Significance
Many cancers (e.g., subpopulations of lung cancer, anaplastic lymphoma, and neuroblastoma) are driven by mutations in the receptor tyrosine kinase ALK (for anaplastic lymphoma kinase). However, the extracellular protein signals that regulate ALK’s activity and its ligand-induced mechanism of activation remain elusive. Here we describe a cytokine designated augmentor-α that binds with high affinity and specificity to ALK’s extracellular glycine-rich region, resulting in robust receptor activation. Augmentor-α also potently activates the related leukocyte tyrosine kinase (LTK) receptor, whereas a previously identified LTK ligand (augmentor-β) only weakly activates ALK. These experiments reveal an important missing link necessary for the regulation of a known oncogenic RTK, providing important insights into its biology and offering new opportunities for therapeutic intervention.
Keywords: cell signaling, surface receptors, phosphorylation, cancer, protein kinases
Abstract
Receptor tyrosine kinases (RTKs) are a class of cell surface receptors that, upon ligand binding, stimulate a variety of critical cellular functions. The orphan receptor anaplastic lymphoma kinase (ALK) is one of very few RTKs that remain without a firmly established protein ligand. Here we present a novel cytokine, FAM150B, which we propose naming augmentor-α (AUG-α), as a ligand for ALK. AUG-α binds ALK with high affinity and activates ALK in cells with subnanomolar potency. Detailed binding experiments using cells expressing ALK or the related receptor leukocyte tyrosine kinase (LTK) demonstrate that AUG-α binds and robustly activates both ALK and LTK. We show that the previously established LTK ligand FAM150A (AUG-β) is specific for LTK and only weakly binds to ALK. Furthermore, expression of AUG-α stimulates transformation of NIH/3T3 cells expressing ALK, induces IL-3 independent growth of Ba/F3 cells expressing ALK, and is expressed in neuroblastoma, a cancer partly driven by ALK. These experiments reveal the hierarchy and specificity of two cytokines as ligands for ALK and LTK and set the stage for elucidating their roles in development and disease states.
Receptor tyrosine kinases (RTKs) are cell surface receptors that serve as a signaling relay across the membrane for growth factors, cytokines, and hormones. They function to coordinate proliferation, differentiation, cell survival, and metabolism in multicellular organisms. The era of RTK study began more than half of a century ago (reviewed in ref. 1) and has significantly advanced over the last 3 decades, with numerous studies shedding light on the function, structure, and regulation of RTKs and their ligands (2–4).
The RTK anaplastic lymphoma kinase (ALK) was originally identified in anaplastic large-cell non-Hodgkin’s lymphoma as an oncogenic fusion protein with nucleophosmin resulting from a 2;5 chromosomal translocation (5, 6). The ALK gene is a hotspot for a variety of chromosomal translocations that result in the formation of fusion proteins that undergo spontaneous dimerization, leading to constitutive activation of the ALK kinase domain (reviewed in refs. 7 and 8). These chimeric ALK proteins were shown to drive numerous human cancers, both in hematopoietic malignancies and in solid tumors (7). Full-length, nonchimeric ALK is a driving force in neuroblastoma (NBL), where genetic studies have identified it as a major target of genetic alterations (i.e., gene amplification and somatic and germ-line mutations) (7, 9–12). The majority of missense mutations in ALK found in NBL are located in the kinase domain and lead to constitutive receptor activation. Amplification of ALK and coamplification with the N-myc proto-oncogene (MYCN) (both genes are located on chromosome 2p) drive and cooperate in NBL progression (13). Collectively, these studies underscore the role of ALK in tumorigenesis, along with approval by the US Food and Drug Administration of an ALK inhibitor, crizotinib, which is used to treat patients with ALK-driven cancers.
As a classical RTK, ALK is composed of an extracellular domain (ECD), a single transmembrane domain, and a cytoplasmic domain (3). Together with another RTK member, leukocyte tyrosine kinase (LTK), ALK constitutes a subfamily of RTKs. The ECDs of both LTK and ALK contain a unique glycine-rich domain and an EGF-like motif (14). In addition, the ECD of ALK also has a heparin-binding N-terminal domain (NTR) and two meprin, A-5 protein, and receptor protein-tyrosine phosphatase mu (MAM) domains separated by a low-density lipoprotein receptor domain class A (LDL-A) domain (15).
The biological role of ALK and LTK in mammals is not well understood. However, they are thought to play a role in the development of the mammalian nervous system. mRNA analysis from different mouse tissues has revealed that ALK mRNA is dominantly expressed in brain and spinal cord during mouse embryogenesis and diminishes after birth (16, 17). ALK−/− mice are viable and fertile, with some alterations in behavioral tests (18). Both LTK and ALK are expressed in the mouse hippocampus and involved in adult neurogenesis (19). In humans, ALK is expressed in the small intestine, testis, and brain (5).
Unlike most RTKs whose ligands are known, there are no established growth factor, cytokine, or hormone ligands for ALK. Two small heparin-binding growth factors, pleiotrophin and midkine, were previously reported as activating ligands for ALK (20, 21). However, subsequent studies were unable to reproduce these results (22–24). We have recently demonstrated that ALK expressed in the NB1 cell line can be activated by heparin (but not by pleiotrophin or midkine), suggesting a proteoglycan may regulate ALK activity and function through binding to its heparin-binding NTR (15). Two small proteins, designated FAM150A and FAM150B, were recently identified in a screening assay for secreted proteins that bind to the recombinant extracellular domain of LTK (25). It was also demonstrated that FAM150A binding results in stimulation of LTK activation, indicating that FAM150A can function as a stimulatory ligand of LTK. As LTK and ALK share unique structural elements, including a homologous glycine-rich domain (which encompasses the majority of the LTK ECD) and represent a distinct subfamily of RTKs, we hypothesized that FAM150B may function as an activating ligand for ALK. Here we show that FAM150B acts as a universal, dual-specific ligand for both ALK and LTK, whereas the previously established LTK ligand FAM150A is a specific ligand for LTK and only weakly stimulates ALK. Finally, because FAM gene designations are temporary symbols assigned by the Human Gene Nomenclature Committee (26), we have named the dual-specific ligand, FAM150B, augmentor-α (AUG-α), and the previously established LTK ligand, FAM150A, augmentor-β (AUG-β).
Results
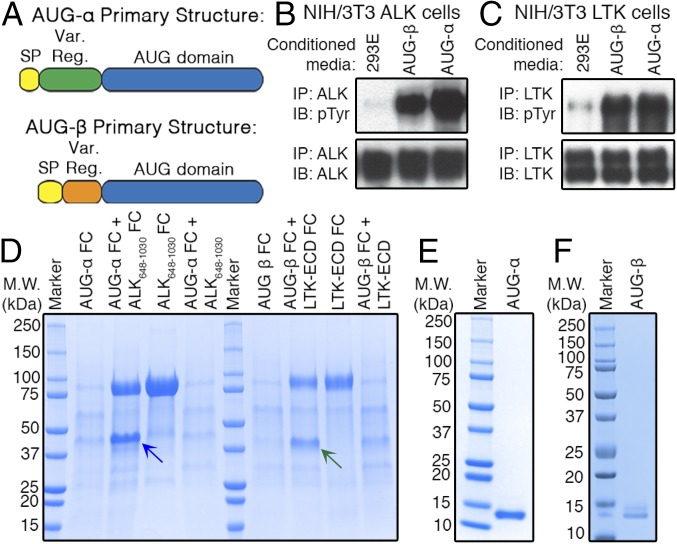
Both AUG-α and AUG-β are very basic, small proteins with theoretical pI values of 9.37 and 10.39, respectively, and predicted molecular weights of 14.5 and 11.5 kDa, respectively (without their signal peptides). Sequence homology analysis (Fig. 1A and Fig. S1) reveals two distinct regions: a variable N-terminal region and a conserved C-terminal domain, designated an “augmentor domain.” The latter is highly conserved between AUG-α and AUG-β, with sequence identity of 65.9% and high conservation of four cysteines in many species (Fig. S1).
Fig. 1.
AUG-α and AUG-β are ligands for ALK and LTK. (A) Schematic representation of AUG-α and AUG-β. Signal peptide (SP), yellow; variable region (Var. Reg.), green (AUG-α) and orange (AUG-β); conserved AUG domain, blue. (B and C) NIH/3T3 cells stably expressing ALK (B) or LTK (C) were stimulated with conditioned medium from 293-E cells (negative control), or 293-E cells expressing AUG-α or AUG-β. Lysates of 3T3 cells were subjected to immunoprecipitation (IP) using anti-ALK (B) or anti-LTK (C) antibodies followed by SDS/PAGE and immunoblotting (IB) with anti-pTyr (IB: pTyr) or anti-ALK (IB: ALK) or anti-LTK (IB: LTK) antibodies (as indicated). (D) Expression of human augmentor ligands. AUG-α, fused to Fc fragment at the C terminus (AUG-α-Fc), was expressed alone or coexpressed with ALK648–1,030 or ALK648–1,030-Fc fusion protein. Similarly, fusion of AUG-β-Fc was expressed alone or coexpressed with LTK-ECD-Fc or with LTK-ECD. Coexpression of ALK648–1,030-Fc and LTK-ECD-Fc with AUG-α-Fc or AUG-β-Fc, respectively, was compared with expression of ALK648–1,030-Fc and LTK-ECD-Fc alone. Fc-tagged proteins were affinity purified using protein A Sepharose, and volume corresponding to 1 mL conditioned medium was loaded on to an SDS/PAGE gel. Blue arrow indicates AUG-α-Fc, and green arrow indicates AUG-β-Fc. Molecular weight (M.W.) marker with corresponding molecular masses is shown on the left side of the gel. (E and F) Reducing SDS/PAGE gel showing final purified preparations of AUG-α (E) and AUG-β (F).
Fig. S1.
(A) Alignment of human AUG-α and AUG-β sequences separated by signal peptide, variable region, and AUG domain. (B) Multiple sequence alignment of AUG-α.
To test whether AUG-α or AUG-β can activate ALK and/or LTK, we generated HEK-293E cell lines stably expressing each augmentor ligand. Because AUG-α and AUG-β are likely secreted proteins (27), we collected conditioned medium from these cells and from untransfected HEK-293E cells. We then applied these media to NIH/3T3 cells stably expressing ALK or LTK and assessed receptor activation by immunoblotting. The conditioned medium from cells expressing AUG-α or AUG-β, but not from untransfected HEK-293E cells, stimulated robust autophosphorylation of both ALK and LTK (Fig. 1 B and C). These preliminary results indicated that AUG-α and AUG-β can act as ligands for ALK and LTK, albeit at unknown concentrations in conditioned media. We therefore next embarked on expression and purification of each ligand to perform detailed ligand–receptor affinity and specificity characterization in vitro and in cells.
Expression of AUG-α and AUG-β.
We used a variety of well-established systems to express human AUG-α and AUG-β without success. We tried to express the bovine, rat, murine, and zebrafish AUG-α and AUG-β homologs also with minimal success. Despite extensive and rigorous trials, it was exceptionally difficult to express enough recombinant AUG-α and AUG-β in either bacterial or mammalian expression systems for purification.
Because the N-terminal region is rich in positively charged amino acids and has no conserved cysteines to stabilize folding, we postulated that poor expression of AUG-α and AUG-β might result from instability and rapid degradation of this region. We reasoned that if AUG-α and AUG-β are high-affinity ligands for ALK and LTK, then coexpression of the ligand together with its receptor could increase the ligand’s stability and protect it from degradation. Therefore, we established a method to produce AUG-α and AUG-β in the form of Ig Fc fusion proteins coexpressed with ALK or LTK ectodomains that were also fused with Fc domains in HEK-293E cells. Using this method, expression was increased significantly compared with in conventional overexpression systems.
This approach yielded high expression of AUG-α when coexpressed with a fragment of the ALK ECD that mimics the full-length LTK ECD (ALK648–1,030), and high expression of AUG-β was achieved when coexpressed with the full-length LTK ECD (Fig. 1D). When expressing each ligand alone, little to no expression was observed (Fig. 1D). When coexpressing ligands with an Fc and receptors without an Fc, no enhancement of expression was observed, indicating that high expression of ligand is dependent on both receptor and ligand having Fc domains (Fig. 1D). This likely indicates that recombinant expression is dependent on the promotion of ligand–receptor interaction via covalently mediated homodimerization of Fc fragments.
After initial purification by protein chromatography, ligand–receptor complexes were subjected to tobacco etch virus protease (TEV) cleavage to remove the Fc-tags, and then free ligands were separated from their receptor fragments using cation exchange and size-exclusion chromatography. The final purification products are depicted in Fig. 1 E and F. Purified AUG-α and AUG-β were then tested for their ability to stimulate kinase activity of the full-length ALK and LTK in 3T3/NIH cells; both ligands were highly active (see following).
Binding Specificities of AUG-α and AUG-β Toward ALK and LTK Stimulation.
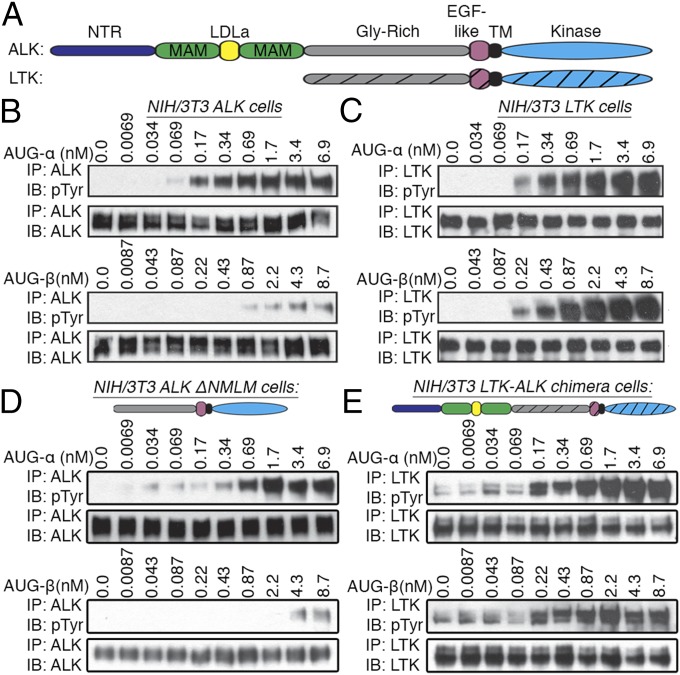
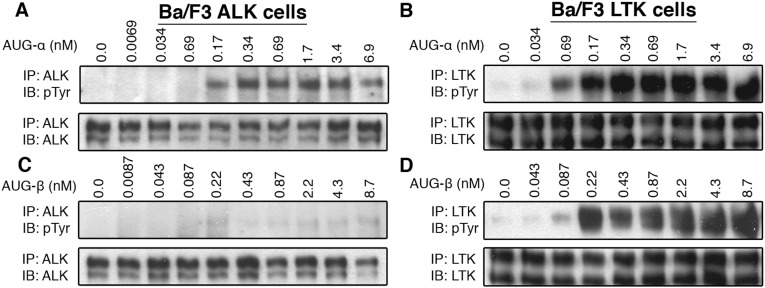
The results of our initial experiments, in which we used conditioned medium from cells expressing AUG-α and AUG-β to activate ALK or LTK indicated that both ligands could stimulate autophosphorylation of ALK and LTK (the domain structure of each receptor is shown in Fig. 2A). To test ligand–receptor specificity, ALK and LTK were stably expressed in NIH/3T3 cells, treated with increasing concentrations of purified AUG-α and AUG-β, and assessed for receptor activation by immunoblotting (Fig. 2 B and C). AUG-α induced robust autophosphorylation of ALK and LTK in picomolar concentrations (Fig. 2 B and C, Top). In contrast, the response of ALK and LTK to AUG-β was quite different. AUG-β stimulated robust activation and autophosphorylation of LTK in the picomolar range, very similar to AUG-α, whereas ALK responded to AUG-β stimulation at only high subnanomolar concentrations (Fig. 2 B and C, Bottom). These experiments were replicated using Ba/F3 cells ectopically expressing either ALK or LTK, and similar results were obtained (Fig. S2). These results indicate that AUG-α acts as a dual-specific ligand for both ALK and LTK, whereas AUG-β acts as a ligand specific for LTK.
Fig. 2.
AUG-α and AUG-β stimulate tyrosine kinase activity of ALK and LTK in a concentration-dependent manner. (A) Schematic representation of ALK and LTK domain organization. N-terminal domain (NTR) colored with dark blue, MAM with green, LDL with yellow, glycine-rich (Gly-Rich) with gray, EGF-like motif with pink, transmembrane (TM) with black, and kinase domain with blue. LTK domains are shown with striations. (B and C) Concentration-dependent stimulation of NIH/3T3 cells stably expressing ALK (B) or LTK (C) with purified augmentor ligands. (D) Ligand-dependent tyrosine kinase activity of an ALK truncation mutant where the NTR, LDL, and both MAM domains were deleted (ΔNMLM) to produce an LTK-like ALK receptor. NIH/3T3 cells stably expressing ΔNMLM ALK were stimulated with various concentrations of AUG-α and AUG-β (as indicated). (E) Ligand-dependent tyrosine kinase activity of an LTK-ALK chimera, where the NTR, LDL, and MAM domains of ALK were fused to the LTK receptor. NIH/3T3 cells stably expressing the LTK-ALK chimera were stimulated with various concentrations of AUG-α and AUG-β (as indicated).
Fig. S2.
(A–D) Stimulation of Ba/F3 cells stably expressing ALK or LTK with the augmentor ligands. Different concentrations of purified AUG-α and AUG-β were used to stimulate tyrosine kinase activity of ALK for 10 min at 37 °C. Lysates of unstimulated or AUG-stimulated cells were subjected to immunoprecipitation (IP), using anti-ALK (A and B) or anti-LTK (C and D) antibodies, followed by SDS/PAGE and immunoblotting (IB) with anti-pTyr (IB: pTyr) or antireceptor antibodies (as indicated).
We then asked whether the N-terminal part of ALK [the NTR, two MAM and LDL-A domains (NMLM)] affects ligand-induced activation. To answer this question, we generated two constructs: an ALK truncation mutant in which the NMLM region was deleted (ALK-ΔNMLM), and a chimera of ALK and LTK, where the NMLM region of ALK was fused in-frame with LTK receptor (see Materials and Methods section for details). These constructs are represented schematically in Fig. 2 D and E.
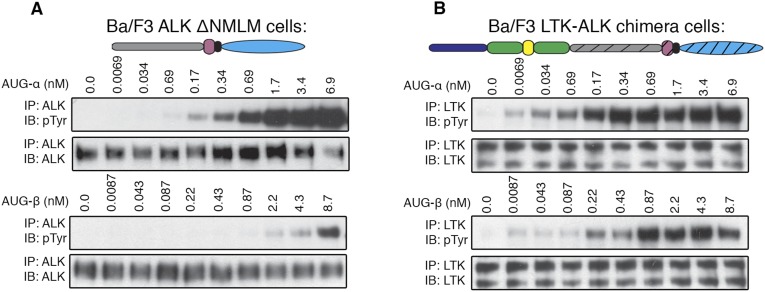
The ALK-ΔNMLM and the LTK-ALK chimera were stably expressed in NIH/3T3 cells and stimulated with various concentrations of AUG-α and AUG-β. The ALK-ΔNMLM receptor, similar to wild-type (WT) ALK, is strongly activated by AUG-α and is barely activated by AUG-β (Fig. 2 B and D). The LTK-ALK chimera, similar to WT LTK, is activated to a similar extent by both ligands (Fig. 2 C and E). Because the NMLM region did not affect ligand-dependent activation or ligand specificity, we concluded that the affinity and specificity of the augmentor ligands is determined by the Gly-R region and EGF-like motif of ALK and LTK. These experiments were repeated, using Ba/F3 cells, and similar results were obtained (Fig. S3).
Fig. S3.
(A) Ligand-dependent tyrosine kinase activity of an ALK truncation mutant where the NTR, LDL, and both MAM domains were deleted (ΔNMLM) to produce an LTK-like ALK receptor. Ba/F3 cells stably expressing ΔNMLM ALK were used. (B) Ligand-dependent tyrosine kinase activity of an LTK-ALK chimera, where the NTR, LDL, and MAM domains of ALK were fused to the LTK receptor. Ba/F3 cells stably expressing the LTK-ALK chimera were used. (Upper) Schematic representation of the ΔNMLM and LTK-ALK chimera constructs.
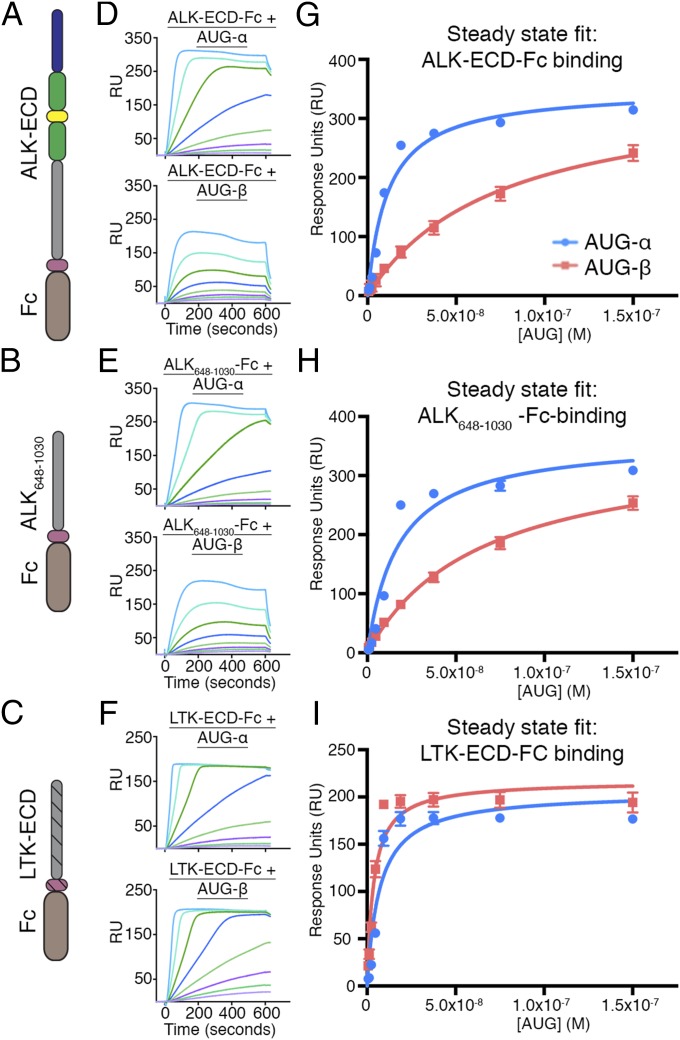
To confirm that stimulation of ALK and LTK by AUG-α and AUG-β in cells results from direct ligand–receptor interactions, and to further establish specificity of these ligands, we performed in vitro binding experiments with purified proteins, using surface plasmon resonance (SPR). Three Fc-fusion receptors were captured on a protein A chip: ALK-ECD-Fc, ALK648–1,030-Fc, and LTK-ECD-Fc (schematically represented in Fig. 3 A–C). Various concentrations of purified AUG-α or AUG-β were then injected over each surface and a reference surface containing only protein A (Fig. 3 D–F). A steady-state 1:1 binding model was used to fit experimental data and to calculate dissociation constants after reference subtraction (Fig. 3 G–I).
Fig. 3.
SPR binding analysis of AUG-α and AUG-β to FC-fusion receptors immobilized on a protein A chip. (A–C) Schematic representation of the domain organization of the ALK-ECD, ALK648–1,030, and LTK-ECD. (D–F) SPR sensograms for binding of AUG-α and AUG-β to immobilized receptors. (D) Binding of AUG-α (Top) and AUG-β (Bottom) to the ALK-ECD surface. (E) Binding of AUG-α (Top) and AUG-β (Bottom) to the ALK648-1,030 surface. (F) Binding of AUG-α (Top) and AUG-β (Bottom) to the LTK-ECD surface. (G–I) Fitting of receptor–augmentor affinity, using the steady-state 1:1 binding model. AUG-α binding is blue, and AUG-β binding is red. (G) For ALK-ECD, the KD for AUG-α binding is 11.4 ± 1.7 nM, and the KD for AUG-β binding is 74.3 ± 9.5 nM. (H) For ALK648–1,030, the KD for AUG-α binding is 17.4 ± 3.9 nM, and the KD for AUG-β binding is 64.0 ± 5.9 nM. (I) For LTK-ECD, the KD for AUG-α binding is 7.1 ± 1.8 nM, and the KD for AUG-β binding is 3.7 ± 0.7 nM.
AUG-α bound to ALK-ECD and to ALK648–1,030 with high and very similar affinities (KD = 11.4 ± 1.7 nM and KD = 17.4 ± 3.9 nM, respectively); in contrast, AUG-β bound with considerably lower affinity (KD = 74.3 ± 9.5 nM and KD = 64.0 ± 5.9 nM, respectively; Fig. 3 D, E, G, and H). Both ligands bound to LTK-ECD with similar high affinities (KD = 7.1 ± 1.8 nM for AUG-α and KD = 3.7 ± 0.7 nM for AUG-β) (Fig. 3 F and I). These results are in agreement with the dose-dependent autophosphorylation of ALK and LTK in live cells stimulated by AUG-α and AUG- β (Fig. 2). Taken together, these results reveal that AUG-α is a high-affinity ligand of both ALK and LTK and that AUG-β is a high-affinity ligand only for LTK, and that its affinity for ALK is relatively low (Figs. 2 and 3). Furthermore, these results indicate that the NMLM region is dispensable for augmentor binding.
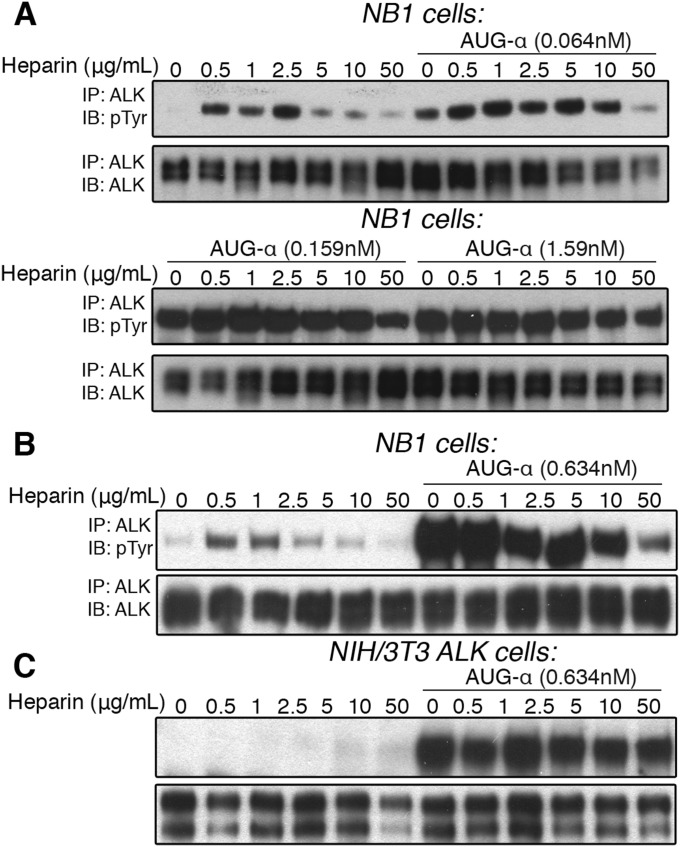
Modulation of AUG-α Induced ALK Activity by Heparin in NB1 Cells.
We have previously shown that heparin binds to the NTR region of ALK and stimulates the receptor’s kinase activity in NB1 cells (15). Therefore, we studied the effect of heparin on ALK in the presence and absence of AUG-α. NB1 cells were treated with various concentrations of heparin in the absence or presence of 0.064, 0.159, or 1.59 nM of AUG-α and assessed receptor activation by immunoblotting (Fig. S4A). These results demonstrate that in cells costimulated with a low AUG-α concentration (0.064 nM) and with heparin (from 0.5 to 10 μg/mL), heparin increased the activation of ALK and its Tyr phosphorylation. However, in cells stimulated with higher concentrations of AUG-α (0.159 and 1.59 nM), the synergistic effect was overshadowed by a much stronger phosphotyrosine (pTyr) signal induced by AUG-α (Fig. S4A, Lower). Interestingly, a higher concentration of heparin (50 μg/mL) appears to inhibit activation of ALK at low concentrations of AUG-α (Fig. S4B). These results indicate that heparin, and probably other sulfated glycosaminoglycans at the cell surface, likely act on ALK in a modulatory fashion, whereas AUG-α acts as the bona fide and major ALK ligand.
Fig. S4.
(A–C) Dose-dependent activation of ALK by heparin in NB1, neuroblastoma cells, endogenously expressing ALK. (A) NB1 cells were treated with increasing concentrations of heparin (as indicated) in the presence or absence of 0.064, 0.159, or 1.59 nM of AUG-α (as indicated). (B) NB1 cells were treated with increasing concentrations of heparin (as indicated) in the presence or absence of 0.634 nM, and an inhibitory effect on AUG-α by heparin was observed at high concentrations of heparin. (C) Dose-dependent activation of ALK by heparin in NIH/3T3 cells exogenously expressing ALK. NIH/3T3 cells were treated with increasing concentrations of heparin (as indicated) in the presence or absence of 0.63 nM of AUG-α. No effect by heparin was observed.
To this end, heparin failed to activate ALK in NIH/3T3 cells stably expressing ALK when treated with increasing concentrations of heparin in the absence or presence of AUG-α (Fig. S4C). Because heparins of sufficient length can bind to ALK with high affinity and induce ALK dimerization and activation in NB1 cells (15), this result suggests that heparin-induced activation is dependent on an unknown factor or factors that exist in NB1 cells but are absent in NIH/3T3 cells ectopically expressing ALK. It is clear that AUG-α can robustly activate ALK in every cell so far tested that expresses ALK containing either its intact ECD or its truncated form, ALK648–1,030, which resembles LTK-ECD lacking the heparin-binding NTR region. The exact role of heparin or sulfated glycosaminoglycans that bind to the ALK NTR region in modulating ALK activity, cellular localization, or other regulatory function remains to be determined.
AUG-α-Induced Cell Proliferation and Transformation Are Dependent upon ALK Activation.
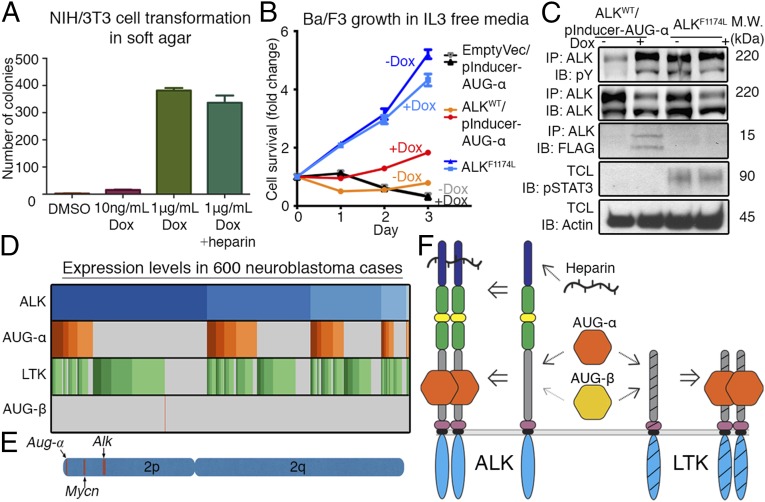
Previous studies have demonstrated that overexpression of an RTK ligand can induce cellular transformation of NIH/3T3 fibroblast cells expressing the cognate RTK (28). We therefore evaluated whether cellular transformation could be induced by AUG-α signals. We engineered NIH/3T3 cells stably expressing ALK with the ability to induce expression of AUG-α, using treatment with doxycycline (Dox). This allowed us to evaluate the transformation properties of AUG-α by treating NIH/3T3 cells with Dox or with a DMSO vehicle control. With induced expression of AUG-α, cells underwent colony formation in soft agar, and therefore, cellular transformation (Fig. 4A). Cells treated with DMSO did not undergo transformation. When cotreated with Dox and heparin, no effect of heparin was observed, further indicating that AUG-α acts as the primary ligand for ALK (Fig. 4A).
Fig. 4.
AUG-α induces transformation of NIH/3T3 cells and proliferation of BaF3 cells in an IL-3-independent manner. (A) NIH/3T3 cells stably transfected with ALKWT and pInducer-AUG-α (Dox inducible) were treated with as indicated. Cells were grown in soft agar for 2 wk and stained with crystal violet. Each experiment was performed in triplicate, colonies were counted and plotted, and SD was calculated and plotted for each experiment. (B) Ba/F3 cells were stably transfected with ALKWT and pInducer-AUG-α (Dox inducible) or were stably transfected with ALKF1174L. They were treated with 1 µg/mL Dox or with a DMSO. Every 24 h, live cell numbers were determined and expressed as a fold-change compared with day 0. Error bars are drawn for each cell type from three independent experiments. (C) Ba/F3 cells were lysed and subjected to immunoprecipitation (IP), using anti-ALK antibodies followed by immunoblotting (IB), as indicated (IB: FLAG was used for detection of AUG-α). Total cell lysate (TCL) was also subjected to IB using pSTAT3 and Actin antibodies. (D) Microarray expression pattern of ALK, AUG-α, LTK, and AUG-β in 600 neuroblastoma cases; each gene is expressed in 99.8%, 38.3%, 74.0%, and 0.2% of cases, respectively. Darker colors represent higher expression. (E) Schematic representation of human chromosome 2 depicting the locations of Aug-α, Mycn, and Alk. (F) Schematic model for the hierarchy and specificity of ligand–receptor interactions. AUG-α binds with high affinity to the glycine-rich regions in ALK and LTK, and AUG-β binds with high affinity to LTK and weakly to ALK. Heparin binds to the N-terminal region of ALK.
Overexpression of oncogenic ALK fusion proteins has previously been shown to induce IL-3-independent survival and growth of Ba/F3 cells (29). We therefore examined the effect of AUG-α on ALK-mediated cell survival and proliferation. We constructed stable Ba/F3 cell lines expressing ALKWT with Dox-inducible expression of AUG-α. AUG-α was fused with a FLAG-tag at the C terminus. As a positive control, Ba/F3 cells with a constitutively active mutant, ALKF1174L, were used. As a negative control, Ba/F3 cells with inducible expression of AUG-α, but lacking exogenous expression of ALK, were used. Cell proliferation was compared in the presence or absence of Dox induction in the absence of IL-3. Here we found that survival and growth of ALKWT-expressing Ba/F3 cells was dependent on Dox-induced expression of AUG-α after removal of IL-3 (Fig. 4B). Appropriately, very robust proliferation was observed in the positive control cells, and all other cells failed to grow or died (Fig. 4B).
Ba/F3 cells were also lysed and subjected to immunoblotting to assess signaling (Fig. 4C). Robust Tyr phosphorylation of ALK was observed in cells coexpressing ALKWT/AUG-α, and in cells expressing the oncogenic ALKF1174L mutant. It is of note that comparable tyrosine phosphorylation of ALK was obtained in cells expressing ALKWT/AUG-α and cells expressing the ALKF1174L mutant, whereas cell proliferation is far more vigorous in cells expressing ALKF1174L than cells expressing ALKWT/AUG-α. This seems to suggest that the ALKF1174L mutation not only activates the kinase but also alters the specificity of downstream signals. Accordingly, we found that STAT3 tyrosine phosphorylation in ALKF1174L cells far exceeded that of ALKWT/AUG-α cells (Fig. 4C). In addition, AUG-α was detected in a coimmunoprecipitation with anti-ALK antibodies in cells expressing ALKWT/AUG-α.
Coexpression of ALK and AUG-α May Play a Significant Role in ALK-Driven NBL.
We next examined the potential role of AUG-α in NBL, an ALK-driven cancer. Expression of 649 previously published NBL cases by microarray were analyzed (30). After background correction, normalization, and removal of outliers, 600 cases were considered. Of these cases, 38.3% expressed AUG-α, the primary ALK ligand, whereas only 0.2% expressed AUG-β. In addition, 99.8% of cases expressed ALK and 74.0% cases expressed LTK (Fig. 4D). Furthermore, AUG-α exists on chromosome 2p, along with ALK and Mycn, a region commonly amplified in neuroblastoma (Fig. 4E).
Taken together, AUG-α’s induction of transformation in NIH/3T3 cells, promotion of IL3-independent growth in Ba/F3 cells, expression in neuroblastoma, and location in a commonly amplified region on chromosome 2 in neuroblastoma reveal a potential role for AUG-α in tumorigenesis.
Discussion
During last 3 decades, tremendous progress has been made in understanding RTK biology, mechanisms of signal transduction, and involvement in pathological states (1). Given this great progress, it is appropriate that very few RTKs without cognate protein ligands remain. Discovery of AUG-α removes ALK from the list of remaining orphan RTKs more than 20 y after its initial discovery.
Both augmentor ligands are basic, small proteins, which are quite unstable and very sensitive to proteolytic cleavage. These features make them very difficult to produce in recombinant form. Therefore, we developed a method to produce AUG-α and AUG-β by coexpression of an Fc-tagged ligand with a Fc-tagged ligand-binding fragment of the receptor, allowing production of highly pure and active ligands. We found that AUG-α is a highly potent ligand for both ALK and for LTK, whereas its family member, AUG-β, is a ligand specific for LTK. AUG-α stimulates robust kinase activity of both ALK and LTK at picomolar concentrations on cells and binds the ECD of ALK and LTK with dissociation constants in the low nanomolar range. We also found that AUG-β, which was previously shown to be a ligand for LTK (25), could stimulate only weak autophosphorylation of ALK at high subnanomolar concentrations, but could strongly stimulate LTK at picomolar concentrations (a schematic model of hierarchy and specificity of ligand–receptor interactions is depicted in Fig. 4F).
Although ALK and LTK receptors are highly related to each other and constitute a subfamily of RTKs, the N-terminal portion (NMLM region) of the ALK ECD (NTR, two MAM, and LDL-A domains) is missing in LTK. Previously we have shown that heparin can bind to the NTR domain of ALK and can activate ALK expressed in NB1 cells (15). However, the role of the two MAM and LDL-A domains remains unclear. Here we tried to understand whether the NMLM region is involved in ligand binding and whether it can alter specificity of ALK and/or LTK. We found that the NMLM region is not necessary for ligand binding, and that both AUG-α and AUG-β could bind to glycine-rich domain and EGF-like motif with affinities very similar to binding to the full-length ECD of ALK. Furthermore, deletion of the NMLM region did not alter the kinase activity of either ALK or LTK in cells.
Given that this region does not affect augmentor specificity or binding affinity, it remains to be determined what role the NMLM region plays in the regulation of ALK. It is, however, interesting that ALK has been reported to be proteolytically processed both in cell culture and in neuronal tissue (16, 23, 31). The most prominent products of this processing is a membrane-bound 140-kDa fragment that remains active on the cell surface and a soluble 80-kDa fragment that contains the heparin-binding NTR region (15, 23). Before cleavage, the full-length receptor is responsive to heparin binding (15), and proteolytic removal of this region may provide a mechanism of regulation, rendering ALK unresponsive to heparins on the cell surface while maintaining fully responsive to AUG-α.
We also found that AUG-α signals could stimulate changes in global cellular function by inducing growth and cell transformation. Our data showed that coexpression of AUG-α together with ALK was able to transform IL-3-dependent murine hematopoietic Ba/F3 cells to cytokine-independent growth. Similarly, coexpression of AUG-α with ALK in NIH/3T3 cells leads to cell transformation. Given these tumorigenic features induced by AUG-α, we probed whether AUG-α could play a role in ALK-driven tumors. Fittingly, we found that AUG-α was expressed in 38.3% of analyzed cases, and 99.8% of cases expressed ALK (Fig. 4D). We also noticed that the Aug-α gene is located on the distal end of human chromosome 2p (Fig. 4E), a common hotspot for amplification in NBL, which is also the location of Alk and Mycn (7, 32). These results may suggest that ALK inhibition may be clinically relevant beyond cases that have activating ALK mutations.
Collectively, these data provide insight and tools for studying these cytokines and receptors in disease and in model organisms. Until now, progress in studying ALK's role in tumorigenesis, neurodevelopment, and neurobiology has been hindered by lack of knowledge about its ligand. Here we describe the first step toward understanding this ligand–receptor interaction. As with the discovery of nearly all novel cytokines, many details of augmentor biology will be determined over the course of the next few years, or even decades. Importantly, establishment of these ligands will allow for in vivo studies that will piece together the physiological and pathogenic roles of these enigmatic receptors and their ligands.
Materials and Methods
Cell Culture.
Cell lines stably expressing WT-ALK and WT-LTK were generated using a retroviral pBABE (for NIH/3T3 cells) or a pMSCVpuro (for Ba/F3 cells) vector. To generate ALK ΔNMLM, the nucleotide sequence coding for amino acids 648–1,620 of human ALK was amplified by PCR and subcloned into pBabe puro vector. ALK’s native signal peptide (amino acids 1–18 of human ALK) was added to the 5′ end. LTK-ALK chimera was designed by in-frame fusion of ALK's NMLM region (amino acids 1–659 of human ALK) with LTK receptor (amino acids 44–864 of human LTK). The corresponding PCR product was subcloned into pBabe puro vector. Before lysis, cells were stimulated with AUG-α or AUG-β and then lysed. Cell lysates were incubated overnight with anti-ALK or anti-LTK antibodies, separated on SDS/PAGE, and immunoblotted with anti-ALK/anti-LTK antibodies or anti-pY antibodies.
AUG-α and AUG-β Expression and Purification.
AUG-α-Fc was coexpressed with ALK648–1,030-Fc, and AUG-β-Fc was coexpressed with LTK-ECD-Fc, using HEK-293EBNA (293-E) cells. Fc-fused complexes of AUG ligands with a corresponding fragment of ALK or LTK receptors were affinity purified, using protein A Sepharose. C-terminal Fc-tag was removed by TEV cleavage, and free AUG ligands were separated from their complexes by cation exchange and size-exclusion chromatography.
SPR Analysis.
SPR was performed using a BiaCore T100 instrument by covalently coupling protein A to a CM5 chip. ALK-ECD-FC, ALK648–1,030-FC, and LTK-ECD-FC were captured on protein A, followed by injection of various concentrations of soluble AUG-α and AUG-β (twofold dilutions from 150 to 0.586 nM) with surface regeneration between cycles. All experiments were performed in duplicate and processed using the BiaCore T100 Evaluation software, with reference subtraction, and plotted using GraphPad prism.
Analysis of Expression of ALK, LTK, and AUG-α and AUG-β in Neuroblastoma Cases.
RNA expression microarray data for 649 neuroblastoma samples were downloaded from ArrayExpress (accession number E-GEOD-45547) (30). After sample quality control and outlier removal, 600 samples remained. For each sample, a gene is deemed expressed if its normalized signal intensity is at least 10% higher than the 95th percentile of negative control probes (33). On the basis of this threshold, ALK is expressed in 599 of 600 samples (99.8%), LTK in 444 samples (74.0%), AUG-α in 230 samples (38.3%), and AUG-β in one sample (0.2%).
NIH 3T3 Cell Transformation Assay.
AUG-α was cloned into a doxycycline-inducible pInducer plasmid. NIH/3T3 ALK cells were then used to generate a double stable cell line with inducible AUG-α. These cells were then plated in 0.3% agar with 5,000 cells per well in a six-well plate for 2 wk with DMSO, 10 ng/mL doxycycline, 1 µg/mL doxycycline, or 1 µg/mL doxycycline with 10 µg/mL heparin. Colonies were then stained with crystal violet and counted.
Ba/F3 Cell Proliferation Assay.
Ba/F3 double stable cell lines were produced using the same protocol as double stable NIH/3T3 cells described earlier. Cells were allowed to proliferate in 96-well plates with or without 1 µg/mL doxycycline for 3 d. Every 24 h, Celltiter-Glo reagent was used to determine the live cell number from one of the plates, and cell proliferation was expressed as fold change compared with day 0. The tyrosine phosphorylation of ALK was determined by Western blot after 24-h doxycycline induction of ligand expression and ALK immunoprecipitation. An anti-FLAG antibody was used to detect AUG-α.
For details see SI Materials and Methods.
Note Added in Proof.
During the review of this manuscript, a related article was published (34).
SI Materials and Methods
Stable NIH 3T3 Cell Lines, Immunoprecipitation, and Immunoblotting Experiments.
NIH/3T3 cell lines stably expressing ALK and LTK were generated using a retroviral pBABE vector containing a puromycin-resistance gene. Corresponding cells were cultured at 37 °C in 5% CO2 in the presence of DMEM containing 10% (vol/vol) FBS, 1% penicillin–streptomycin, and 1 mg/mL puromycin. Cells expressing ALK or LTK were grown in 150-mm cell culture dishes (Falcon) until 80–90% confluency was reached. Then cells were treated with AUG-α or AUG-β (from 0.1 to 100 ng/mL or conditioned medium) for 10 min at 37 °C, washed with ice-cold PBS, and lysed in the lysis buffer [50 mM Hepes, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10% (vol/vol) glycerol, 1% (vol/vol) Triton-X100] containing protease (1 mM PMSF and 1× Roche mixture) and phosphatase inhibitors (25 mM NaF, 10 μM ZnCl2, and 1 mM N3VO4). Lysates were incubated with appropriate antibodies and protein A Sepharose (Invitrogen) overnight at +4 °C. The immunocomplexes were washed with lysis buffer, boiled for 5 min with Laemmli sample buffer (BioRad), separated on SDS/PAGE, and then immunoblotted with anti-ALK, anti-LTK, or anti-pTyr antibodies.
Cloning and Construction of ALK ΔNMLM and LTK-ALK Chimera Cell Lines.
To generate ALK ΔNMLM, the nucleotide sequence coding for amino acids 648–1,620 of human ALK was amplified by PCR and subcloned into pBabe puro vector. ALK’s native signal peptide (amino acids 1–18 of human ALK) was added to the 5′ end.
LTK-ALK chimera was designed by in-frame fusion of ALK’s NMLM region (amino acids 1–659 of human ALK) with LTK receptor (amino acids 44–864 of human LTK). This chimera was constructed by PCR overlap extension, using ALK and LTK cDNAs as a template. Corresponding PCR product was subcloned into pBABE-puro vector. Corresponding constructs of ALK ΔNMLM and LTK-ALK chimera were used to generate NIH 3T3 and Ba/F3 stable cell lines, using a retroviral infection.
Production of AUG-α and AUG-β into Conditioned Medium.
Human AUG-α and AUG-β were subcloned into pCEP4 vector. The corresponding constructs of AUG-α and AUG-β were transfected into HEK293-EBNA (293-E) cells using lipofectamine 2000 according to manufacturer's protocol (Invitrogen). Stable cell lines were selected in DMEM, 10% (vol/vol) FBS, 1% penicillin–streptomycin, in the presence of 200 mg/mL hygromycin and 250 mg/mL G418. For protein production, medium was changed to DMEM and 5% (vol/vol) FBS, and supernatants were collected after 4 d of incubation.
AUG-α Expression and Purification.
For coexpression of AUG-α-FC with ALK648–1,030-FC, two vectors were designed and cotransfected. AUG-α and ALK648–1,030 were cloned into pCEP4 with TEV-cleavable, C-terminal FC-tags (derived from human IgG1). AUG-α-FC and ALK648–1,030-FC were then mixed at a 1:1 ratio and cotransfected into HEK-293EBNA (293-E) cells using the standard lipofectamine protocol (Invitrogen) and incubated for 6 d in Opti-mem (Gibco) supplemented with 1% penicillin–streptomycin at 37 °C at 5% CO2. Media were collected, clarified, and supplemented with 50 mM Hepes and incubated with protein A Sepharose (Invitrogen) with agitation for 24 h. Beads were then washed with 50 column volumes PBS, and complexes were eluted with a buffer consisting of 100 mM Glycine at pH 2.5 and 10% (vol/vol) glycerol. Eluates were neutralized using a 10× neutralization buffer (1 M Tris pH 8, 1.5 M NaCl) and subjected to TEV cleavage to remove the FC tags. Complexes were then loaded on a MonoS 5/50 GL (GE Healthcare) column equilibrated in a 25 mM Hepes at pH 7.4 buffer, and AUG-α-Fc was separated from its complex with ALK648–1,030-Fc, using linear gradient to 1 M NaCl in equilibration buffer. Fractions containing AUG-α (as determined by SDS/PAGE) were combined, concentrated, and subjected to gel filtration using a Superdex 75 10/300 GL (GE Healthcare) column equilibrated in 25 mM Hepes at pH 7.4, 300 mM NaCl, and 10% (vol/vol) glycerol.
AUG-β Expression and Purification.
For coexpression of AUG-β-Fc with LTK-ECD-Fc, a bicistronic vector was designed. The construct consisted of AUG-β with a TEV-cleavable Fc-tag followed by an internal ribosome entry site, followed by the full-length LTK-ECD with a TEV-cleavable Fc-tag and was generated by PCR overlap extension and subcloned into a pCEP4 vector. 293-E cells were transfected with this vector using lipofectamine 2000, and cells stably expressing AUG-β-Fc and LTK-ECD-Fc were selected in the presence of 200 mg/mL hygromycin and 250 mg/mL G418. Stable cells were seeded in HYPERFlasks (Corning), and the medium was changed to DMEM, 5% (vol/vol) FBS, and 1% penicillin–streptomycin upon reaching confluency. Conditioned medium was collected and clarified after 6 d of incubation and applied onto protein A Sepharose (Invitrogen), equilibrated with PBS buffer. The column was washed with 50 column volumes of PBS, and protein complex of AUG-β-Fc with LTK-ECD-Fc was eluted with 100 mM glycine⋅HCl buffer at pH 2.9. Pooled fractions were immediately loaded onto MonoS 5/50 GL (GE Healthcare) equilibrated in 10 mM Hepes buffer at pH 7.4. The column was washed with equilibration buffer, and AUG-β-Fc was separated from its complex with LTK-ECD-Fc, using linear gradient to 1 M NaCl in equilibration buffer. Fractions containing AUG-β-Fc (as determined by SDS/PAGE) were pooled, concentrated, and subjected to TEV cleavage to remove Fc tag. An AUG-β was separated from Fc tag and other contaminating proteins by anion exchange chromatography, using MonoS 5/50 GL (as described earlier), and size-exclusion chromatography, using Superdex 75 10/300 GL (GE Healthcare) equilibrated in 10 mM Hepes at pH 7.4, 150 mM NaCl, and 10% (vol/vol) glycerol.
SPR Analysis.
SPR was performed using a BiaCore T100 instrument (GE Healthcare) at 25 °C. All experiments were performed using 1× HBSP+ buffer at pH 7.4 (GE Healthcare, catalog no. BR100671) and a Series S CM5 chip (GE Healthcare, catalog no. BR100530). All four CM5 surfaces on the chip were prepared by covalently coupling protein A, using an amine coupling kit (GE Healthcare, catalog no. BR100050). ALK-ECD-FC, ALK648–1,030-FC, and LTK-ECD-FC were then captured on the protein A surface. Soluble AUG-α and Aug-β were injected over these three surfaces and a reference surface at various concentrations (twofold dilutions from 150 to 0.586 nM). After each injection of AUG-α or Aug-β, surfaces were regenerated using 10 mM glycine at pH 2.0, receptors were then recaptured, and then soluble AUG-α or Aug-β was reinjected. Data were processed using the BiaCore T100 Evaluation software, with reference subtraction, and plotted using GraphPad prism. In addition, direct immobilizing of untagged receptors or untagged augmentors by amine coupling to the dextran surface destroyed their activity. In vitro biotinylation and capture on a NeutrAvidin chip of untagged receptors or untagged augmentors destroyed their activity as well.
NIH 3T3 Cell Transformation Assay.
AUG-α was cloned into a doxycycline-inducible pInducer plasmid. Lentivirus was produced using this construct and transduced into ALK expressing NIH/3T3 cells. Stable expressing cells were selected using 1 mg/mL G418 for 2 wk. These double stable cells were then plated in 0.3% agar with 5,000 cells per well in a six-well plate for 2 wk with DMSO, 10 ng/mL doxycycline, 1 µg/mL doxycycline, or 1 µg/mL doxycycline with 10 µg/mL heparin. Colonies were then stained with crystal violet and imaged using an 8-megapixel iSight camera with 1.5 µpixels.
Analysis of Expression of ALK, LTK, and AUG-α and AUG-β in Neuroblastoma Cases.
RNA expression microarray data for 649 neuroblastoma samples were downloaded from ArrayExpress (accession number: E-GEOD-45547) (30). Raw expression files were converted into an R software-compatible format and subsequently processed, including background correction and quantile normalization, using limma package in R (33). After sample quality control and outlier removal, 600 samples remained. For each sample, a gene is deemed expressed if its normalized signal intensity is at least 10% higher than the 95th percentile of negative control probes (33). On the basis of this threshold, ALK is expressed in 599 of 600 samples (99.8%), LTK in 444 samples (74.0%), AUG-α in 230 samples (38.3%), and AUG-β in one sample (0.2%).
Acknowledgments
We thank the members of the J.S. laboratory for valuable discussions and critical comments, along with Ewa Folta-Stogniew for assistance in the Surface Plasmon Resonance analysis. This work was supported by The Yale Gilead Collaboration. The T100 Biacore instrumentation was supported by NIH Award S10RR026992-0110.
Footnotes
The authors declare no conflict of interest.
See Commentary on page 15783.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520099112/-/DCSupplemental.
References
- 1.Schlessinger J. Receptor tyrosine kinases: Legacy of the first two decades. Cold Spring Harb Perspect Biol. 2014;6(3):a008912. doi: 10.1101/cshperspect.a008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411(6835):355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 3.Lemmon MA, Schlessinger J. 2010. Cell signaling by receptor tyrosine kinases. Cell 141(7):1117–1113.
- 4.Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19(49):5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- 5.Morris SW, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263(5151):1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 6.Shiota M, et al. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene. 1994;9(6):1567–1574. [PubMed] [Google Scholar]
- 7.Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13(10):685–700. doi: 10.1038/nrc3580. [DOI] [PubMed] [Google Scholar]
- 8.Roskoski R., Jr Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacol Res. 2013;68(1):68–94. doi: 10.1016/j.phrs.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Carén H, Abel F, Kogner P, Martinsson T. High incidence of DNA mutations and gene amplifications of the ALK gene in advanced sporadic neuroblastoma tumours. Biochem J. 2008;416(2):153–159. doi: 10.1042/bj20081834. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, et al. 2008. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 455(7215):971–974.
- 11.George RE, et al. 2008. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 455(7215):975–978.
- 12.Janoueix-Lerosey I, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455(7215):967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 13.Zhu S, et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21(3):362–373. doi: 10.1016/j.ccr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJ. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murray PB, et al. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015;8(360):ra6. doi: 10.1126/scisignal.2005916. [DOI] [PubMed] [Google Scholar]
- 16.Iwahara T, et al. 1997. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 14(4):439–449.
- 17.Vernersson E, et al. Characterization of the expression of the ALK receptor tyrosine kinase in mice. Gene Expr Patterns. 2006;6(5):448–461. doi: 10.1016/j.modgep.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Bilsland JG, et al. Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology. 2008;33(3):685–700. doi: 10.1038/sj.npp.1301446. [DOI] [PubMed] [Google Scholar]
- 19.Weiss JB, et al. Anaplastic lymphoma kinase and leukocyte tyrosine kinase: Functions and genetic interactions in learning, memory and adult neurogenesis. Pharmacol Biochem Behav. 2012;100(3):566–574. doi: 10.1016/j.pbb.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Stoica GE, et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276(20):16772–16779. doi: 10.1074/jbc.M010660200. [DOI] [PubMed] [Google Scholar]
- 21.Stoica GE, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem. 2002;277(39):35990–35998. doi: 10.1074/jbc.M205749200. [DOI] [PubMed] [Google Scholar]
- 22.Mathivet T, Mazot P, Vigny M. 2007. In contrast to agonist monoclonal antibodies, both C-terminal truncated form and full length form of Pleiotrophin failed to activate vertebrate ALK (anaplastic lymphoma kinase)? Cell Signal 19(12):2434–2443. [DOI] [PubMed]
- 23.Moog-Lutz C, et al. Activation and inhibition of anaplastic lymphoma kinase receptor tyrosine kinase by monoclonal antibodies and absence of agonist activity of pleiotrophin. J Biol Chem. 2005;280(28):26039–26048. doi: 10.1074/jbc.M501972200. [DOI] [PubMed] [Google Scholar]
- 24.Motegi A, Fujimoto J, Kotani M, Sakuraba H, Yamamoto T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J Cell Sci. 2004;117(Pt 15):3319–3329. doi: 10.1242/jcs.01183. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, et al. Deorphanization of the human leukocyte tyrosine kinase (LTK) receptor by a signaling screen of the extracellular proteome. Proc Natl Acad Sci USA. 2014;111(44):15741–15745. doi: 10.1073/pnas.1412009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wain HM, et al. Guidelines for human gene nomenclature. Genomics. 2002;79(4):464–470. doi: 10.1006/geno.2002.6748. [DOI] [PubMed] [Google Scholar]
- 27.Clark HF, et al. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: A bioinformatics assessment. Genome Res. 2003;13(10):2265–2270. doi: 10.1101/gr.1293003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velu TJ, et al. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- 29.Soda M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 30.Kocak H, et al. Hox-C9 activates the intrinsic pathway of apoptosis and is associated with spontaneous regression in neuroblastoma. Cell Death Dis. 2013;4:e586. doi: 10.1038/cddis.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degoutin J, Brunet-de Carvalho N, Cifuentes-Diaz C, Vigny M. ALK (Anaplastic Lymphoma Kinase) expression in DRG neurons and its involvement in neuron-Schwann cells interaction. Eur J Neurosci. 2009;29(2):275–286. doi: 10.1111/j.1460-9568.2008.06593.x. [DOI] [PubMed] [Google Scholar]
- 32.Stallings RL, et al. Evolution of unbalanced gain of distal chromosome 2p in neuroblastoma. Cytogenet Genome Res. 2004;106(1):49–54. doi: 10.1159/000078560. [DOI] [PubMed] [Google Scholar]
- 33.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guan J, et al. 2015. FAM150A and FAM150B are activating ligands for Anaplastic Lymphoma Kinase. eLife, e09811.