Abstract
The Eukaryotic Linear Motif (ELM) resource (http://elm.eu.org) is a manually curated database of short linear motifs (SLiMs). In this update, we present the latest additions to this resource, along with more improvements to the web interface. ELM 2016 contains more than 240 different motif classes with over 2700 experimentally validated instances, manually curated from more than 2400 scientific publications. In addition, more data have been made available as individually searchable pages and are downloadable in various formats.
INTRODUCTION
Short Linear motifs (SLiMs) are small protein-interaction-mediating modules that have unique properties (1,2). They play an important role in biological systems (3–6) and the analysis of motifs in protein sequences remains an important step in protein research. The ELM database provides manually curated classes of motifs as well as instances thereof. Furthermore, it provides a web interface that allows users to explore possible instances of annotated classes in proteins of interest. More than 10 years since its inception (7,8) it remains a popular resource being continually used by scientists worldwide. It has proved invaluable for investigating interactions mediated by short linear motifs, be it for individual proteins (9–11), large-scale analyses (12–14), algorithm development (15), prediction of novel motif-mediated interactions (16) or investigation of host–virus interactions (17–20). Here we give an overview of the latest developments and new features introduced to the ELM resource (see Figure 1) since the last update (21).
Figure 1.
ELM history showing the creation date (red line) and date of latest modification (orange line) of each ELM motif class (light blue line) and motif instance (dark blue line) annotated in the ELM database. Note that for each entry only the very latest modification time is recorded, not every modification timestamp.
RESOURCE DESCRIPTION
The ELM resource provides two main services, a database of short linear motif annotation and a tool that uses this information to explore possible instances of motifs in any given protein sequence. The main database content are annotations of motif classes, hand-curated from the scientific literature and enriched with links to resources such as PSI-MI (22), UniProt (23), GO (24), PDB (25), switches.ELM (6), Interpro (26), iELM (27), Pfam (28) and KEGG (29).
An ELM motif class is described by a unique regular expression pattern, such as ‘...([ST])P.’ (meaning any three residues followed by a serine or threonine, followed by proline and another wildcard residue) for the DOC_WW_Pin1_4 class. Ideally, each motif class has multiple example instances of this motif annotated, whereby an instance is described as a match to the regular expression pattern of the ELM motif class in a protein sequence. For each instance entry, ideally, multiple sources of experimental evidence are recorded (identifying participant, detecting motif presence and detecting interaction), and, following annotation best practices, a reliability score is given by the annotator.
NEW FEATURES AND FUNCTIONALITY
Interface
The HTML interface has been updated and new functionality has been added. A multi-tier navigation menu has been implemented to provide improved accessibility to the individual pages. Also, on each page, a unified search box is available, providing auto-completion and faster access to the ELM database content via simple keyword search (see Figure 2).
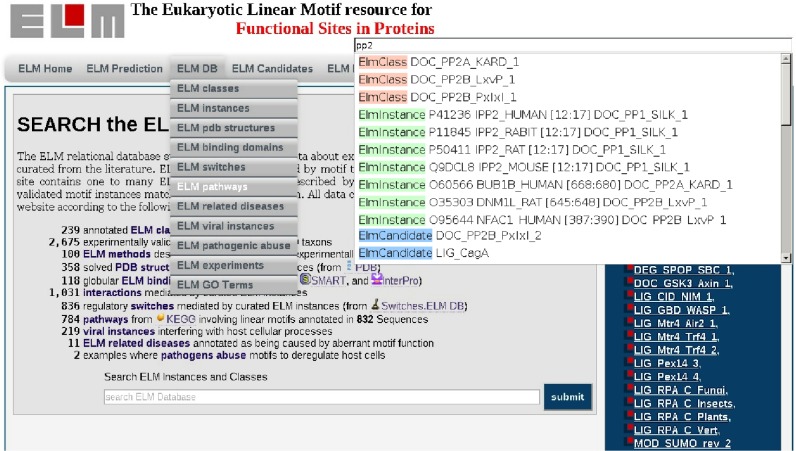
Figure 2.
Screenshot of the ELM home page showing autocompletion of keyword search and expanded menu bar. The dropdown menu lists individual pages of ELM database content; the top right shows a dropdown of the autocompletion search for the keyword ‘pp2’, different content type is highlighted in different colors (ELM classes: orange, ELM instances: green, ELM class candidates: blue); a click on a link directs to the details page of the entry.
Several new webpages have been introduced to allow easier access to the database content. There are now individual pages for interaction domains, motif-mediated switches, PDB structures, ELM methods and GO terms. This allows easy access to individual content searching and selecting data by user provided keywords, fostering data dissemination and re-use.
Database content
The ELM database has been updated and existing data types have been enriched with more data, see Tables 1 and 2, Figures 1 and 3 for an overview of data on ELM classes and instances, as well as Figures 4 and 5 for information about the taxonomic distribution of ELM instances and the experimental methods annotated for these instances. Furthermore, new data types have been added: Many motif instances have been annotated from large-scale studies (30) for which known mutations in the motif contribute to diseases. These can be collectively viewed at the ELM disease page (http://elm.eu.org/infos/diseases.html). Each annotated disease is described by a short abstract, links to the sequence variation causing the condition (linked to swissprot (31)) in the the motif-bearing protein as well as reference article(s). Each entry is linked to the corresponding ELM instances page.
Table 1. Summary of data stored in the ELM database1.
| Functional sites | ELM classes | ELM instances | PDB structures | GO terms | PubMed links | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 159 | 246 | 2702 | 348 | 549 | 2439 | |||||
| By category | LIG | 137 | Human | 1594 | |||||||
| MOD | 31 | Mouse | 253 | Biological Process | 283 | From class | 1174 | ||||
| DEG | 25 | Rat | 130 | ||||||||
| DOC | 22 | Yeast | 94 | Cellular Compartment | 119 | From instance | 1746 | ||||
| TRG | 20 | Fly | 90 | ||||||||
| CLV | 11 | Other | 541 | Molecular Function | 147 | ||||||
1 as of November 2015.
Table 2. 49 novel1 ELM classes that have been added since the last ELM publication (21), together with the number of associated instances (middle column) and a short description.
| ELM class identifier | Instances | ELM class description |
|---|---|---|
| DEG_Kelch_KLHL3_1 | 4 | An acidic degron motif present in wnk kinases necessary to interact with kelch domain of KLHL2 and KLHL3 proteins for efficient ubiquitination degradation. |
| DEG_Kelch_Keap1_1 | 13 | Motif that binds to the Kelch domain of KEAP1 with high affinity. This high affinity motif is required for the |
| DEG_Kelch_Keap1_2 | 1 | efficient recruitment of target proteins to the Cul3-based E3 ligase. |
| DEG_Kelch_actinfilin_1 | 1 | A hydrophobic degron motif present in some kainate receptors necessary to interact with kelch domain of actinfilin protein for efficient ubiquitination and degradation. |
| DEG_Nend_Nbox_1 | 0 | N-terminal motif that initiates protein degradation by binding to the N-box of N-recognins. This N-degron variant comprises a bulky hydrophobic residue as destabilizing residue. |
| DEG_Nend_UBRbox_1 | 0 | N-terminal motifs that initiate protein degradation by binding to the UBR-box of N-recognins. Four different |
| DEG_Nend_UBRbox_2 | 0 | N-degron variants comprise different N-terminal residues. Type I destabilizing residues can either occur as primary |
| DEG_Nend_UBRbox_3 | 0 | destabilizing residues, which are positively charged amino acids directly recognized by N-recognins, or as |
| DEG_Nend_UBRbox_4 | 8 | secondary and tertiary destabilizing amino acids, which can be conjugated to a primary destabilizing residue. |
| DEG_SPOP_SBC_1 | 8 | The S/T rich motif known as the SPOP-binding consensus (SBC) of the MATH-BTB protein, SPOP, is present in substrates that undergo SPOP/Cul3-dependant ubiquitination. |
| DOC_CKS1_1 | 8 | Phospho-dependent motif that mediates docking of CDK substrates and regulators to cyclin-CDK-bound Cks1. |
| DOC_GSK3_Axin_1 | 6 | Docking motif present in Axin protein binds the GSK-3β kinase and aids the phosphorylation of components in the APC destruction complex. |
| DOC_PP1_MyPhoNE_1 | 9 | Docking motif that binds to the catalytic subunit of Protein Phosphatase 1 (PP1c). |
| DOC_PP1_SILK_1 | 14 | Protein phosphatase 1 catalytic subunit (PP1c) interacting motif that often cooperates with and is located N-terminal to the RVXF motif to dock proteins to PP1c. |
| DOC_PP2A_KARD_1 | 1 | Protein Phosphatase 2A (PP2A)-binding motif found in BubR1 for docking to the regulatory subunit B56 of PP2A. |
| DOC_PP2B_LxvP_1 | 8 | Docking motif in calcineurin substrates that binds at the interface of the catalytic CNA and regulatory CNB subunits. |
| DOC_USP7_UBL2_3 | 0 | The USP7 CTD domain binding motif variant based on the ICP0 and DNMT1 interactions. |
| LIG_Axin_LRP6_1 | 0 | Motif in LRP6, which in its phosphorylated form binds Axin in a pseudo-substrate manner. |
| LIG_CID_NIM_1 | 1 | The NIM motif in Trf4 interacts with the CTD-interacting domain (CID) of Nrd1. |
| LIG_CNOT1_NIM_1 | 10 | The CNOT1-interacting motif (NIM) found in Nanos proteins mediates recruitment of the CCR4-NOT deadenylase complex. |
| LIG_CaMK_CASK_1 | 6 | Motif mediating binding to the calmodulin-dependent protein kinase (CaMK) domain of the membrane protein CASK/Lin2. |
| LIG_DCNL_PONY_1 | 2 | DCNL PONY domain binding motif variant based on the UBE2M and UBE2F interactions. |
| LIG_EF_ALG2_ABM_1 | 9 | This isoform-specific ALG-2-binding motif binds to the EF hand domains of the proapoptotic Ca2 +-binding |
| LIG_EF_ALG2_ABM_2 | 3 | ALG-2 protein in a calcium-dependent manner. |
| LIG_FZD_DVL_PDZ | 0 | A short internal motif near the C-terminus of Frizzleds, which interacts with the PDZ domain of DVL in Wnt pathway. |
| LIG_GBD_WASP_1 | 4 | A hydrophobic motif of double function – it acts as an autoinhibitory element of the GTPase- binding domain (GDB), as well as mediating the protein's interactions with the Arp2/3 complex. |
| LIG_GSK3_LRP6_1 | 8 | Motif present five times on membrane receptor LRP6, responsible for GSK3 binding and inhibition when phosphorylated. |
| LIG_LIR_Apic_2 | 1 | Apicomplexa-specific variant of the canonical LIR motif that binds to Atg8 protein family members. |
| LIG_LIR_Gen_1 | 21 | Canonical LIR motif that binds to Atg8 protein family members to mediate processes involved in autophagy. |
| LIG_LIR_LC3C_4 | 1 | Non-canonical variant of the LIR motif that binds to Atg8 protein family members to mediate processes involved in autophagy. |
| LIG_LIR_Nem_3 | 1 | Nematode-specific variant of the canonical LIR motif that binds to Atg8 protein family members. |
| LIG_LRP6_Inhibitor_1 | 0 | Short motif present in extracellular of some Wnt antagonists recognized by the N-terminal β-propeller domain of LRP5/6 and thus inhibits the Wnt pathway. |
| LIG_Mtr4_Air2_1 | 3 | This motif on Air2 interacts with the DExH core of Mtr4, forming a part of the nucleus-located TRAMP complex. |
| LIG_Mtr4_Trf4_1 | 4 | This motif on Trf4 interacts with the DExH core of Mtr4, forming a part of the nucleus-located TRAMP complex. |
| LIG_Mtr4_Trf4_2 | 3 | This motif on PAPD5 interacts with the DExH core of SKIV2L2, forming a part of the nucleus-located TRAMP complex. |
| LIG_Pex14_3 | 1 | Motif in Pex5 interacting with the N-terminal domain (NTD) of Pex14 |
| LIG_Pex14_4 | 0 | Fungal motif in Pex5 interacting with the N-terminal domain of Pex14 |
| LIG_RPA_C_Fungi | 1 | Fungi version of the RPA interacting motif. |
| LIG_RPA_C_Insects | 0 | Insect version of the RPA interacting motif. |
| LIG_RPA_C_Plants | 0 | Plant version of the RPA interacting motif, which is located on DNA replication and repair proteins UNG2, XPA, TIPIN, SMARCAL1 and RAD14 and interacts with Replication Protein A (RPA), a DNA binding protein. |
| LIG_RPA_C_Vert | 4 | The RPA interacting motif is located on DNA replication and repair proteins UNG2, XPA, TIPIN, SMARCAL1 and RAD14 and interacts with Replication Protein A (RPA), a DNA binding protein. |
| LIG_SUFU_1 | 5 | A hydrophobic motif in GLI transcription factors required for binding to SUFU protein, which inhibits their activity and hence negatively regulates hedgehog signalling. |
| LIG_UBA3_1 | 2 | UBA3 adenylation domain binding motif variant based on the UBE2M and UBE2F interactions. |
| LIG_WD40_WDR5_VDV_1 | 3 | This WDR5-binding motif binds to a cleft between blades 5 and 6 of the WD40 repeat domain of WDR5, opposite |
| LIG_WD40_WDR5_VDV_2 | 2 | of the Win motif-binding site, to mediate assembly of histone modification complexes. |
| LIG_WD40_WDR5_WIN_1 | 7 | Known as the Win (WDR5 interaction) motif, this peptide contains an invariant arginine residue that inserts into the |
| LIG_WD40_WDR5_WIN_2 | 4 | central tunnel of the WD40 repeat domain of WDR5 to mediate assembly of histone modification complexes. |
| LIG_WD40_WDR5_WIN_3 | 3 | Surrounding this arginine are small residues that fit tightly at the entrance of the arginine-binding pocket. |
| MOD_SUMO_rev_2 | 20 | Inverted version of SUMOylation motif recognized for modification by SUMO-1 |
1 as of November 2015.
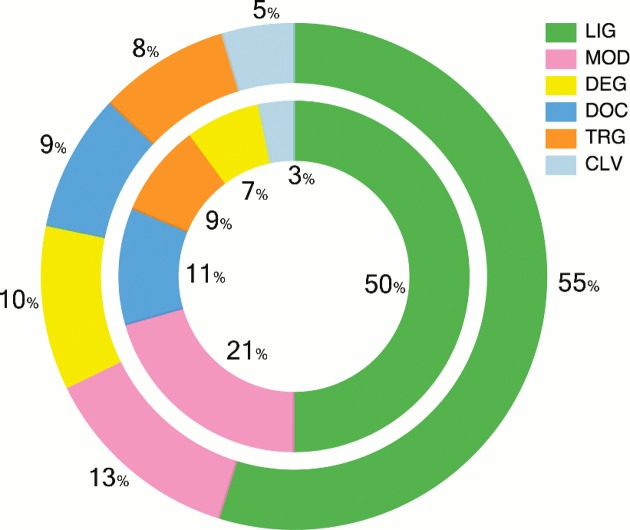
Figure 3.
Percentages of ELM classes (outer ring) and instances (inner ring) by type.
Figure 4.
Taxons annotated in the ELM database ordered by number of instances (in percentages).
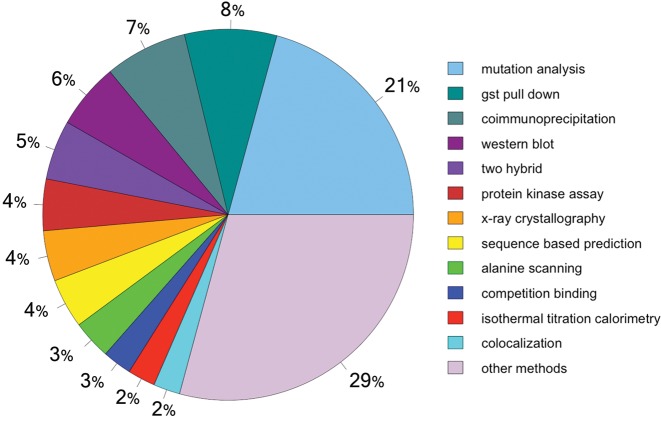
Figure 5.
Methods used for ELM annotation, ordered by number of motif instances for which the method has been annotated (in percentages). A full list can be found at http://elm.eu.org/infos/browse_elm_methods.html, which includes links to instances and PSI-MI vocabulary (22).
Database and web server optimization
The ELM resource is implemented using a PostgreSQL (https://postgresql.org) relational database as a backend to store all annotations and associated data, while the frontend web interface makes use of the Django web-framework (https://djangoproject.com). Recent improvements include an updated annotation system allowing easy annotation and correction of entries. This aids annotators in inserting novel entries and updating existing ones. Figure 1 illustrates how this annotation system has helped increasing the database content: since its implementation (in the year 2014) many new ELM classes and instances have been annotated, and a large number of classes and instances have been revised.
The high degree of data integration and interconnectedness caused some ELM database queries to become slow. To remedy this and thus increase user experience, an HTTP Cache/Reverse Proxy has been employed caching rendered HTML pages, which significantly speeds up page delivery and increases user experience.
Downloads
The data annotated in the ELM database is freely available to the scientific community and the ELM team tries to make this data as easily accessible as possible. Several new pages are now available providing more download formats/options; the best starting point for looking for ELM downloads is the web page http://elm.eu.org/downloads.html. Novel pages include the experimental methods used during annotation, the PDB structures associated with motif–domain interactions, or all linked GO-terms. All of these are also available for download in computer-parseable tab-separated format. Also, a simple timestamp has been implemented, which allows clients to update their data only when newer data is available. Further formats and options can be implemented upon request; the authors welcome suggestions to implement user-suggested features.
SHORT LINEAR MOTIFS IN BIOLOGICAL PATHWAYS
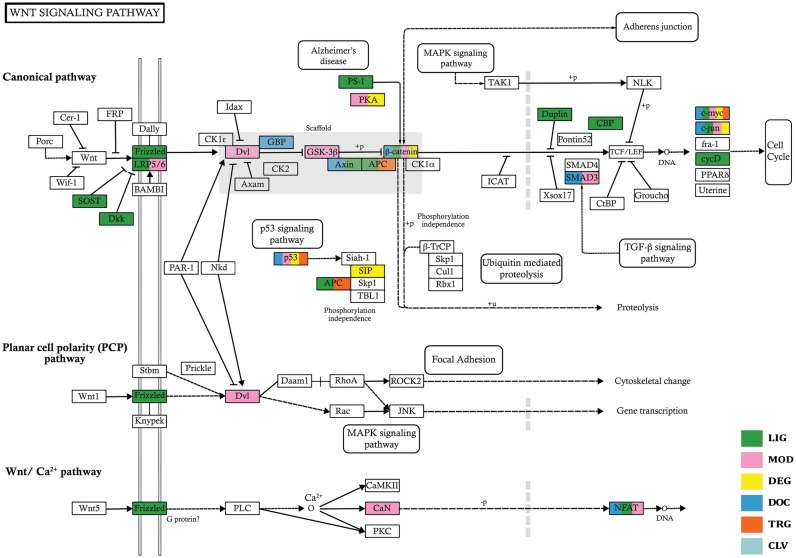
Relevance of linear motifs is well known in signaling pathways (32). Furthermore, accumulation of more experimental evidence demands a systematic analysis of all biological pathways for the presence of linear motifs. The ELM resource assists in this endeavor by providing a distinct color mapping of different ELM classes on the proteins involved in different pathways, information of which is obtained from KEGG (29). We have used the Wnt signaling pathway (hsa04310) to illustrate this feature (Figure 6).
Figure 6.
‘Wnt Signaling pathway’ from KEGG (29) (ID hsa04310). The color scheme has been modified for the cases having more than one ELM class in order to provide additional clarity. The original figure can be reproduced on the ELM web server (http://elm.eu.org/pathways/index.html?q=wnt)
Signaling in this pathway starts from Wnt proteins that transduce the signal from the extracellular part of the cell to the interior via the Frizzled receptor, which in turn transmits it to other regulatory proteins. A key component of Wnt signaling is β-catenin which needs to accumulate in the cytoplasm in order to be translocated into the nucleus, where it subsequently induces a cellular response via gene transduction (33). This accumulation however is regulated by APC/Axin1 destruction complex in which β-catenin is ubiquitinylated (34). Thereafter, modified β-catenin is degraded via proteosomal machinery. Short linear motifs play a prominent role in this process as exemplified by the presence of at least four different motifs in β-catenin (annotated at the ELM resource): It contains modification sites for Casein kinase 1 (CK1) and glycogen synthase kinase 3 (GSK3) and the sequential modification of these sites by CK1 and GSK3 generates a phosphodegron at the N-terminus of this protein. This phospho-regulated degron acts as a recognition site for β-TrCP, a subunit of the SCF-β-TrCP E3 ligase enzyme (35). After binding to the activated degron, β-TrCP ubiquitinylates β-catenin, which gets subsequently degraded by the proteosomal machinery (36). Among other classes, β-catenin contains ligand binding (LIG) motifs for 14-3-3 proteins and the binding of 14-3-3 along with Chibby protein has been suggested to facilitate the nuclear export of β-catenin, which puts an end to its signaling (37). The presence of each of these motifs is necessary for modulation of this pathway and the regulation of β-catenin by different motif classes clearly underlines the important role of short linear motifs in signaling pathways.
CONCLUSION AND FUTURE DIRECTIONS
The number of motifs that have been experimentally validated up to today is still small, with annotated instance numbers in the low thousands, compared to the estimated total number, which might exceed a million (38); hence, we expect many more motifs and motif-mediated interactions to be discovered (for guidelines on motif discovery see (39)). The ELM resource will continue to provide support to the scientific community with a repository of high quality annotations and facilitate the linear motif analysis of protein sequences.
Acknowledgments
The authors would like to thank the users of the ELM resource for their longstanding support as well as colleagues and annotators for their contributions and feedback to the ELM database.
FUNDING
European Community‘s Seventh Framework Programme FP7/2009 (SyBoSS) [242129 to K.V.R.]; EMBL International PhD Program (to B.U.); SFI Starting investigator Research Grant [13/SIRG/2193 to N.E.D]. Funding for open access charge: EMBL.
Conflict of interest statement. None declared.
REFERENCES
- 1.Davey N. E., Van Roey K., Weatheritt R.J., Toedt G., Uyar B., Altenberg B., Budd A., Diella F., Dinkel H., Gibson T.J. Attributes of short linear motifs. Mol. Biosyst. 2012;8:268–281. doi: 10.1039/c1mb05231d. [DOI] [PubMed] [Google Scholar]
- 2.Van Roey K., Uyar B., Weatheritt R.J., Dinkel H., Seiler M., Budd A., Gibson T.J., Davey N.E. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem. Rev. 2014;114:6733–6778. doi: 10.1021/cr400585q. [DOI] [PubMed] [Google Scholar]
- 3.Dyson H.J., Wright P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 4.Uversky V.N., Dunker A.K. Understanding protein non-folding. Biochim. Biophys. Acta. 2010;1804:1231–1264. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beltrao P., Albanèse V., Kenner L.R., Swaney D.L., Burlingame A., Villén J., Lim W.A., Fraser J.S., Frydman J., Krogan N.J. Systematic functional prioritization of protein posttranslational modifications. Cell. 2012;150:413–425. doi: 10.1016/j.cell.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Roey K., Dinkel H., Weatheritt R.J., Gibson T.J., Davey N.E. The switches.ELM resource: a compendium of conditional regulatory interaction interfaces. Sci. Signal. 2013;6:rs7. doi: 10.1126/scisignal.2003345. [DOI] [PubMed] [Google Scholar]
- 7.Puntervoll P., Linding R., Gemünd C., Chabanis-Davidson S., Mattingsdal M., Cameron S., Martin D. M.A., Ausiello G., Brannetti B., Costantini A., et al. ELM server: a new resource for investigating short functional sites in modular eukaryotic proteins. Nucleic Acids Res. 2003;31:3625–3630. doi: 10.1093/nar/gkg545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinkel H., Michael S., Weatheritt R.J., Davey N.E., Van Roey K., Altenberg B., Toedt G., Uyar B., Seiler M., Budd A., et al. ELM–the database of eukaryotic linear motifs. Nucleic Acids Res. 2012;40:D242–D251. doi: 10.1093/nar/gkr1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.García-Santisteban I., Bañuelos S., Rodríguez J.A. A global survey of CRM1-dependent nuclear export sequences in the human deubiquitinase family. Biochem. J. 2012;441:209–217. doi: 10.1042/BJ20111300. [DOI] [PubMed] [Google Scholar]
- 10.Vandergaast R., Mitchell J.K., Byers N.M., Friesen P.D. Insect inhibitor-of-apoptosis (IAP) proteins are negatively regulated by signal-induced N-terminal degrons absent within viral IAP proteins. J. Virol. 2015;89:4481–4493. doi: 10.1128/JVI.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundell G.N., Ivarsson Y. Interaction analysis through proteomic phage display. Biomed. Res. Int. 2014;2014:2542–2547. doi: 10.1155/2014/176172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reimand J., Bader G.D. Systematic analysis of somatic mutations in phosphorylation signaling predicts novel cancer drivers. Mol. Syst. Biol. 2013;9:637. doi: 10.1038/msb.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivarsson Y., Arnold R., McLaughlin M., Nim S., Joshi R., Ray D., Liu B., Teyra J., Pawson T., Moffat J., et al. Large-scale interaction profiling of PDZ domains through proteomic peptide-phage display using human and viral phage peptidomes. Proc. Natl. Acad. Sci. U.S.A. 2014;111:2542–2547. doi: 10.1073/pnas.1312296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tovo-Rodrigues L., Roux A., Hutz M.H., Rohde L.A., Woods A.S. Functional characterization of G-protein-coupled receptors: a bioinformatics approach. Neuroscience. 2014;277:764–779. doi: 10.1016/j.neuroscience.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan W., Duffy F., Pollastri G., Shields D.C., Mooney C. Predicting binding within disordered protein regions to structurally characterised peptide-binding domains. PLoS One. 2013;8:e72838. doi: 10.1371/journal.pone.0072838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T.S., Petrey D., Garzon J.I., Honig B. Predicting peptide-mediated interactions on a genome-wide scale. PLoS Comput. Biol. 2015;11:e1004248. doi: 10.1371/journal.pcbi.1004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chemes L.B., de Prat-Gay G., Sánchez I.E. Convergent evolution and mimicry of protein linear motifs in host-pathogen interactions. Curr. Opin. Struct. Biol. 2015;32:91–101. doi: 10.1016/j.sbi.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Via A., Uyar B., Brun C., Zanzoni A. How pathogens use linear motifs to perturb host cell networks. Trends Biochem. Sci. 2015;40:36–48. doi: 10.1016/j.tibs.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Graf L., Webel R., Wagner S., Hamilton S.T., Rawlinson W.D., Sticht H., Marschall M. The cyclin-dependent kinase ortholog pUL97 of human cytomegalovirus interacts with cyclins. Viruses. 2013;5:3213–3230. doi: 10.3390/v5123213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagai T., Azia A., Babu M.M., Andino R. Use of host-like peptide motifs in viral proteins is a prevalent strategy in host-virus interaction. Cell Rep. 2014;7:1729–1739. doi: 10.1016/j.celrep.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinkel H., Van Roey K., Michael S., Davey N.E., Weatheritt R.J., Born D., Speck T., Krüger D., Grebnev G., Kuban M., et al. The eukaryotic linear motif resource ELM: 10 years and counting. Nucleic Acids Res. 2014;42:D259–D266. doi: 10.1093/nar/gkt1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerrien S., Orchard S., Montecchi-Palazzi L., Aranda B., Quinn A.F., Vinod N., Bader G.D., Xenarios I., Wojcik J., Sherman D., et al. Broadening the horizon–level 2.5 of the HUPO-PSI format for molecular interactions. BMC Biol. 2007;5:44. doi: 10.1186/1741-7007-5-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The UniProt Consortium. UniProt: a hub for protein information. Nucleic Acids Res. 2015;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein F.C., Koetzle T.F., Williams G.J., Meyer E. Jr, Brice M.D., Rodgers J.R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J. Mol. Biol. 1977;112:535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell A., Chang H.-Y., Daugherty L., Fraser M., Hunter S., Lopez R., McAnulla C., McMenamin C., Nuka G., Pesseat S., et al. The InterPro protein families database: the classification resource after 15 years. Nucleic Acids Res. 2015;43:D213–D221. doi: 10.1093/nar/gku1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weatheritt R.J., Jehl P., Dinkel H., Gibson T.J. iELM–a web server to explore short linear motif-mediated interactions. Nucleic Acids Res. 2012;40:W364–W369. doi: 10.1093/nar/gks444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punta M., Coggill P.C., Eberhardt R.Y., Mistry J., Tate J., Boursnell C., Pang N., Forslund K., Ceric G., Clements J., et al. The Pfam protein families database. Nucleic Acids Res. 2012;40:D290–D301. doi: 10.1093/nar/gkr1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanabe M., Kanehisa M. Using the KEGG database resource. Curr. Protoc. Bioinformatics. 2012;2012 doi: 10.1002/0471250953.bi0112s38. doi:10.1002/0471250953.bi0112s38. [DOI] [PubMed] [Google Scholar]
- 30.Uyar B., Weatheritt R.J., Dinkel H., Davey N.E., Gibson T.J. Proteome-wide analysis of human disease mutations in short linear motifs: neglected players in cancer. Mol. Biosyst. 2014;10:2626–2642. doi: 10.1039/c4mb00290c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mottaz A., David F. P.A., Veuthey A.-L., Yip Y.L. Easy retrieval of single amino-acid polymorphisms and phenotype information using SwissVar. Bioinformatics. 2010;26:851–852. doi: 10.1093/bioinformatics/btq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diella F., Haslam N., Chica C., Budd A., Michael S., Brown N.P., Trave G., Gibson T. Understanding eukaryotic linear motifs and their role in cell signaling and regulation. Front. Biosci. 2008;13:6580–6603. doi: 10.2741/3175. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li V. S.W., Ng S.S., Boersema P.J., Low T.Y., Karthaus W.R., Gerlach J.P., Mohammed S., Heck A.J.R., Maurice M.M., Mahmoudi T., et al. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245–1256. doi: 10.1016/j.cell.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Winston J.T., Strack P., Beer-Romero P., Chu C.Y., Elledge S.J., Harper J.W. The SCFbeta-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IkappaBalpha and beta-catenin and stimulates IkappaBalpha ubiquitination in vitro. Genes Dev. 1999;13:270–283. doi: 10.1101/gad.13.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He X., Semenov M., Tamai K., Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 37.Takemaru K.-I., Fischer V., Li F.-Q. Fine-tuning of nuclear-catenin by Chibby and 14-3-3. Cell Cycle. 2009;8:210–213. doi: 10.4161/cc.8.2.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tompa P., Davey N.E., Gibson T.J., Babu M.M. A million peptide motifs for the molecular biologist. Mol. Cell. 2014;55:161–169. doi: 10.1016/j.molcel.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 39.Gibson T.J., Dinkel H., Van Roey K., Diella F. Experimental detection of short regulatory motifs in eukaryotic proteins: tips for good practice as well as for bad. Cell Commun. Signal. 2015;13:42. doi: 10.1186/s12964-015-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]