Abstract
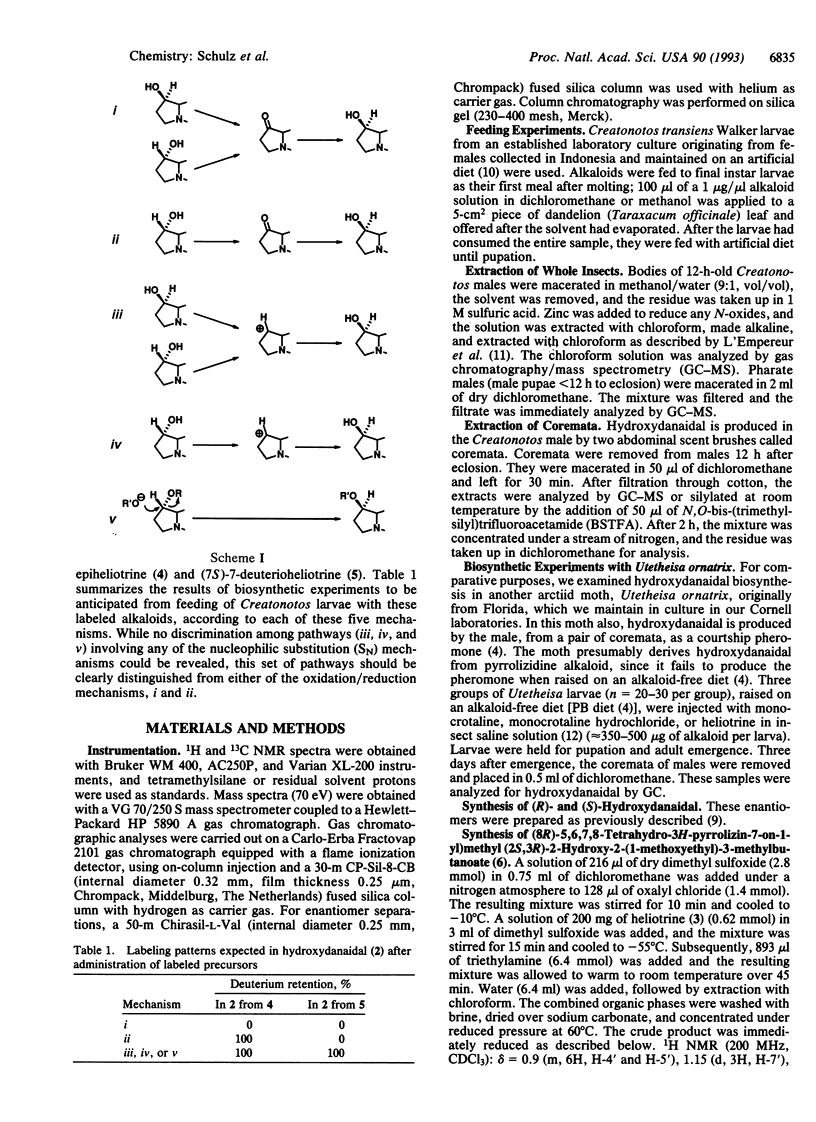
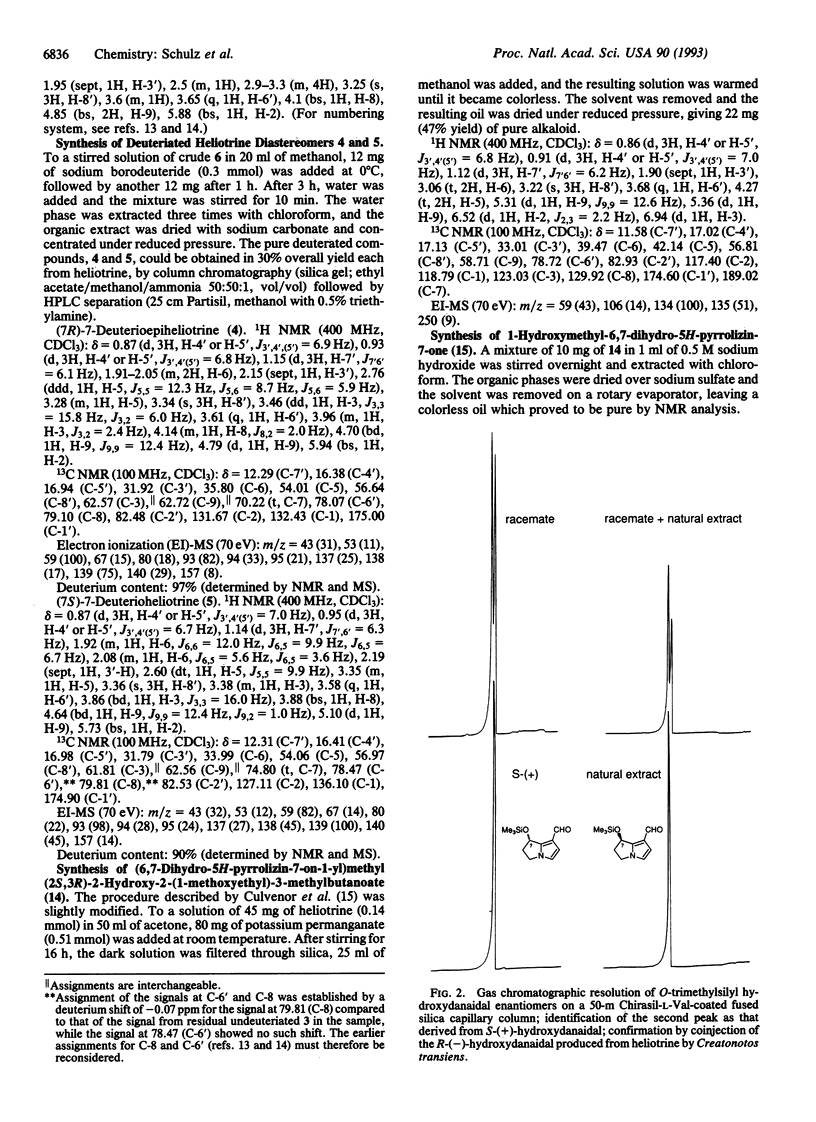
The mechanism by which the moth Creatonotos transiens produces its male pheromone, (7R)-hydroxydanaidal, from heliotrine, an alkaloidal precursor of opposite (7S) stereochemistry, was investigated. Specifically deuteriated samples of heliotrine and epiheliotrine were prepared and fed to C. transiens larvae, and the steps in the biosynthetic process were monitored by gas chromatography/mass spectrometry. These analyses indicate that heliotrine is initially epimerized to (7S)-epiheliotrine by oxidation to the corresponding ketone followed by stereospecific reduction. The order of the subsequent steps is (i) aromatization of the dihydropyrrole ring, (ii) ester hydrolysis, and (iii) oxidation of the resulting primary alcohol to the final aldehyde. The ecological implications of this insect's ability (and the inability of another moth, Utetheisa ornatrix) to use representatives of two stereochemical families of alkaloids as pheromone precursors are discussed.
Full text
PDF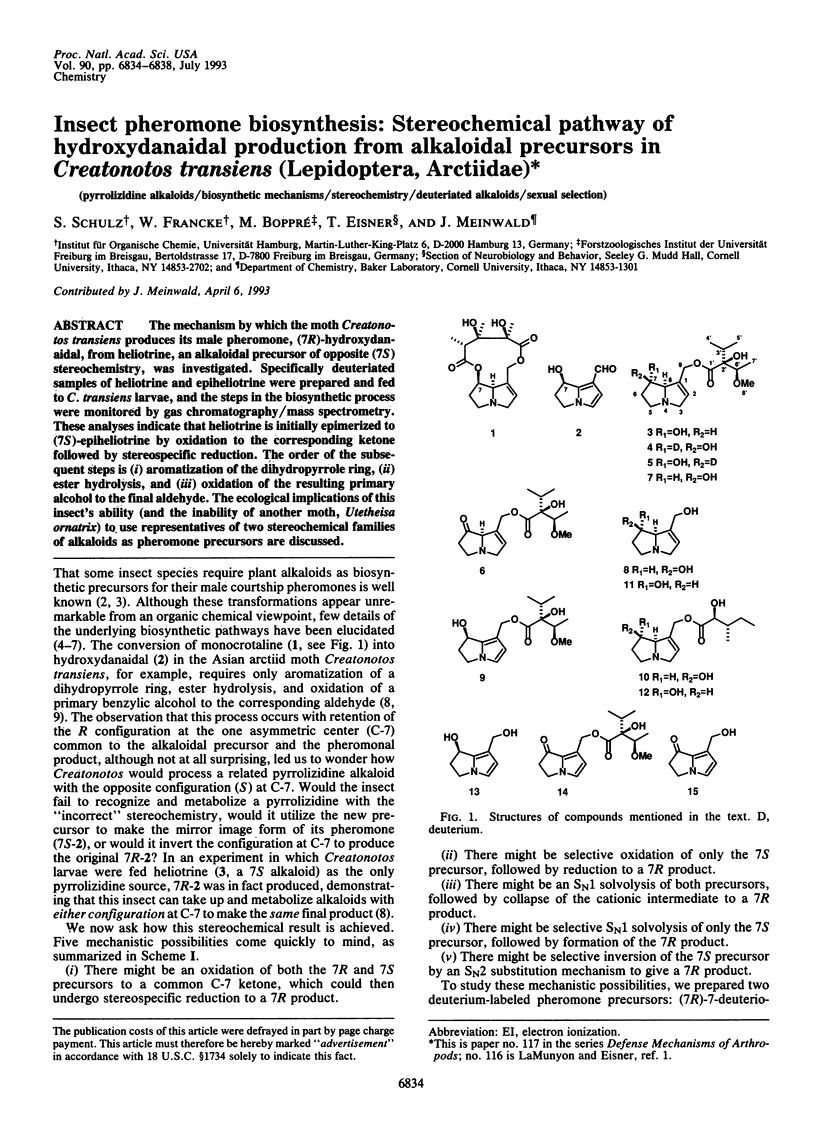


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell T. W., Boppré M., Schneider D., Meinwald J. Stereochemical course of pheromone biosynthesis in the arctiid moth, Creatonotos transiens. Experientia. 1984 Jul 15;40(7):713–714. doi: 10.1007/BF01949738. [DOI] [PubMed] [Google Scholar]
- LaMunyon C. W., Eisner T. Postcopulatory sexual selection in an arctiid moth (Utetheisa ornatrix). Proc Natl Acad Sci U S A. 1993 May 15;90(10):4689–4692. doi: 10.1073/pnas.90.10.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattocks A. R. Dihydropyrrolizine derivatives from unsaturated pyrrolizidine alkaloids. J Chem Soc Perkin 1. 1969;8:1155–1162. doi: 10.1039/j39690001155. [DOI] [PubMed] [Google Scholar]



