ABSTRACT
Severe fever with thrombocytopenia syndrome (SFTS) virus is a newly recognized member of the genus Phlebovirus in the family Bunyaviridae. The virus was isolated from patients presenting with hemorrhagic manifestations and an initial case fatality rate of 12 to 30% was reported. Due to the recent emergence of this pathogen, there is limited knowledge on the molecular virology of SFTS virus. Recently, we reported that the SFTS virus NSs protein inhibited the activation of the beta interferon (IFN-β) promoter. Furthermore, we also found that SFTS virus NSs relocalizes key components of the IFN response into NSs-induced cytoplasmic structures. Due to the important role these structures play during SFTS virus replication, we conducted live cell imaging studies to gain further insight into the role and trafficking of these cytoplasmic structures during virus infection. We found that some of the SFTS virus NSs-positive cytoplasmic structures were secreted to the extracellular space and endocytosed by neighboring cells. We also found that these secreted structures isolated from NSs-expressing cells and SFTS virus-infected cells were positive for the viral protein NSs and the host protein CD63, a protein associated with extracellular vesicles. Electron microscopy studies also revealed that the isolated CD63-immunoprecipitated extracellular vesicles produced during SFTS virus infection contained virions. The virions harbored within these structures were efficiently delivered to uninfected cells and were able to sustain SFTS virus replication. Altogether, these results suggest that SFTS virus exploits extracellular vesicles to mediate virus receptor-independent transmission to host cells and open the avenue for novel therapeutic strategies against SFTS virus and related pathogens.
IMPORTANCE SFTS virus is novel bunyavirus associated with hemorrhagic fever illness. Currently, limited information is available about SFTS virus. In the present study, we demonstrated that extracellular vesicles produced by SFTS virus-infected cells harbor infectious virions. We sought to determine whether these “infectious” extracellular vesicles can mediate transmission of the virus and confirmed that the SFTS virions were efficiently transported by these secreted structures into uninfected cells and were able to sustain efficient replication of SFTS virus. These results have significant impact on our understanding of how the novel tick-borne phleboviruses hijack cellular machineries to establish infection and point toward a novel mechanism for virus replication among arthropod-borne viruses.
INTRODUCTION
The Bunyaviridae family comprises five genera, including Orthobunyavirus, Phlebovirus, Nairovirus, Hantavirus, and Tospovirus. The majority of viruses within this family (with the exception of Hantaviruses) are considered arthropod-borne viruses and are important causes of morbidity and mortality around the world. These viruses are associated with a range of clinical symptoms characterized by febrile illness and in the most severe cases fatal hepatitis, hemorrhagic fever, or neurological manifestations requiring intensive care have been reported.
Due to advances in genomic and virus identification approaches, novel bunyaviruses have been discovered and identified as important causes of human disease during recent years (1–3). One example is severe fever with thrombocytopenia syndrome (SFTS) virus, a new member of the family Bunyaviridae, genus Phlebovirus (1, 4). The virus was first isolated in China in 2009 from patients presenting with a hemorrhagic fever illness (1, 5). The initial case fatality rate reported for SFTS was 12 to 30%, and a recent serosurvey among persons living in rural Jiangsu Province found that 3.6% of residents had neutralizing antibodies to SFTS virus (6). Evidence has also been obtained about the possibility of person-to-person transmission (7, 8). Furthermore, hemorrhagic fever cases with mortality rates as high as 50% have now been recognized in Japan and Korea, further highlighting the emerging potential of this pathogen (1, 2, 9–11). Therefore, SFTS virus is a highly pathogenic phlebovirus, and due to its recent emergence, the mechanism of disease pathogenesis is still unclear.
Like other members of the family Bunyaviridae, SFTS virus possesses a negative sense tripartite genome consisting of the S, M, and L segments. The L segment encodes the viral RNA polymerase (L), the M segment encodes glycoproteins (Gn/Gc), and the S segment uses an ambisense coding strategy to encode a nonstructural protein (NSs) and a nucleocapsid protein (NP) (12). Although many bunyaviruses, including the prototype virus in the Bunyaviridae family Bunyamwera virus (BUNV), also encodes the nonstructural protein NSm within the M segment, some members of the Phlebovirus genus, including SFTS and Uukuniemi viruses (UUKV) do not encode this viral protein (1, 13). The BUNV NSm is known to serve as a scaffold protein that associates to globular and tubular structures derived from the Golgi apparatus (14–16). These structures have been shown to harbor the ribonucleoprotein (RNP), a complex essential for the transcription and replication of viral RNA (14). Although SFTS virus does not encode the NSm protein, it has been recently suggested that the SFTS virus NSs may exert some of the NSm's function by serving as a scaffold protein and forming viral replication factories (17). Colocalization of the early endosomal marker Rab5 with the viral factories induced by SFTS virus NSs suggests that these structures are of endosomal origin and not derived from the Golgi apparatus (18). Additionally, the SFTS virus NSs protein has also been shown to play a critical role in the inhibition of host innate immunity (18, 19). Although these findings are consistent with previous studies on bunyavirus NSs proteins describing the NSs as a major virulence factor that acts as a global inhibitor of host cell transcription and antagonist of the IFN system (20–22), our previous studies have shown that, unlike any other bunyavirus NSs, the SFTS virus NSs interacts with and relocalizes TBK1, RIG-I, and TRIM25 into endosome-like structures (18). Thus, SFTS virus appears to use a different mechanism for virus replication and inhibition of IFN responses than those described for other bunyaviruses.
Studies aimed at characterizing early events of the phlebovirus replication cycle have shown that the prototype member, UUKV, enters the cells through a clathrin-independent mechanism. Specifically, UUKV has been shown to use Rab5a+ early endosomes and later Rab7a+ and LAMP-1+ endosomes, suggesting that after entry the virus is directed toward the classical endosomal pathway (23). Interestingly, our studies have also shown that the SFTS virus NSs-positive cytoplasmic structures colocalize with Rab5, but not with Rab4 (18). Furthermore, we found that LC3, an important marker for autophagy, also colocalizes with these NSs-cytoplasmic structures; however, these structures were still observed in cells lacking Atg7, a gene essential for conventional autophagy (18, 24). These results led us to hypothesize that these SFTS virus NSs-positive structures were not conventional autophagosomes but rather they are derived from the endosomal pathway. Due to the important role that these structures play in viral replication and evasion of host innate immunity, we have investigated the sources and the trafficking of these structures within the cells. Surprisingly, we observed that some of the SFTS virus NSs-positive structures were secreted into the extracellular space and were taken up by neighboring cells. Furthermore, we also demonstrated that these structures possess markers associated with extracellular vesicles and, more importantly, they contain infectious virions that were efficiently transported by these secreted structures into uninfected cells and were able to sustain efficient replication of the SFTS virus. Altogether, the data suggest that SFTS virus exploits extracellular vesicles to mediate receptor-independent transmission of the virus.
MATERIALS AND METHODS
Cells, plasmids, and viruses.
HeLa and Vero76 cells were obtained from ATCC and maintained with minimal essential Eagle medium (Lonza) supplemented with l-glutamine, 1% penicillin-streptomycin (Gibco), and 10% fetal bovine serum. Cells used in the isolation of secreted vesicles were grown in media containing 10% fetal bovine serum depleted of endogenous vesicles by ultracentrifugation at 100,000 × g for 16 h. Human embryonic kidney cells (HEK 293T) were obtained from the American Type Culture Collection and maintained with Dulbecco minimal essential medium (Lonza) supplemented with l-glutamine, 1% penicillin-streptomycin, and 10% fetal bovine serum. The SFTS virus NSs plasmid was constructed by PCR using overlapping deoxyoligonucleotides corresponding to the published GenBank sequence (NC_018137.1) and has been described elsewhere (18). The SFTS virus NSs-mCherry was constructed using standard cloning techniques (18). The mCherry and the SFTS virus NSs-mCherry genes were then cloned into a third-generation lentivirus vector and used to generate lentiviruses. HeLa cells were transduce with the lentivirus particles and the mCherry and SFTS virus NSs-mCherry stable cell lines were generated by antibiotic selection and cloning of the mCherry fluorescent cells to select those with high level of protein expression as determined by confocal microscopy and Western blot analyses.
The SFTS virus strain used in this study was provided by the Chinese Center for Disease Control and Prevention and passaged twice in Vero76 cells to generate viral stocks for this study. Generation of viral stocks was performed in Vero76 cells, with titers determined by plaque assay as previously described (18, 25). A multiplicity of infection (MOI) of ∼0.01 was used in all experiments involving virus infection, unless stated otherwise.
Transfections and immunoblotting.
All transfections were carried using 500 ng of plasmid DNA and Lipofectamine 3000 (Invitrogen) according to manufacturer's established protocol. Transfected cells were lysed with NP-40 lysis buffer (150 mM NaCl, 1.0% NP-40, 50 nM Tris-Cl [pH 8.0]) containing complete protease inhibitor cocktail (Roche) at 16 to 24 h posttransfection. For immunoblotting, proteins were resolved by SDS-PAGE and subsequently transferred onto a 0.2-μm-pore size polyvinylidene difluoride (PVDF) membrane (Thermo Scientific). PVDF membranes were blocked for 1 h with 5% nonfat dry milk or 5% bovine serum albumin (BSA; Fisher) in Tris-buffered saline with 1% Tween 20 (TBS-T). Membranes were then incubated for 16 to 18 h at 4°C with primary antibodies. After incubation, membranes were washed three times and incubated with anti-mouse or anti-rabbit secondary antibodies conjugated with horseradish peroxidase (HRP) for 1 h. Lastly, blots were developed by using Western Lightning ECL (Perkin-Elmer) substrate according to the manufacturer's protocol. The following primary antibodies were used for immunoblotting: rabbit anti-SFTS virus NSs (1:500; GenScript), mouse anti-SFTS virus NP (1:500), rabbit anti-CD63 (1:100; Abcam), mouse anti-β Tubulin (1:1,000; Abcam), rabbit anti-LC3 (1:1,000; Abcam), and rabbit anti-Rab5 (1:1,000; Abcam). The secondary antibodies used were donkey anti-rabbit IgG HRP-conjugated antibody (1:5,000) and sheep anti-mouse IgG HRP-conjugated antibody (1:5,000) from GE Healthcare. For the detection of the exosomal marker CD63, the purified extracellular vesicles were lysed and resolved under nondenaturing conditions in order to detect the glycosylated forms of CD63 according to the manufacturer's recommendations. For comparison purposes, both reduced and nonreduced samples were transferred onto PVDF membrane and Western blotting performed as indicated above.
Immunofluorescence.
HeLa cells were seeded onto coverslips treated with 50 μg/ml mouse laminin I (Cutler) and infected according to standard procedures. The cells were then incubated overnight at 37°C in 5% CO2. The cells prepared for infection were infected with SFTS virus (MOI = 0.5) for 24, 48, or 72 h, fixed with 4% paraformaldehyde for 30 min, and then permeabilized with 0.1% Triton-X (Sigma) for 10 min. The cells were then washed, and a blocking incubation step with 10% goat serum (Sigma) and 3% BSA (Thermo Scientific) was carried out. Next, cells were incubated with primary antibodies for 1 h. Cell nuclei were visualized with Hoechst 33342 (1:1,000; Invitrogen) or with TO-PRO-3 Iodide (Invitrogen) according to the manufacturer's protocol. The following Alexa Fluor-conjugated antibodies from Invitrogen were used: Alexa Fluor 488-goat anti-mouse or Alexa Fluor 594-goat anti-rabbit antibodies. All secondary antibodies were used at a 1:1,000 concentration, and samples were visualized with a Zeiss LSM510META laser scanning confocal microscope or Olympus spinning disc confocal microscope.
Live cell imaging.
HeLa cells stably expressing the SFTS virus NSs-mCherry were plated on 35-mm glass bottom culture dishes (MatTek Corp.) and incubated overnight at 37°C in 5% CO2. Prior to live cell imaging, the cell culture medium was removed, cells washed with Dulbecco phosphate-buffered saline (DPBS), and live cell imaging solution (Invitrogen) was added. Cells were visualized for 16 h using a Prairie Technologies/Nikon multimodal live cell imaging system.
Isolation and purification of extracellular vesicles.
Isolation of SFTS virus NSs-positive vesicles was first standardized in the stable cell line expressing the NSs fused to mCherry fluorescent protein. Cells were grown to 90% confluence for approximately 3 days, and supernatants were later collected and clarified by centrifugation. Cleared supernatant was concentrated using a 3,000 molecular weight cutoff value column (Sartorius) and voided of any cellular debris by centrifugation at 10,000 × g for 30 min. Vesicles were then pelleted at 100,000 × g for 90 min. To further purify the vesicles and remove any contaminant protein, the pellet containing the vesicles was washed with ice-cold phosphate-buffered saline (PBS) and repelleted at 100,000 × g for 90 min. The pellets used for electron microscopy were resuspended in 100 μl of molecular-grade water. For Western blot analysis, the pellets were resuspended in 100 μl of NP-40 lysis buffer and sonicated for 1 min. For isolation and purification of vesicles produced during SFTS virus infection, the same centrifugation procedure was used. However, the final pellet was subjected to an immunoprecipitation step using magnetic beads coated with anti-CD63 antibody at 4°C overnight. Beads were then washed with ice-cold PBS, and the CD63+ vesicles were released by resuspension in elution buffer (100 mM glycine-HCl [pH 2.8]). To further ensure that the CD63+ vesicles were free of SFTS virions not packaged into the vesicles, we carried out an immunoprecipitation (negative selection) step using magnetic beads coated with antibodies against SFTS virus, and the mix was incubated overnight. The supernatant containing the CD63+ vesicles was then removed and incubated at 4°C for 4 h with SFTS virus mouse hyperimmune ascitic fluid at a 1:1 ratio. The virus-antibody complex was then removed, and the clarified supernatant was used to infect HeLa cells as described below to determine the capacity of the CD63+ vesicles in mediating transmission of SFTS virus. To verify that the above procedures were effective at removing SFTS virions that were not packaged within the extracellular vesicles, we used the same methodology (immunoprecipitation and incubation with antibodies against SFTS virus) using an SFTS virus stock (titer of 106 PFU/ml). The resulting preparation was then used to infect HeLa cells as described below.
Infection of HeLa cells with purified extracellular vesicles.
Adsorption of the purified vesicles was performed by overlaying the purified preparation onto cells and incubated at 37°C for 1 h. Where indicated, HeLa cells were pretreated with 2 μg/ml of mouse anti-CD63, mouse IgG1, or mouse anti-SFTS virus antibodies for 1 h prior to overlaying the cells with the purified vesicles or infecting them with SFTS virus. Supernatants were collected at 0, 24, 48, and 72 h postinfection (hpi) and assayed by plaque assay.
Transmission electron microscopy (TEM).
For ultrastructural analysis in ultrathin sections, infected cells were fixed for at least 1 h in a mixture of 2.5% formaldehyde prepared from paraformaldehyde powder and 0.1% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.3) to which 0.03% picric acid and 0.03% CaCl2 were added. The monolayers were washed in 0.1 M cacodylate buffer, and the cells were scraped off and processed further as a pellet. The pellets were postfixed in 1% OsO4 in 0.1 M cacodylate buffer (pH 7.3) for 1 h, washed with distilled water, and en bloc stained with 2% aqueous uranyl acetate for 20 min at 60°C. The pellets were dehydrated in ethanol, processed through propylene oxide, and embedded in Poly/Bed 812 (Polysciences, Warrington, PA). Ultrathin sections were cut on Leica EM UC7 ultramicrotome (Leica Microsystems, Buffalo Grove, IL), stained with lead citrate, and examined in a Philips 201 transmission electron microscope at 60 kV.
For immunogold labeling of thin sections, infected cells were fixed for at least 2 h in a mixture of 2.5% formaldehyde prepared from paraformaldehyde powder and 0.1% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.3), to which 0.03% picric acid and 0.03% CaCl2 were added. The monolayers were washed in 0.1 M cacodylate buffer, and the cells were scraped off and processed further as a pellet. The pellets were en bloc stained with 2% aqueous uranyl acetate for 20 min at 60°C. The pellets were dehydrated in ethanol, processed through propylene oxide, and embedded in LR White (Polysciences). Ultrathin sections were then cut on a Leica EM UC7 ultramicrotome (Leica Microsystems). The sections were then labeled and incubated with mouse anti-SFTS virus NSs and rabbit anti-LC3 (1:20) or mouse anti-SFTS virus NSs and rabbit anti-Rab5 (1:20) primary antibodies for 1 h at room temperature and overnight at 4°C. The sections were next washed three times with 1% BSA in TBS and incubated with secondary antibody goat anti-rabbit labeled with 6 nm colloidal gold (1:20) and goat anti-mouse labeled with 15-nm colloidal gold (1:20) for 1 h. After being washed with water, the grids were fixed with glutaraldehyde for 5 min, washed with water again, and negatively stained with 2% aqueous uranyl acetate for 5 min. A final wash with water was performed three times, followed by staining with lead citrate for 30 s. They were examined with a Philips CM-100 transmission electron microscope at 60 kV.
For visualization of isolated microvesicles by electron microscopy, purified vesicles were adsorbed onto Formvar-carbon coated nickel grids for 15 min, washed three times with molecular-grade water, and negatively stained with 2% aqueous uranyl acetate. For immunogold labeling, the sample was first adsorbed onto nickel grids as previously described (18) and then incubated with rabbit anti-SFTS virus NSs and mouse anti-CD63 (1:10) antibodies for 1 h at room temperature in a wet chamber. The grids were next washed three times with 1% BSA in TBS and incubated with secondary antibody goat anti-mouse labeled with 6-nm colloidal gold (1:20) and goat anti-rabbit labeled with 15-nm colloidal gold (1:20) for 1 h. After being washed with water, the grids were fixed with glutaraldehyde for 10 min, washed with water again, and negatively stained with 2% aqueous uranyl acetate. The samples were examined with a Philips CM-100 transmission electron microscope at 60 kV.
Additionally, for ultrastructural analysis in ultrathin sections, purified vesicles were fixed overnight at 4°C in a mixture of 2.5% formaldehyde prepared from paraformaldehyde powder and 0.1% glutaraldehyde in 0.05 M cacodylate buffer. The pellets were washed in cacodylate buffer, followed by postfixation in 1% OsO4 for 1 h, washed, and en bloc stained with 2% aqueous uranyl acetate for 20 min at 60°C. The pellets were dehydrated in ethanol, processed through propylene oxide, and embedded in Poly/Bed 812 (Polysciences). For conventional TEM, SFTS virus-infected cells were fixed and processed the same way. For immunoelectron microscopy on ultrathin sections, postfixation was omitted and, after dehydration in 75% ethanol, the pellets were processed and embedded in LR White resin. Ultrathin sections were cut on a Leica EM UC7 ultramicrotome (Leica Microsystems), stained with lead citrate, and examined in a Philips CM-100 transmission electron microscope at 60 kV. Grids where then processed as mentioned above for immunostaining.
Statistical analysis.
Statistical analyses were carried out using two-way analysis of variance for multiple comparisons to determine statistical differences in virus titers by plaque assay. The results of the electron microscopy experiments were analyzed by performing Student t tests. All analyses were done by using GraphPad Prism version 6.05 (GraphPad Software). A P value of <0.05 was considered significant.
RESULTS
SFTS virus infection induces the formation of cytoplasmic structures reminiscent of early endosomes.
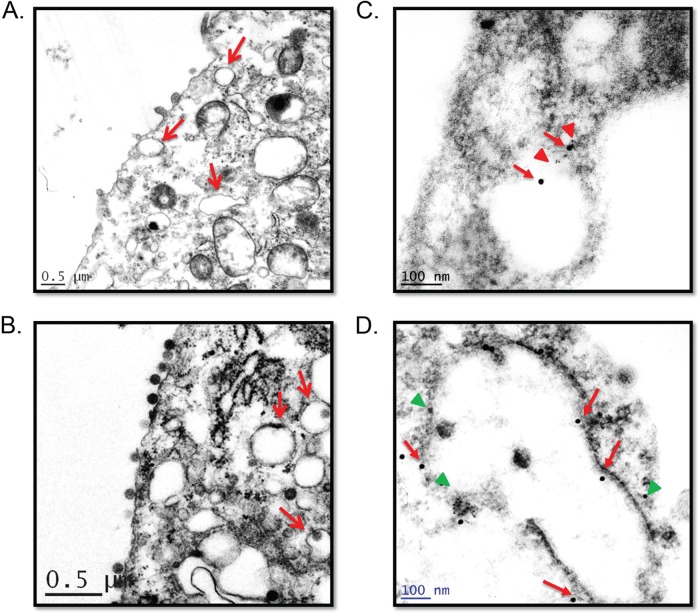
We, and others, have recently reported that SFTS virus infection induces the formation of cytoplasmic structures that play a critical role during SFTS virus replication and for evasion of innate immune responses (17, 18). Furthermore, we reported that these structures colocalized with the early endosomal marker Rab-5 and the autophagy marker LC3, but not with the endosomal marker Rab-4 (18). It was also reported that the formation of these cytoplasmic structures in SFTS virus-infected cells was dependent on lipid metabolism and that lipid droplets may play a role during SFTS virus infection (17). Therefore, we conducted TEM studies to gain further insight into the sources, morphology, and composition of these cytoplasmic structures in HeLa cells stably expressing mCherry or SFTS virus NSs-mCherry. Electron microscopy studies were also conducted in SFTS virus-infected or mock-infected Vero cells. Consistent with our previous observations (18), TEM revealed the formation of structures reminiscent of early endosomes in cells stably expressing SFTS virus NSs, as well as in virus-infected cells (Fig. 1A and B, respectively). Similar structures have also been described during UUKV infection (23). Additionally, we conducted immunogold electron microscopy on ultrathin sections of infected cells to substantiate that these structures were of endosomal origin. In correlation with our previous findings, we observed the colocalization of Rab5 and LC3 with the SFTS virus NSs-positive structures (Fig. 1C and D, respectively).
FIG 1.
SFTS virus NSs induces the formation of endosome-like structures. Ultrastructure analyses of SFTS virus NSs-expressing cells (A) and SFTS virus-infected cells (B) show cytoplasmic structures reminiscent of early endosomes (arrows) in ultrathin sections. Immunogold staining of distinct ultrathin sections shows cytoplasmic structures positive for SFTS virus NSs and Rab5 (C) or SFTS virus NSs and LC3B (D). A solid red arrows indicate SFTS virus NSs in panels C and D, while a red triangle (in panel C) and a green triangle (in panel D) indicate the detection of Rab5 and LC3B, respectively. Ultrathin sections of mock-infected cells were also labeled, as indicated above (not shown), to ensure the specificity of antibody. Representatives images are shown.
SFTS virus NSs-positive structures are released into the extracellular space.
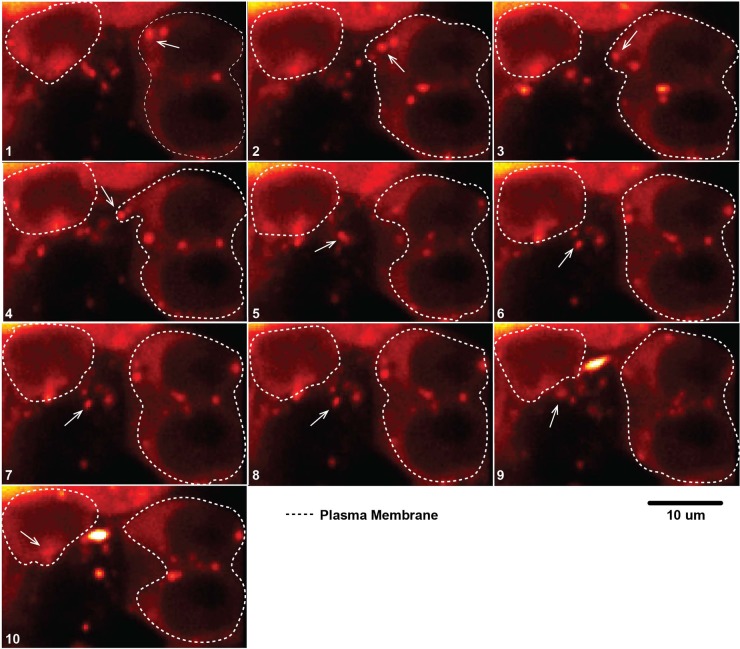
In order to gain a better understanding on how these SFTS virus NSs-positive structures traffic within the cells, we conducted live cell imaging of HeLa cells expressing SFTS virus NSs-mCherry. This approach allows the direct observation of the SFTS virus NSs-cytoplasmic structures due to the fluorescent signal. Cells were plated and monitored for 16 h using a Prairie Technologies/Nikon multimodal live cell imaging system. Interestingly, we observed that a portion of the SFTS virus NSs structures were secreted into the extracellular space and were taken up by neighboring cells (Fig. 2 and see also the supplemental material). These data suggest that the released SFTS virus NSs cytoplasmic structures may be extracellular vesicles.
FIG 2.
Cytoplasmic vesicles containing SFTS virus NSs-mCherry are secreted into the extracellular space and are endocytosed by neighboring cells. Live cell imaging was carried out in HeLa cell line stably expressing SFTS virus NSs-mCherry. Cells were visualized for 16 h using a Prairie Technologies/Nikon multimodal live cell imaging system. The arrow highlights the movement of the vesicle from cell to cell.
SFTS virus NSs-positive secreted structures contain markers found in extracellular vesicles.
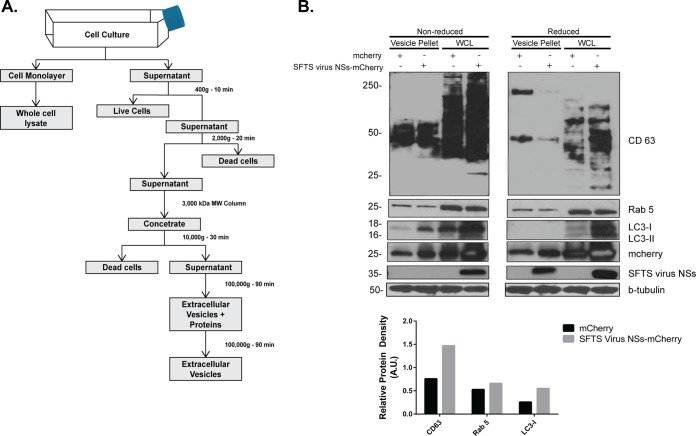
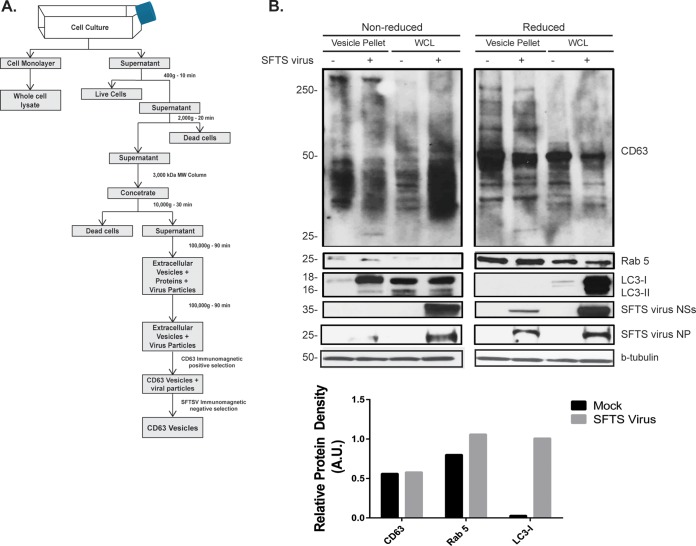
The release of extracellular vesicles has been shown to be an important mechanism for intercellular communication. These vesicles are generally referred to as exosomes (if they originated from multivesicular endosome) or microvesicles (if they originate from the plasma membrane) (26). In light of the results described above related to the involvement of the endosomal pathway in the formation of SFTS virus NSs-induced structures, as well as the active transfer of secreted SFTS virus NSs-positive structures into neighboring cells, we hypothesized that the cytoplasmic structures produced by SFTS virus NSs-expressing cells were exosomes. To test this hypothesis, we initially purified the extracellular vesicles produced by HeLa cells expressing mCherry and SFTS virus NSs-mCherry (as described in Fig. 3A) and carried out SDS-PAGE coupled with Western blotting to investigate the presence of tetraspanins such as CD63 that are known to be abundant in exosomes (27). Consistent with our hypothesis, the presence of CD63 was confirmed in the purified extracellular vesicles produced by SFTS virus NSs-mCherry expressing cells, as well as in cells expressing the mCherry protein (Fig. 3B). The presence of both Rab5 and LC3-I was also detected in the extracellular vesicles produced by both mCherry and SFTS virus NSs-mCherry expressing cells. It was also evident that in the presence of SFTS virus NSs, there was an increased amount of LC3-I and CD63 protein and a minor increase in the amount of Rab5 being incorporated into the extracellular vesicles. Densitometry analyses confirmed these observations (Fig. 3B, bottom panel). The increased detection of LC3-I also correlates with the increased amount of mCherry protein being detected in SFTS virus NSs-expressing cells, which may indicate that the SFTS virus NSs induces or enhances the production of extracellular vesicles (Fig. 3B). Furthermore, the mCherry protein was also detected in the extracellular vesicles, which could indicate that the mCherry protein may be mediating the incorporation of SFTS virus NSs into these vesicles rather than the viral protein. Therefore, we proceeded to investigate whether or not extracellular vesicles are produced during SFTS virus infection and determine if the SFTS virus NSs protein was incorporated within these vesicles, similar to what was found in NSs-expressing cells. The approach for the isolation and purification of the extracellular vesicles is described in Fig. 4A. Consistent with the results obtained in HeLa cells expressing the viral protein NSs, Western blot analyses revealed the presence of SFTS virus NSs, LC3-I as well as the endosomal marker Rab5 within the extracellular purified vesicles produced by SFTS virus-infected Vero cells (Fig. 4B). Densitometry analyses confirmed that there was increased amount of LC3 and Rab5 being incorporated into the vesicles but no CD63 (Fig. 4B, bottom panel). Furthermore, the viral nucleoprotein NP was also detected (Fig. 4B). These data provide evidence that SFTS virus NSs is incorporated within extracellular vesicles produced in NSs-expressing cells, as well as those produced during SFTS virus infection.
FIG 3.
Isolation and characterization of SFTS virus NSs-positive secreted extracellular vesicles. (A) Schematic representation of the protocol for the isolation of secreted extracellular vesicles by ultracentrifugation. (B) Supernatants from cell lines expressing the mCherry and SFTS virus NSs-mCherry proteins were collected, and isolation of extracellular microvesicles was performed as indicated in panel A. The final pellet was resuspended in lysis buffer, sonicated, resolved by SDS-PAGE electrophoresis, transferred to a PVDF membrane, and blotted for SFTS virus NSs, LC3B, and common markers for microvesicles such as Rab5, β-tubulin, and CD63 (core protein, 26 kDa; glycosylated protein, 30 to 60 kDa). The cell monolayer was used to generate the whole-cell lysate (WCL) and assayed for the detection of the proteins indicated above. Densitometry analysis of CD63, LC3-I, and Rab 5 present in extracellular vesicles isolated from mCherry or SFTS virus NSs-mCherry expressing cells was also conducted. The band signal intensity of each protein was normalized to the signal intensity of β-tubulin and expressed as arbitrary units (A.U.). Signal intensities were obtained by using ImageJ software.
FIG 4.
Characterization of extracellular microvesicles secreted during SFTS virus infection. HeLa cells were mock infected or infected with SFTS virus for 72 h. (A) Supernatant was collected, and isolation of extracellular microvesicles was performed as indicated. (B) The final pellet was resuspended in lysis buffer, sonicated, resolved by SDS-PAGE electrophoresis, transferred to a PVDF membrane, and blotted for SFTS virus NSs, SFTS virus NP, and LC3B, in addition to common markers for microvesicles such as Rab5, CD63, and β-tubulin. The cell monolayer was used to generate the WCL and assayed for the detection of proteins indicated above. Densitometry analysis of CD63, LC3-I, and Rab 5 present in extracellular vesicles isolated from mock infected or SFTS virus-infected cells was carried out. The band signal intensity of each protein was normalized to the signal intensity of β-tubulin and expressed as arbitrary units (A.U.). Signal intensities were obtained by using ImageJ software.
Ultrastructural analysis of purified SFTS virus NSs-positive extracellular vesicles.
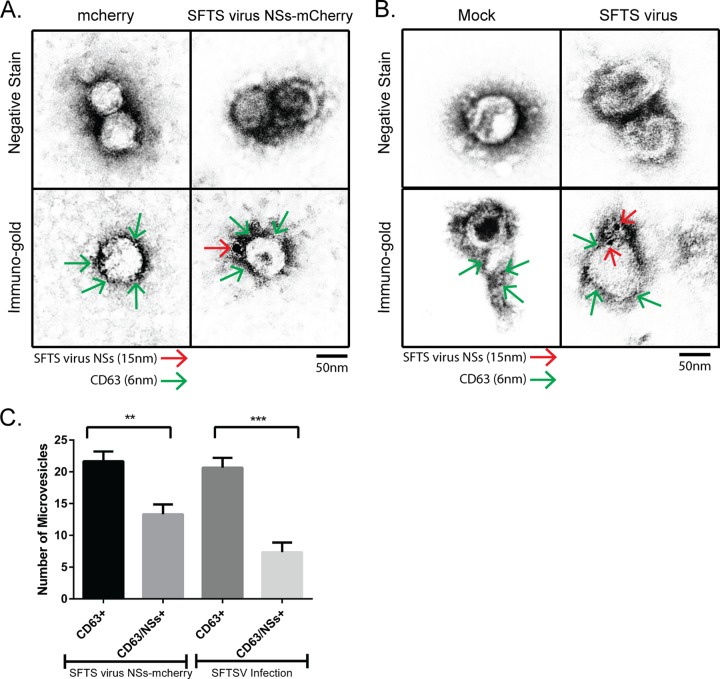
Extracellular vesicles are known to be secreted by most cell types (28). Thus, we next explored the possibility that the majority of the extracellular vesicles released in SFTS virus NSs-mCherry expressing cells and SFTS virus-infected cells harbor the NSs viral protein, which could suggest that SFTS virus directly targets the secretory multivesicular endosomal pathway. To evaluate this possibility, extracellular vesicles produced by HeLa cells expressing the mCherry or SFTS virus NSs-mCherry and SFTS virus-infected or mock-infected HeLa cells were purified and examined by electron microscopy and immunogold electron microscopy using antibodies against the SFTS virus NSs and CD63. Discrimination between these proteins after immunogold staining was done based on the size of the gold beads. The extracellular vesicles isolated from mCherry and mock-infected cells were only positive for the exosomal marker CD63 and were 50 to 100 nm in size, which is consistent with the normal 30- to 150-nm size range of exosomes (29, 30) (Fig. 5A and B, left panels). The extracellular vesicles isolated from SFTS virus NSs-mCherry and SFTS virus-infected cells were positive for CD63 (Fig. 5A and B, right bottom panels). Interestingly, the SFTS virus NSs protein (Fig. 5A and B, right bottom panels) was detected in approximately 35 to 50% of these CD63+ vesicles produced by NSs-expressing cells and SFTS virus-infected cells (Fig. 5C). No significant difference in size was observed among the extracellular vesicles whether they were positive for SFTS virus NSs or not. These results suggest that SFTS virus efficiently targets the secretory multivesicular endosomal pathway.
FIG 5.
Isolated extracellular vesicles are positive for SFTS virus NSs and the exosomal marker CD63. Supernatant was collected from HeLa cells stably expressing mCherry or SFTS virus NSs-mCherry (A) and from HeLa cells mock-infected or infected with SFTS virus (B), and isolation of extracellular microvesicles was performed as depicted in Fig. 3A. The final pellet was resuspended in molecular-grade water. The sample was adsorbed onto Ni grids and negatively stained with 2% aqueous uranyl acetate. Additional grids were incubated with primary antibodies against SFTS virus NSs (rabbit) and CD63 (mouse) and then secondary goat anti-mouse IgG couple to 6-nm colloidal gold and goat anti-rabbit IgG coupled to 15-nm colloidal gold antibodies and then negatively stained with 2% aqueous uranyl acetate. SFTS virus NSs is indicated by red arrows, and CD63 is indicated by green arrows. (C) The number of CD63-positive and CD63/SFTS virus NSs-positive extracellular vesicles was quantified based on immunogold labeling observed from 10 fields of view. A total of 22 vesicles were observed in each experiment, of which an average of 14 were positive for both SFTS virus NSs and CD63. All electron microscopy experiments were repeated three times. Results are expressed as means plus the standard errors of the mean (SEM). Asterisks specify statistically significant difference (P < 0.05) between the indicated groups.
SFTS virus NSs-positive extracellular vesicles contain SFTS virions.
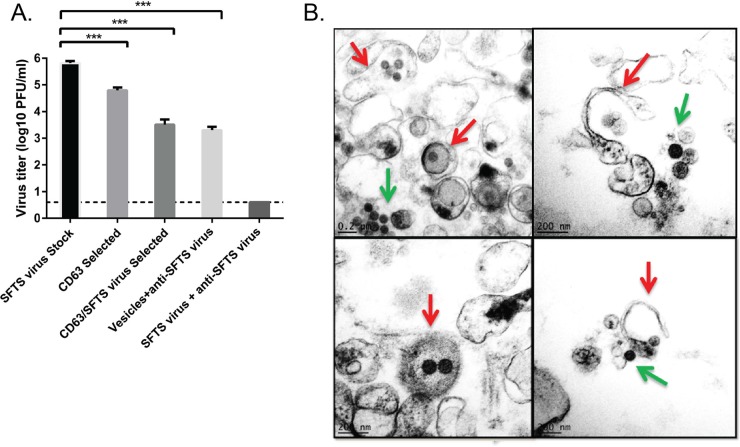
Several studies have implicated the role of extracellular vesicles in many cellular processes, including tissue injury and immune responses, and for the transport of proteins, mRNA, and microRNAs (miRNAs) between cells (31). More recently, evidence has also been obtained for the role of extracellular vesicles in the transmission of infectious agents, as well as in the modulation of host immune responses to many pathogens (32–37). With regard to SFTS virus, a recent study suggested that the SFTS virus NSs-positive cytoplasmic structures play a role during SFTS virus replication on the basis of colocalization with viral RNPs and double-stranded RNA (17). In light of the results described above and the role of SFTS virus NSs cytoplasmic structures during virus replication, we next investigated if the extracellular vesicles released by SFTS virus-infected cells contained infectious virions. Extracellular vesicles produced during SFTS virus infection were purified as shown in Fig. 4A. Briefly, due to the possibility that the virions and extracellular vesicles may have similar sizes and densities and that our procedure would not completely devoid the extracellular vesicles from free virions (28, 38), we carried out an immune-selection step using anti-CD63 beads (Fig. 6A) as previously described (39), followed by a second immune(-negative) selection using magnetic beads coated with antibodies against SFTS virus (Fig. 6A). As a final step and to ensure removal of contaminant SFTS virions in our sample, the purified vesicles were incubated with SFTS virus mouse hyperimmune ascitic fluid at a 1:1 ratio (Fig. 6A). The antibody-virus complex was then removed with magnetic beads. The resulting purified vesicles were then used for plaque assay (Fig. 6A) and to infect HeLa cells (Fig. 7). Notably we observed that the CD63-purified extracellular vesicles that underwent only immune-negative magnetic selection and those that were incubated with anti-SFTS virus hyperimmune ascitic fluid after immune-negative selection were able to produce viral titers of 4.5 × 103 PFU/ml and 2.5 × 103 PFU/ml, respectively (Fig. 6A). In contrast, the SFTS virus stock produced a titer of 7.5 × 105 PFU/ml whereas the extracellular vesicles that solely underwent CD63 positive immuno-magnetic selection produced a titer of 7.5 × 104 PFU/ml. This indicates that (i) centrifugation and CD63-positive immunoselection are not sufficient enough to void the vesicle preparation of free SFTS virions and (ii) purified extracellular vesicles produced by SFTS virus-infected cells are capable of mediating productive infection. We confirmed that our immune-negative selection step and further incubation with anti-SFTS virus antibodies was successful in removing virus particles not packaged into vesicles because our virus stock (titer of 106 PFU/ml) was subjected to the same procedure and subsequently assayed by plaque assay and we were unable to detect viral plaques (Fig. 6A). Lastly, these data suggest that the extracellular vesicles contribute as much as 2 logs of infectious virus particles to the total viral titer (compare SFTS virus stock titer to CD63/SFTS virus selected vesicles). To further confirm that the extracellular vesicles contain infectious virions, vesicles were purified from SFTS virus-infected cells, as shown in Fig. 4A, and analyzed by electron microscopy, which revealed the presence of “virus-like particles” within the isolated vesicles (Fig. 6B). These data indicate that SFTS virus hijacks the secretory multivesicular endosomal pathway to possibly mediate transmission of the virus.
FIG 6.
SFTS virus NSs-positive extracellular vesicles harbor infectious SFTS virus virions. (A) Extracellular vesicles were isolated as indicated in Fig. 4A, and samples were collected at each step of the purification process for evaluation by plaque assay. The sample resulting from the immune-selection step using anti-CD63 beads is depicted in the figure as the CD63 selected, whereas those resulting from the subsequent immune-(negative) selection step are shown as the CD63/SFTSV selected. As a final step, the purified vesicles were incubated with SFTS virus mouse hyperimmune ascitic fluid at a 1:1 ratio, and the antibody-virus complex was then removed with magnetic beads. A plaque assay of the resulting purified vesicles was performed, and the results are depicted in the figure as vesicles + anti-SFTS virus. The results of a plaque assay of the virus stock (infected cells from which the extracellular vesicles were derived) are shown as the SFTS virus stock. As a control, a virus stock was also subjected to the immune-negative selection step, followed by incubation with the SFTS virus antibody as indicated above, and a plaque assay was carried out (labeled “SFTS virus+anti-SFTS virus”). A dashed line indicates the limit of detection. The results are expressed as means + the SEM. Asterisks specify statistically significant differences (P < 0.05) between the indicated groups. (B) An electron micrograph of isolated extracellular vesicles reveals the presence of virus-like particles contained within the vesicles (red arrow). The presence of free virions from broken vesicles was also observed (green arrow).
FIG 7.
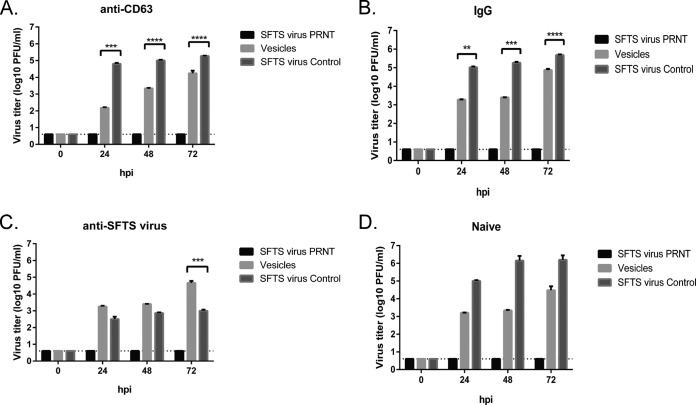
SFTS virus NSs-positive extracellular vesicles can mediate receptor-independent transmission of SFTS virus. HeLa cells were pretreated with anti-CD63 antibody (A), IgG control (B), anti-SFTS virus antibody (C), or PBS (D) prior to infection with the purified extracellular vesicles, SFTS virus, or the SFTS virus preparation subjected to the immune-negative selection and the antibody incubation step described in Fig. 6. Supernatants were harvested at 0, 24, 48, and 72 hpi, and a plaque assay was performed. The dashed line indicates the limit of detection (4 PFU/ml). All experiments were repeated three times with consistent results. The results presented are expressed as means + the SEM. Asterisks indicate statistically significant differences (P < 0.05) between the indicated groups.
Extracellular vesicles produced during SFTS virus infection mediate receptor-independent transmission of SFTS virus.
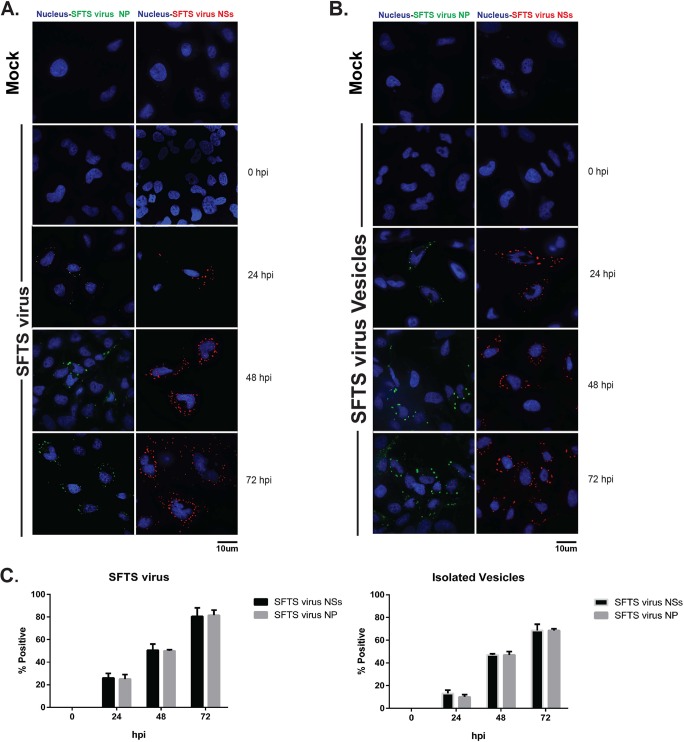
It has been previously shown that hepatitis C virus (HCV) hijacks secreted vesicles for receptor independent transmission of viral RNA (32, 39). Furthermore, hepatitis A virus (HAV) is released cloaked in host membranes in a release mechanism resembling those of exosomes (40, 41). Additionally, coxsackievirus B3 (CVB3) has been shown to target secreted vesicles for virus dissemination (33). We therefore hypothesized that extracellular vesicles produced during SFTS virus infection may mediate transmission of SFTS virus between cells. Extracellular vesicles were purified, subjected to immune selection using anti-CD63 and anti-SFTS virus antibodies, and incubated with SFTS virus mouse hyper-immune ascitic fluid as indicated above. Purified vesicles were overlaid onto uninfected HeLa cells pretreated with 2 μg/ml of either mouse anti-CD63 (Fig. 7A), mouse IgG1 (Fig. 7B), or mouse anti-SFTS virus antibodies (Fig. 7C). As a control, PBS was used for the Naive group (Fig. 7D). Supernatants were collected at 0, 24, 48, and 72 hpi and virus titer assayed by plaque assay. Consistent with our hypothesis, the purified extracellular vesicles were able to mediate productive infection of SFTS virus with titers at 24 hpi of 1.5 × 103 PFU/ml (Fig. 7D) and 1.85 × 103 PFU/ml (Fig. 7B) in the PBS- and IgG-treated HeLa cells, respectively. Interestingly, when cells were treated with anti-SFTS virus antibodies, we observed up to 1,000-fold reduction in viral titers in the SFTS virus control group; however, there was minimal effect on viral titers mediated by the purified vesicles (Fig. 7C). In contrast, we observed a 10-fold reduction in virus titer in cells treated with anti-CD63 antibody that were infected with the purified vesicles but no effect on viral titers mediated by SFTS virus (Fig. 7A). These data suggest that the extracellular vesicles produced during SFTS virus infection can mediate receptor independent transmission of SFTS virus. Lastly, to further confirm that the SFTS virus particles produced as a result of the infection with the extracellular vesicles can mediate additional rounds of replication, supernatants collected from cells infected with the purified, CD63 immune-selected extracellular vesicles described in Fig. 7D were used to infect HeLa cells. At 0, 24, 48, and 72 hpi, immunofluorescence was performed in the infected cells using antibodies against SFTS virus NP and NSs. As predicted, we were able to detect SFTS virus proteins at 24 to 72 hpi (Fig. 8). Altogether, our results suggest that SFTS virus hijacks the secretory multivesicular endosomal pathway to mediate receptor-independent transmission of the virus.
FIG 8.
Virions harbored within SFTS virus NSs-positive extracellular vesicles are capable of establishing productive infection. HeLa cells were mock infected (top of panels A and B), infected with SFTS virus (A), or infected with supernatants collected from cells infected with the purified, CD63 immune-selected extracellular vesicles described in Fig. 7D (B). Cells were fixed at 0, 24, 48, and 72 hpi. Immunofluorescence was performed using primary antibodies against SFTS virus NP or NSs and Alexa Fluor 488 as the secondary antibody. Nuclei were visualized with Hoechst 33342. Representative images for the mock-infected groups are shown. (C) Percentages of cells positive for SFTS virus NP or NSs calculated by cell counts in 10 fields of view. A total of 150 cells were counted, and the percentage was calculated by dividing the total cells positive for SFTS virus NP or NSs by 150.
DISCUSSION
SFTS is a newly emerging viral hemorrhagic fever that was first described in China and has now been recognized in Japan and South Korea (1, 2, 9–11). Although human cases caused by SFTS virus have only been reported in Asia, the recent emergence of another tick-borne phlebovirus, Heartland virus, a close relative of SFTS virus, responsible for serious and fatal cases in the United States (3, 42), and the recognition of another tick-borne phlebovirus with zoonotic potential in Australia (43), named Hunter Island Group virus, have only underscored the need to increase our knowledge of how these novel phleboviruses cause disease and establish infection.
Recent studies conducted by us and others have determined that this pathogen counteracts innate immune responses via mechanisms distinct from those described for other bunyaviruses. Unlike any other bunyavirus nonstructural protein NSs, the SFTS virus NSs interacts with and relocalizes multiple components of the IFN response into cytoplasmic structures (18, 19, 44, 45). With regard to SFTS virus replication, it has been recently shown that these cytoplasmic structures might also play a role in virus replication because double-stranded RNA and the viral proteins NP and L that are known to be involved in virus replication colocalize within these structures (17). These structures were also found to colocalize with lipid droplets. Moreover, inhibitors affecting the synthesis of fatty acids negatively impacted the formation of these cytoplasmic structures, as well as virus replication (17). In an attempt to identify the source of these structures, we found that they were most likely of endosomal origin because the early endosomal marker Rab5, but no markers associated with the Golgi apparatus colocalized with these cytoplasmic structures (18).
Although these initial investigations provided preliminary critical knowledge on the source of these structures, it was still unclear whether these structures containing viral RNA traffic within the cells to incorporate the glycoproteins to form infectious virions. Thus, in order to provide further insights into the intracellular trafficking of these structures, we initially conducted live cell imaging studies on cells expressing SFTS virus NSs fused to the mCherry protein. Surprisingly, these investigations revealed that a portion of the SFTS virus NSs-expressing cytoplasmic structures were released into the extracellular space and were taken up by neighboring cells. Subsequent studies carried out in SFTS virus-infected cells further confirmed that the SFTS virus NSs was incorporated in extracellular vesicles produced by these cells and, more importantly, they carried virions capable of sustaining transmission of the virus to neighboring cells. Furthermore, the extracellular vesicles produced from cells expressing the SFTS virus NSs were not cellular debris released from dying cells because live cell imaging studies clearly showed that these structures were released from cells that were still alive and without noticeable damage. Additionally, SFTS virus does not induce a cytopathic effect on infected cells and, more importantly, these extracellular vesicles were detected in significant amount only 3 days after infection.
The extracellular vesicles produced by SFTS virus NSs-expressing cells and SFTS virus-infected cells displayed markers characteristic of exosomes, such as being positive for the tetraspanin CD63, a widely used exosome marker (46). Interestingly, we also found that the extracellular vesicles preferentially contain LC3-I rather than LC3-II. The limited detection of LC3-II lipidated form, which is known to associate with membranes upon the induction of autophagy (47, 48) and during infection with several different viruses, including poliovirus, rhinovirus, enterovirus 71, CVB3, and foot-and-mouth disease virus, among others (49–51), suggests that these structures are not derived from the autophagy pathway. Further, the shedding mechanism is distinct from the previously described autophagosome-mediated exit without lysis (AWOL) model for poliovirus release and also differs from a similar model described recently for CVB3 (33, 52). Since the nonlipidated form of LC3 is preferentially incorporated into the extracellular vesicles secreted by SFTS virus-infected cells, it is likely that this represents another example of a role for LC3 that is unrelated to autophagy. It has been previously reported that the nonlipidated form of LC3, referred as LC3-I, is also associated with membranes of the endoplasmic reticulum-associated degradation (ERAD) tuning vesicles (or EDEMosomes) and recent studies have suggested that these structures may serve as scaffold for positive-strand RNA virus replication complexes (53, 54). In our attempts to determine the source of the cytoplasmic structures induced by SFTS virus, we previously explored the possibility that they might be derived from the ERAD tuning pathway; however, we did not find any evidence supporting this possibility (18). In contrast, our data suggest that these structures are derived from the multivesicular endosomal pathway and might be classified as exosomes. Thus, our studies suggest that the nonlipidated form of LC3 is incorporated into extracellular vesicles of endosomal origin and may facilitate replication of negative-strand RNA viruses (such as SFTS virus) as well.
It has been recently shown that HCV hijacks exosomes to incorporate infectious RNA into these structures that are then capable of mediating receptor-independent transmission of the virus (32, 39). Here, we describe another model for subversion of exosome-like structures to mediate receptor-independent transmission involving the novel bunyavirus SFTS virus. Similar to the CVB3, but in contrast to HCV, we were able to detect one to five virions harbored within the exosome-like structures that were capable of establishing productive infection of cells that received them. These findings are quite remarkable because there have not been prior reports describing the localization of bunyavirus or any other arthropod-borne viruses within extracellular vesicles to mediate receptor-independent transmission of the virus. Thus, our findings highlight an elegant strategy by which the recently recognized SFTS virus subverts exosome-like structures for virus dissemination. Our data also suggest that this mechanism of infection is likely beneficial for SFTS virus because it provides a degree of protection against neutralizing antibodies and therefore contributes to the immune evasion properties of the virus. Future studies are needed to define the role of these “infectious exosome-like structures” in expanding the tropism of the virus and their contribution to viral pathogenesis. Additional studies are also needed to define exactly how these structures deliver the virus and viral RNA into the cells and the fusion mechanisms that probably occur between the viral and cellular vesicles membranes for infection to occur. Furthermore, studies are needed to determine whether the infectious extracellular vesicles are also produced during infection of the arthropod host and whether they play a significant role during the transmission cycle involving host and vector.
Supplementary Material
ACKNOWLEDGMENTS
We thank Linsey Yeager and Alan Barrett for critically reading the manuscript and for helpful suggestions. Additionally, we thank Mohammad Jamaluddin for providing the protocol for vesicle isolation.
This research was supported by grants from the Institute for Human Infections and Immunity and the National Institute of Allergy and Infectious Diseases (NIAID) through the Western Regional Center for Excellence for Biodefense and Emerging Infectious Disease Research, by National Institutes of Health (NIH) grant U54AI057156 (P.V.A.), by NIH grant AI115286-01A1, by NIH contract HHSN27220100 0040I/HHSN27200004/D04, by NIH grant 5UC7AI094660, and by start-up funds from the Department of Pathology to P.V.A.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02490-15.
REFERENCES
- 1.Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, Zhang L, Zhang QF, Popov VL, Li C, Qu J, Li Q, Zhang YP, Hai R, Wu W, Wang Q, Zhan FX, Wang XJ, Kan B, Wang SW, Wan KL, Jing HQ, Lu JX, Yin WW, Zhou H, Guan XH, Liu JF, Bi ZQ, Liu GH, Ren J, Wang H, Zhao Z, Song JD, He JR, Wan T, Zhang JS, Fu XP, Sun LN, Dong XP, Feng ZJ, Yang WZ, Hong T, Zhang Y, Walker DH, Wang Y, Li DX. 2011. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med 364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Q, He B, Huang SY, Wei F, Zhu XQ. 2014. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis 14:763–772. doi: 10.1016/S1473-3099(14)70718-2. [DOI] [PubMed] [Google Scholar]
- 3.Muehlenbachs A, Fata CR, Lambert AJ, Paddock CD, Velez JO, Blau DM, Staples JE, Karlekar MB, Bhatnagar J, Nasci RS, Zaki SR. 2014. Heartland virus-associated death in Tennessee. Clin Infect Dis 59:845–850. doi: 10.1093/cid/ciu434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yun SM, Lee WG, Ryou J, Yang SC, Park SW, Roh JY, Lee YJ, Park C, Han MG. 2014. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg Infect Dis 20:1358–1361. doi: 10.3201/eid2008.131857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ding F, Zhang W, Wang L, Hu W, Soares Magalhaes RJ, Sun H, Zhou H, Sha S, Li S, Liu Q, Li Q, Yang W, Huang L, Li C, Yin W. 2013. Epidemiologic features of severe fever with thrombocytopenia syndrome in China, 2011-2012. Clin Infect Dis 56:1682–1683. doi: 10.1093/cid/cit100. [DOI] [PubMed] [Google Scholar]
- 6.Jiao Y, Zeng X, Guo X, Qi X, Zhang X, Shi Z, Zhou M, Bao C, Zhang W, Xu Y, Wang H. 2012. Preparation and evaluation of recombinant severe Fever with thrombocytopenia syndrome virus nucleocapsid protein for detection of total antibodies in human and animal sera by double-antigen sandwich enzyme-linked immunosorbent assay. J Clin Microbiol 50:372–377. doi: 10.1128/JCM.01319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao CJ, Guo XL, Qi X, Hu JL, Zhou MH, Varma JK, Cui LB, Yang HT, Jiao YJ, Klena JD, Li LX, Tao WY, Li X, Chen Y, Zhu Z, Xu K, Shen AH, Wu T, Peng HY, Li ZF, Shan J, Shi ZY, Wang H. 2011. A family cluster of infections by a newly recognized bunyavirus in eastern China, 2007: further evidence of person-to-person transmission. Clin Infect Dis 53:1208–1214. doi: 10.1093/cid/cir732. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Li Q, Hu W, Wu J, Wang Y, Mei L, Walker DH, Ren J, Yu XJ. 2012. Person-to-person transmission of severe fever with thrombocytopenia syndrome virus. Vector Borne Zoonotic Dis 12:156–160. doi: 10.1089/vbz.2011.0758. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YZ, Zhou DJ, Xiong Y, Chen XP, He YW, Sun Q, Yu B, Li J, Dai YA, Tian JH, Qin XC, Jin D, Cui Z, Luo XL, Li W, Lu S, Wang W, Peng JS, Guo WP, Li MH, Li ZJ, Zhang S, Chen C, Wang Y, de Jong MD, Xu J. 2011. Hemorrhagic fever caused by a novel tick-borne Bunyavirus in Huaiyangshan, China. Zhonghua Liu Xing Bing Xue Za Zhi 32:209–220. (In Chinese.) [PubMed] [Google Scholar]
- 10.Chang MS, Woo JH. 2013. Severe Fever with thrombocytopenia syndrome: tick-mediated viral disease. J Korean Med Sci 28:795–796. doi: 10.3346/jkms.2013.28.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, Choe PG, Kim NJ, Lee JK, Oh MD. 2013. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis 19:1892–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guu TS, Zheng W, Tao YJ. 2012. Bunyavirus: structure and replication. Adv Exp Med Biol 726:245–266. doi: 10.1007/978-1-4614-0980-9_11. [DOI] [PubMed] [Google Scholar]
- 13.Palacios G, Savji N, Travassos da Rosa A, Guzman H, Yu X, Desai A, Rosen GE, Hutchison S, Lipkin WI, Tesh R. 2013. Characterization of the Uukuniemi virus group (Phlebovirus: Bunyaviridae): evidence for seven distinct species. J Virol 87:3187–3195. doi: 10.1128/JVI.02719-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana J, Lopez-Montero N, Elliott RM, Fernandez JJ, Risco C. 2008. The unique architecture of Bunyamwera virus factories around the Golgi complex. Cell Microbiol 10:2012–2028. doi: 10.1111/j.1462-5822.2008.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Novoa RR, Calderita G, Cabezas P, Elliott RM, Risco C. 2005. Key Golgi factors for structural and functional maturation of bunyamwera virus. J Virol 79:10852–10863. doi: 10.1128/JVI.79.17.10852-10863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salanueva IJ, Novoa RR, Cabezas P, Lopez-Iglesias C, Carrascosa JL, Elliott RM, Risco C. 2003. Polymorphism and structural maturation of bunyamwera virus in Golgi and post-Golgi compartments. J Virol 77:1368–1381. doi: 10.1128/JVI.77.2.1368-1381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Qi X, Liang M, Li C, Cardona CJ, Li D, Xing Z. 2014. Roles of viroplasm-like structures formed by nonstructural protein NSs in infection with severe fever with thrombocytopenia syndrome virus. FASEB J 28:2504–2516. doi: 10.1096/fj.13-243857. [DOI] [PubMed] [Google Scholar]
- 18.Santiago FW, Covaleda LM, Sanchez-Aparicio MT, Silvas JA, Diaz-Vizarreta AC, Patel JR, Popov V, Yu XJ, García-Sastre A, Aguilar PV. 2014. Hijacking of RIG-I signaling proteins into virus-induced cytoplasmic structures correlates with the inhibition of type I interferon responses. J Virol 88:4572–4585. doi: 10.1128/JVI.03021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ning YJ, Wang M, Deng M, Shen S, Liu W, Cao WC, Deng F, Wang YY, Hu Z, Wang H. 2014. Viral suppression of innate immunity via spatial isolation of TBK1/IKKε from mitochondrial antiviral platform. J Mol Cell Biol 6:324–337. doi: 10.1093/jmcb/mju015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bridgen A, Weber F, Fazakerley JK, Elliott RM. 2001. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis. Proc Natl Acad Sci U S A 98:664–669. doi: 10.1073/pnas.98.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blakqori G, Delhaye S, Habjan M, Blair CD, Sánchez-Vargas I, Olson KE, Attarzadeh-Yazdi G, Fragkoudis R, Kohl A, Kalinke U, Weiss S, Michiels T, Staeheli P, Weber F. 2007. La Crosse bunyavirus nonstructural protein NSs serves to suppress the type I interferon system of mammalian hosts. J Virol 81:4991–4999. doi: 10.1128/JVI.01933-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliott RM, Weber F. 2009. Bunyaviruses and the type I interferon system. Viruses 1:1003–1021. doi: 10.3390/v1031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozach PY, Mancini R, Bitto D, Meier R, Oestereich L, Overby AK, Pettersson RF, Helenius A. 2010. Entry of bunyaviruses into mammalian cells. Cell Host Microbe 7:488–499. doi: 10.1016/j.chom.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, Kominami E, Tanaka K, Chiba T. 2005. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol 169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brennan B, Li P, Zhang S, Li A, Liang M, Li D, Elliott RM. 2015. Reverse genetics system for severe fever with thrombocytopenia syndrome virus. J Virol 89:3026–3037. doi: 10.1128/JVI.03432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raposo G, Stoorvogel W. 2013. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escola JM, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. 1998. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 28.Lai FW, Lichty BD, Bowdish DM. 2015. Microvesicles: ubiquitous contributors to infection and immunity. J Leukoc Biol 97:237–245. doi: 10.1189/jlb.3RU0513-292RR. [DOI] [PubMed] [Google Scholar]
- 29.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. 1985. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol 101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harding C, Heuser J, Stahl P. 1984. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: demonstration of a pathway for receptor shedding. Eur J Cell Biol 35:256–263. [PubMed] [Google Scholar]
- 31.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 32.Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin Raj V, Jenster G, Kwekkeboom J, Tilanus HW, Haagmans BL, Baumert TF, van der Laan LJ. 2013. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A 110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson SM, Tsueng G, Sin J, Mangale V, Rahawi S, McIntyre LL, Williams W, Kha N, Cruz C, Hancock BM, Nguyen DP, Sayen MR, Hilton BJ, Doran KS, Segall AM, Wolkowicz R, Cornell CT, Whitton JL, Gottlieb RA, Feuer R. 2014. Coxsackievirus B exits the host cell in shed microvesicles displaying autophagosomal markers. PLoS Pathog 10:e1004045. doi: 10.1371/journal.ppat.1004045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalamvoki M, Du T, Roizman B. 2014. Cells infected with herpes simplex virus 1 export to uninfected cells exosomes containing STING, viral mRNAs, and microRNAs. Proc Natl Acad Sci U S A 111:E4991–E4996. doi: 10.1073/pnas.1419338111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mack M, Kleinschmidt A, Brühl H, Klier C, Nelson PJ, Cihak J, Plach[1290] J, Stangassinger M, Erfle V, Schlöndorff D. 2000. Transfer of the chemokine receptor CCR5 between cells by membrane-derived microparticles: a mechanism for cellular human immunodeficiency virus 1 infection. Nat Med 6:769–775. doi: 10.1038/77498. [DOI] [PubMed] [Google Scholar]
- 36.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ. 1996. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Würdinger T, Middeldorp JM. 2010. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A 107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Liu Y, Zhao L, Li B, Yu H, Wen H, Yu XJ. 2013. An emerging hemorrhagic fever in China caused by a novel bunyavirus SFTSV. Sci China Life Sci 56:697–700. doi: 10.1007/s11427-013-4518-9. [DOI] [PubMed] [Google Scholar]
- 39.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. 2014. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog 10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colombo M, Raposo G, Théry C. 2014. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 41.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. 2013. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, Metcalfe MG, Batten BC, Albariño CG, Zaki SR, Rollin PE, Nicholson WL, Nichol ST. 2012. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med 367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Selleck P, Yu M, Ha W, Rootes C, Gales R, Wise T, Crameri S, Chen H, Broz I, Hyatt A, Woods R, Meehan B, McCullough S, Wang LF. 2014. Novel phlebovirus with zoonotic potential isolated from ticks, Australia. Emerg Infect Dis 20:1040–1043. doi: 10.3201/eid2006.140003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Qi X, Qu B, Zhang Z, Liang M, Li C, Cardona CJ, Li D, Xing Z. 2014. Evasion of antiviral immunity through sequestering of TBK1/IKKε/IRF3 into viral inclusion bodies. J Virol 88:3067–3076. doi: 10.1128/JVI.03510-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ning YJ, Feng K, Min YQ, Cao WC, Wang M, Deng F, Hu Z, Wang H. 2015. Disruption of type I interferon signaling by the nonstructural protein of severe fever with thrombocytopenia syndrome virus via the hijacking of STAT2 and STAT1 into inclusion bodies. J Virol 89:4227–4236. doi: 10.1128/JVI.00154-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Natasha G, Gundogan B, Tan A, Farhatnia Y, Wu W, Rajadas J, Seifalian AM. 2014. Exosomes as immunotheranostic nanoparticles. Clin Ther 36:820–829. doi: 10.1016/j.clinthera.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. 2000. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N, Mizushima N, Tanida I, Kominami E, Ohsumi M, Noda T, Ohsumi Y. 2000. A ubiquitin-like system mediates protein lipidation. Nature 408:488–492. doi: 10.1038/35044114. [DOI] [PubMed] [Google Scholar]
- 49.O'Donnell V, Pacheco JM, LaRocco M, Burrage T, Jackson W, Rodriguez LL, Borca MV, Baxt B. 2011. Foot-and-mouth disease virus utilizes an autophagic pathway during viral replication. Virology 410:142–150. doi: 10.1016/j.virol.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang SC, Chang CL, Wang PS, Tsai Y, Liu HS. 2009. Enterovirus 71-induced autophagy detected in vitro and in vivo promotes viral replication. J Med Virol 81:1241–1252. doi: 10.1002/jmv.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klein KA, Jackson WT. 2011. Human rhinovirus 2 induces the autophagic pathway and replicates more efficiently in autophagic cells. J Virol 85:9651–9654. doi: 10.1128/JVI.00316-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bird SW, Maynard ND, Covert MW, Kirkegaard K. 2014. Nonlytic viral spread enhanced by autophagy components. Proc Natl Acad Sci U S A 111:13081–13086. doi: 10.1073/pnas.1401437111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reggiori F, Monastyrska I, Verheije MH, Cal[1279] T, Ulasli M, Bianchi S, Bernasconi R, de Haan CA, Molinari M. 2010. Coronaviruses hijack the LC3-I-positive EDEMosomes, ER-derived vesicles exporting short-lived ERAD regulators, for replication. Cell Host Microbe 7:500–508. doi: 10.1016/j.chom.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reggiori F, de Haan CA, Molinari M. 2011. Unconventional use of LC3 by coronaviruses through the alleged subversion of the ERAD tuning pathway. Viruses 3:1610–1623. doi: 10.3390/v3091610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.