Abstract
We analyzed data of 263 women with at least one genital or anorectal sexually transmitted infection from a cross-sectional study conducted in rural South Africa. We provide new insights concerning the concurrence of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, and Trichomonas vaginalis infections as well as the characteristics of bacterial loads.
TEXT
Chlamydia trachomatis and Neisseria gonorrhoeae are the most prevalent bacterial sexually transmitted infections (STI) in the world (1). The course of these infections is unpredictable and diverse (2–7). Most infections in women are asymptomatic and frequently remain unrecognized, which increases the risk for reproductive tract complications (6, 8–10). Insight into the mechanisms that drive the burden of these infections is essential for disease control. In addition to age, HIV infection, and behavioral factors, microbiological characteristics, including bacterial load, may play an important role in the risk of transmission, clinical presentation, and course of infection (6, 11–14). Chlamydial load has been associated with clinical presentation, severity of infection, and transmissibility in animal models and in patients with ocular C. trachomatis infection (15–17). Gonorrheal loads have been shown to differ between anatomic locations and associated clinical presentations in men (18). Although real-time PCR and quantitation of DNA load have the potential of revealing new insights into the characteristics of infection, there is only limited literature about the relevance of bacterial load and frequency of concurrent STI in women (19, 20). Knowledge about microbiological characteristics of infection could possibly help to improve understanding of the differences in STI prevalences at the population level. This study aimed to evaluate the concurrence and bacterial loads of genital and anorectal C. trachomatis and N. gonorrhoeae infections in South African women from a setting of high HIV prevalence.
This study was a subanalysis of a previously described cross-sectional study of 604 women in rural Mopani District, South Africa (21). In brief, consenting women 18 to 49 years of age who reported sexual activity during the previous 6 months were eligible. Questionnaires were completed and vaginal, anorectal and pharyngeal swabs (Copan Diagnostics, Brescia, Italy) were obtained by health care workers and stored at −20°C. Menses on the day of recruitment and refusal to have all three anatomic sites tested were exclusion criteria. Symptomatic women were treated the same day according to local treatment protocols, which include a notification slip for the partner. Asymptomatic women with an STI proven by molecular detection were called to return to the clinic for specific treatment. For the evaluation presented in this article, we selected all women (n = 263) vaginally and/or anorectally infected with at least one of the following pathogens: C. trachomatis (n = 107), N. gonorrhoeae (n = 66), Mycoplasma genitalium (n = 66), and Trichomonas vaginalis (n = 116). As we observed only one woman with an oropharyngeal infection, we excluded this anatomical location from further analyses. The Human Ethics Research Committee of the University of the Witwatersrand, South Africa, approved the study (reference no. M110726), including the M. genitalium and T. vaginalis testing.
Dry swabs were transported on dry ice in order to perform laboratory analysis at the Laboratory of Immunogenetics, VU University Medical Center, Amsterdam, the Netherlands. Bacterial DNA was extracted using a High Pure PCR template preparation kit (Roche Diagnostics, Indianapolis, IN) followed by real-time PCR detection of C. trachomatis, N. gonorrhoeae, and T. vaginalis using a PrestoPluS CT-NG-TV assay (Microbiome Ltd., Amsterdam, the Netherlands) and a LightMix real-time PCR kit (TIB Molbiol, Berlin, Germany) for M. genitalium as described elsewhere (21, 22) (D. J. de Waaij, J. H. Dubbink, R. P. H. Peters, S. Ouburg, and S.A. Morré, unpublished data). The bacterial DNA load of C. trachomatis and N. gonorrhoeae was calculated as follows. The Cp (crossing-point) value, i.e., the number of amplification cycles before a standardized threshold is reached, was related to a calibration curve of a known C. trachomatis DNA quantity and N. gonorrhoeae DNA quantity to determine the amount of bacterial DNA. In our analysis, the bacterial load is provided as the logarithm of the number of inclusion-forming units (IFU) per nanoliter for C. trachomatis and as the logarithm of the number of copies per microliter for N. gonorrhoeae. Statistical analyses were performed using the chi-square test or Fisher's exact test and Mann-Whitney U test to compare dichotomous and continuous variables between groups. A P value of less than 0.05 was considered statistically significant for all tests.
Of the 263 women with a genital and/or anorectal STI, 247 (94%) had a genital STI and 73 (27%) an anorectal STI. The median age of all women (n = 263) was 29 (range, 18 to 49) years and HIV infection was reported by 33%, whereas 44% reported testing HIV negative in the last 6 months and 24% did not know their status. Fellatio was reported by 13% of the women and receptive anal intercourse (RAI) by 5.5%.
The distribution of genital microorganisms is shown in Fig. 1. Anorectal infection was caused in 64 (88%)/73 women by a single microorganism: C. trachomatis in 34/43, N. gonorrhoeae in 9/15, M. genitalium in 16/17, and T. vaginalis in 5/7. Single infections were significantly more common at the anorectal than at the genital location (88% versus 74%, P = 0.013). Among the 107 women with a C. trachomatis infection, 32 (30%) women had a double infection (combined genital and rectal infection). Among the 66 women with a N. gonorrhoeae infection, 9 (14%) women had a double infection. Within the 66 and 116 women with a M. genitalium infection and a T. vaginalis infection, 3 (4.5%) and 4 (3.4%) women had a double infection, respectively.
FIG 1.
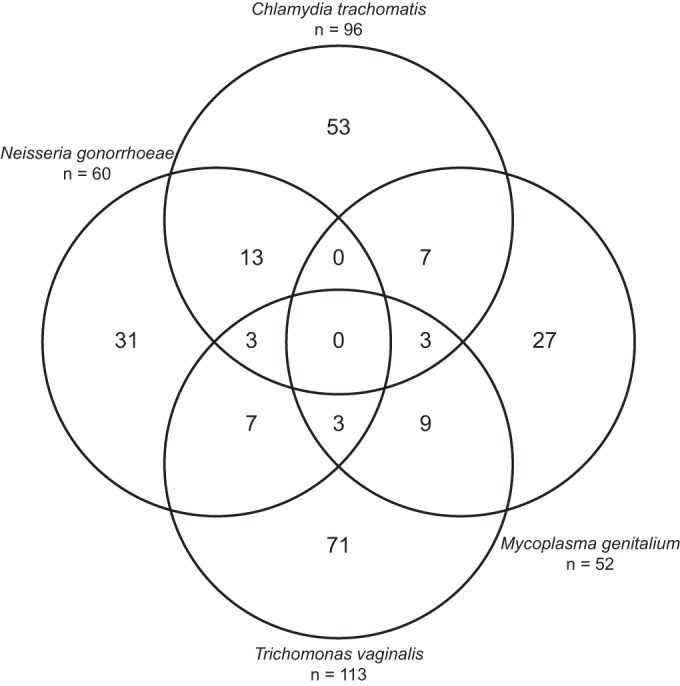
Venn diagram of multiple genital infections. Note that the double infections of Chlamydia trachomatis with Trichomonas vaginalis (n = 17) and Neisseria gonorrhoeae with Mycoplasma genitalium (n = 3) are not represented in the figure. The number of infections in each circle equals the total amount (n) given.
The median load of genital C. trachomatis was 2.7 log10 IFU/nl (range, 0.36 to 4.5), and that of anorectal C. trachomatis was 2.5 log10 IFU/nl (range, 0.58 to 3.6). For N. gonorrhoeae, the median load of genital infection was 2.7 log10 copies/μl (range, 1.6 to 8.0) and that of rectal infection was 4.4 log10 copies/μl (range, 2.2 to 6.3). Characteristics and associations of genital and rectal bacterial load are presented in Table 1. In multivariate analyses, age of ≤24 years (P = 0.009) and pregnancy (P = 0.023) were independently associated with a higher C. trachomatis load and same age group (P < 0.0001) and having had more than 1 sexual partner in last 6 months (P = 0.029) with a higher N. gonorrhoeae load. The association of a concurrent relationship in the last year with N. gonorrhoeae load was no longer manifest in multivariate analysis (P = 0.32).
TABLE 1.
Characteristics of and associations with bacterial loada
| Patient category |
Chlamydia trachomatis |
Neisseria gonorrhoeae |
||
|---|---|---|---|---|
| Median log10 IFU/nl | P | Median log10 copies/μl | P | |
| Characteristics of genital bacterial load | ||||
| Single genital infection | 2.7 | 2.9 | ||
| Mixed genital infection withb: | ||||
| Chlamydia trachomatis | 2.6 | 0.55 | ||
| Neisseria gonorrhoeae | 1.6 | 0.06 | ||
| Mycoplasma genitalium | 2.9 | 0.60 | 3.1 | 0.23 |
| Trichomonas vaginalis | 2.7 | 0.70 | 3.6 | 0.16 |
| Reported HIV infectionc | ||||
| Yes | 2.6 | 0.52 | 2.5 | 0.56 |
| No | 2.7 | 3.0 | ||
| Genital infection statusd | ||||
| Symptomatic | 2.6 | 0.54 | 3.3 | 0.16 |
| Asymptomatic | 2.7 | 2.7 | ||
| Infection category | ||||
| Single (genital only) | 2.3 | 0.002 | 2.7 | 0.004 |
| Double | 3.2 | 5.8 | ||
| Characteristics of rectal bacterial load | ||||
| Infection category | ||||
| Single (anorectal only) | 2.7 | 0.63 | 4.0 | 0.39 |
| Double | 2.1 | 4.9 | ||
| Associations with genital bacterial load | ||||
| Age | ||||
| ≤24 yrs | 3.0 | 0.010 | 5.5 | <0.001 |
| >24 yrs | 2.5 | 2.5 | ||
| Currently pregnant | ||||
| Yes | 3.4 | 0.017 | 3.0 | 0.29 |
| No | 2.5 | 2.5 | ||
| Hormonal injections | ||||
| Yes | 2.7 | 0.96 | 3.0 | 0.45 |
| No | 2.6 | 2.7 | ||
| Intravaginal cleansing | ||||
| Yes | 3.0 | 0.29 | 2.5 | 0.11 |
| No | 2.7 | 3.0 | ||
| No. of sexual partners in last 6 mo | ||||
| ≤1 partner | 2.7 | 0.66 | 2.7 | 0.028 |
| >1 partners | 3.0 | 5.2 | ||
| Days since last sex act | ||||
| ≤7 days | 2.7 | 0.70 | 2.7 | 0.61 |
| >7 days | 2.5 | 2.9 | ||
| Concurrent relationship <1 yr earlier | ||||
| Yes | 2.7 | 0.58 | 4.9 | 0.021 |
| No | 2.7 | 2.6 | ||
Boldface data indicate statistically significant results.
Mixed infection per pathogen given, with the P value related to single-infection data as a reference.
HIV (human immunodeficiency virus), HIV-positive status compared with negative status in the previous 6 months.
Genital infection is defined as symptomatic when abnormal vaginal discharge, intramenstrual bleeding, or vaginal blood loss during or after sexual intercourse was reported.
This report describes the concurrence of STI and bacterial DNA load in South African women with genital and anorectal Chlamydia trachomatis and Neisseria gonorrhoeae infection. Only a few reports of studies on these microbiological characteristics of infection have been published, and to our knowledge, this is the first report of a study conducted in a rural setting of high HIV prevalence. We observed that nearly half of the women with genital C. trachomatis infection (47%) and N. gonorrhoeae infection (48%) had a concomitant genital infection. Although there is limited knowledge about the significance of mixed infections, these might theoretically increase susceptibility to long-term complications and transmissibility for individual STI. Mixed rectal infections were significantly less common among women than genital STI, as 88% of these infections were caused by a single microorganism. Potential explanations would be less-frequent exposure to infection, as RAI was not common in our cohort, and different susceptibilities of the anorectal mucosa for certain microorganisms.
Women with a double infection had significantly higher C. trachomatis and N. gonorrhoeae genital loads than women with genital infection only. To our knowledge, this has not been reported before and might support the hypothesis of autoinoculation through cervicovaginal fluids from the vagina to the rectum. This possible role of spillover might explain the high prevalence of anorectal infection despite the low frequency of RAI in our study (23, 24).
We observed no significant difference between the bacterial loads of single and mixed infections for any of the STI. In line with two other nucleic acid amplification test (NAAT)-based studies, we did not observe a relation between bacterial load and genital symptomatology (25, 26). Vodstrcil and colleagues observed in a recent review that culture-based studies are more likely to find an association with signs and symptoms than NAAT-based studies (89% versus 38%) (27). This might be reflected by the tendency of viable organisms to be more closely associated with clinical manifestations. Younger age and pregnancy were independently associated with a higher bacterial load of C. trachomatis and younger age and having had more than one sexual partner in the last 6 months with a higher load of N. gonorrhoeae. The same relation with age (for C. trachomatis) in several NAAT studies has been described before and might be due to changes in the cervical epithelium or to younger women being more sexually active and exhibiting riskier behavior or might reflect acquired immunity in adults (28–30). There were several limitations to our study. First, we did not account for sampling variability by reporting loads corresponding to the number of eukaryotic cells. However, as inflammatory cells which are attracted to the site of infection would be included among the sampled cells, the load per eukaryotic cell would be decreased (25). A second limitation may be the use of stored specimens, but the literature does not show a clinically relevant degradation impact (31). A last limitation may be represented by women using antimicrobials, a factor which might have caused us to underestimate our results to some extent, as this was not an exclusion criterion.
In conclusion, this study revealed frequent concurrent infections of C. trachomatis and N. gonorrhoeae and differences in bacterial loads in a setting of high HIV prevalence. These new insights might help to explain differences in STI prevalence at a population level where increased efforts are needed to control the burden of C. trachomatis and N. gonorrhoeae infections.
ACKNOWLEDGMENTS
We thank all women who participated in this study. Our further gratitude goes to the staff of Anova Health Institute for their invaluable contribution, efforts, and support.
This work was supported by the Dutch Society for Tropical Medicine (NVTG), the Netherlands, and TubaScan Ltd., the Netherlands. The Anova Health Institute is supported by the US President's Emergency Plan for AIDS Relief program via the U.S. Agency for International Development under cooperative agreement no. AID-674-A-12-00015. We thank Roche Molecular Systems, Inc., Pleasanton, CA, USA, for partial funding of this study.
S.A.M. is a full-time employee of the VU University Medical Center Amsterdam and has been involved in the technical development of the PRESTO-Plus CT-NG-TV assay (Microbiome Ltd., Amsterdam, the Netherlands). S.A.M. is cofounder and codirector of Microbiome Ltd., a spin-in company of the VU University Medical Center, Amsterdam, the Netherlands.
The rest of us declare that we have no potential conflicts of interest.
The views expressed in the manuscript do not necessarily reflect those of the President's Emergency Plan for AIDS Relief or the U.S. Agency for International Development.
REFERENCES
- 1.World Health Organization. 2012. Global incidence and prevalence of selected curable sexually transmitted infections—2008. Methods and results used by WHO to generate 2008 estimates World Health Organization; Geneva, Switzerland: http://apps.who.int/iris/bitstream/10665/75181/1/9789241503839_eng.pdf Accessed 1 August 2015. [Google Scholar]
- 2.Mascellino MT, Boccia P, Oliva A. 2011. Immunopathogenesis in Chlamydia trachomatis infected women. ISRN Obstet Gynecol 2011:436936. doi: 10.5402/2011/436936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geisler WM, Lensing SY, Press CG, Hook EW III. 2013. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis 207:1850–1856. doi: 10.1093/infdis/jit094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards JL, Apicella MA. 2004. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin Microbiol Rev 17:965–981. doi: 10.1128/CMR.17.4.965-981.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottlieb SL, Martin DH, Xu F, Byrne GI, Brunham RC. 2010. Summary: the natural history and immunobiology of Chlamydia trachomatis genital infection and implications for Chlamydia control. J Infect Dis 201(Suppl 2):S190–S204. [DOI] [PubMed] [Google Scholar]
- 6.Detels R, Green AM, Klausner JD, Katzenstein D, Gaydos C, Handsfield H, Pequegnat W, Mayer K, Hartwell TD, Quinn TC. 2011. The incidence and correlates of symptomatic and asymptomatic Chlamydia trachomatis and Neisseria gonorrhoeae infections in selected populations in five countries. Sex Transm Dis 38:503–509. [PMC free article] [PubMed] [Google Scholar]
- 7.Mayor MT, Roett MA, Uduhiri KA. 2012. Diagnosis and management of gonococcal infections. Am Fam Physician 86:931–938. [PubMed] [Google Scholar]
- 8.Walker CK, Sweet RL. 2011. Gonorrhea infection in women: prevalence, effects, screening, and management. Int J Womens Health 3:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anschuetz GL, Asbel L, Spain CV, Salmon M, Lewis F, Newbern EC, Goldberg M, Johnson CC. 2012. Association between enhanced screening for Chlamydia trachomatis and Neisseria gonorrhoeae and reductions in sequelae among women. J Adolesc Health 51:80–85. doi: 10.1016/j.jadohealth.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. 2010. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis 201(Suppl 2):S134–S155. [DOI] [PubMed] [Google Scholar]
- 11.Geisler WM. 2010. Duration of untreated, uncomplicated Chlamydia trachomatis genital infection and factors associated with chlamydia resolution: a review of human studies. J Infect Dis 201(Suppl 2):S104–S113. [DOI] [PubMed] [Google Scholar]
- 12.Boyer CB, Shafer MA, Pollack LM, Canchola J, Moncada J, Schachter J. 2006. Sociodemographic markers and behavioral correlates of sexually transmitted infections in a nonclinical sample of adolescent and young adult women. J Infect Dis 194:307–315. doi: 10.1086/506328. [DOI] [PubMed] [Google Scholar]
- 13.Huffam S, Chow EP, Fairley CK, Hocking J, Peel J, Chen M. 2015. Chlamydia infection in individuals reporting contact with sexual partners with chlamydia: a cross-sectional study of sexual health clinic attendees. Sex Transm Infect 91:434–439. doi: 10.1136/sextrans-2015-052068. [DOI] [PubMed] [Google Scholar]
- 14.Ghebremichael M, Paintsil E, Larsen U. 2009. Alcohol abuse, sexual risk behaviors, and sexually transmitted infections in women in Moshi urban district, northern Tanzania. Sex Transm Dis 36:102–107. doi: 10.1097/OLQ.0b013e31818b20e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carey AJ, Cunningham KA, Hafner LM, Timms P, Beagley KW. 2009. Effects of inoculating dose on the kinetics of Chlamydia muridarum genital infection in female mice. Immunol Cell Biol 87:337–343. doi: 10.1038/icb.2009.3. [DOI] [PubMed] [Google Scholar]
- 16.Pal S, Hui W, Peterson EM, de la Maza LM. 1998. Factors influencing the induction of infertility in a mouse model of Chlamydia trachomatis ascending genital tract infection. J Med Microbiol 47:599–605. doi: 10.1099/00222615-47-7-599. [DOI] [PubMed] [Google Scholar]
- 17.West SK, Munoz B, Mkocha H, Holland MJ, Aguirre A, Solomon AW, Foster A, Bailey RL, Mabey DC. 2005. Infection with Chlamydia trachomatis after mass treatment of a trachoma hyperendemic community in Tanzania: a longitudinal study. Lancet 366:1296–1300. doi: 10.1016/S0140-6736(05)67529-0. [DOI] [PubMed] [Google Scholar]
- 18.Bissessor M, Tabrizi SN, Fairley CK, Danielewski J, Whitton B, Bird S, Garland S, Chen MY. 2011. Differing Neisseria gonorrhoeae bacterial loads in the pharynx and rectum in men who have sex with men: implications for gonococcal detection, transmission, and control. J Clin Microbiol 49:4304–4306. doi: 10.1128/JCM.05341-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hocking JS, Guy R, Walker J, Tabrizi SN. 2013. Advances in sampling and screening for chlamydia. Future Microbiol 8:367–386. doi: 10.2217/fmb.13.3. [DOI] [PubMed] [Google Scholar]
- 20.Stupiansky NW, Van Der Pol B, Williams JA, Weaver B, Taylor SE, Fortenberry JD. 2011. The natural history of incident gonococcal infection in adolescent women. Sex Transm Dis 38:750–754. [DOI] [PubMed] [Google Scholar]
- 21.Peters RP, Dubbink JH, van der Eem L, Verweij SP, Bos ML, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morre SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal Chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. doi: 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 22.Hay B, Dubbink JH, Ouburg S, Le RC, Pereyre S, van der Eem L, Morre SA, Bebear C, Peters RP. 2015. Prevalence and macrolide resistance of Mycoplasma genitalium in South African women. Sex Transm Dis 42:140–142. doi: 10.1097/OLQ.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 23.Ding A, Challenor R. 2013. Rectal Chlamydia in heterosexual women: more questions than answers. Int J STD AIDS 25:587–592. [DOI] [PubMed] [Google Scholar]
- 24.van Liere GA, Dirks JA, Hoebe CJ, Wolffs PF, Dukers-Muijrers NH. 2015. Anorectal Chlamydia trachomatis load is similar in men who have sex with men and women reporting anal sex. PLoS One 10:e0134991. doi: 10.1371/journal.pone.0134991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jalal H, Verlander NQ, Kumar N, Bentley N, Carne C, Sonnex C. 2011. Genital chlamydial infection: association between clinical features, organism genotype and load. J Med Microbiol 60:881–888. doi: 10.1099/jmm.0.028076-0. [DOI] [PubMed] [Google Scholar]
- 26.Gomes JP, Borrego MJ, Atik B, Santo I, Azevedo J, Brito de Sá A, Nogueira P, Dean D. 2006. Correlating Chlamydia trachomatis infectious load with urogenital ecological success and disease pathogenesis. Microbes Infect 8:16–26. doi: 10.1016/j.micinf.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Vodstrcil LA, McIver R, Huston WM, Tabrizi SN, Timms P, Hocking JS. 2015. The epidemiology of Chlamydia trachomatis organism load during genital infection: a systematic review. J Infect Dis 211:1628–1645. doi: 10.1093/infdis/jiu670. [DOI] [PubMed] [Google Scholar]
- 28.Wiggins R, Graf S, Low N, Horner PJ. 2009. Real-time quantitative PCR to determine chlamydial load in men and women in a community setting. J Clin Microbiol 47:1824–1829. doi: 10.1128/JCM.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Batteiger BE, Xu F, Johnson RE, Rekart ML. 2010. Protective immunity to Chlamydia trachomatis genital infection: evidence from human studies. J Infect Dis 201(Suppl 2):S178–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnes RC, Katz BP, Rolfs RT, Batteiger B, Caine V, Jones RB. 1990. Quantitative culture of endocervical Chlamydia trachomatis. J Clin Microbiol 28:774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Dommelen L, Wolffs PF, van Tiel FH, Dukers N, Herngreen SB, Bruggeman CA, Hoebe CJ. 2013. Influence of temperature, medium, and storage duration on Chlamydia trachomatis DNA detection by PCR. J Clin Microbiol 51:990–992. doi: 10.1128/JCM.02631-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


