ABSTRACT
Epstein-Barr virus (EBV) is a ubiquitous gammaherpesvirus associated with both B cell and epithelial cell malignancies. EBV infection of B cells triggers activation of several signaling pathways that are critical for cell survival, virus latency, and growth transformation. To identify EBV proteins important for regulating cell signaling, we used a proteomic approach to screen viral proteins for AP-1 and NF-κB promoter activity in AP-1– and NF-κB–luciferase reporter assays. We found that EBV BGLF2 activated AP-1 but not NF-κB reporter activity. Expression of EBV BGLF2 in cells activated p38 and c-Jun N-terminal kinase (JNK), both of which are important for mitogen-activated protein kinase (MAPK) signaling. Deletion of the carboxyl-terminal 66 amino acids of BGLF2 reduced the ability of BGLF2 to activate JNK and p38. Expression of BGLF2 enhanced BZLF1 expression in latently EBV-infected lymphoblastoid cell lines, and knockdown of BGLF2 reduced EBV reactivation induced by IgG cross-linking. Expression of BGLF2 induced BZLF1 expression and virus production in EBV-infected gastric carcinoma cells. BGLF2 enhanced BZLF1 expression and EBV production by activating p38; chemical inhibition of p38 and MAPK/ERK kinases 1 and 2 (MEK1/2) reduced expression of BZLF1 and virus production induced by BGLF2. In summary, the EBV tegument protein BGLF2, which is delivered to the cell at the onset of virus infection, activates the AP-1 pathway and enhances EBV reactivation and virus production.
IMPORTANCE Epstein-Barr virus (EBV) is associated with both B cell and epithelial cell malignancies, and the virus activates multiple signaling pathways important for its persistence in latently infected cells. We identified a viral tegument protein, BGLF2, which activates members of the mitogen-activated protein kinase signaling pathway. Expression of BGLF2 increased expression of EBV BZLF1, which activates a switch from latent to lytic virus infection, and increased production of EBV. Inhibition of BGFL2 expression or inhibition of p38/MAPK, which is activated by BGLF2, reduced virus reactivation from latency. These results indicate that a viral tegument protein which is delivered to cells upon infection activates signaling pathways to enhance virus production and facilitate virus reactivation from latency.
INTRODUCTION
Epstein-Barr virus (EBV) is a cause of infectious mononucleosis and is associated with both B lymphocyte and epithelial cell malignancies. EBV encodes a number of proteins that trigger cell signaling pathways, such as AP-1, JAK-STAT, NF-κB, and phosphatidylinositol 3-kinase (PI3K)/Akt, which are critical for cell survival, virus latency, and growth transformation (1–6). For example, EBV latent membrane protein 1 (LMP1) mimics CD40 signaling and is important for EBV-induced B cell growth proliferation, inhibition of apoptosis, and EBV transformation. LMP1 interacts with tumor necrosis factor receptor-associated factors (TRAFs), leading to activation of NF-κB, c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), PI3K/Akt, and STAT3 (7–12). EBV LMP2A mimics B cell receptor signaling and contributes to the long-term survival of B cells (13–16). LMP2A activates extracellular signal-related protein kinase (ERK), PI3K/Akt, and JNK (17) and downregulates NF-κB and STAT signaling pathways (18). The EBV immediate early protein BZLF1 activates virus reactivation from latency (19–21). BZLF1 activates p38 and JNK to turn on the ATF2 transcription factor (22). BRLF1, another EBV immediate early protein, activates the AP-1 pathway by increasing the levels of phosphorylated p38, JNK, and ERK (22, 23) and induces phosphorylation of Akt through the PI3K pathway (24). Activation of Akt, ERK, and p38 signaling is required for EBV reactivation from latency (25–28).
To identify additional EBV proteins important for regulating cell signaling, we used a proteomic approach to screen viral proteins for AP-1 promoter activity in AP-1–luciferase reporter assays. We found that the EBV tegument protein BGLF2 induced AP-1 reporter activity and activated p38 and JNK. BGLF2 promoted EBV reactivation by enhancing BZLF1 expression and EBV production, and p38 activation by BGLF2 was required for these activities.
MATERIALS AND METHODS
Cells.
Human embryonic kidney (HEK293T) cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS), EBV-infected Akata and Raji Burkitt lymphoma cells were grown in RPMI medium with 15% FBS, and EBV-infected AGS-EBV-GFP cells (29) were grown in Ham's F-12 medium with 15% FBS.
Plasmids and cosmids.
Individual EBV open reading frames (ORFs) were amplified by PCR from DNAs of five cosmids that encompass the complete EBV genome (30) and were inserted into the multiple-cloning site of pcDNA3.1 (Invitrogen). The following ORFs were cloned: BVRF2, BRRF2, BCRF1, BBLF4, BBRF1, BWRF1, BSRF1, BKRF2, BRRF1, BBRF2, BKRF4, BALF1, BNLF2a, BBRF3, K15, BHRF1, BFLF2, BFRF2, BFRF3, BaRF1, BLLF3, BLRF1, BLRF2, BZLF1, BBLF1, BGLF4, BGLF3, BGLF2, BDLF4, BDLF3, BDLF2, BXLF1, BXRF1, BVRF1, BVLF1, BILF1, BALF4, BARF1, LMP-1, BALF2, BDLF1, BcLF1, and BXLF2. The plasmids express the EBV proteins tagged with a V5 epitope (found in the P and V proteins of the simian virus 5 [SV5] paramyxovirus) at their carboxyl termini. All plasmid inserts were sequenced, and protein expression was verified by transfection followed by immunoblotting with anti-V5 antibody (AbD Serotec). Plasmid pDK24 (which contains the EBV BamHI Z fragment) and cosmids EBV SalI-EC and EBV EcoRI-B each contain the BZLF1 gene and its promoter (30, 31). A plasmid expressing varicella-zoster virus (VZV) ORF12 was constructed previously (32), and a plasmid expressing the catalytic domain of MEK kinase 1 (MEKK1) was obtained from Stratagene.
Luciferase reporter assays.
293T cells were transfected using GeneJuice (Novagen) and a combination of (i) 20 ng of plasmid expressing an EBV open reading frame, (ii) 20 ng of plasmid with an AP-1 (Stratagene)- or NF-κB-responsive element driving firefly luciferase expression (kindly provided by Katherine Fitzgerald, University of Massachusetts), and (iii) 5 μg of plasmid expressing Renilla luciferase under the control of the herpes simplex virus (HSV) thymidine kinase promoter (Promega). At 24 h posttransfection, cells in 96-well plates were incubated for 12 min with 50 μl of 1× passive lysis buffer (Promega), cell lysates (30 μl) were transferred to a luciferase assay plate (Costar), 30 μl of Dual-Glo substrate was added, and firefly and Renilla luciferase activities were assayed using a dual-luciferase assay system (Promega) as described previously (33). The ratio of firefly to Renilla luciferase activity was calculated. The reporter activity was expressed as the fold induction of the luciferase reporter after normalization to green fluorescent protein (GFP) expression in plasmid-transfected cells.
Lentivirus production.
Lentiviral vectors were constructed to express FLAG-tagged BGLF2 (pLenti6/Ubc-BGLF2-Flag), V5-tagged BGLF2 (pLenti6/Ubc-BGLF2-V5 or pSILK-TTmiR-BGLF2-V5, which expresses BGLF2 under the control of a doxycycline-inducible promoter [34]), or shBGLF2 (pSILK-shBGLF2). The shBGLF2 sequences used were as follows: shBGLF2-1, 5′-GCCCGAGGTTCTCTTCACTAAG; and shBGLF2-2, 5′-ACCTGCTAACCAGATGTGATAA. These sequences correspond to BGLF2 nucleotides 257 to 280 and 598 to 620, respectively, and were cloned as described previously (35, 36). HEK293T cells in 10-cm dishes were transfected with 7.5 μg of lentiviral vectors expressing BGLF2 along with 5 μg of a plasmid expressing vesicular stomatitis virus glycoprotein G (pVSV-G) and 7.5 μg each of the two packaging plasmids (pMDLg/pRRE and pRSVREV) (34) diluted in Opti-MEM (Gibco Invitrogen) by using Lipofectamine 2000 reagent (Invitrogen). The medium was replaced with DMEM containing 10% FBS 16 h after transfection, and 48 h after transfection, the supernatant (containing virus) was centrifuged, filtered, and stored at −80°C.
Cell line construction.
HEK293T, AGS-EBV-GFP, and Akata-EBV cells were transduced with lentiviruses expressing V5- or FLAG-tagged BGLF2 or BGLF2ΔC and selected with blasticidin to obtain cell lines stably expressing BGLF2, termed HEK293T-BGLF2-V5, HEK293T-TTmiR-BGLF2-V5, AGS-EBV-GFP-BGLF2-Flag, AGS-EBV-GFP-BGLF2ΔC, and Akata-EBV-TTmiR-BGLF2-V5. Induction of BGLF2 expression in cells transduced with lentivirus from plasmid pSILK-TTmiR-BGLF2-V5 was achieved by adding doxycycline to the medium for 48 to 72 h.
Immunoblotting, antibodies, and inhibitors.
Cell lysates were fractionated in polyacrylamide gels, transferred to nitrocellulose membranes, and incubated with mouse anti-EBV BZLF1 (sc-53904; Santa Cruz), anti-BMRF1 (MAB8186; Millipore), anti-FLAG (M2; Sigma), rabbit anti-p-ERK1/2, anti-ERK1/2, anti-p-p38, anti-p38, anti-p-JNK, anti-JNK, and anti-GFP antibodies (Cell Signaling Technology), or mouse anti-actin antibody (Sigma). The MEK1/2 inhibitor U0126 (EMD Chemicals), the p38 inhibitor SB202190 (Gibco), and the JNK1/2 inhibitor SP600125 (Gibco) were added to cells where appropriate.
Detection of infectious EBV.
Supernatants from AGS-EBV-GFP cells were collected 2 or 3 days after treatment with methotrexate (MTX) or a p38, JNK, or ERK inhibitor and filtered through 0.45-μm filters (Corning). Two milliliters of filtered supernatant was used to infect Raji cells, and 3 days later the cells were collected for immunoblotting to detect GFP for quantitation of the amount of infectious EBV in the supernatants.
RESULTS
BGLF2 activates the AP-1 pathway.
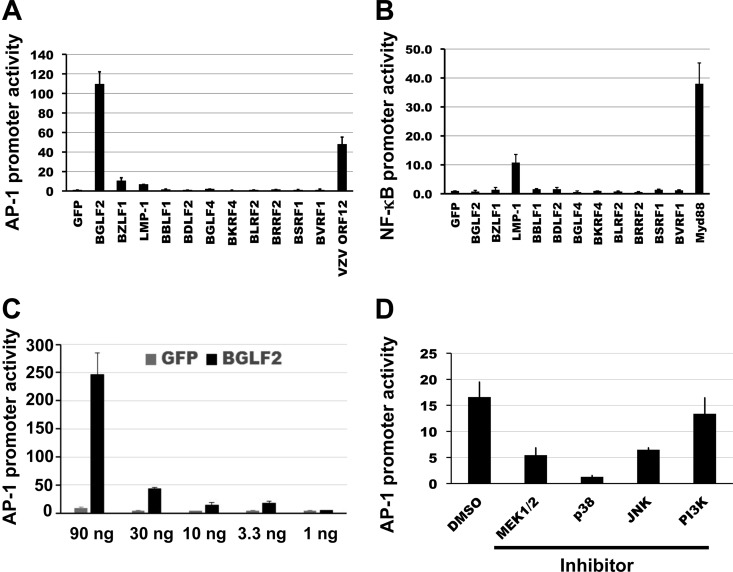
Since several EBV proteins, including LMP1, LMP2, and BZLF1, activate the MAPK pathway, and since this pathway is important for EBV lytic infection and virus reactivation, we searched for additional viral proteins that activate this pathway by using a proteomic approach. We constructed an EBV protein expression library and screened viral proteins for activation of the MAPK pathway by using an AP-1 reporter assay. 293T cells were transfected with (i) individual plasmids encoding EBV open reading frames, (ii) an AP-1 reporter plasmid expressing firefly luciferase under the control of an AP-1-responsive promoter, and (iii) a plasmid expressing Renilla luciferase under the control of the HSV-1 thymidine kinase promoter (pRL-TK), which allowed us to control for the efficiency of transfection. Of the EBV open reading frames tested, BGLF2 strongly enhanced AP-1 reporter activity, with a level approximately 100-fold higher than that of a control plasmid expressing GFP (Fig. 1A). The level of activation of the AP-1 reporter by EBV BGLF2 was about 2-fold higher than that observed with a plasmid expressing VZV ORF12, which we previously showed to activate the AP-1 pathway (32). We also confirmed that EBV LMP1 and BZLF1 activate the AP-1 pathway as reported previously (22, 37), although the level of activation was lower than that with BGLF2. In contrast, expression of other EBV proteins, including the tegument proteins BBLF1, BDLF2, BGLF4, BKRF4, BLRF2, BRRF2, BSRF1, and BVRF1, did not activate the AP-1 reporter; expression of each of these tegument proteins was confirmed by using an antibody to the V5 epitope tag. To ensure that BGLF2 activation of the AP-1 reporter was specific and not due to a general effect on the luciferase reporter, we tested the ability of BGLF2 to activate an NF-κB–luciferase reporter. Transfection of the BGLF2-expressing plasmid did not activate the NF-κB reporter, while LMP1 expression activated NF-κB reporter activity as expected (Fig. 1B). AP-1 reporter activity increased in response to larger amounts of transfected BGLF2 plasmid (Fig. 1C).
FIG 1.
BGLF2 activates AP-1 promoter activity. An AP-1–firefly luciferase reporter plasmid (A) or NF-κB–firefly luciferase reporter plasmid (B), along with a Renilla luciferase plasmid and plasmids expressing EBV proteins, the VZV ORF12 protein, Myd88, or GFP, were transfected into 293T cells, and AP-1 and NF-κB reporter activities were plotted as luciferase units normalized for transfection efficiency. (C) AP-1 reporter activity was determined in 293T cells transfected with different amounts of BGLF2 or GFP plasmid. (D) 293T cells were transfected with an AP-1 reporter plasmid, a control pRL-TK plasmid, and a BGLF2 expression plasmid for 20 h. Cells were then treated with a MEK1/2 inhibitor (U0126; 20 μM), a p38 inhibitor (SB202190; 20 μM), a JNK inhibitor (SP600125; 50 μM), or a PI3K inhibitor (LY294002; 10 μM), and luciferase activity was measured as described as above. The errors bars represent the standard errors.
Activation of AP-1 is regulated by upstream MAPKs, including ERK1/2, p38, and JNK. To determine which MAPK(s) is activated by BGLF2, 293T cells were transfected with the BGLF2 plasmid, the AP-1 reporter plasmid, and the pRL-TK plasmid, and 20 h later cells were treated with a MEK1/2 inhibitor (U0126), a p38 inhibitor (SB202190), a JNK inhibitor (SP600125), or a PI3K inhibitor (LY2940001). Measurement of luciferase activity 6 h after treatment showed that BGLF2 activation of the AP-1 reporter was markedly reduced by the p38 inhibitor and, to a lesser extent, by the JNK and MEK1/2 inhibitors, but not by the PI3K inhibitor (Fig. 1D).
Expression of BGLF2 induces p38 and JNK phosphorylation.
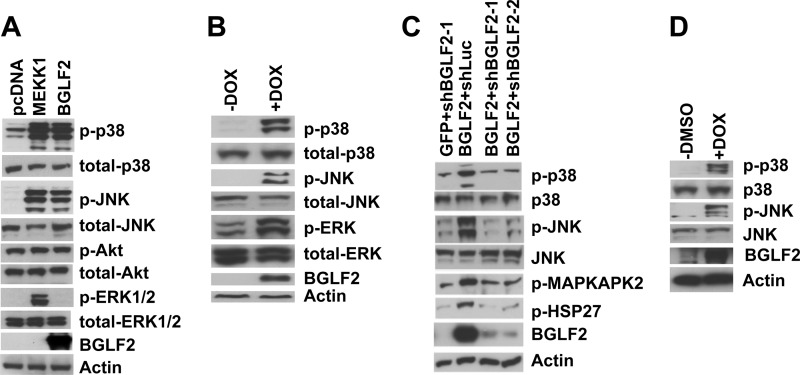
To verify that the increased AP-1 reporter activity was due to BGLF2 activation of MAPKs, we measured the levels of phosphorylated p38, JNK, Akt, and ERK1/2 in 293T cells transfected with a plasmid expressing BGLF2 tagged with the V5 epitope. Expression of BGLF2 strongly activated p38 and JNK, similar to the activation observed with a positive-control plasmid expressing the catalytic portion of MEKK1, while expression of BGLF2 did not activate Akt or ERK (Fig. 2A). BGLF2 was expressed, and the levels of actin were similar for all the samples. To ensure that BGLF2 activation of p38 and JNK was not due to the transfection procedure, we constructed a cell line expressing BGLF2 under the control of a doxycycline-inducible promoter (293T-BGLF2-V5), and the addition of doxycycline induced BGLF2 expression (Fig. 2B). Expression of BGLF2 increased both p38 and JNK phosphorylation, while the total levels of p38 and JNK did not increase with increased BGLF2. BGLF2 expression also activated ERK, although ERK was constitutively phosphorylated in the cell line (Fig. 2B).
FIG 2.
Expression of BGLF2 induces p38 and JNK phosphorylation. (A) 293T cells were transfected with a plasmid expressing the BGLF2 protein with a V5 epitope tag, a plasmid expressing MEKK1 (positive control), or pcDNA3.1 (negative control), and after 48 h, cell lysates were immunoblotted with an antibody to p38, p-p38, JNK, p-JNK, Akt, p-Akt, ERK1/2, p-ERK1/2, actin, or V5 (to detect BGLF2). (B) 293T-BGLF2 cells, which express BGLF2 under the control of a doxycycline (DOX)-inducible promoter, were treated with or without DOX for 2 days to induce expression of BGLF2, and cell lysates were immunoblotted for signaling proteins. (C) 293T-lenti6-BGLF2 cells, which constitutively express BGLF2, were transduced with a lentivirus-mediated shRNA against BGLF2 (shBGLF2) or luciferase (shLuc), and after 4 days, cell lysates were collected for immunoblotting. (D) Akata-EBV cells were transduced with a lentivirus expressing BGLF2 under the control of a DOX-inducible promoter and were treated with or without DOX for 4 days, and cell lysates were collected to detect p-p38, p38, p-JNK, JNK, BGLF2 (using an antibody to V5), and actin.
Transduction of 293T-BGLF2 cells with lentiviruses constitutively expressing BGLF2-specific short hairpin RNAs (shRNAs) (shBGLF2-1 and -2) resulted in reduced levels of BGLF2; in contrast, the level of BGLF2 remained elevated after transduction with a control lentivirus expressing luciferase-specific shRNA (Fig. 2C). Reduction of the BGLF2 level in 293T-BGLF2 cells after lentivirus transduction with BGLF2 shRNAs resulted in reduced phosphorylation of both p38 and JNK. Activated p38 affects a number of downstream effector molecules, such as heat shock protein 27 (HSP27) and MAP kinase-activated protein kinase 2 (MAPKAPK-2). Activation of p38 by EBV BGLF2 resulted in phosphorylation of HSP27 and MAPKAPK-2, while reduced expression of BGLF2 by shBGLF2 resulted in less phosphorylation of these proteins. Thus, BGLF2 activation of p38 affected its downstream targets.
We also examined the effects of BGLF2 expression in EBV-positive Burkitt lymphoma cells latently infected with EBV. Transduction of EBV-positive Akata cells with a lentivirus expressing BGLF2 resulted in phosphorylation of p38 and JNK (Fig. 2D). Taken together, these results indicate that BGLF2 activates p38 and JNK in both epithelial (293T) cells and B lymphoma cells and that BGLF2 activation of p38 results in phosphorylation of its downstream targets.
The carboxyl-terminal domain of BGLF2 is required for activation of AP-1 and JNK.
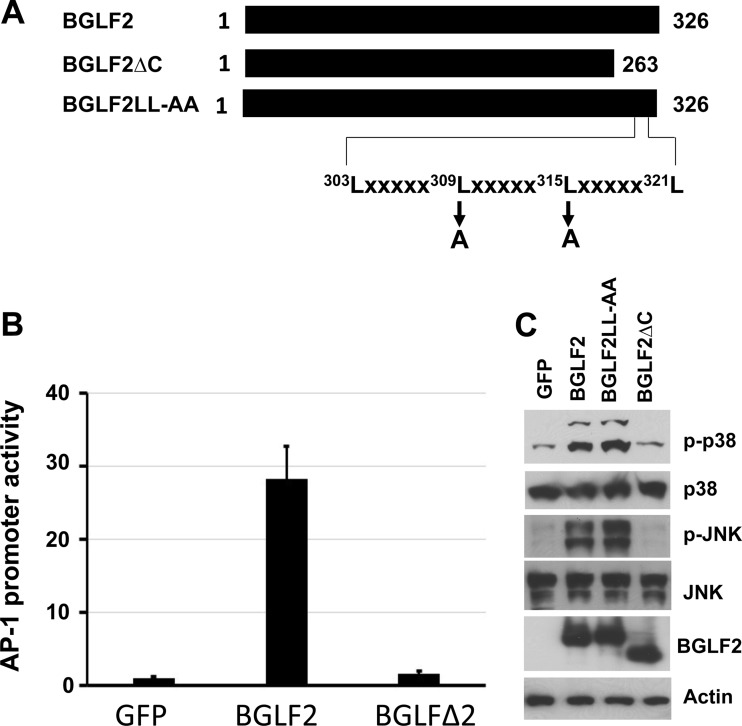
To map the elements in BGLF2 for activation of AP-1 signaling, we constructed amino- and carboxyl-terminal deletion mutants of the protein. When these mutants were expressed in 293T cells, only a carboxyl-terminal 66-amino-acid deletion mutant of BGLF2, BGLF2ΔC (Fig. 3A), expressed levels of protein similar to those of wild-type BGLF2. Transfection of a plasmid expressing BGLF2ΔC into 293T cells showed that the mutant protein had lost the ability to activate AP-1 signaling (Fig. 3B) and failed to activate p38 and JNK (Fig. 3C). Examination of the sequence of the carboxyl-terminal 66 amino acids of BGLF2 showed a leucine zipper-like motif in which the leucine residues are separated by 5 amino acids (303Lxxxxx309Lxxxxx315Lxxxxx321L). While leucine zipper motifs have leucine residues separated by 6 amino acids, leucine zipper-like motifs have been reported in which leucine residues are separated by 5 amino acids (38). To determine the importance of the leucine residues in the carboxyl-terminal region of BGLF2, we mutated leucines 309 and 315 to alanine (BGLF2LL-AA) (Fig. 3A) and found that the mutant retained the ability to activate p38 and JNK similarly to wild-type BGLF2 (Fig. 3C). Thus, while the carboxyl terminus of BGLF2 is required for phosphorylation of p38 and JNK, the leucine zipper-like motif is not required for this activity.
FIG 3.
The C-terminal domain of BGLF2 is important for activation of AP-1 and JNK. (A) Map of BGLF2 C-terminal domain mutants. (B) AP-1 reporter activity was measured as described in the legend to Fig. 1. (C) 293T cells were transfected with plasmids expressing GFP, BGLF2, BGLF2LL-AA, or BGLF2ΔC with a V5 epitope tag, and after 48 h, cell lysates were prepared and immunoblotted for signaling proteins.
BGLF2 forms homodimers.
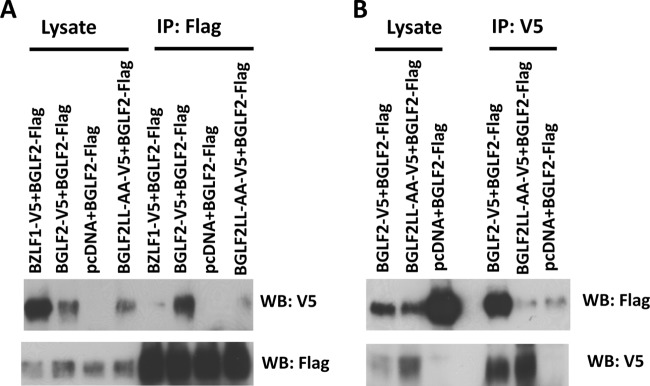
Since BGLF2 contains a possible leucine zipper-like motif and proteins with leucine zipper or leucine zipper-like motifs dimerize (38, 39), we determined whether BGLF2 forms homodimers. 293T cells were cotransfected with V5-tagged and FLAG-tagged BGLF2, and lysates were immunoprecipitated with anti-FLAG or anti-V5 agarose beads and immunoblotted with anti-V5 antibody or anti-FLAG antibody, respectively. V5-tagged BGLF2 immunoprecipitated with FLAG-tagged BGLF2, and FLAG-tagged BGLF2 immunoprecipitated with V5-tagged BGLF2 (Fig. 4A and B). Therefore, BGLF2 forms homodimers. As a control, 293 cells were cotransfected with FLAG-tagged BGLF2 and V5-tagged BZLF1. BGLF2 did not form heterodimers with BZLF1.
FIG 4.
Dimerization of BGLF2. 293T cells were cotransfected with FLAG-tagged BGLF2 (BGLF2-Flag) and V5-tagged BGLF2, BGLF2LL-AA, or BZLF1 or with pcDNA3.1 empty vector. Twenty-four hours after transfection, cell lysates were prepared in RIPA buffer and immunoprecipitated (IP) with anti-FLAG agarose beads (A) or anti-V5 agarose beads (B). Immune complexes were Western blotted (WB) with an antibody to the V5 or FLAG epitope.
To determine if the leucine zipper-like motif in the carboxyl-terminal region of BGLF2 is required for dimerization of the protein, we cotransfected 293T cells with V5-tagged and FLAG-tagged BGLF2LL-AA, in which leucines 309 and 315 of the leucine zipper-like motif are replaced with alanines. Unlike BGLF2, BGLF2LL-AA was unable to dimerize with wild-type BGLF2 (Fig. 4A and B). Thus, the leucine zipper-like motif in the carboxyl-terminal region of BGLF2 is required for the BGLF2 protein to dimerize.
BGLF2 enhances EBV BZLF1 expression.
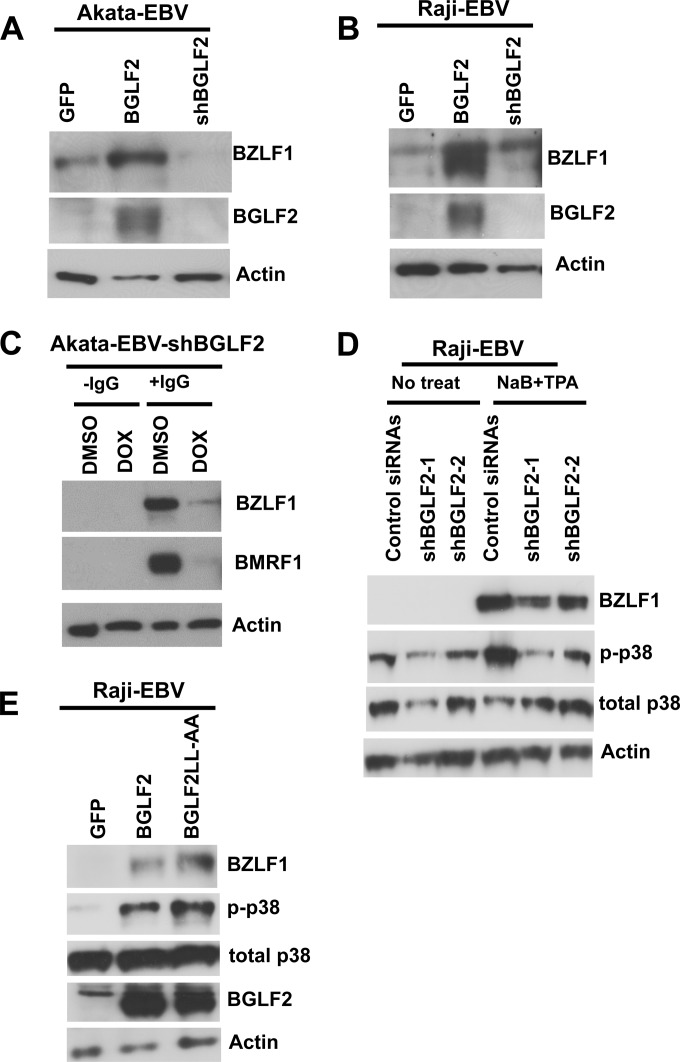
EBV can be reactivated from latently infected B cells by cytotoxic chemotherapeutic agents, such as methotrexate (MTX); by histone deacetylase inhibitors, such as sodium butyrate; and, in some Burkitt lymphoma cell lines, by cross-linking of surface IgG. Induction of lytic EBV infection by MTX requires activation of p38, PI3K, and MEK pathways and the EBV immediate early promoters, BZLF1 and BRLF1 (29). BZLF1 is the major switch for lytic reactivation of EBV in B cells, and expression of BZLF1 is regulated by the AP-1 transcription factor (28). Phosphorylation of p38 also plays a key role in reactivation of EBV from latency (27). Since BGLF2 activated AP-1 and AP-1 regulates BZLF1 (25, 28, 40), we postulated that BGLF2 may be important for EBV reactivation. To test this hypothesis, we transduced EBV-positive Akata and Raji cells with lentiviruses expressing BGLF2, an shRNA against BGLF2, or GFP and measured BZLF1 expression as an indicator of EBV reactivation. BGLF2 induced BZLF1 expression in both Akata and Raji cells (Fig. 5A and B). Transduction of EBV-Akata cells with the lentivirus expressing shRNA against BGLF2 followed by cross-linking of surface IgG to induce EBV replication showed that knockdown of BGLF2 reduced EBV reactivation as measured by BZLF1 and BMRF1 expression (Fig. 5C). Taken together, these results indicate that BGLF2 can enhance EBV reactivation in lymphoma cell lines and that BGLF2 is important for virus reactivation by cross-linking of surface IgG.
FIG 5.
BGLF2 induces BZLF1 expression in lymphoblastoid cell lines. Akata-EBV (A) or Raji-EBV (B) cells were transduced with a lentivirus expressing GFP, with lenti6-BGLF2 (expressing BGLF2), or with a lentivirus expressing shBGLF2. After 4 days, cell lysates were prepared and immunoblotted for detection of the EBV immediate early protein BZLF1, BGLF2, or actin. (C) Akata-EBV cells were transduced with a lentivirus expressing shRNA against BGLF2 (shBGLF2) under the control of a doxycycline (DOX)-inducible promoter and treated with or without rabbit anti-human IgG (10 μg/ml). After 2 days, cell lysates were collected and immunoblotted for BZLF1, the EBV early antigen BMRF1, or actin. (D) Raji-EBV cells were transfected with plasmids expressing two different BGLF2 shRNAs (targeting BGLF2 nucleotides 257 to 280 or 598 to 620) or a control siRNA pool. Two days later, cell lysates were treated with sodium butyrate (NaB) and 12-O-tetradecanoylphorbol 13-acetate (TPA) to induce EBV reactivation, and after 2 more days, cell lysates were prepared and immunoblotted for detection of BZLF1, p-p38, p38, or actin. (E) Raji-EBV cells were transfected with a plasmid expressing GFP, BGLF2, or BGLF2LL-AA, and after 3 days, lysates were immunoblotted for BZLF1, p-p38, p38, BGLF2, and actin.
To verify that the knockdown of BGLF2 was specific, Raji cells were transfected with plasmids expressing two different shRNAs against BGLF2 or with a nonspecific small interfering RNA (siRNA) pool, and virus replication was induced. Treatment with either BGLF2 shRNA reduced expression of BZLF1 and activation of p38 (Fig. 5D). We also determined whether the leucine zipper-like motif of BGLF2 was important for the ability of the protein to activate expression of BZLF1. BGLF2LL-AA, in which two leucines in the leucine zipper-like motif are replaced with alanines, was not impaired for upregulation of BZFL1 (Fig. 5E).
BGLF2 enhances the release of infectious EBV from AGS-EBV-GFP cells.
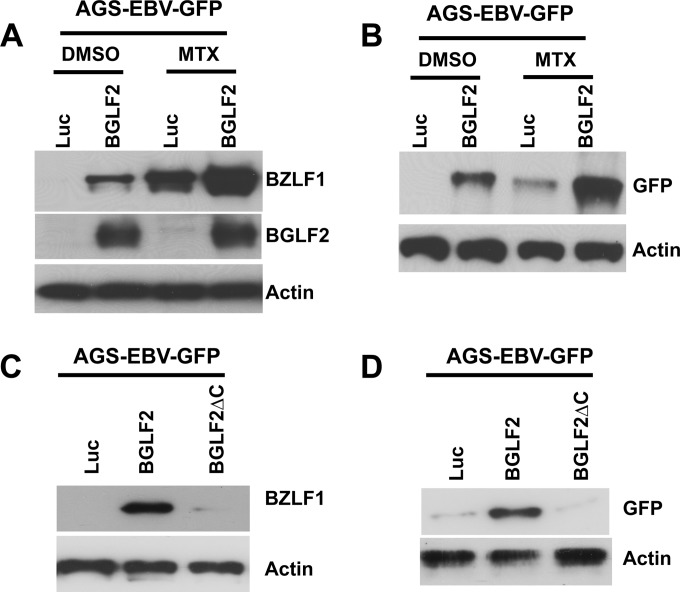
Since BGLF2 induced BZLF1 expression in Akata and Raji cells, we determined whether BGLF2 could induce expression of BZLF1 and enhance lytic cycle replication and production of infectious virus in epithelial cells. AGS-EBV-GFP cells stably expressing BGLF2 or luciferase were treated with MTX for 3 days, cell lysates were analyzed for BZLF1 expression, and supernatants were collected to test for EBV production. Supernatants were filtered and used to infect Raji cells, and immunoblotting of infected Raji cell lysates for GFP was performed 3 days after infection to detect the presence of infectious progeny virus released from AGS-EBV-GFP cells. BGLF2 induced BZLF1 expression in AGS-EBV-GFP cells, and it enhanced the ability of MTX to induce BZLF1 expression (Fig. 6A). BGLF2 enhanced the release of infectious progeny virus production from AGS-EBV-BGLF2 cells, as determined by increased GFP levels in Raji cells (Fig. 6B, lanes 1 and 2). BGLF2 also increased the yield of EBV from AGS-EBV-GFP cells after treatment of the cells with MTX (Fig. 6B, lanes 3 and 4).
FIG 6.
BGLF2 induces EBV reactivation in AGS-EBV cells. (A) AGS-EBV-GFP cells stably expressing BGLF2 or luciferase were treated with DMSO or methotrexate (MTX; 50 μg/ml) for 2 days, and then cell lysates were collected and immunoblotted with an antibody to BZLF1, BGLF2, or actin. (B) Supernatants from the cells in panel A were filtered through a 0.45-μm filter and used to infect Raji cells. After 3 days, the cells were lysed and immunoblotted for GFP to determine if virus had been produced from the cells in panel A. (C) AGS-EBV-GFP cells stably expressing BGLF2, BGLF2ΔC, or luciferase were immunoblotted for BZLF1 and actin. (D) Supernatants from the cells in panel C were collected and processed as described for panel B.
To determine if the carboxyl-terminal 66 amino acids of BGLF2 are important for its ability to enhance production of EBV, AGS-EBV-GFP cells stably expressing BGLF2 or BGLF2ΔC were immunoblotted for BZLF1, and supernatants were assayed for production of EBV. Cells expressing BGLF2ΔC, unlike those expressing wild-type BGLF2, expressed little or no BZLF1 (Fig. 6C) and did not produce infectious EBV (Fig. 6D).
Inhibition of p38 and MEK1/2 activity reduces the ability of BGLF2 to enhance EBV reactivation.
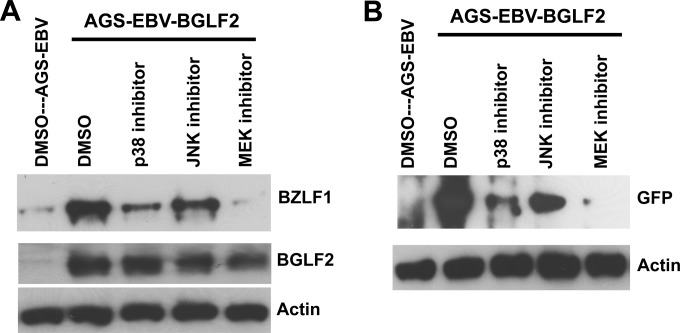
Because BGLF2 promotes EBV reactivation with production of infectious EBV and activates both p38 and JNK, we determined if BGLF2 activation of p38 and/or JNK was necessary for enhanced reactivation of EBV by BGLF2. AGS-EBV-BGLF2 cells were treated with an inhibitor of p38 (SB202192), JNK (SP600125), or MEK1/2 (U0126) or with dimethyl sulfoxide (DMSO) for 48 h, BZLF1 was measured in cell lysates, and EBV released into the supernatant was assayed in Raji cells. Inhibition of p38 and MEK1/2 markedly reduced BZLF1 expression and production of infectious EBV (Fig. 7A and B). In contrast, inhibition of JNK had a more modest effect. Therefore, p38 and MEK1/2 are critical for the ability of BGLF2 to enhance EBV reactivation.
FIG 7.
Inhibition of p38 reduces BGLF2-induced EBV reactivation. (A) AGS-EBV-BGLF2 cells were treated with an inhibitor of p38 (SB202190; 20 μM), JNK (SP600125; 50 μM), or MEK1/2 (U0126; 20 μM) or with DMSO for 2 days, and cell lysates were immunoblotted for BZLF1, BGLF2, and actin. (B) EBV released into the supernatant from the cells in panel A was used to infect Raji cells, and cell lysates were immunoblotted for GFP to measure virus production.
BGLF2 activates the BZLF1 promoter.
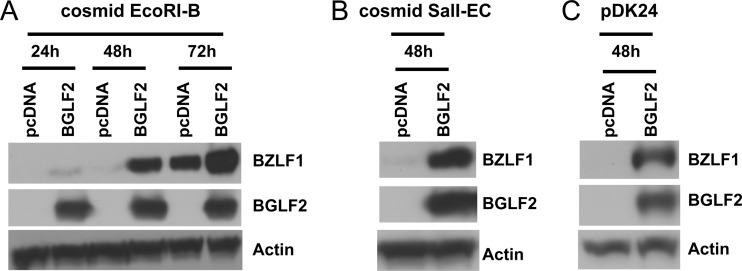
Since BGLF2 enhances BZLF1 expression and promotes the release of infectious EBV in latently infected cells, we determined whether BGLF2 can upregulate expression of the immediate early BZLF1 protein. 293T cells were cotransfected with pcDNA or BGLF2 and the EBV cosmid EcoRI-B, EBV cosmid SalI-EC (31), or plasmid pDK24 (30). Each of the two cosmids and plasmid pDK24 contain the BZLF1 gene with its promoter; plasmid pDK24 does not contain any other full-length EBV ORFs. Expression of BGLF2 induced BZLF1 expression in cells transfected with each of the cosmids or with the plasmid containing BZLF1 (Fig. 8), suggesting that BGLF2 can upregulate the expression of BZLF1 in the absence of other viral proteins.
FIG 8.
BGLF2 enhances expression of BZLF1 in the absence of expression of the full EBV genome. 293T cells were cotransfected with EBV cosmid EcoRI-B (A), EBV cosmid SalI-EC (B), or plasmid pDK24, which contains the BamHI Z fragment of EBV (C), and either pcDNA or a plasmid expressing the BGLF2 protein with a V5 epitope tag. Cell lysates were collected at different times after transfection, and immunoblots were performed for BZLF1, BGLF2, or actin.
DISCUSSION
In this study, we found that the EBV tegument protein BGLF2 activates AP-1 and induces phosphorylation of both p38 and JNK MAPKs. Expression of BGLF2 in cells latently infected with EBV enhances EBV reactivation by induction of BZLF1 expression, resulting in production of infectious EBV. p38 and MEK1/2 were required for enhancement of EBV reactivation by BGLF2, and inhibition of p38 or MEK1/2 reduced EBV reactivation and virus production.
BGLF2 is a tegument protein (41) that is expressed with late kinetics and localizes to the nuclei of virus-infected cells undergoing lytic infection (42). BGLF2 interacts with NIMA-related protein kinase (NEK9) and GEM-interacting protein (GMIP), induces expression of p21, and induces cell cycle arrest at the G1/S transition (43). Several orthologs of BGLF2, such as Kaposi's sarcoma-associated herpesvirus ORF33 (44), varicella-zoster virus ORF44 (45, 46), and cytomegalovirus (CMV) UL94 (47), are essential for virus replication; however, herpes simplex virus UL16 is not essential for replication, but deletion of the gene impairs virus growth (48, 49). HSV UL16 is important for DNA encapsidation and virus assembly (50, 51), and CMV UL94 is required for secondary envelopment of nucleocapsids in the endoplasmic reticulum and the Golgi apparatus (47). Our finding that EBV BGLF2 activates AP-1 adds a potentially new function for BGLF2's orthologs in other herpesviruses, in addition to their known activities in virus morphology and virion maturation.
We found that BGLF2 induces phosphorylation of p38 MAPK and JNK. MAPKs regulate a number of cellular functions, such as gene expression, cell differentiation, proliferation, and death. JNK is important for cell stress responses, cellular apoptosis pathways, and inflammatory responses (52, 53). Two EBV immediate early proteins, BZLF1 and BRFL1, and two latent proteins, LMP1 and LMP2, have previously been shown to activate JNK (9, 17, 22), and BZLF1, BRLF1, and LMP1 have also been reported to activate p38 (22, 37). Since BGLF2 is a tegument protein in the virion, it would be released on entry of the virus into the cell and could activate these signaling pathways even before viral gene expression is initiated.
We found that BGLF2 activates expression of BZLF1. Cross-linking of the B cell receptor in Akata cells induces BZLF1 expression. Cross-linking of the B cell receptor results in activation of phospholipase C, activation of MAPK, and binding of transcription factors, including AP-1, to the ZII element on the BZLF1 promoter (reviewed in reference 40). Similarly, transforming growth factor beta (TGF-β) induces BZLF1 expression by activating MAPK, resulting in binding of AP-1 to the ZII element on the BZLF1 promoter (54, 55). Since BGLF2 activates AP-1 via p38 MAPK, BGLF2 likely uses the same pathways as B cell receptor cross-linking and TGF-β to enhance expression of BZLF1. Another EBV tegument protein, BNRF1, was also shown to enhance expression of BZLF1 (56).
The EBV tegument protein BGLF2 enhanced the production and release of infectious EBV from latently infected AGS cells. Other herpesvirus tegument proteins, such as HSV VP16 (57) and CMV pp71 (58, 59), are also important for initiating lytic gene expression and reactivation from latency. HSV VP16 and CMV pp71 are excluded from the nuclei of sensory neurons and CD34 hematopoietic progenitor cells, respectively, during infection, allowing establishment of latency (60, 61). EBV BGLF2 might also be excluded from the nucleus during infection so that it does not activate expression of BZLF1. Both HSV and CMV tegument proteins are required for activating virus immediate early gene expression. HSV VP16 forms a complex with HCF and Oct1 to interact with TAATGARAT motifs on immediate early gene promoters (62). Expression of CMV IE1 and IE2 from the major immediate early CMV promoter is dependent on viral tegument proteins, including pp71 (63). Thus, our finding that the EBV tegument protein BGLF2 enhances expression of BZLF1 and increases release of infectious EBV has parallels with other herpesvirus tegument proteins.
We found that inhibition of p38 and MEK1/2 activity reduced the ability of BGLF2 to enhance EBV reactivation and resulted in release of less EBV from infected cells. Taken together, our results indicate that the EBV tegument protein BGLF2 contributes to virus production and that the p38/MAPK and MEK signaling pathways are required for this activity. Further studies are needed to determine whether BGLF2 is sequestered in the cytoplasm and/or inactivated in newly infected B cells that are destined for latency and what additional cellular and viral proteins interact with BGLF2 to mediate its effects.
REFERENCES
- 1.Damania B, Choi JK, Jung JU. 2000. Signaling activities of gammaherpesvirus membrane proteins. J Virol 74:1593–1601. doi: 10.1128/JVI.74.4.1593-1601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveira DE, Ballon G, Cesarman E. 2010. NF-kappaB signaling modulation by EBV and KSHV. Trends Microbiol 18:248–257. doi: 10.1016/j.tim.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Hatzivassiliou E, Mosialos G. 2002. Cellular signaling pathways engaged by the Epstein-Barr virus transforming protein LMP1. Front Biosci 7:d319–d329. doi: 10.2741/hatziva. [DOI] [PubMed] [Google Scholar]
- 4.Miller CL, Lee JH, Kieff E, Burkhardt AL, Bolen JB, Longnecker R. 1994. Epstein-Barr virus protein LMP2A regulates reactivation from latency by negatively regulating tyrosine kinases involved in sIg-mediated signal transduction. Infect Agents Dis 3:128–136. [PubMed] [Google Scholar]
- 5.Saha A, Robertson ES. 2011. Epstein-Barr virus-associated B-cell lymphomas: pathogenesis and clinical outcomes. Clin Cancer Res 17:3056–3063. doi: 10.1158/1078-0432.CCR-10-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorley-Lawson DA. 2001. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol 1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 7.Kieser A, Kilger E, Gires O, Ueffing M, Kolch W, Hammerschmidt W. 1997. Epstein-Barr virus latent membrane protein-1 triggers AP-1 activity via the c-Jun N-terminal kinase cascade. EMBO J 16:6478–6485. doi: 10.1093/emboj/16.21.6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eliopoulos AG, Blake SM, Floettmann JE, Rowe M, Young LS. 1999. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J Virol 73:1023–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliopoulos AG, Young LS. 1998. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1). Oncogene 16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 10.Kilger E, Kieser A, Baumann M, Hammerschmidt W. 1998. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J 17:1700–1709. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieser A, Kaiser C, Hammerschmidt W. 1999. LMP1 signal transduction differs substantially from TNF receptor 1 signaling in the molecular functions of TRADD and TRAF2. EMBO J 18:2511–2521. doi: 10.1093/emboj/18.9.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busch LK, Bishop GA. 2001. Multiple carboxyl-terminal regions of the EBV oncoprotein, latent membrane protein 1, cooperatively regulate signaling to B lymphocytes via TNF receptor-associated factor (TRAF)-dependent and TRAF-independent mechanisms. J Immunol 167:5805–5813. doi: 10.4049/jimmunol.167.10.5805. [DOI] [PubMed] [Google Scholar]
- 13.Brinkmann MM, Schulz TF. 2006. Regulation of intracellular signalling by the terminal membrane proteins of members of the Gammaherpesvirinae. J Gen Virol 87:1047–1074. doi: 10.1099/vir.0.81598-0. [DOI] [PubMed] [Google Scholar]
- 14.Longnecker R, Miller CL. 1996. Regulation of Epstein-Barr virus latency by latent membrane protein 2. Trends Microbiol 4:38–42. [DOI] [PubMed] [Google Scholar]
- 15.Merchant M, Swart R, Katzman RB, Ikeda M, Ikeda A, Longnecker R, Dykstra ML, Pierce SK. 2001. The effects of the Epstein-Barr virus latent membrane protein 2A on B cell function. Int Rev Immunol 20:805–835. doi: 10.3109/08830180109045591. [DOI] [PubMed] [Google Scholar]
- 16.Pang MF, Lin KW, Peh SC. 2009. The signaling pathways of Epstein-Barr virus-encoded latent membrane protein 2A (LMP2A) in latency and cancer. Cell Mol Biol Lett 14:222–247. doi: 10.2478/s11658-008-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen SY, Lu J, Shih YC, Tsai CH. 2002. Epstein-Barr virus latent membrane protein 2A regulates c-Jun protein through extracellular signal-regulated kinase. J Virol 76:9556–9561. doi: 10.1128/JVI.76.18.9556-9561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart S, Dawson CW, Takada K, Curnow J, Moody CA, Sixbey JW, Young LS. 2004. Epstein-Barr virus-encoded LMP2A regulates viral and cellular gene expression by modulation of the NF-kappaB transcription factor pathway. Proc Natl Acad Sci U S A 101:15730–15735. doi: 10.1073/pnas.0402135101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Speck SH, Chatila T, Flemington E. 1997. Reactivation of Epstein-Barr virus: regulation and function of the BZLF1 gene. Trends Microbiol 5:399–405. doi: 10.1016/S0966-842X(97)01129-3. [DOI] [PubMed] [Google Scholar]
- 20.Amon W, Farrell PJ. 2005. Reactivation of Epstein-Barr virus from latency. Rev Med Virol 15:149–156. doi: 10.1002/rmv.456. [DOI] [PubMed] [Google Scholar]
- 21.Miller G, El-Guindy A, Countryman J, Ye J, Gradoville L. 2007. Lytic cycle switches of oncogenic human gammaherpesviruses. Adv Cancer Res 97:81–109. doi: 10.1016/S0065-230X(06)97004-3. [DOI] [PubMed] [Google Scholar]
- 22.Adamson AL, Darr D, Holley-Guthrie E, Johnson RA, Mauser A, Swenson J, Kenney S. 2000. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol 74:1224–1233. doi: 10.1128/JVI.74.3.1224-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee YH, Chiu YF, Wang WH, Chang LK, Liu ST. 2008. Activation of the ERK signal transduction pathway by Epstein-Barr virus immediate-early protein Rta. J Gen Virol 89:2437–2446. doi: 10.1099/vir.0.2008/003897-0. [DOI] [PubMed] [Google Scholar]
- 24.Darr CD, Mauser A, Kenney S. 2001. Epstein-Barr virus immediate-early protein BRLF1 induces the lytic form of viral replication through a mechanism involving phosphatidylinositol-3 kinase activation. J Virol 75:6135–6142. doi: 10.1128/JVI.75.13.6135-6142.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata T. 2014. Regulation of Epstein-Barr virus reactivation from latency. Microbiol Immunol 58:307–317. doi: 10.1111/1348-0421.12155. [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Wang H, Sairenji T. 2004. Inhibition of Epstein-Barr virus (EBV) reactivation by short interfering RNAs targeting p38 mitogen-activated protein kinase or c-myc in EBV-positive epithelial cells. J Virol 78:11798–11806. doi: 10.1128/JVI.78.21.11798-11806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matusali G, Arena G, De Leo A, Di Renzo L, Mattia E. 2009. Inhibition of p38 MAP kinase pathway induces apoptosis and prevents Epstein Barr virus reactivation in Raji cells exposed to lytic cycle inducing compounds. Mol Cancer 8:18. doi: 10.1186/1476-4598-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenney SC, Mertz JE. 2014. Regulation of the latent-lytic switch in Epstein-Barr virus. Semin Cancer Biol 26:60–68. doi: 10.1016/j.semcancer.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng WH, Cohen JI, Fischer S, Li L, Sneller M, Goldbach-Mansky R, Raab-Traub N, Delecluse HJ, Kenney SC. 2004. Reactivation of latent Epstein-Barr virus by methotrexate: a potential contributor to methotrexate-associated lymphomas. J Natl Cancer Inst 96:1691–1702. doi: 10.1093/jnci/djh313. [DOI] [PubMed] [Google Scholar]
- 30.Tomkinson B, Robertson E, Yalamanchili R, Longnecker R, Kieff E. 1993. Epstein-Barr virus recombinants from overlapping cosmid fragments. J Virol 67:7298–7306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dambaugh T, Beisel C, Hummel M, King W, Fennewald S, Cheung A, Heller M, Raab-Traub N, Kieff E. 1980. Epstein-Barr virus (B95-8) DNA VII: molecular cloning and detailed mapping. Proc Natl Acad Sci U S A 77:2999–3003. doi: 10.1073/pnas.77.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Li Q, Dowdell K, Fischer ER, Cohen JI. 2012. Varicella-zoster virus ORF12 protein triggers phosphorylation of ERK1/2 and inhibits apoptosis. J Virol 86:3143–3151. doi: 10.1128/JVI.06923-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Fitzgerald K, Kurt-Jones E, Finberg R, Knipe DM. 2008. Herpesvirus tegument protein activates NF-kappaB signaling through the TRAF6 adaptor protein. Proc Natl Acad Sci U S A 105:11335–11339. doi: 10.1073/pnas.0801617105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. 1998. A third-generation lentivirus vector with a conditional packaging system. J Virol 72:8463–8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shin KJ, Wall EA, Zavzavadjian JR, Santat LA, Liu J, Hwang JI, Rebres R, Roach T, Seaman W, Simon MI, Fraser ID. 2006. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc Natl Acad Sci U S A 103:13759–13764. doi: 10.1073/pnas.0606179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu X, Santat LA, Chang MS, Liu J, Zavzavadjian JR, Wall EA, Kivork C, Simon MI, Fraser ID. 2007. A versatile approach to multiple gene RNA interference using microRNA-based short hairpin RNAs. BMC Mol Biol 8:98. doi: 10.1186/1471-2199-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eliopoulos AG, Gallagher NJ, Blake SM, Dawson CW, Young LS. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem 274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 38.Okamoto S, Terao Y, Hasuike K, Hamada S, Kawabata S. 2008. A novel streptococcal leucine zipper protein (Lzp) binds to human immunoglobulins. Biochem Biophys Res Commun 377:1128–1134. doi: 10.1016/j.bbrc.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 39.Landschulz WH, Johnson PF, McKnight SL. 1988. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 40.Murata T, Tsurumi T. 2014. Switching of EBV cycles between latent and lytic states. Rev Med Virol 24:142–153. doi: 10.1002/rmv.1780. [DOI] [PubMed] [Google Scholar]
- 41.Johannsen E, Luftig M, Chase MR, Weicksel S, Cahir-McFarland E, Illanes D, Sarracino D, Kieff E. 2004. Proteins of purified Epstein-Barr virus. Proc Natl Acad Sci U S A 101:16286–16291. doi: 10.1073/pnas.0407320101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen MR, Hsu TY, Lin SW, Chen JY, Yang CS. 1991. Cloning and characterization of cDNA clones corresponding to transcripts from the BamHI G region of the Epstein-Barr virus genome and expression of BGLF2. J Gen Virol 72:3047–3055. doi: 10.1099/0022-1317-72-12-3047. [DOI] [PubMed] [Google Scholar]
- 43.Paladino P, Marcon E, Greenblatt J, Frappier L. 2014. Identification of herpesvirus proteins that contribute to G1/S arrest. J Virol 88:4480–4492. doi: 10.1128/JVI.00059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Budt M, Hristozova T, Hille G, Berger K, Brune W. 2011. Construction of a lytically replicating Kaposi's sarcoma-associated herpesvirus. J Virol 85:10415–10420. doi: 10.1128/JVI.05071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Selariu A, Warden C, Huang G, Huang Y, Zaccheus O, Cheng T, Xia N, Zhu H. 2010. Genome-wide mutagenesis reveals that ORF7 is a novel VZV skin-tropic factor. PLoS Pathog 6:e1000971. doi: 10.1371/journal.ppat.1000971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadaoka T, Serada S, Kato J, Hayashi M, Gomi Y, Naka T, Yamanishi K, Mori Y. 2014. Varicella-zoster virus ORF49 functions in the efficient production of progeny virus through its interaction with essential tegument protein ORF44. J Virol 88:188–201. doi: 10.1128/JVI.02245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips SL, Bresnahan WA. 2012. The human cytomegalovirus (HCMV) tegument protein UL94 is essential for secondary envelopment of HCMV virions. J Virol 86:2523–2532. doi: 10.1128/JVI.06548-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starkey JL, Han J, Chadha P, Marsh JA, Wills JW. 2014. Elucidation of the block to herpes simplex virus egress in the absence of tegument protein UL16 reveals a novel interaction with VP22. J Virol 88:110–119. doi: 10.1128/JVI.02555-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baines JD, Roizman B. 1991. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J Virol 65:938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meckes DG Jr, Wills JW. 2007. Dynamic interactions of the UL16 tegument protein with the capsid of herpes simplex virus. J Virol 81:13028–13036. doi: 10.1128/JVI.01306-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nalwanga D, Rempel S, Roizman B, Baines JD. 1996. The UL16 gene product of herpes simplex virus 1 is a virion protein that colocalizes with intranuclear capsid proteins. Virology 226:236–242. doi: 10.1006/viro.1996.0651. [DOI] [PubMed] [Google Scholar]
- 52.Coffey ET. 2014. Nuclear and cytosolic JNK signalling in neurons. Nat Rev Neurosci 15:285–299. doi: 10.1038/nrn3729. [DOI] [PubMed] [Google Scholar]
- 53.Tournier C. 2013. The 2 faces of JNK signaling in cancer. Genes Cancer 4:397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fahmi H, Cochet C, Hmama Z, Opolon P, Joab I. 2000. Transforming growth factor beta 1 stimulates expression of the Epstein-Barr virus BZLF1 immediate-early gene product ZEBRA by an indirect mechanism which requires the MAPK kinase pathway. J Virol 74:5810–5818. doi: 10.1128/JVI.74.13.5810-5818.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang CL, Chen JL, Hsu YP, Ou JT, Chang YS. 2002. Epstein-Barr virus BZLF1 gene is activated by transforming growth factor-beta through cooperativity of Smads and c-Jun/c-Fos proteins. J Biol Chem 277:23345–23357. doi: 10.1074/jbc.M107420200. [DOI] [PubMed] [Google Scholar]
- 56.Tsai K, Thikmyanova N, Wojcechowskyj JA, Delecluse HJ, Lieberman PM. 2011. EBV tegument protein BNRF1 disrupts DAXX-ATRX to activate viral early gene transcription. PLoS Pathog 7:e1002376. doi: 10.1371/journal.ppat.1002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson RL, Preston CM, Sawtell NM. 2009. De novo synthesis of VP16 coordinates the exit from HSV latency in vivo. PLoS Pathog 5:e1000352. doi: 10.1371/journal.ppat.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Homer EG, Rinaldi A, Nicholl MJ, Preston CM. 1999. Activation of herpesvirus gene expression by the human cytomegalovirus protein pp71. J Virol 73:8512–8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Preston CM, Nicholl MJ. 2005. Human cytomegalovirus tegument protein pp71 directs long-term gene expression from quiescent herpes simplex virus genomes. J Virol 79:525–535. doi: 10.1128/JVI.79.1.525-535.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saffert RT, Penkert RR, Kalejta RF. 2010. Cellular and viral control over the initial events of human cytomegalovirus experimental latency in CD34+ cells. J Virol 84:5594–5604. doi: 10.1128/JVI.00348-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Penkert RR, Kalejta RF. 2011. Tegument protein control of latent herpesvirus establishment and animation. Herpesviridae 2:3. doi: 10.1186/2042-4280-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Preston CM, Frame MC, Campbell ME. 1988. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell 52:425–434. doi: 10.1016/S0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- 63.Schierling K, Stamminger T, Mertens T, Winkler M. 2004. Human cytomegalovirus tegument proteins ppUL82 (pp71) and ppUL35 interact and cooperatively activate the major immediate-early enhancer. J Virol 78:9512–9523. doi: 10.1128/JVI.78.17.9512-9523.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]