ABSTRACT
Receptor-interacting protein kinase 3 (RIP3) and its substrate mixed-lineage kinase domain-like protein (MLKL) are core regulators of programmed necrosis. The elimination of pathogen-infected cells by programmed necrosis acts as an important host defense mechanism. Here, we report that human herpes simplex virus 1 (HSV-1) and HSV-2 had opposite impacts on programmed necrosis in human cells versus their impacts in mouse cells. Similar to HSV-1, HSV-2 infection triggered programmed necrosis in mouse cells. However, neither HSV-1 nor HSV-2 infection was able to induce programmed necrosis in human cells. Moreover, HSV-1 or HSV-2 infection in human cells blocked tumor necrosis factor (TNF)-induced necrosis by preventing the induction of an RIP1/RIP3 necrosome. The HSV ribonucleotide reductase large subunit R1 was sufficient to suppress TNF-induced necrosis, and its RIP homotypic interaction motif (RHIM) domain was required to disrupt the RIP1/RIP3 complex in human cells. Therefore, this study provides evidence that HSV has likely evolved strategies to evade the host defense mechanism of programmed necrosis in human cells.
IMPORTANCE This study demonstrated that infection with HSV-1 and HSV-2 blocked TNF-induced necrosis in human cells while these viruses directly activated programmed necrosis in mouse cells. Expression of HSV R1 suppressed TNF-induced necrosis of human cells. The RHIM domain of R1 was essential for its association with human RIP3 and RIP1, leading to disruption of the RIP1/RIP3 complex. This study provides new insights into the species-specific modulation of programmed necrosis by HSV.
INTRODUCTION
Necrotic cell death characterized by the disruption of the plasma membrane has been observed in a variety of physiological and pathological processes, including in mammalian development, in tissue damage, and in pathogen infection (1–3). Inhibition of apoptosis is known to facilitate programmed necrosis in cells. Proteins of the tumor necrosis factor (TNF) family of cytokines, including TNF-α, TRAIL (TNF-related apoptosis-inducing ligand), and FasL, are classic inducers of programmed necrosis, also known as necroptosis (4). In TNF-α-triggered necrosis, receptor-interacting protein kinase 1 (RIP1) (5) forms a protein complex, called the necrosome (6), with receptor-interacting protein kinase 3 (RIP3) (7–9) through the RIP homotypic interaction motif (RHIM) domains of both proteins (10). Deubiquitination of RIP1 by cylindromatosis (CYLD) is required to mediate necrosome formation and activation (11, 12). Active RIP3 subsequently phosphorylates its substrate, mixed-lineage kinase domain-like protein (MLKL), to trigger membrane localization of MLKL and downstream events for the induction of membrane rupture (13–17).
Additionally, the recognition of pathogen-associated molecular patterns by the Toll-like receptor (TLR) proteins triggers programmed necrosis. TLR3 and TLR4 specifically recognize, respectively, viral double-stranded RNA (dsRNA) [or a synthesized analog of dsRNA poly(I·C)], and bacteria lipopolysaccharide (LPS), respectively (18). Activation of TLR3 and TLR4 by these ligands induces the interaction of the Toll/interleukin-1 (IL-1) receptor domain-containing adaptor inducing beta interferon (IFN-β) (TRIF) with RIP3. TRIF, RIP3, and MLKL are all known to be essential components in the regulation of TLR-mediated necrosis (19, 20).
Recent studies have revealed that programmed necrosis acts as an effective mechanism to control viral replication and pathogenesis. Vaccinia virus (VV) is known to encode the caspase inhibitor B13R (21, 22) that confers the ability to block apoptosis. Infection of vaccinia virus (VV) in mouse embryonic fibroblasts (MEFs) sensitizes the cells to TNF-α-induced necrosis (7). RIP3 knockout mice exert reduced necrosis and succumb to VV infection (7). In contrast, murine cytomegalovirus (MCMV) infection suppresses both TNF receptor (TNFR)- and TLR3-mediated necrosis in mouse cells via the RHIM-containing viral protein M45/vIRA (19, 23). M45/vIRA mutant MCMV triggers programmed necrosis by inducing an interaction between RIP3 and the DNA-dependent activator of IFN regulatory factor (DAI) (24). Unlike VV and MCMV, herpes simplex virus 1 (HSV-1) infection naturally activates mouse RIP3 (mRIP3)/mMLKL-dependent necrosis in mouse cells independently of TNFR, TLR3, and DAI (25, 26). During HSV-1 infection, RIP3 is activated by the assembly of a complex with the RHIM-containing viral protein ICP6, the large subunit (R1) of ribonucleotide reductase (RR), leading to MLKL activation and necrosis of host cells (25, 26). RIP3-deficient mice showed severely impaired control of HSV-1 replication and pathogenesis (25). Although HSV-1 is a common human herpesvirus, it remains unclear precisely how HSV-1 modulates programmed necrosis in human cells. In the present study, we demonstrate that HSV-1 and HSV-2 modulate programmed necrosis by distinct mechanisms in murine cells and human cells, leading to opposite consequences in these two species. Both HSV-1 and HSV-2 trigger the formation of the mRIP3/mMLKL complex and programmed necrosis in mouse cells. In human cells, in contrast, HSV-1 or HSV-2 infection not only fails to activate programmed necrosis but also effectively subverts TNF-induced necrosis. HSV R1 is sufficient to prevent the recruitment of human RIP1 (hRIP1) to hRIP3 and TNF-induced necrosis of human cells. Together, our work reveals dual roles of HSV R1 in modulating programmed necrosis via the RHIM-dependent activation or suppression of RIP3 signaling in a species-specific manner.
MATERIALS AND METHODS
Reagents.
Human TNF-α recombinant protein and an Smac mimetic were generated as previously described (8). Z-VAD (benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone) was purchased from Bachem. Mouse anti-RIPK1 monoclonal antibody (MAb) was purchased from BD Biosciences (Shanghai, China). Rabbit anti-mRIP3 polyclonal antibody was purchased from ProSci (San Diego, CA). Mouse anti-VP16 MAb was purchased from Abcam (Shanghai, China). Anti-Flag MAb, anti-Myc MAb, antihemagglutinin (anti-HA) MAbs, and rabbit anti-β-actin polyclonal antibody were purchased from Sigma (Shanghai, China). Rabbit anti-hRIP3 polyclonal antibody was generated as previously described (8). HSV-1 ICP6 (25) polyclonal antibody was generated as previously reported.
Cell culture and viral infection.
HEK-293T cells, mouse fibrosarcoma L929 cells, and African green monkey kidney (Vero) cells were from ATCC. MEFs were isolated from day 14.5 to day 15.5 embryos. HEK-293T, L929, MEF, and Vero cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher). HT-29 cells were cultured in McCoy's 5A culture medium (Invitrogen). All media were supplemented with 10% fetal bovine serum (FBS; Gibco) and 100 U/ml penicillin-streptomycin (HyClone). HSV-1(KOS), HSV-1 with a deletion of ICP6 (ICP6Δ HSV-1), and HSV-2 were grown in Vero cells.
Stable cell lines.
HeLa-hRIP3 cells were generated as described previously (27). HeLa cells stably expressing Tet repressor (HeLa-tetR stable cells) were generated after transfection with pcDNA6/TR (Invitrogen) and selection with 10 μg/ml blasticidin. HeLa-tetR stable cells were further transfected with a DNA plasmid expressing HA-Flag-hRIP3 and then selected with 1 mg/ml G418. HT-29 cells stably expressing a short hairpin RNA targeting RIP3 (HT-29-RIP3-shRNA cells) were generated as described previously (8).
Plasmids and siRNA oligonucleotides.
cDNAs of ICP6 and ICP10 were amplified from total RNA of cells infected with HSV-1 and HSV-2, respectively, by reverse transcription-PCR (RT-PCR). ICP6 and ICP10 were cloned into the pCDNA3.1 plasmid. The ICP6 RHIM mutant (residues 73 through 76 mutated to four alanine residues) and the ICP10 RHIM mutant were generated using a QuikChange Lightning site-directed mutagenesis kit (Stratagene). Mouse RIP3 and the MLKL small interfering RNAs (siRNAs) were synthesized by Shanghai GenePharma Co., Ltd. The following siRNA oligonucleotides were used: mRIP3, CCCGACGAUGUCUUCUGUCAA; mMLKL, GAGAUCCAGUUCAACGAUA; and negative-control siRNA, AACGUACGCGGAAUACUUCGA. Lipofectamine 2000 (Invitrogen) and INTERFERin (Polyplus) were used for the transfection of DNA plasmids and siRNA oligonucleotides, respectively.
Cell viability assay.
Cell survival analysis was performed by measuring intracellular ATP levels using a Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) according to the manufacturer's instructions. Data are represented as the means ± standard deviations of duplicates. All experiments were repeated at least three times with similar results.
Western blot analysis and immunoprecipitation.
The cells were harvested and resuspended in lysis buffer containing 20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 10% glycerol, 25 mM β-glycerol-phosphate, 1 mM Na3VO4, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), a complete protease inhibitor set (Roche), and a phosphatase inhibitor set (Sigma). After incubation on ice for 20 min, the total cell lysates were centrifuged at 20,000 × g for 20 min. The supernatant was then collected and used for subsequent Western blot or immunoprecipitation analysis. For Flag immunoprecipitation, cell lysates were incubated overnight with anti-Flag agarose beads (Sigma) at 4°C. Agarose beads were washed four to six times with lysis buffer, and the immunoprecipitants were eluted by the addition of a low-pH elution buffer (Pierce) or Flag peptide (Sigma). Myc pulldown was performed using anti-Myc agarose beads (Sigma) with a similar procedure. Finally, acid elution was neutralized by the addition of 1/20 volume of 1 M Tris-HCl (pH 9.4). Immunocomplexes were further examined by Western blot analysis.
RESULTS
HSV-2 as well as HSV-1 infection directly activates RIP3/MLKL-dependent necrosis in mouse cells.
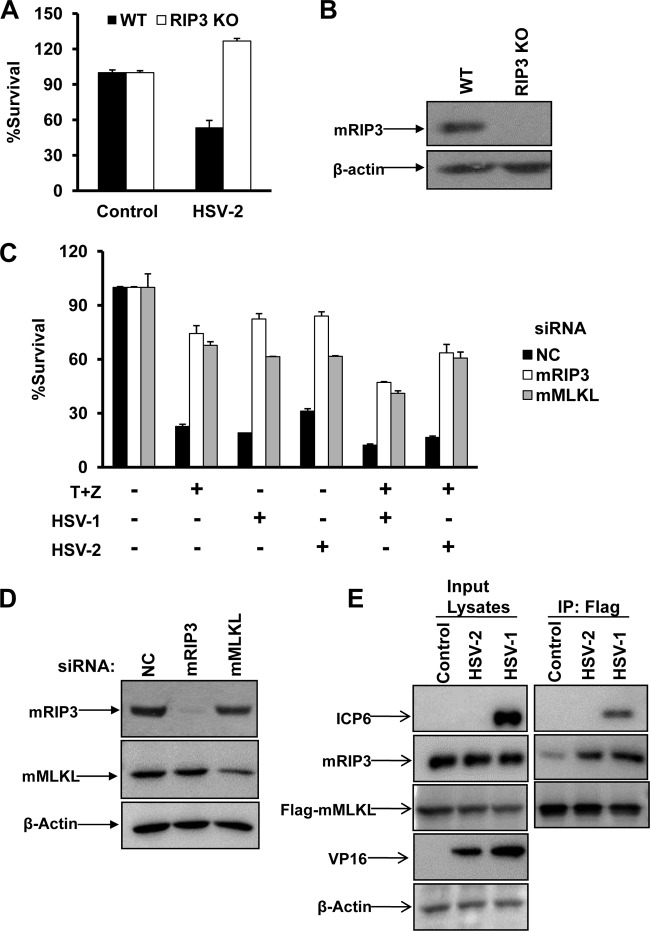
Our recent work has demonstrated that HSV-1 activated RIP3-dependent necrosis in mouse cells. We subsequently examined the effect of HSV-2 on RIP3-dependent necrosis in MEFs. As shown in Fig. 1A and B, HSV-2 infection triggered necrosis in MEFs, and this cell death could be blocked by the lack of RIP3. It is known that the addition of TNF-α/Smac mimetic/Z-VAD triggers TNF-mediated necrosis in MEFs. As expected, reducing the expression of mRIP3 or mMLKL by RNA interference (RNAi) provided effective protection against the cell death induced both by HSV alone and by HSV infection plus TNF-α/Smac mimetic/Z-VAD treatment (Fig. 1C and D). As activated RIP3 is known to recruit its substrate MLKL, we further examined whether HSV-2 infection could trigger the mRIP3/mMLKL complex. As shown in Fig. 1E, the mRIP3/mMLKL complex was apparently induced in response to infection of HSV-2 as well as HSV-1.
FIG 1.
HSV-2 as well as HSV-1 infection directly activates RIP3/MLKL-dependent necrosis in mouse cells. (A) Wild type (WT) and RIP3 knockout (KO) MEFs were infected with a control or HSV-2 at a multiplicity of infection (MOI) of 10 for about 20 h. Identical MOIs were used in MEFs in later experiments. Cell viability analysis was performed as described in Materials and Methods. (B) WT or RIP3 knockout MEF lysates were prepared and subjected to Western blot analysis. (C) L929 cells were transfected with a negative control (NC), mRIP3, or mMLKL siRNA oligonucleotide for 48 h. Then cells were treated as indicated for 15 h, and cell viability was determined. The HSV-1 MOI was 5. T, TNF-α (10 ng/ml); Z, Z-VAD (10 μM). (D) L929 cells were transfected with the indicated siRNA oligonucleotides for 48 h. Then cell lysates were prepared and subjected to Western blot analysis. (E) MEFs stably expressing Flag- and HA-tagged mMLKL were infected with HSV-1 or HSV-2 for 6 h. Cell lysates were prepared for immunoprecipitation with anti-Flag agarose beads. The Flag-mMLKL immunocomplex was then determined by Western blotting of the indicated proteins. IP, immunoprecipitation.
HSV-1 or HSV-2 infection subverts necroptosis in human cells.
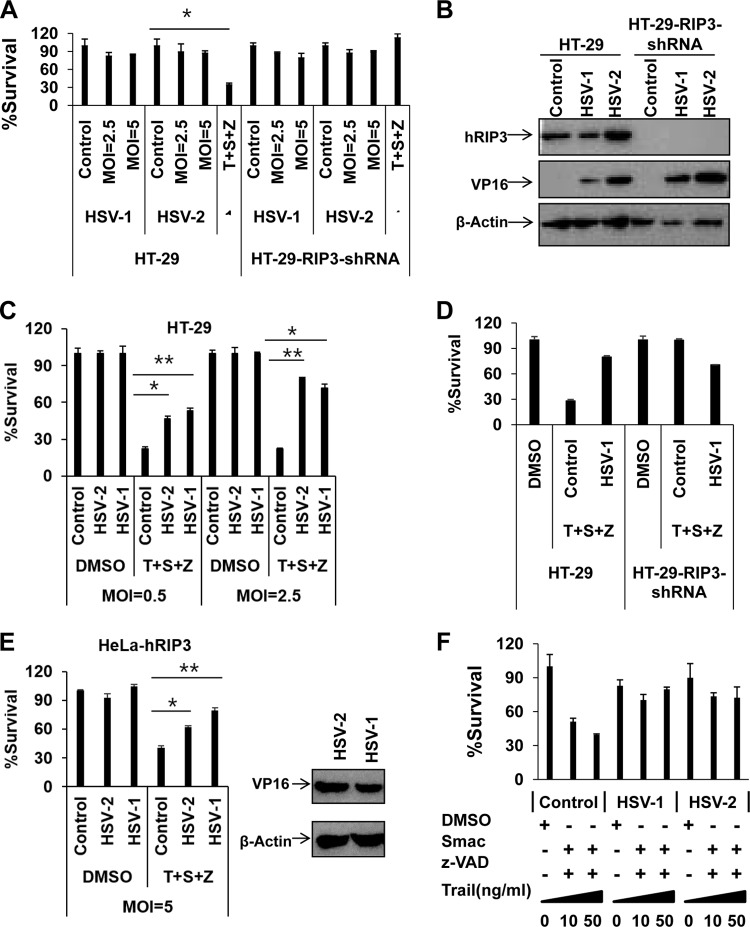
As HSV-1 and HSV-2 are common human pathogens, we further investigated the ability of HSV-1 and HSV-2 to induce programmed necrosis in human colon cancer HT-29 cells, a widely used cell model for the induction of programmed necrosis. RIP3-dependent necrosis was determined by comparing the cell survival rates between parental and RIP3 shRNA-expressing HT-29 cells in response to HSV infection. To our surprise, HSV-1 or HSV-2 infection failed to induce RIP3-dependent necrosis in HT-29 cells even at a high multiplicity of infection (MOI) of 5 (Fig. 2A). However, these cells underwent RIP3-dependent programmed necrosis in response to treatment with TNF-α/Smac mimetic/Z-VAD (Fig. 2A). The viral protein VP16 was detected in the infected cells (Fig. 2B), indicating successful infection by HSV-1 and HSV-2. Strikingly, both HSV-1 and HSV-2 infection exerted dose-dependent suppression of TNF-induced necrosis in HT-29 cells (Fig. 2C and D). Consistently, both HSV-1 and HSV-2 infection resulted in the inhibition of TNF-induced necrosis in HeLa cells ectopically expressing hRIP3 (HeLa-hRIP3) (Fig. 2E and F). Furthermore, we found that both HSV-1 and HSV-2 infection abolished TRAIL-induced programmed necrosis as well (Fig. 2F). Taken together, these results suggest that both HSV-1 and HSV-2 interfere with the necroptotic signaling pathway in human cells.
FIG 2.
HSV-1 or HSV-2 infection subverts necroptosis in human cells. (A) HT-29 or HT-29-RIP3-shRNA cells were infected with HSV-1 or HSV-2 or treated with TNF-α/Smac mimetic/Z-VAD (40 ng/ml TNF-α, 100 nM Smac mimetic, and 20 μM Z-VAD) for 16 h, and then cell viability was determined. *, P < 0.05 versus the control. (B) HT-29 or HT-29-RIP3-shRNA cells were treated with the indicated virus (MOI of 2.5). Cell lysates were collected at 6 h postinfection and then subjected to Western blot analysis. (C) HT-29 cells were infected with HSV-1 and HSV-2 at the indicated MOI. At 2 h postinfection, cells were treated with dimethyl sulfoxide (DMSO) or TNF-α/Smac mimetic/Z-VAD for 16 h, and then cell viability was determined. *, P < 0.05; **, P < 0.001 versus the control. (D) HT-29 or HT-29-RIP3-shRNA cells were infected with HSV-1 at an MOI of 2.5. At 2 h postinfection, cells were treated with dimethyl sulfoxide or TNF-α/Smac mimetic/Z-VAD for 48 h, and then cell viability was determined. (E) HeLa-hRIP3 cells were infected with the indicated virus. At 2 h postinfection, cells were treated with dimethyl sulfoxide or TNF-α/Smac mimetic/Z-VAD for 16 h, and then cell viability was determined. HeLa-hRIP3 cell lysates were collected after treatment with HSV-1 or HSV-2 (MOI of 2.5). The expression of viral protein VP16 was detected by Western blotting using an anti-VP16 antibody. *, P < 0.05; **, P < 0.001 versus control (F) HT-29 cells were treated with the indicated virus (MOI of 2.5). At 2 h postinfection, cells were treated with dimethyl sulfoxide or TRAIL/Smac mimetic/Z-VAD for 16 h, and then cell viability was determined.
HSV R1 is required to disrupt necrosome formation during necroptosis in human cells.
Since the R1 subunits of HSV-1 and HSV-2 are RHIM-containing proteins (called ICP6 and ICP10, respectively), we sought to characterize the role of the HSV R1 subunits in the suppression of TNF-induced necrosis of human cells. We infected HT-29 cells with wild-type (WT) HSV-1 or an ICP6 deletion mutant (ICP6Δ HSV-1). Compared to WT virus, ICP6Δ HSV-1 lost the ability to block the necrosis induced by TNF-α/Smac mimetic/Z-VAD even though these viruses replicated to similar levels (Fig. 3A and B). Moreover, we found that HSV-1 infection decreased the modification of hRIP3 and resulted in the reduced phosphorylation of hMLKL following treatment with TNF-α/Smac mimetic/Z-VAD (Fig. 3C), indicating that the activation of RIP3 was strongly attenuated in the WT HSV-1-infected cells. In contrast, ICP6Δ HSV-1 failed to limit the phosphorylation of both hRIP3 and hMLKL during TNF-induced necrosis (Fig. 3C). As induction of the necrosome (the RIP1/RIP3 complex) is critical for RIP3 activation during TNF-induced necrosis, we examined necrosome formation in HSV-infected HeLa-hRIP3 cells. As shown in Fig. 3D, HSV-1 and HSV-2 infections disrupted the formation of the necrosome following TNF-α/Smac mimetic/Z-VAD treatment, whereas this complex was induced in cells infected with ICP6Δ HSV-1. These results demonstrate that the HSV R1 subunit is critical for preventing necrosome formation during necroptosis in human cells.
FIG 3.
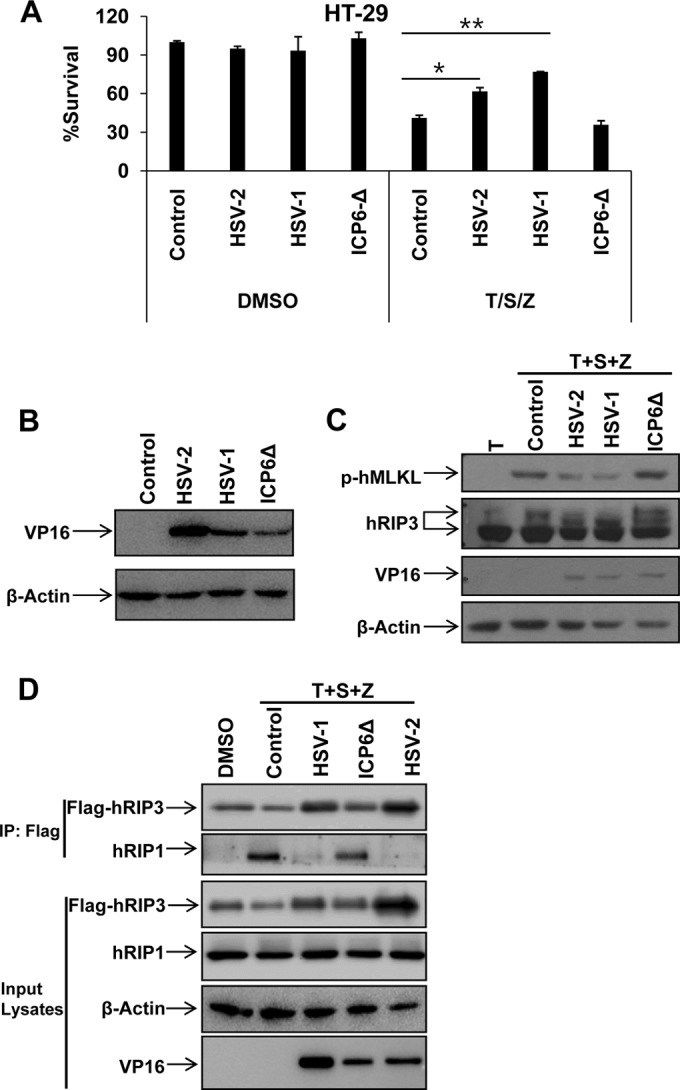
HSV R1 is required to disrupt necrosome formation during necroptosis in human cells. (A) HT-29 cells were infected with the indicated virus (MOI of 2.5). At 2 h postinfection, cells were treated with dimethyl sulfoxide (DMSO) or TNF-α/Smac mimetic/Z-VAD for 16 h, and then cell viability was determined. *, P < 0.05; **, P < 0.001 versus the control. (B) HT-29 cells were infected with the indicated virus (MOI of 2.5). Cell lysates were collected at 6 h postinfection and then subjected to Western blot analysis. (C) HT-29 cells were infected as indicated (MOI of 2.5). At 2 h postinfection, cells were treated with TNF-α/Smac mimetic/Z-VAD for an additional 6 h. Then cell lysates were collected and subjected to Western blot analysis. (D) HeLa-hRIP3 cells were infected as indicated (MOI of 2.5). At 2 h postinfection, cells were treated with TNF-α/Smac mimetic/Z-VAD for an additional 6 h. Cell lysates were collected and used for anti-Flag immunoprecipitation. The Flag-RIP3 immunocomplex was analyzed by Western blotting with the indicated antibody.
Ectopic expression of HSV R1 is sufficient to block TNF-induced necrosis of human cells depending on both RHIM and RR domains.
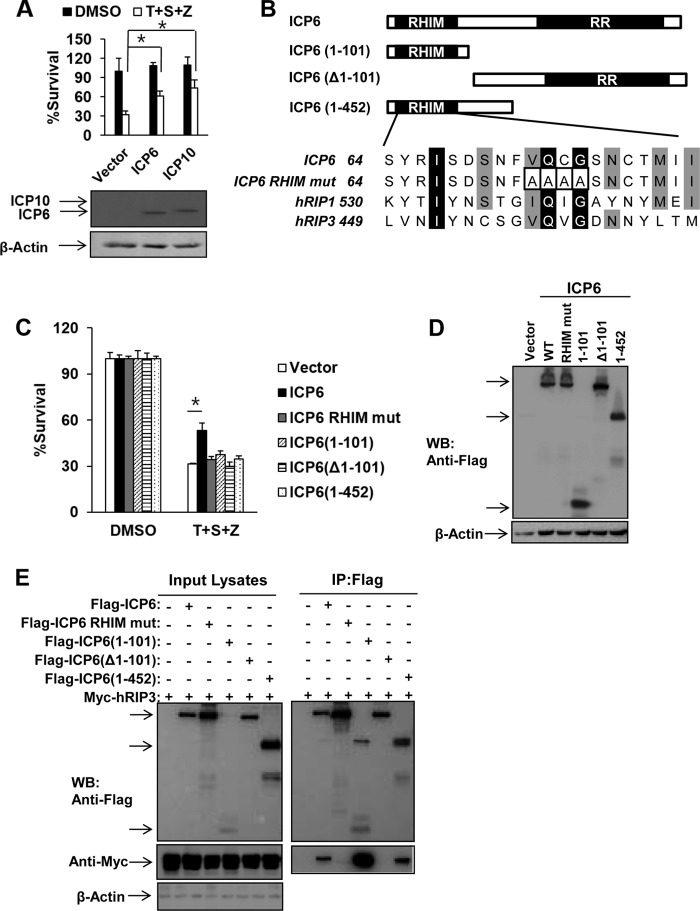
We next investigated whether either ICP6 or ICP10 was sufficient to abolish TNF-induced necrosis in human cells by ectopically expressing ICP6 or ICP10 in HeLa-hRIP3 cells. As shown in Fig. 4A, expression of ICP6 or ICP10 efficiently reduced TNF-induced necrosis. Moreover, we examined the roles of the RHIM and RR domains of ICP6 by evaluating truncated forms of ICP6 lacking the N-terminal RHIM domain or C-terminal RR domain in human cells (Fig. 4B). None of the truncated forms of ICP6, including ICP6 consisting of amino acids 1 to 101 [ICP(1–101)], ICP6 with a deletion of residues 1 to 101 [ICP6(Δ1–101)], and ICP6(1–452), was able to block TNF-induced necrosis although their expression levels were similar to the level of full-length ICP6 in HeLa-RIP3 cells (Fig. 4C and D). Additionally, a RHIM mutant form of ICP6, in which residues 73 to 76 were mutated to four alanines (Fig. 4B), failed to affect TNF-induced necrosis in the transfected HeLa-hRIP3 cells (Fig. 4C and D). Although RHIM-containing truncated proteins such as ICP6(1–101) and ICP6(1–452) retained the ability to interact with hRIP3, they failed to influence necroptosis (Fig. 4E). These results indicate that both the RHIM and the RR domains of ICP6 are required for its proper function in modulating TNF-induced necrosis in human cells.
FIG 4.
Ectopic expression of HSV R1 is sufficient to block TNF-induced necrosis of human cells depending on both RHIM and RR domains. (A) HeLa-hRIP3 cells were transfected with empty vector or an ICP6 or ICP10 DNA plasmid for 24 h. Cells were treated with TNF-α/Smac mimetic/Z-VAD for an additional 36 h, and then cell viability was measured. Data represent the averages ± standard errors for three independent experiments. *, P < 0.05, versus results for the vector. Cell lysates were collected at 24 h posttransfection and subjected to Western blot analysis. (B) Domain structure of ICP6. Full-length ICP6 (amino acids 1 to 1137) contains the N-terminal RHIM domain and the C-terminal RR domain. ICP6(1–101) and ICP6(1–452) contain the N-terminal 101 residues and 452 residues, respectively. ICP6(Δ1–101) lacks residues 1 to 101. The residues 73 to 76 of ICP6 were mutated to four alanine residues. (C and D) HeLa-hRIP3 cells were transfected with the indicated plasmids for 24 h. Cells were treated with TNF-α/Smac mimetic/Z-VAD for an additional 36 h, and then cell viability was measured (C). Data represent the averages ± standard errors for three independent experiments. *, P < 0.05, versus results for the vector. Cell lysates were collected at 24 h posttransfection and subjected to Western blot analysis (D). (E) 293T cells were transfected with the indicated plasmids for 48 h. Cell lysates were collected and immunoprecipitated with anti-Flag agarose beads. The immunoprecipitate was analyzed by Western blotting (WB).
HSV R1 prevents the recruitment of hRIP1 to hRIP3.
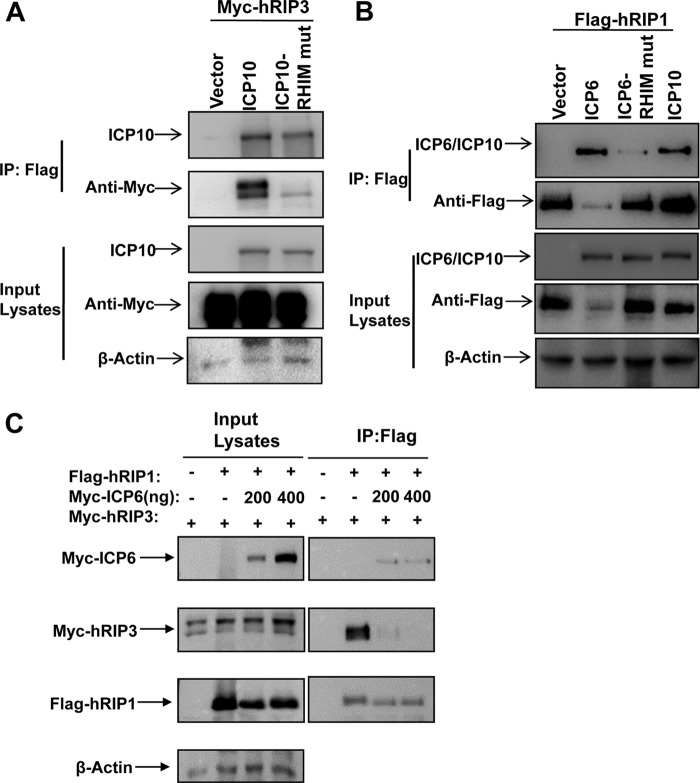
Since the RHIM domain of ICP6 is essential for the suppression of TNF-induced necrosis of human cells, we examined the association of ICP6 and ICP10 with hRIP1 and hRIP3. As shown in Fig. 4E and 5A, hRIP3 was pulled down by either Flag-tagged ICP6 or ICP10. We also found that ICP6 and ICP10 were pulled down by Flag-tagged hRIP1 (Fig. 5B). Furthermore, we found that the RHIM mutant form of ICP6 lost the ability to bind hRIP3 and hRIP1 (Fig. 5A and B). These findings suggest that HSV R1 has the capacity to form complexes with both hRIP3 and hRIP1 through RHIM domains. We then investigated whether ectopic expression of ICP6 and ICP10 could influence the recruitment of hRIP1 to hRIP3. The hRIP1/hRIP3 complex was detected in 293T cells cotransfected with DNA plasmids expressing hRIP1 and hRIP3 (Fig. 5C). However, the formation of the hRIP1/hRIP3 complex decreased in the presence of ICP6 (Fig. 5C). The reduced hRIP1/hRIP3 complex level correlated with the increased level of ICP6 (Fig. 5C). These results demonstrate that HSV R1 is able to disrupt the binding of hRIP1 to hRIP3 in an RHIM-dependent manner.
FIG 5.
HSV R1 prevents the recruitment of hRIP1 to hRIP3. (A) 293T cells were cotransfected with the Myc-tagged hRIP3 plasmid and a plasmid expressing Flag-tagged ICP10 and the RHIM mutant form of ICP10 (RHIM mut). The residues from 64 to 67 of ICP10 were mutated to four alanine residues. At 48 h posttransfection, cell lysates were collected and used for anti-Flag immunoprecipitation. The Flag-hRIP3 immunocomplex was analyzed by Western blotting with the indicated antibodies. (B) 293T cells were cotransfected with the Flag-tagged hRIP1 plasmid and a plasmid expressing Myc-tagged ICP6, the RHIM mutant, or ICP10. At 48 h posttransfection, cell lysates were collected and used for anti-Flag immunoprecipitation. The Flag-hRIP1 immunocomplex was analyzed by Western blotting with the indicated antibodies. (C) 293T cells were cotransfected with plasmids as indicated. At 48 h posttransfection, cell lysates were collected for anti-Flag immunoprecipitation. The immunocomplex was subjected to Western blot analysis.
DISCUSSION
Initiation of programmed cell death (PCD) in host cells is a critical strategy to prevent pathogen replication. Extensive studies have shown that many pathogens encode apoptotic suppressors such as caspase inhibitors to circumvent apoptosis, a major form of PCD in mammals. Inhibition of apoptosis has been shown to facilitate the activation of programmed necrosis, a process that is mediated by RIP3 and MLKL. We recently demonstrated that RIP3/MLKL-dependent necrosis is activated in HSV-1-infected mouse cells through the recruitment of viral ICP6 to RIP3, a result supporting the importance of programmed necrosis in the control of HSV-1 replication. In the current study, we demonstrate a negative regulation of programmed necrosis by both HSV-1 and HSV-2 infection in human cells. The R1 subunits of HSV-1 and HSV-2 are sufficient to disrupt TNF-induced necrosis of human cells. Although HSV-2 as well as HSV-1 infection directly activates the formation of the mRIP3/mMLKL complex in mouse cells, the recruitment of HSV R1 with hRIP3 failed to trigger hRIP3/hMLKL signaling and also disrupted the binding of hRIP1 to hRIP3 in human cells. Thus, this study uncovers dual roles of HSV R1 in modulating programmed necrosis through either activation or inactivation of RIP3 signaling in a species-specific manner. During the preparation of the present manuscript, similar work has been published (28).
RIP3 plays a central role in the regulation of programmed necrosis initiated by death ligands, TLR ligands, or viral infection. The recruitment of an RHIM-containing protein to RIP3 is a crucial process for the activation of RIP3. For example, TNFR- and TLR-mediated necrosis requires the formation of the RIP1/RIP3 complex and the TRIF/RIP3 complex, respectively. Moreover, the ICP6/RIP3 complex and the DAI/RIP3 complex are essential for HSV-1 and M45/vIRA mutant MCMV-associated necrosis, respectively. Thus, RIP3 acts as a cellular necrotic sensor in the recognition of RHIM-containing proteins, leading to the activation of the MLKL substrate. Notably, RHIM-dependent modulation of RIP3 is utilized by MCMV M45/vIRA to block programmed necrosis during viral infection. The present study shows that HSV-1 ICP6 and HSV-2 ICP10 manipulate programmed necrosis of human cells through RHIM-dependent suppression of RIP3 signaling. These findings provide strong evidence that pathogens have likely evolved strategies to modulate the necrotic sensor RIP3 via disruption of the RHIM-dependent activation of RIP3, leading to inactivation of the programmed necrosis responses in hosts.
Interestingly, our work suggests that HSV R1 has an opposite impact on programmed necrosis in mouse cells versus that in human cells. Although HSV R1 is able to interact with both hRIP3 and mRIP3 through the RHIM domains, we found that the recruitment of HSV R1 to mRIP3 directly activated the formation of the mRIP3/mMLKL complex in mouse cells but not in human cells. We found that the RHIM-dependent association of HSV R1 with hRIP3 or hRIP1 prevented the recruitment of hRIP1 to hRIP3. Previous studies have shown that mMLKL is unable to bind to hRIP3 and that mRIP3 cannot interact with hMLKL (29). The phosphorylation of S227 in hRIP3 is critical for its interaction with hMLKL, while the interaction between mRIP3 and mMLKL requires the phosphorylation of the conserved S232 residue and an additional T231 residue in mRIP3 (14, 29). Although the RIP3-MLKL interaction is functionally conserved for programmed necrosis, differential sequences and phosphorylation sites of RIP3 control the species specificity of this RIP3-MLKL interaction. It is tempting to speculate that HSV R1-mediated species-specific modulation of programmed necrosis is determined by the differential manipulation of RIP3/MLKL signaling. Further structure-based studies on HSV R1/RIP3 complexes are required to understand the precise molecular mechanism for this species-specific modulation.
ACKNOWLEDGMENTS
We thank Xiaodong Wang (National Institute of Biological Sciences [NIBS], Beijing, China) for anti-phosphor-MLKL antibody and Smac mimetic. We also thank Zhigao Wang (University of Texas Southwestern Medical Center, Dallas, TX, USA) for HeLa-hRIP3 cells, Sandra K. Weller (University of Connecticut Health Center, Farmington, CT, USA) for the HSV-1 KOS strain and the ICP6 deletion mutant (ICP6Δ).
This work was supported by the National Basic Research Program of China (2013CB910102), the National Natural Science Foundation of China (grants 31222036 and 31471303), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, Natural Science Foundation of Jiangsu Province (grants BK2012004 and BK2011287), the National Institutes of Health (1R01CA166413), and the Undergraduate Training Programs for Innovation and Entrepreneurship (201410285046).
REFERENCES
- 1.Sridharan H, Upton JW. 2014. Programmed necrosis in microbial pathogenesis. Trends Microbiol 22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Han J, Zhong CQ, Zhang DW. 2011. Programmed necrosis: backup to and competitor with apoptosis in the immune system. Nat Immunol 12:1143–1149. doi: 10.1038/ni.2159. [DOI] [PubMed] [Google Scholar]
- 3.Moriwaki K, Chan FK. 2013. RIP3: a molecular switch for necrosis and inflammation. Genes Dev 27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. 2005. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 5.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. 2000. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol 1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 6.Declercq W, Vanden Berghe T, Vandenabeele P. 2009. RIP kinases at the crossroads of cell death and survival. Cell 138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. 2009. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. 2009. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 9.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. 2009. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 10.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. 2002. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem 277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 11.Moquin DM, McQuade T, Chan FK. 2013. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One 8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. 2008. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. 2012. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A 109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. 2012. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. 2014. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. 2014. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. 2014. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. 2013. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem 288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He S, Liang Y, Shao F, Wang X. 2011. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A 108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobbelstein M, Shenk T. 1996. Protection against apoptosis by the vaccinia virus SPI-2 (B13R) gene product. J Virol 70:6479–6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kettle S, Alcami A, Khanna A, Ehret R, Jassoy C, Smith GL. 1997. Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1beta-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1beta-induced fever. J Gen Virol 78:677–685. doi: 10.1099/0022-1317-78-3-677. [DOI] [PubMed] [Google Scholar]
- 23.Upton JW, Kaiser WJ, Mocarski ES. 2010. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Upton JW, Kaiser WJ, Mocarski ES. 2012. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe 11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Li Y, Liu S, Yu X, Li L, Shi C, He W, Li J, Xu L, Hu Z, Yu L, Yang Z, Chen Q, Ge L, Zhang Z, Zhou B, Jiang X, Chen S, He S. 2014. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A 111:15438–15443. doi: 10.1073/pnas.1412767111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Z, Wu SQ, Liang Y, Zhou X, Chen W, Li L, Wu J, Zhuang Q, Chen C, Li J, Zhong CQ, Xia W, Zhou R, Zheng C, Han J. 2015. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe 17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Jiang H, Chen S, Du F, Wang X. 2012. The mitochondrial phosphatase PGAM5 functions at the convergent point of multiple necrotic death pathways. Cell 148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 28.Guo H, Omoto S, Harris PA, Finger JN, Bertin J, Gough PJ, Kaiser WJ, Mocarski ES. 2015. Herpes simplex virus suppresses necroptosis in human cells. Cell Host Microbe 17:243–251. doi: 10.1016/j.chom.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W, Zhou Z, Li L, Zhong CQ, Zheng X, Wu X, Zhang Y, Ma H, Huang D, Li W, Xia Z, Han J. 2013. Diverse sequence determinants control human and mouse receptor interacting protein 3 (RIP3) and mixed lineage kinase domain-like (MLKL) interaction in necroptotic signaling. J Biol Chem 288:16247–16261. doi: 10.1074/jbc.M112.435545. [DOI] [PMC free article] [PubMed] [Google Scholar]