006602. doi: 10.1002/14651858.CD006602.pub4. [DOI] [PubMed] [Google Scholar]
- 3.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N Engl J Med 360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Committee on Infectious Diseases. 1996. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 97:137–140. [PubMed] [Google Scholar]
- 5.Chu HY, Englund JA. 2013. Respiratory syncytial virus disease: prevention and treatment. Curr Top Microbiol Immunol 372:235–258. doi: 10.1007/978-3-642-38919-1_12. [DOI] [PubMed] [Google Scholar]
- 6.DeVincenzo J, Lambkin-Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, Meyers R, Gollob J, Vaishnaw A. 2010. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc Natl Acad Sci U S A 107:8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeVincenzo JP, Whitley RJ, Mackman RL, Scaglioni-Weinlich C, Harrison L, Farrell E, McBride S, Lambkin-Williams R, Jordan R, Xin Y, Ramanathan S, O'Riordan T, Lewis SA, Li X, Toback SL, Lin SL, Chien JW. 2014. Oral GS-5806 activity in a respiratory syncytial virus challenge study. N Engl J Med 371:711–722. doi: 10.1056/NEJMoa1401184. [DOI] [PubMed] [Google Scholar]
- 8.Plant H, Stacey C, Tiong-Yip CL, Walsh J, Yu Q, Rich K. 2015. High-throughput hit screening cascade to identify respiratory syncytial virus (RSV) inhibitors. J Biomol Screen 20:597–605. doi: 10.1177/1087057115569428. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee S, Lukacs NW. 2013. Innate immune responses to respiratory syncytial virus infection. Curr Top Microbiol Immunol 372:139–154. doi: 10.1007/978-3-642-38919-1_7. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg HF, Domachowske JB. 2012. Inflammatory responses to respiratory syncytial virus (RSV) infection and the development of immunomodulatory pharmacotherapeutics. Curr Med Chem 19:1424–1431. doi: 10.2174/092986712799828346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bem RA, Domachowske JB, Rosenberg HF. 2011. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol 301:L148–156. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer KD, Garcia-Crespo KE, Glineur S, Domachowske JB, Rosenberg HF. 2012. The pneumonia virus of mice (PVM) model of acute respiratory infection. Viruses 4:3494–3510. doi: 10.3390/v4123494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JE, Gonzales RA, Olson SJ, Wright PF, Graham BS. 2007. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod Pathol 20:108–119. doi: 10.1038/modpathol.3800725. [DOI] [PubMed] [Google Scholar]
- 14.Bondue B, Vosters O, de Nadai P, Glineur S, De Henau O, Luangsay S, Van Gool F, Communi D, De Vuyst P, Desmecht D, Parmentier M. 2011. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog 7:e1002358. doi: 10.1371/journal.ppat.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonville CA, Lau VK, DeLeon JM, Gao JL, Easton AJ, Rosenberg HF, Domachowske JB. 2004. Functional antagonism of chemokine receptor CCR1 reduces mortality in acute pneumovirus infection in vivo. J Virol 78:7984–7989. doi: 10.1128/JVI.78.15.7984-7989.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonville CA, Rosenberg HF, Domachowske JB. 2006. Ribavirin and cysteinyl leukotriene-1 receptor blockade as treatment for severe bronchiolitis. Antiviral Res 69:53–59. doi: 10.1016/j.antiviral.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Walsh KB, Teijaro JR, Brock LG, Fremgen DM, Collins PL, Rosen H, Oldstone MB. 2014. Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J Virol 88:6281–6293. doi: 10.1128/JVI.00464-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esposito S, Rigante D, Principi N. 2014. Do children's upper respiratory tract infections benefit from probiotics? BMC Infect Dis 14:194. doi: 10.1186/1471-2334-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozen M, Kocabas Sandal G, Dinleyici EC. 2015. Probiotics for the prevention of pediatric upper respiratory tract infections: a systematic review. Expert Opin Biol Ther 15:9–20. doi: 10.1517/14712598.2015.980233. [DOI] [PubMed] [Google Scholar]
- 20.Gabryszewski SJ, Bachar O, Dyer KD, Percopo CM, Killoran KE, Domachowske JB, Rosenberg HF. 2011. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J Immunol 186:1151–1161. doi: 10.4049/jimmunol.1001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia-Crespo KE, Chan CC, Gabryszewski SJ, Percopo CM, Rigaux P, Dyer KD, Domachowske JB, Rosenberg HF. 2013. Lactobacillus priming of the respiratory tract: heterologous immunity and protection against lethal pneumovirus infection. Antiviral Res 97:270–279. doi: 10.1016/j.antiviral.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Percopo CM, Dyer KD, Garcia-Crespo KE, Gabryszewski SJ, Shaffer AL III, Domachowske JB, Rosenberg HF. 2014. B cells are not essential for Lactobacillus-mediated protection against lethal pneumovirus infection. J Immunol 192:5265–5272. doi: 10.4049/jimmunol.1400087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ifrim DC, Quintin J, Joosten LA, Jacobs C, Jansen T, Jacobs L, Gow NA, Williams DL, van der Meer JW, Netea MG. 2014. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin Vaccine Immunol 21:534–545. doi: 10.1128/CVI.00688-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locati M, Mantovani A, Sica A. 2013. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol 120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. [DOI] [PubMed] [Google Scholar]
- 25.Wissinger E, Goulding J, Hussell T. 2009. Immune homeostasis in the respiratory tract and its impact on heterologous infection. Semin Immunol 21:147–155. doi: 10.1016/j.smim.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Wiley JA, Richert LE, Swain SD, Harmsen A, Barnard DL, Randall TD, Jutila M, Douglas T, Broomell C, Young M, Harmsen A. 2009. Inducible bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PLoS One 4:e7142. doi: 10.1371/journal.pone.0007142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Easton AJ, Scott PD, Edworthy NL, Meng B, Marriott AC, Dimmock NJ. 2011. A novel broad-spectrum treatment for respiratory virus infections: influenza-based defective interfering virus provides protection against pneumovirus infection in vivo. Vaccine 29:2777–2784. doi: 10.1016/j.vaccine.2011.01.102. [DOI] [PubMed] [Google Scholar]
- 28.Schnoeller C, Roux X, Sawant D, Raze D, Olszewska W, Locht C, Openshaw PJ. 2014. Attenuated Bordetella pertussis vaccine protects against respiratory syncytial virus disease via an IL-17-dependent mechanism. Am J Respir Crit Care Med 189:194–202. doi: 10.1164/rccm.201307-1227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shcherbo D, Murphy CS, Ermakova GV, Solovieva EA, Chepurnykh TV, Shcheglov AS, Verkhusha VV, Pletnev VZ, Hazelwood KL, Roche PM, Lukyanov S, Zaraisky AG, Davidson MW, Chudakov DM. 2009. Far-red fluorescent tags for protein imaging in living tissues. Biochem J 418:567–574. doi: 10.1042/BJ20081949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotard AL, Shaikh FY, Lee S, Yan D, Teng MN, Plemper RK, Crowe JE Jr, Moore ML. 2012. A stabilized respiratory syncytial virus reverse genetics system amenable to recombination-mediated mutagenesis. Virology 434:129–136. doi: 10.1016/j.virol.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dochow M, Krumm SA, Crowe JE Jr, Moore ML, Plemper RK. 2012. Independent structural domains in paramyxovirus polymerase protein. J Biol Chem 287:6878–6891. doi: 10.1074/jbc.M111.325258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dibben O, Easton AJ. 2007. Mutational analysis of the gene start sequences of pneumonia virus of mice. Virus Res 130:303–309. doi: 10.1016/j.virusres.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Buchholz UJ, Finke S, Conzelmann KK. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J Virol 73:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kozak M. 1987. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol 196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- 35.Krempl CD, Wnekowicz A, Lamirande EW, Nayebagha G, Collins PL, Buchholz UJ. 2007. Identification of a novel virulence factor in recombinant pneumonia virus of mice. J Virol 81:9490–9501. doi: 10.1128/JVI.00364-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Percopo CM, Dubovi EJ, Renshaw RW, Dyer KD, Domachowske JB, Rosenberg HF. 2011. Canine pneumovirus replicates in mouse lung tissue and elicits inflammatory pathology. Virology 416:26–31. doi: 10.1016/j.virol.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed LJ, Muench H. 1938. A simple method of estimating fifty per cent endpoint. Am J Hyg 27:493–497. [Google Scholar]
- 38.Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. 2000. The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J Immunol 165:2677–2682. doi: 10.4049/jimmunol.165.5.2677. [DOI] [PubMed] [Google Scholar]
- 39.Van den Broeck W, Derore A, Simoens P. 2006. Anatomy and nomenclature of murine lymph nodes: descriptive study and nomenclatory standardization in BALB/cAnNCrl mice. J Immunol Methods 312:12–19. doi: 10.1016/j.jim.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Percopo CM, Dyer KD, Karpe KA, Domachowske JB, Rosenberg HF. 2014. Eosinophils and respiratory virus infection: a dual-standard curve qRT-PCR-based method for determining virus recovery from mouse lung tissue. Methods Mol Biol 1178:257–266. doi: 10.1007/978-1-4939-1016-8_22. [DOI] [PubMed] [Google Scholar]
- 41.Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. 2010. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem 25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- 42.Davidson S, Kaiko G, Loh Z, Lalwani A, Zhang V, Spann K, Foo SY, Hansbro N, Uematsu S, Akira S, Matthaei KI, Rosenberg HF, Foster PS, Phipps S. 2011. Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway. J Immunol 186:5938–5948. doi: 10.4049/jimmunol.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morales-Nebreda L, Misharin AV, Perlman H, Budinger GRS. 2015. The heterogeneity of lung macrophages in the susceptibility to disease. Eur Respir Rev 24:505–509. doi: 10.1183/16000617.0031-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S, Deswarte K, Malissen B, Hammad H, Lambrecht BN. 2013. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp Med 210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kopf M, Schneider C, Nobs SP. 2015. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol 16:36–44. doi: 10.1038/ni.3052. [DOI] [PubMed] [Google Scholar]
- 46.Misharin AV, Morales-Nebreda L, Mutlu GM, Budinger GR, Perlman H. 2013. Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am J Respir Cell Mol Biol 49:503–510. doi: 10.1165/rcmb.2013-0086MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins PL, Fearns R, Graham BS. 2013. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol 372:3–38. doi: 10.1007/978-3-642-38919-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tischer BK, Kaufer BB. 2012. Viral bacterial artificial chromosomes: generation, mutagenesis, and removal of mini-F sequences. J Biomed Biotechnol 2012:472537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krempl C, Murphy BR, Collins PL. 2002. Recombinant respiratory syncytial virus with the G and F genes shifted to the promoter-proximal positions. J Virol 76:11931–11942. doi: 10.1128/JVI.76.23.11931-11942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dyer KD, Schellens IM, Bonville CA, Martin BV, Domachowske JB, Rosenberg HF. 2007. Efficient replication of pneumonia virus of mice (PVM) in a mouse macrophage cell line. Virol J 4:48. doi: 10.1186/1743-422X-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rigaux P, Killoran KE, Qiu Z, Rosenberg HF. 2012. Depletion of alveolar macrophages prolongs survival in response to acute pneumovirus infection. Virology 422:338–345. doi: 10.1016/j.virol.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guilliams M, Lambrecht BN, Hammad H. 2013. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal Immunol 6:464–473. doi: 10.1038/mi.2013.14. [DOI] [PubMed] [Google Scholar]
- 53.Mercer J, Greber UF. 2013. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol 21:380–388. doi: 10.1016/j.tim.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Short KR, Brooks AG, Reading PC, Londrigan SL. 2012. The fate of influenza A virus after infection of human macrophages and dendritic cells. J Gen Virol 93:2315–2325. doi: 10.1099/vir.0.045021-0. [DOI] [PubMed] [Google Scholar]
- 55.Waithman J, Mintern JD. 2012. Dendritic cells and influenza A virus infection. Virulence 3:603–608. doi: 10.4161/viru.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Graaff PM, de Jong EC, van Capel TM, van Dijk ME, Roholl PJ, Boes J, Luytjes W, Kimpen JL, van Bleek GM. 2005. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J Immunol 175:5904–5911. doi: 10.4049/jimmunol.175.9.5904. [DOI] [PubMed] [Google Scholar]
- 57.Jones A, Morton I, Hobson L, Evans GS, Everard ML. 2006. Differentiation and immune function of human dendritic cells following infection by respiratory syncytial virus. Clin Exp Immunol 143:513–522. doi: 10.1111/j.1365-2249.2005.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson TR, Johnson CN, Corbett KS, Edwards GC, Graham BS. 2011. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS One 6:e16458. doi: 10.1371/journal.pone.0016458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bender A, Albert M, Reddy A, Feldman M, Sauter B, Kaplan G, Hellman W, Bhardwaj N. 1998. The distinctive features of influenza virus infection of dendritic cells. Immunobiology 198:552–567. doi: 10.1016/S0171-2985(98)80078-8. [DOI] [PubMed] [Google Scholar]
- 60.Ioannidis LJ, Verity EE, Crawford S, Rockman SP, Brown LE. 2012. Abortive replication of influenza virus in mouse dendritic cells. J Virol 86:5922–5925. doi: 10.1128/JVI.07060-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smed-Sörensen A, Chalouni C, Chatterjee B, Cohn L, Blattmann P, Nakamura N, Delamarre L, Mellman I. 2012. Influenza A virus infection of human primary dendritic cells impairs their ability to cross-present antigen to CD8 T cells. PLoS Pathog 8:e1002572. doi: 10.1371/journal.ppat.1002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fach SJ, Meyerholz DK, Gallup JM, Ackermann MR, Lehmkuhl HD, Sacco RE. 2007. Neonatal ovine pulmonary dendritic cells support bovine respiratory syncytial virus replication with enhanced interleukin (IL)-4 And IL-10 gene transcripts. Viral Immunol 20:119–130. doi: 10.1089/vim.2006.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Midulla F, Villani A, Panuska JR, Dab I, Kolls JK, Merolla R, Ronchetti R. 1993. Respiratory syncytial virus lung infection in infants: immunoregulatory role of infected alveolar macrophages. J Infect Dis 168:1515–1519. doi: 10.1093/infdis/168.6.1515. [DOI] [PubMed] [Google Scholar]
- 64.Becker S, Soukup J, Yankaskas JR. 1992. Respiratory syncytial virus infection of human primary nasal and bronchial epithelial cell cultures and bronchoalveolar macrophages. Am J Respir Cell Mol Biol 6:369–374. doi: 10.1165/ajrcmb/6.4.369. [DOI] [PubMed] [Google Scholar]
- 65.Becker S, Quay J, Soukup J. 1991. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J Immunol 147:4307–4312. [PubMed] [Google Scholar]
- 66.Goritzka M, Makris S, Kausar F, Durant LR, Pereira C, Kumagai Y, Culley FJ, Mack M, Akira S, Johansson C. 2015. Alveolar macrophage-derived type I interferons orchestrate innate immunity to RSV through recruitment of antiviral monocytes. J Exp Med 212:699–714. doi: 10.1084/jem.20140825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Divangahi M, King IL, Pernet E. 2015. Alveolar macrophages and type I IFN in airway homeostasis and immunity. Trends Immunol 36:307–314. doi: 10.1016/j.it.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Percopo CM, Rice TA, Brenner TA, Dyer KD, Luo JL, Kanakabandi K, Sturdevant DE, Porcella SF, Domachowske JB, Keicher JD, Rosenberg HF. 2015. Immunobiotic Lactobacillus administered post-exposure averts the lethal sequelae of respiratory virus infection. Antiviral Res 121:109–119. doi: 10.1016/j.antiviral.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sadler AJ, Williams BR. 2008. Interferon-inducible antiviral effectors. Nat Rev Immunol 8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Li F, Sun R, Gao X, Wei H, Li LJ, Tian Z. 2013. Bacterial colonization dampens influenza-mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun 4:2106. doi: 10.1038/ncomms3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolf AI, Strauman MC, Mozdzanowska K, Williams KL, Osborne LC, Shen H, Liu Q, Garlick D, Artis D, Hensley SE, Caton AJ, Weiser JN, Erikson J. 2014. Pneumolysin expression by Streptococcus pneumoniae protects colonized mice from influenza virus-induced disease. Virology 462–463:254–265. doi: 10.1016/j.virol.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mosser DM, Edwards JP. 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bayer C, Varani S, Wang L, Walther P, Zhou S, Straschewski S, Bachem M, Soderberg-Naucler C, Mertens T, Frascaroli G. 2013. Human cytomegalovirus infection of M1 and M2 macrophages triggers inflammation and autologous T-cell proliferation. J Virol 87:67–79. doi: 10.1128/JVI.01585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller JL, de Wet BJ, Martinez-Pomares L, Radcliffe CM, Dwek RA, Rudd PM, Gordon S. 2008. The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathog 4:e17. doi: 10.1371/journal.ppat.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Frey S, Krempl CD, Schmitt-Graff A, Ehl S. 2008. Role of T cells in virus control and disease after infection with pneumonia virus of mice. J Virol 82:11619–11627. doi: 10.1128/JVI.00375-08. [DOI] [PMC free article] [PubMed] [Google Scholar]