Significance
We characterize the channel properties, distribution, and behavioral function of hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels in the mollusc Aplysia. Aplysia has one HCN gene, which has overall similarities to HCN homologs from other species. The acHCN channel also has functional properties that closely resemble vertebrate homologs and is expressed in neurons including siphon motor neurons. HCN channels contribute to an associative form of learning (classical conditioning) but not to two nonassociative forms of learning (intermediate-term sensitization and unpaired training) of the siphon withdrawal reflex. The HCN current is enhanced by NO and in turn enhances the NMDA-like current in the motor neurons, suggesting that HCN channels contribute to conditioning through this pathway.
Keywords: HCN channels, learning and memory, NMDA, nitric oxide, Aplysia
Abstract
Hyperpolarization-activated, cyclic nucleotide-gated cation (HCN) channels are critical regulators of neuronal excitability, but less is known about their possible roles in synaptic plasticity and memory circuits. Here, we characterized the HCN gene organization, channel properties, distribution, and involvement in associative and nonassociative forms of learning in Aplysia californica. Aplysia has only one HCN gene, which codes for a channel that has many similarities to the mammalian HCN channel. The cloned acHCN gene was expressed in Xenopus oocytes, which displayed a hyperpolarization-induced inward current that was enhanced by cGMP as well as cAMP. Similarly to its homologs in other animals, acHCN is permeable to K+ and Na+ ions, and is selectively blocked by Cs+ and ZD7288. We found that acHCN is predominantly expressed in inter- and motor neurons, including LFS siphon motor neurons, and therefore tested whether HCN channels are involved in simple forms of learning of the siphon-withdrawal reflex in a semiintact preparation. ZD7288 (100 μM) significantly reduced an associative form of learning (classical conditioning) but had no effect on two nonassociative forms of learning (intermediate-term sensitization and unpaired training) or baseline responses. The HCN current is enhanced by nitric oxide (NO), which may explain the postsynaptic role of NO during conditioning. HCN current in turn enhances the NMDA-like current in the motor neurons, suggesting that HCN channels contribute to conditioning through this pathway.
Hyperpolarization-activated, cyclic nucleotide-gated (HCN), cation nonselective ion channels generate hyperpolarization-activated inward currents (Ih) and thus tend to stabilize membrane potential (1–3). In addition, binding of cyclic nucleotides (cAMP and cGMP) to the C-terminal cyclic nucleotide binding domain (CNBD) enhances Ih and thus couples membrane excitability with intracellular signaling pathways (2, 4). HCN channels are widely important for numerous systemic functions such as hormonal regulation, heart contractility, epilepsy, pain, central pattern generation, sensory perception (4–15), and learning and memory (16–24).
However, in previous studies it has been difficult to relate the cellular effects of HCN channels directly to their behavioral effects, because of the immense complexity of the mammalian brain. We have therefore investigated the role of HCN channels in Aplysia, which has a numerically simpler nervous system (25). We first identified and characterized an HCN gene in Aplysia, and showed that it codes for a channel that has many similarities to the mammalian HCN channel. We found that the Aplysia HCN channel is predominantly expressed in motor neurons including LFS neurons in the siphon withdrawal reflex circuit (26, 27). We therefore investigated simple forms of learning of that reflex in a semiintact preparation (28–30) and found that HCN current is involved in classical conditioning and enhances the NMDA-like current in the motor neurons. These results provide a direct connection between HCN channels and behavioral learning and suggest a postsynaptic mechanism of that effect. HCN current in turn is enhanced by nitric oxide (NO), a transmitter of facilitatory interneurons, and thus may contribute to the postsynaptic role of NO during conditioning.
Results
The acHCN, 626 aa [GenBank: AY924397.3; NCBI RefSeq (National Center for Biotechnology Information Reference Sequence): NM_001204707.1 (LOC100533424)], contains all canonical sequence features attributed to the HCN family (Fig. 1 and SI Appendix, Fig. S2), including six conserved transmembrane segments with the voltage sensor and pore region as well as a C linker to the intracellular cyclic nucleotide binding domain (CNBD), suggesting overall similarities in their properties with HCN homologs across other species studied (4, 31). There are also several predicted phosphorylation sites consistent with findings in mammals. However, we found a few amino acid differences in the CNBD region between acHCN and their homologs in vertebrates (SI Appendix, Table S1). These results suggest some difference in cyclic nucleotide dependence, which was confirmed by expression analysis in Xenopus oocytes (see below).
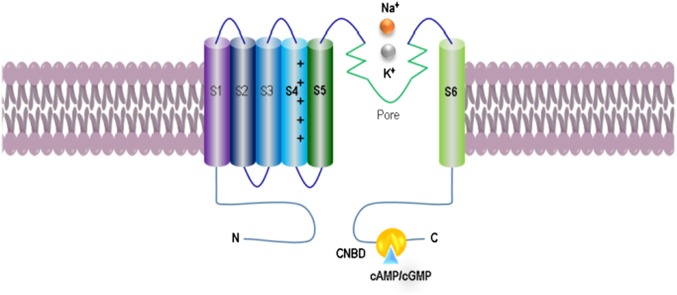
Fig. 1.
Organization and membrane topology of Aplysia HCN channels. The acHCN contains canonical sequence features attributed to the HCN family including six conserved transmembrane segments with the voltage sensor and pore region as well as a C linker to the intracellular cyclic nucleotide binding domain (CNBD).
HCN Channels Expressed in Xenopus Oocytes Share Overall Similarity to Their Vertebrate Homologs.
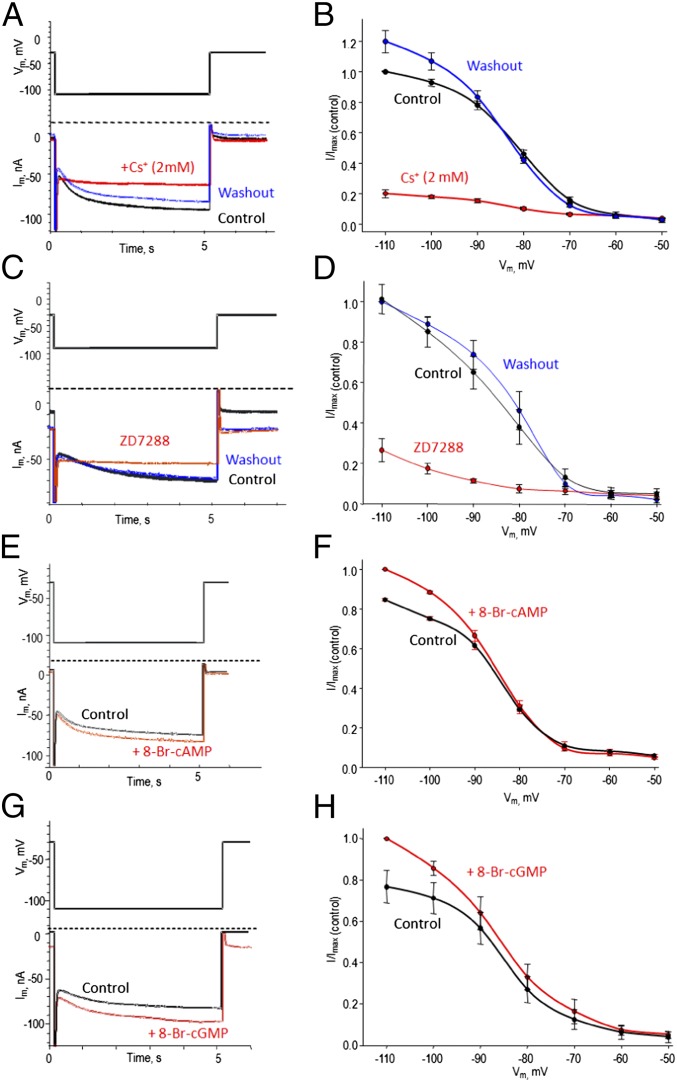
To characterize the biophysical and pharmacological properties of Aplysia HCN channels, we expressed acHCN in Xenopus laevis oocytes (Fig. 2 and SI Appendix, Figs. S3–S9). The heterologous expression of acHCN resulted in the appearance of a hyperpolarization-activated inward current (Ih) that was absent in vehicle-injected control oocytes. acHCN-mediated Ih had slightly slower kinetics compared with its vertebrate homologs but a similar voltage dependence (half-maximum activation, V1/2 = −83.8 ± 0.8 mV with a slope factor 7.2 ± 0.7, n = 4; the reversal potential was −25.8 ± 1.1 mV, n = 17) and relative permeability to Na+ and K+ ions determined as 0.4 (see details in SI Appendix). The time course of activation (τ) was strongly dependent on the voltage step with τ value ranging from 6,229 ± 584 ms at −80 mV to 2,330 ± 156 ms at −110 mV (n = 18). The acHCN-mediated currents were blocked by Cs+ ions (Fig. 2 A and B) and, importantly, by ZD7288 [4-(N-ethyl-N-phenylamino)-1,2-dimethyl-6-(methylamono) pyridinum chloride] (Fig. 2 C and D and SI Appendix, Fig. S9), a selective inhibitor of these channels in insects and mammals (32). Inhibition of the acHCN channel by ZD7288 was concentration dependent with a Ki of 4.7 ± 1.5 µM (n = 4). Applying 100 µM ZD7288 decreased Ih by 83.9 ± 2.4% (n = 4), but did not change the voltage of half-maximal activation (V1/2 = −83.8 ± 0.8 mV in control vs. −83.9 ± 3.7 mV in the presence of ZD7288). Furthermore, acHCN currents were increased in the presence of soluble analogs of both cAMP and cGMP (Fig. 2 E–H).
Fig. 2.
Biophysical and pharmacological properties of the Aplysia HCN channel expressed in Xenopus laevis oocytes. (A and B) Inhibition of the acHCN channel by low concentrations of Cs+. (A) Ih current traces generated upon hyperpolarization from −30 mV to −110 mV in media with 2 mM Cs+ (red) or following washout (blue) were superimposed on the current trace generated under the same conditions in media without cesium (black). (B) I/V relationships for acHCN-mediated Ih in control conditions (black), in the presence of 2 mM Cs+ (red) or following washout (blue). The currents were normalized to the current value at −110 mV under the control conditions. Applying 2 mM Cs+ decreased Ih amplitude by 79.9 ± 2.7%, n = 4. (C and D) Applying 100 μM ZD7288 decreased the amplitude of Ih by 83.9 ± 2.4%; n = 4. (E and F) Applying 1 mM 8-Br-cAMP increased Ih amplitude by 18.0 ± 0.7%; n = 4. (G and H) Applying 1 mM 8-Br-cGMP increased Ih amplitude by 35.7 ± 20.3%; n = 4.
However, the Aplysia channels appear to have two differences compared with mammals (4, 6, 8, 31). First, in Aplysia the gating of the acHCN channels by cyclic nucleotides was associated primarily with an increase in Ih amplitude rather than in a voltage-dependence shift (e.g., a positive shift of 2.2 mV in the presence of 1 mM 8-Br-cAMP and 1.5 mV in the presence of 8-Br-cGMP; n = 4 in each test). Mammalian HCN1 channels undergo a similar cAMP- or cGMP-mediated shift of +2–5 mV, but HCN2 and HCN4 channels respond with a greater shift of +15–20 mV (32–35).
Second, acHCN-mediated Ih was more sensitive to cGMP than cAMP (Fig. 2 E–H and SI Appendix, Fig. S8). Both cAMP and cGMP shift the activation curve of the mammalian HCN channels, but they can require a higher cGMP concentration (∼10-fold or more) to achieve the effects seen with cAMP (31). However, some mammalian HCN channel types also display high sensitivity to cGMP (23, 36). Both the cAMP and cGMP/NO signal transduction pathways contribute to sensitization and classical conditioning of the siphon-withdrawal reflex (25, 29, 37, 38), suggesting that they might have HCN as one of their targets in relevant neurons during those forms of learning. Based on our results, one might expect the cGMP/NO pathway to play a relatively broader role in Aplysia than in mammals, consistent with a broad range of functions of nitrergic (NO releasing) neurons in molluscs (e.g., refs. 39–41). However, both pathways contribute to learning in both types of animals (25, 42).
Widespread Expression of HCN Channels in the Aplysia CNS.
Both in situ hybridization and transcriptome profiling were used to characterize and quantify HCN expression in the CNS and peripheral tissues. The CNS has the highest level of HCN expression (Fig. 3B). Although the majority of Aplysia neurons express HCN mRNAs, the most prominent expression was found in several groups of buccal and pedal motor neurons as well as in a population of interneurons associated with both feeding (e.g., the metacerebral cells) and defensive circuits, with lower expression in mechanosensory neurons. The greatest overall expression level was found in the pedal ganglia including serotonergic neurons controlling locomotion. Illustrative examples are shown in Fig. 3A, and SI Appendix, Fig. S10 shows a schematic HCN expression map of the entire CNS. All HCN-expressing neurons show spontaneous electrical activity (SI Appendix, Figs. S11–S13) (26, 27). ZD7288 reduced that activity in neurons expressing HCN but not in other neurons, supporting the specificity of the inhibitor in Aplysia.
Fig. 3.
Expression of transcripts encoding HCN channels in Aplysia. (A) In situ hybridization labeling of HCN mRNAs in the central ganglia. The red circle indicates the general location of neurons involved in the siphon-withdrawal reflex (LE, LFS, L29). (B and C) Quantification of HCN expression using RNA-seq data for peripheral tissues and individual identified neurons. See schematic diagram of the distribution of HCN channels in SI Appendix, Fig. S10.
In situ hybridization labeling was correlated with quantitative RNA-seq measurements. When we performed direct single-cell transcriptome profiling of several identified neurons, we also confirmed highly differential expression of HCN channels across the CNS with one of the highest levels in LFS motor neurons of the siphon-withdrawal circuit (Fig. 3C).
HCN Channels Contribute to Behavioral Conditioning but Not Intermediate-Term Sensitization, Unpaired Training, or Baseline Responses.
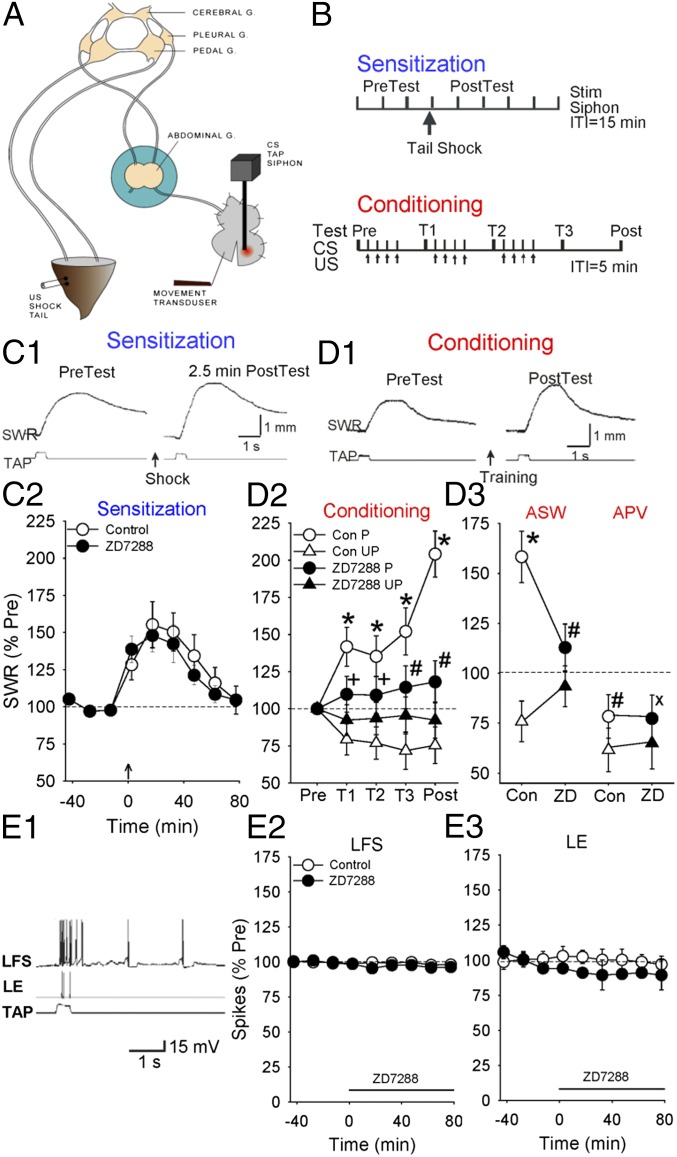
The finding that HCN channels are highly expressed in LFS siphon motor neurons suggested that they might play a role in simple forms of learning of the siphon withdrawal reflex. We therefore investigated intermediate-term sensitization and classical conditioning in a semiintact preparation (Fig. 4 A and B) in which the reflex is mediated in part by monosynaptic excitatory postsynaptic potentials (EPSPs) from LE siphon sensory neurons to LFS siphon motor neurons in the abdominal ganglion (30). Sensitization is due in part to presynaptic facilitation of those EPSPs by PKA (43, 44) and conditioning to activity-dependent facilitation of the EPSPs (28), which involves two mechanisms that interact (29): activity-dependent enhancement of the facilitation due to Ca2+ priming of adenylyl cyclase and greater production of cAMP and activation of PKA in the sensory neurons (43–51), and Hebbian potentiation due to Ca2+ influx through NMDA-like receptor channels in the motor neurons (52–55).
Fig. 4.
ZD7288 reduces behavioral conditioning but not intermediate-term sensitization or baseline responding in the siphon-withdrawal preparation. (A) The preparation. (B) Behavioral protocols. See the text for details. (C1) Examples of siphon withdrawal before (pretest) and 2.5 min after (posttest) tail shock (sensitization). (C2) Average results from experiments like the ones shown in C1 with the abdominal ganglion bathed in normal saline (control, n = 6) or ZD7288 (n = 5). The amplitude of siphon withdrawal has been normalized to the average value on the three pretests in each experiment. The overall average pretest value was 2.3 mm, not significantly different in experiments with ZD7288. The average response to the tail shock was 5.4 mm. (D1) Examples of siphon withdrawal before (pretest) and 45 min after (posttest) conditioning. (D2 and D3) Average siphon withdrawal in groups that received paired or unpaired training with the abdominal ganglion bathed in either normal seawater (control, n = 5 paired and 8 unpaired), ZD7288 (n = 6 paired and 8 unpaired), APV (n = 7 paired and 7 unpaired), or ZD7288 and APV (n = 6 paired and 5 unpaired). Responses have been normalized to the value on the pretest in each experiment. The overall average pretest value was 3.1 mm and the average response to the first tail shock US was 6.8 mm, not significantly different in experiments with ZD7288 or APV. Error bars indicate SEMs, *P < 0.01 for the difference between the paired and unpaired groups, #P < 0.05 and +P < 0.05 one tail for the reduction of that difference by each drug, and xP < 0.05 one tail for the APV × ZD7288 × pairing interaction. (D2) Average withdrawal on each test with the ganglion bathed in seawater or ZD7288. (D3) Average withdrawal overall illustrating occlusion of APV and ZD7288. (E) ZD7288 does not affect baseline evoked firing of LFS siphon motor neurons or LE siphon sensory neurons. (E1) Example of spikes in an LFS motor neuron and an LE sensory neuron evoked by a tap to the siphon. (E2 and E3) Average number of evoked spikes in LFS neurons and LE neurons with the abdominal ganglion perfused with seawater (control, n = 6) or ZD7288 (n = 5) after the third test. The number of spikes has been normalized to the average value on the three pretests in each experiment. The overall average pretest value was 15.7 for LFS neurons and 4.0 for LE neurons, not significantly different in experiments with ZD7288.
A train of four shocks to the tail produced behavioral sensitization lasting about 1 h, which was not significantly affected by bathing the ganglion in ZD7288 (100 μM) [F[1,9] = 0.13, not significant (NS)] (Fig. 4C). To examine the effect of ZD7288 on classical conditioning, we compared changes in the withdrawal reflex in groups that received either paired or unpaired training with a siphon tap conditioned stimulus (CS) and a tail shock unconditioned stimulus (US), while the abdominal ganglion was bathed in either normal seawater (control) or ZD7288 (Fig. 4D). The response to the CS was tested before, during, and 45 min after training, which is in an intermediate-term time range roughly similar to the sensitization. In the control group, paired training produced an increase in the response to the CS compared with either the pretest or unpaired training (F[1,44] = 25.10, P < 0.001 for the effect of pairing overall and P < 0.01 at each test), demonstrating classical conditioning. ZD7288 (100 μM) did not have significant effects on the amplitude of the initial siphon withdrawal in response to either the siphon tap CS or tail shock US. ZD7288 also did not have a significant effect on the change in response to the CS following unpaired training (F[1,44] = 1.48, NS overall and at each test), which is a nonassociative protocol that produces results intermediate between training with the CS alone (habituation) or US alone (sensitization), and can be thought of as a combination of the two (56, 57). However, ZD7288 significantly reduced the difference between paired and unpaired training, or conditioning (average = 23% of control, F[1,44] = 7.26, P < 0.01 for the interaction of drug and pairing overall and P < 0.05 one-tail at each test). This effect began at the first test (T1), 30 min after the beginning of training, when ZD7288 had no significant effect on sensitization (Fig. 4C). These results suggest that HCN channels do not contribute to two nonassociative forms of learning (intermediate-term sensitization and unpaired training) of the withdrawal reflex but do contribute to an associative form of learning (conditioning), although differences in the temporal patterns of the different types of learning may also be important.
HCN channels can affect membrane potential and resistance, and thus might act by affecting the excitability and firing of neurons in the reflex pathway. However, ZD7288 did not have a significant effect on the baseline-evoked firing of LFS siphon motor neurons or LE siphon sensory neurons (Fig. 4E) or the peak amplitude of the baseline monosynaptic LE-LFS EPSP (Fig. 5B2). These results suggest that HCN channels do not play an important role in behavioral or cellular responses or transmission in the reflex pathway under baseline conditions, but rather play a selective role in changes in those properties during conditioning.
Fig. 5.
ZD7288 reduces HCN current in LFS motor neurons and the NMDA-like component of the LE-LFS EPSP. (A) Evidence for HCN current in LFS siphon motor neurons, reduction by ZD7288, and enhancement by DEA-NO. (A1) Example of hyperpolarization of an LFS neuron by a 6-s constant current injection before (control) and after perfusing the ganglion with ZD7288. ZD7288 reduced the “sag” or depolarization from the beginning to the end of the current injection, which is characteristic of HCN current. (A2) Average results from experiments like the one shown in A1 (n = 4), each with four different amplitudes of current injection. (A3) The nitric oxide donor DEA-NO enhanced the sag, normalized to the value before DEA-NO perfusion (control) in each experiment (n = 6). The average control value was 18.6 mV. (B) ZD7288 did not affect the baseline monosynaptic LE-LFS EPSP, but reduced the NMDA-like component of the EPSP. (B1) Example of the monosynaptic LE-LFS EPSP in artificial sea water (ASW) (control) and CNQX. (B2) Average peak and late (50–75 ms after peak) amplitude of the LE-LFS EPSP in ASW with the abdominal ganglion perfused with ZD7288 after the second test, normalized to the average value on the pretests in each experiment (n = 3). The average pretest values were 12.5 (peak) and 5.4 (late) mV. (B3) Average amplitude of the late component of the EPSP in ASW (control), CNQX, and CNQX + ZD7288, normalized to the average control value in each experiment (n = 3). There was a significant overall effect of drug (F[2,4] = 54.21, P < 0.01). The average control value was 2.7 mV. (B4) Similar experiments with ASW (control), CNQX, CNQX + APV, and CNQX + APV + ZD7288 (n = 3). There was a significant overall effect of drug (F[3,6] = 62.18, P < 0.01). The average control value was 3.6 mV. (C) Hypothetical model of postsynaptic mechanisms by which HCN channels contribute to classical conditioning of the siphon-withdrawal reflex. Nitric oxide (NO) released by L29 interneurons acts directly in the LFS motor neurons to stimulate production of cGMP and activation of HCN channels, which in turn enhances activation of NMDA-like channels and Hebbian potentiation at LE-LFS synapses.
HCN Current Is Expressed in LFS Motor Neurons, Where It Is Enhanced by Nitric Oxide.
Single cell RNAseq revealed HCN transcript in LFS motor neurons, but very little in LE sensory neurons or L29 interneurons (Fig. 3C). We investigated whether LFS neurons also express HCN current by intracellular injection of different magnitudes of hyperpolarizing current, which activates HCN channels. LFS neurons but not most other neurons in the vicinity exhibited the slow depolarization or “sag” that is characteristic of HCN current (Fig. 5A). The sag was greater with larger hyperpolarizing steps (F[3,9] = 18.47, P < 0.001) and was reduced on average 82% by ZD7288 (F[1,3] = 96.85, P < 0.01), supporting the idea that LFS neurons express HCN current. In addition, the sag was enhanced by a donor of nitric oxide [2-(N,N-diethylamino)-diazenolate-2-oxide (DEA-NO), 10 µM] (t[5] = 3.80, P < 0.05), which can act through cGMP to enhance HCN current in mammalian cells (23, 36, 58, 59).
As in Aplysia metacerebral cells and pedal motor neurons (SI Appendix, Figs. S12 and S13) and some but not all mammalian neurons that also express HCN channels (23, 60–62), ZD7288 reduced the spontaneous firing of LFS neurons (64.9 ± 1.2%, t[4] = 27.79, P < 0.001). However, it did not have a significant effect on either the resting membrane potential (100.7 ± 2.2%, t[11] = 0.32) or input resistance (98.7 ± 1.7%, t[11] = 0.71) of the LFS neurons. One possible explanation of the reduced firing is that activation of HCN current during spike afterhyperpolarizations may normally contribute to faster repolarization and generation of subsequent spikes.
HCN Current Enhances NMDA Receptor Current in LFS Neurons Involved in Conditioning.
How might HCN current in LFS neurons contribute selectively to associative learning (conditioning) but not to two nonassociative forms of learning or baseline responses, transmission, and membrane properties? One possibility was suggested by experiments in mammalian hippocampus in which inhibition of HCN current by ZD7288 reduced the NMDA component of the EPSP, which is critical for Hebbian or associative long-term potentiation (LTP) (23). Sensory-motor neuron EPSPs in Aplysia have both AMPA-like and NMDA-like components (29, 63–65), and conditioning involves both pre- and postsynaptic mechanisms including Hebbian potentiation induced by activation of an NMDA-like current in the LFS neurons (29, 66–68).
To examine whether inhibition of HCN current acts by reducing activation of NMDA-like receptors, we first tested whether the NMDA receptor antagonist DL-2-amino-5-phosphonovaleric acid (APV) occludes the effect of the HCN antagonist ZD7288 on conditioning (Fig. 4D3). APV (10 µM) did not have significant effects on the amplitude of the initial siphon withdrawal in response to either the siphon tap CS or tail shock US, or the change in response to the CS following unpaired training (F[1,44] = 0.91, NS overall and at each test). However, APV produced a decrease in the difference between paired and unpaired training (or conditioning) roughly similar to the decrease by ZD7288 (average = 20% of control, F[1,44] = 8.46, P < 0.01 overall and P < 0.05 at each test). Furthermore, in the presence of APV, ZD7288 produced little additional decrease (average = 73% of APV, F[1,44] = 0.03, NS overall and at each test), which was significantly less than the decrease by ZD7288 in the absence of APV (F[1,44] = 3.23, P < 0.05 one tail for the APV × ZD7288 × pairing interaction overall).
We next examined whether inhibition of HCN current also reduces the NMDA-like current in LFS neurons. We fired a single action potential in an LE neuron and measured the late (50–75 ms after peak) component of the monosynaptic EPSP in an LFS neuron, which previous experiments suggested is predominantly NMDA (29) (Fig. 5B1). ZD7288 produced a small but significant reduction in the late component of the EPSP (average = 86% of control, F[1,2] = 21.33, P < 0.05) (Fig. 5B2). To further enrich for the NMDA component we perfused the ganglion with the AMPA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (50 µM), which partially reduced the peak of the EPSP (average = 59% of control, F[1,2] = 34.12, P < 0.05) with little effect on the late component (average = 99%, NS) (Fig. 5B3). When we then added ZD7288 in the presence of CNQX it reduced the late component much more (average = 33% of control (F[1,2] = 78.30, P < 0.05). In addition, although CNQX reduced the peak more than the late component (F[1,2] = 127.18, P < 0.01 for the peak/late × Con/CNQX interaction), ZD7288 reduced the late component more than the peak (F[1,2] = 53.29, P < 0.05), similar to APV (29). The reduction was not due to homosynaptic depression because there was no depression with the 15- to 20-min interval between trials within each drug condition (F[2,4] = 0.63, NS). The NMDA receptor antagonist APV (10 µM) produced a similar reduction in the late component of the EPSP in the presence of CNQX (average = 25% of control, F[1,2] = 107.21, P < 0.01) (Fig. 5B4). When we then added ZD7288 there was little additional reduction (average = 80% of APV, F[1,2] = 2.08, NS), which was significantly less than the reduction by ZD7288 in the absence of APV (F[1,4] = 8.69, P < 0.05). Moreover, the magnitudes of the reductions in the late component by ZD7288 and APV were similar to the analogous reductions in conditioning (Fig. 4D3). These results suggest that inhibition of HCN current substantially reduces the NMDA-like current in LFS neurons, and are consistent with the idea that NMDA-like receptors act at least in part downstream of the HCN channels during conditioning.
Discussion
Although HCN ion channels are known to play important roles in the excitability, integrative properties, and plasticity of neurons, less is known about their possible roles in behavioral learning, in part because of the immense complexity of the mammalian brain. Our results provide evidence for a direct link between HCN current and learning in the semiintact siphon withdrawal preparation of Aplysia. In this case the role of HCN current appears to be similar to its role during LTP at Schaffer collateral-CA1 synapses in hippocampus (23), presenting an interesting example of convergent evolution in memory circuits: HCN enhances NMDA receptor current required for Hebbian potentiation, which contributes to classical conditioning of the withdrawal reflex in Aplysia (29, 52–55). In neither preparation is it known how HCN current enhances NMDA current, but it might act either by depolarizing the postsynaptic neuron or by a receptor–receptor interaction. The conditioning also involves NO, which appears to be released from L29 facilitatory interneurons and acts directly in both the sensory neurons and the motor neurons to strengthen the synaptic connection between them (37). Because NO is necessary but not sufficient for PKA-dependent presynaptic effects, it is hypothesized to gate or enhance activation of PKA in the sensory neurons, but how it acts in the motor neurons has not been known.
Our results suggest a plausible mechanism for the postsynaptic role of NO during conditioning (Fig. 5C). As in hippocampus (23), NO may stimulate soluble guanylyl cyclase leading to production of cGMP, activation of HCN current, and enhancement of NMDA current. Consistent with that idea, a NO donor enhances the HCN current in LFS motor neurons, and the pattern of results with ZD7288 during conditioning (a partial blockade that becomes larger with additional training) is similar to the pattern with injection of the NO scavenger oxymyoglobin into the motor neuron, but not the sensory neuron (37). The pattern is also similar with the NMDA receptor antagonist APV (29). Furthermore, ZD7288 and APV (10 µM) are about equally effective in blocking both conditioning and the NMDA-like component of the EPSP, and APV occludes the effect of ZD7288 for both. These results support the idea that NO may act through the HCN current to gate or enhance the NMDA receptor pathway in the motor neuron. If so, that effect could be analogous to the hypothesized role of NO in gating the PKA pathway in the sensory neuron (37). NO is also thought to act both presynaptically and postsynaptically in hippocampus, where it may engage additional molecular mechanisms as well (58, 69, 70).
Materials and Methods
The methods for cloning and sequencing (71), in situ hybridization (40), and behavior and electrophysiology (28–30, 37, 43) were generally the same as described previously. See SI Appendix for details. This research did not need institutional approval because Aplysia are not covered by the animal use regulations of Columbia University, New York State Psychiatric Institute, or the NIH.
Supplementary Material
Acknowledgments
We thank Anagha Nagaraj and David Castillejos for their help with the behavioral experiments and Steve Siegelbaum for his comments on the manuscript. This research was supported by NIH Grant GM097502 and, in part, by National Science Foundation (NSF) Grants NSF-0744649 and NSF CNS-0821622, NIH Grant MH097062, NASA Grant NNX13AJ31G, and the McKnight Brain Research Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.H.B. is a guest editor invited by the Editorial Board.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database [accession no. AY924397.3; NCBI RefSeq: NM_001204707.1 (LOC100533424)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1501731113/-/DCSupplemental.
References
- 1.Accili EA, Proenza C, Baruscotti M, DiFrancesco D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002;17:32–37. doi: 10.1152/physiologyonline.2002.17.1.32. [DOI] [PubMed] [Google Scholar]
- 2.Surges R, et al. Regulated expression of HCN channels and cAMP levels shape the properties of the h current in developing rat hippocampus. Eur J Neurosci. 2006;24(1):94–104. doi: 10.1111/j.1460-9568.2006.04880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frère SG, Kuisle M, Lüthi A. Regulation of recombinant and native hyperpolarization-activated cation channels. Mol Neurobiol. 2004;30(3):279–305. doi: 10.1385/MN:30:3:279. [DOI] [PubMed] [Google Scholar]
- 4.Biel M, Wahl-Schott C, Michalakis S, Zong X. Hyperpolarization-activated cation channels: From genes to function. Physiol Rev. 2009;89(3):847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- 5.Wahl-Schott C, Fenske S, Biel M. HCN channels: New roles in sinoatrial node function. Curr Opin Pharmacol. 2014;15:83–90. doi: 10.1016/j.coph.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 6.He C, Chen F, Li B, Hu Z. Neurophysiology of HCN channels: From cellular functions to multiple regulations. Prog Neurobiol. 2014;112:1–23. doi: 10.1016/j.pneurobio.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Benarroch EE. HCN channels: Function and clinical implications. Neurology. 2013;80(3):304–310. doi: 10.1212/WNL.0b013e31827dec42. [DOI] [PubMed] [Google Scholar]
- 8.Postea O, Biel M. Exploring HCN channels as novel drug targets. Nat Rev Drug Discov. 2011;10(12):903–914. doi: 10.1038/nrd3576. [DOI] [PubMed] [Google Scholar]
- 9.Noam Y, Bernard C, Baram TZ. Towards an integrated view of HCN channel role in epilepsy. Curr Opin Neurobiol. 2011;21(6):873–879. doi: 10.1016/j.conb.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baruscotti M, Bottelli G, Milanesi R, DiFrancesco JC, DiFrancesco D. HCN-related channelopathies. Pflugers Arch. 2010;460(2):405–415. doi: 10.1007/s00424-010-0810-8. [DOI] [PubMed] [Google Scholar]
- 11.Wickenden AD, Maher MP, Chaplan SR. HCN pacemaker channels and pain: A drug discovery perspective. Curr Pharm Des. 2009;15(18):2149–2168. doi: 10.2174/138161209788489122. [DOI] [PubMed] [Google Scholar]
- 12.Nusser Z. Variability in the subcellular distribution of ion channels increases neuronal diversity. Trends Neurosci. 2009;32(5):267–274. doi: 10.1016/j.tins.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Dunlop J, Vasilyev D, Lu P, Cummons T, Bowlby MR. Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and pain. Curr Pharm Des. 2009;15(15):1767–1772. doi: 10.2174/138161209788186281. [DOI] [PubMed] [Google Scholar]
- 14.Bender RA, Baram TZ. Hyperpolarization activated cyclic-nucleotide gated (HCN) channels in developing neuronal networks. Prog Neurobiol. 2008;86(3):129–140. doi: 10.1016/j.pneurobio.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson HA, Marshall CR, Accili EA. Evolution and structural diversification of hyperpolarization-activated cyclic nucleotide-gated channel genes. Physiol Genomics. 2007;29(3):231–245. doi: 10.1152/physiolgenomics.00142.2006. [DOI] [PubMed] [Google Scholar]
- 16.Nolan MF, et al. The hyperpolarization-activated HCN1 channel is important for motor learning and neuronal integration by cerebellar Purkinje cells. Cell. 2003;115(5):551–564. doi: 10.1016/s0092-8674(03)00884-5. [DOI] [PubMed] [Google Scholar]
- 17.Koga K, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron. 2015;85(2):377–389. doi: 10.1016/j.neuron.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129(2):397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Thuault SJ, et al. Prefrontal cortex HCN1 channels enable intrinsic persistent neural firing and executive memory function. J Neurosci. 2013;33(34):13583–13599. doi: 10.1523/JNEUROSCI.2427-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nolan MF, et al. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119(5):719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 21.Tsay D, Dudman JT, Siegelbaum SA. HCN1 channels constrain synaptically evoked Ca2+ spikes in distal dendrites of CA1 pyramidal neurons. Neuron. 2007;56(6):1076–1089. doi: 10.1016/j.neuron.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matt L, et al. HCN2 channels in local inhibitory interneurons constrain LTP in the hippocampal direct perforant path. Cell Mol Life Sci. 2011;68(1):125–137. doi: 10.1007/s00018-010-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neitz A, et al. Postsynaptic NO/cGMP increases NMDA receptor currents via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus. Cereb Cortex. 2014;24(7):1923–1936. doi: 10.1093/cercor/bht048. [DOI] [PubMed] [Google Scholar]
- 24.Giocomo LM, et al. Grid cells use HCN1 channels for spatial scaling. Cell. 2011;147(5):1159–1170. doi: 10.1016/j.cell.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 25.Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Science. 2001;294(5544):1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 26.Frost WN, Kandel ER. Structure of the network mediating siphon-elicited siphon withdrawal in Aplysia. J Neurophysiol. 1995;73(6):2413–2427. doi: 10.1152/jn.1995.73.6.2413. [DOI] [PubMed] [Google Scholar]
- 27.Kandel ER. 1976. Cellular Basis of Behavior (W. H. Freeman, San Francisco)
- 28.Antonov I, Antonova I, Kandel ER, Hawkins RD. The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia. J Neurosci. 2001;21(16):6413–6422. doi: 10.1523/JNEUROSCI.21-16-06413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Antonov I, Antonova I, Kandel ER, Hawkins RD. Activity-dependent presynaptic facilitation and hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron. 2003;37(1):135–147. doi: 10.1016/s0896-6273(02)01129-7. [DOI] [PubMed] [Google Scholar]
- 30.Antonov I, Kandel ER, Hawkins RD. The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex. J Neurosci. 1999;19(23):10438–10450. doi: 10.1523/JNEUROSCI.19-23-10438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Craven KB, Zagotta WN. CNG and HCN channels: Two peas, one pod. Annu Rev Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- 32.BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol. 1993;110(1):343–349. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: From molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 34.Altomare C, et al. Heteromeric HCN1-HCN4 channels: A comparison with native pacemaker channels from the rabbit sinoatrial node. J Physiol. 2003;549(Pt 2):347–359. doi: 10.1113/jphysiol.2002.027698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393(6685):587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 36.Wilson GW, Garthwaite J. Hyperpolarization-activated ion channels as targets for nitric oxide signalling in deep cerebellar nuclei. Eur J Neurosci. 2010;31(11):1935–1945. doi: 10.1111/j.1460-9568.2010.07226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Antonov I, Ha T, Antonova I, Moroz LL, Hawkins RD. Role of nitric oxide in classical conditioning of siphon withdrawal in Aplysia. J Neurosci. 2007;27(41):10993–11002. doi: 10.1523/JNEUROSCI.2357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lewin MR, Walters ET. Cyclic GMP pathway is critical for inducing long-term sensitization of nociceptive sensory neurons. Nat Neurosci. 1999;2(1):18–23. doi: 10.1038/4520. [DOI] [PubMed] [Google Scholar]
- 39.Bodnárová M, Martásek P, Moroz LL. Calcium/calmodulin-dependent nitric oxide synthase activity in the CNS of Aplysia californica: Biochemical characterization and link to cGMP pathways. J Inorg Biochem. 2005;99(4):922–928. doi: 10.1016/j.jinorgbio.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 40.Moroz LL. Localization of putative nitrergic neurons in peripheral chemosensory areas and the central nervous system of Aplysia californica. J Comp Neurol. 2006;495(1):10–20. doi: 10.1002/cne.20842. [DOI] [PubMed] [Google Scholar]
- 41.Moroz LL, Norekian TP, Pirtle TJ, Robertson KJ, Satterlie RA. Distribution of NADPH-diaphorase reactivity and effects of nitric oxide on feeding and locomotory circuitry in the pteropod mollusc, Clione limacina. J Comp Neurol. 2000;427(2):274–284. [PubMed] [Google Scholar]
- 42.Hawkins RD. NO Honey, I don’t remember. Neuron. 1996;16(3):465–467. doi: 10.1016/s0896-6273(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 43.Antonov I, Kandel ER, Hawkins RD. Presynaptic and postsynaptic mechanisms of synaptic plasticity and metaplasticity during intermediate-term memory formation in Aplysia. J Neurosci. 2010;30(16):5781–5791. doi: 10.1523/JNEUROSCI.4947-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Byrne JH, Kandel ER. Presynaptic facilitation revisited: State and time dependence. J Neurosci. 1996;16(2):425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abrams TW, Yovell Y, Onyike CU, Cohen JE, Jarrard HE. Analysis of sequence-dependent interactions between transient calcium and transmitter stimuli in activating adenylyl cyclase in Aplysia: Possible contribution to CS--US sequence requirement during conditioning. Learn Mem. 1998;4(6):496–509. doi: 10.1101/lm.4.6.496. [DOI] [PubMed] [Google Scholar]
- 46.Bao JX, Kandel ER, Hawkins RD. Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1998;18(1):458–466. doi: 10.1523/JNEUROSCI.18-01-00458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eliot LS, Hawkins RD, Kandel ER, Schacher S. Pairing-specific, activity-dependent presynaptic facilitation at Aplysia sensory-motor neuron synapses in isolated cell culture. J Neurosci. 1994;14(1):368–383. doi: 10.1523/JNEUROSCI.14-01-00368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: Activity-dependent amplification of presynaptic facilitation. Science. 1983;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- 49.Kandel ER, et al. Classical conditioning and sensitization share aspects of the same molecular cascade in Aplysia. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):821–830. doi: 10.1101/sqb.1983.048.01.085. [DOI] [PubMed] [Google Scholar]
- 50.Ocorr KA, Walters ET, Byrne JH. Associative conditioning analog selectively increases cAMP levels of tail sensory neurons in Aplysia. Proc Natl Acad Sci USA. 1985;82(8):2548–2552. doi: 10.1073/pnas.82.8.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walters ET, Byrne JH. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- 52.Bao JX, Kandel ER, Hawkins RD. Involvement of pre- and postsynaptic mechanisms in posttetanic potentiation at Aplysia synapses. Science. 1997;275(5302):969–973. doi: 10.1126/science.275.5302.969. [DOI] [PubMed] [Google Scholar]
- 53.Jin I, Hawkins RD. Presynaptic and postsynaptic mechanisms of a novel form of homosynaptic potentiation at aplysia sensory-motor neuron synapses. J Neurosci. 2003;23(19):7288–7297. doi: 10.1523/JNEUROSCI.23-19-07288.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin XY, Glanzman DL. Hebbian induction of long-term potentiation of Aplysia sensorimotor synapses: Partial requirement for activation of an NMDA-related receptor. Proc Biol Sci. 1994;255(1344):215–221. doi: 10.1098/rspb.1994.0031. [DOI] [PubMed] [Google Scholar]
- 55.Lin XY, Glanzman DL. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: Regulation by postsynaptic voltage. Proc Biol Sci. 1994;255(1343):113–118. doi: 10.1098/rspb.1994.0016. [DOI] [PubMed] [Google Scholar]
- 56.Carew TJ, Walters ET, Kandel ER. Classical conditioning in a simple withdrawal reflex in Aplysia californica. J Neurosci. 1981;1(12):1426–1437. doi: 10.1523/JNEUROSCI.01-12-01426.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hawkins RD. A biologically based computational model for several simple forms of learning. Psychol Learn Motiv. 1989;23:65–108. [Google Scholar]
- 58.Neitz A, Mergia E, Eysel UT, Koesling D, Mittmann T. Presynaptic nitric oxide/cGMP facilitates glutamate release via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus. Eur J Neurosci. 2011;33(9):1611–1621. doi: 10.1111/j.1460-9568.2011.07654.x. [DOI] [PubMed] [Google Scholar]
- 59.Kopp-Scheinpflug C, Pigott BM, Forsythe ID. Nitric oxide selectively suppresses IH currents mediated by HCN1-containing channels. J Physiol. 2015;593(7):1685–1700. doi: 10.1113/jphysiol.2014.282194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bender RA, et al. Synchronized network activity in developing rat hippocampus involves regional hyperpolarization-activated cyclic nucleotide-gated (HCN) channel function. Eur J Neurosci. 2005;22(10):2669–2674. doi: 10.1111/j.1460-9568.2005.04407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rusznák Z, et al. The hyperpolarization-activated non-specific cation current (Ih) adjusts the membrane properties, excitability, and activity pattern of the giant cells in the rat dorsal cochlear nucleus. Eur J Neurosci. 2013;37(6):876–890. doi: 10.1111/ejn.12116. [DOI] [PubMed] [Google Scholar]
- 62.Elgueta C, Köhler J, Bartos M. Persistent discharges in dentate gyrus perisoma-inhibiting interneurons require hyperpolarization-activated cyclic nucleotide-gated channel activation. J Neurosci. 2015;35(10):4131–4139. doi: 10.1523/JNEUROSCI.3671-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dale N, Kandel ER. L-glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc Natl Acad Sci USA. 1993;90(15):7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glanzman DL. Postsynaptic regulation of the development and long-term plasticity of Aplysia sensorimotor synapses in cell culture. J Neurobiol. 1994;25(6):666–693. doi: 10.1002/neu.480250608. [DOI] [PubMed] [Google Scholar]
- 65.Conrad P, Wu F, Schacher S. Changes in functional glutamate receptors on a postsynaptic neuron accompany formation and maturation of an identified synapse. J Neurobiol. 1999;39(2):237–248. [PubMed] [Google Scholar]
- 66.Murphy GG, Glanzman DL. Enhancement of sensorimotor connections by conditioning-related stimulation in Aplysia depends upon postsynaptic Ca2+ Proc Natl Acad Sci USA. 1996;93(18):9931–9936. doi: 10.1073/pnas.93.18.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy GG, Glanzman DL. Mediation of classical conditioning in Aplysia californica by long-term potentiation of sensorimotor synapses. Science. 1997;278(5337):467–471. doi: 10.1126/science.278.5337.467. [DOI] [PubMed] [Google Scholar]
- 68.Murphy GG, Glanzman DL. Cellular analog of differential classical conditioning in Aplysia: Disruption by the NMDA receptor antagonist DL-2-amino-5-phosphonovalerate. J Neurosci. 1999;19(23):10595–10602. doi: 10.1523/JNEUROSCI.19-23-10595.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawkins RD. 2008. Trans-synaptic signaling by NO during learning-related synaptic plasticity. Learning and Memory: A Comprehensive Reference, ed Byrne JH. Elsevier, Oxford, UK.
- 70.Wang HG, et al. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45(3):389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 71.Moroz LL, et al. Neuronal transcriptome of Aplysia: Neuronal compartments and circuitry. Cell. 2006;127(7):1453–1467. doi: 10.1016/j.cell.2006.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.