Significance
Skeletal muscle is increasingly recognized as a secretory organ. Revealing the identity and function of myokines can improve our understanding of skeletal muscle function under sedentary or exercise conditions, as well as its coordination with other organs, tissues, and overall body metabolism. This study identifies musclin as an exercise-responsive myokine critical for skeletal muscle adaptation to physical activity. We develop a musclin-encoding gene (Ostn) knockout mouse, which allows us to determine a previously unrecognized physiologic function of musclin in regulation of skeletal muscle mitochondrial biogenesis and physical endurance. The demonstrated molecular mechanism for musclin-dependent skeletal muscle adaptation to exercise also transforms the perspective on natriuretic peptide signaling, particularly as it relates to physical activity and exercise-induced remodeling in different tissues.
Keywords: osteocrin, mitochondria, skeletal muscle, exercise, natriuretic peptide
Abstract
Exercise remains the most effective way to promote physical and metabolic wellbeing, but molecular mechanisms underlying exercise tolerance and its plasticity are only partially understood. In this study we identify musclin—a peptide with high homology to natriuretic peptides (NP)—as an exercise-responsive myokine that acts to enhance exercise capacity in mice. We use human primary myoblast culture and in vivo murine models to establish that the activity-related production of musclin is driven by Ca2+-dependent activation of Akt1 and the release of musclin-encoding gene (Ostn) transcription from forkhead box O1 transcription factor inhibition. Disruption of Ostn and elimination of musclin secretion in mice results in reduced exercise tolerance that can be rescued by treatment with recombinant musclin. Reduced exercise capacity in mice with disrupted musclin signaling is associated with a trend toward lower levels of plasma atrial NP (ANP) and significantly smaller levels of cyclic guanosine monophosphate (cGMP) and peroxisome proliferator-activated receptor gamma coactivator 1-α in skeletal muscles after exposure to exercise. Furthermore, in agreement with the established musclin ability to interact with NP clearance receptors, but not with NP guanyl cyclase-coupled signaling receptors, we demonstrate that musclin enhances cGMP production in cultured myoblasts only when applied together with ANP. Elimination of the activity-related musclin-dependent boost of ANP/cGMP signaling results in significantly lower maximum aerobic capacity, mitochondrial protein content, respiratory complex protein expression, and succinate dehydrogenase activity in skeletal muscles. Together, these data indicate that musclin enhances physical endurance by promoting mitochondrial biogenesis.
The ability to sustain physical activity is necessary for both quality and longevity of life. Regular exposure to exercise is associated with reduced rates of all-cause mortality (1). There are multiple mechanisms by which physical activity promotes health; however, recently there has been an interest in defining the contribution of circulating proteins secreted by skeletal muscle, termed myokines (2, 3). Myokines are autocrine, paracrine, or endocrine stimuli that may guide local skeletal muscle remodeling, repair, and maintenance or steer systemic adaptation related to physical activity (2). Understanding the functional role and the signaling pathways of myokines, particularly as they relate to exercise, may reveal new therapeutic targets to promote health and augment the benefits of physical activity.
This study is focused on the recently discovered myokine musclin (4, 5). Two groups initially identified this peptide: one as bone-derived osteocrin (5) and the second as muscle-secreted musclin (4). Musclin mRNA expression has been linked to insulin-induced activation of protein kinase B (Akt) that phosphorylates forkhead box O1 transcription factor (FOXO1), causing it to be exported from the nucleus and thus releasing the musclin-encoding gene from transcriptional inhibition (4, 6). This pathway has been demonstrated to regulate musclin transcription in both cell culture and skeletal muscles (4, 6). Musclin contains two KKKR putative serine protease cleavage sites and a region homologous to members of the natriuretic peptide (NP) family (4, 5). However, musclin does not have two cysteine residues needed to form the Ω-like structure characteristic for NPs (4, 5). In line with these structural characteristics, it has been demonstrated that musclin binds to the NP clearance receptor, NPRC, with affinity comparable to NPs, but exhibits only weak binding to NPRA and NPRB without activating the linked guanyl cyclase that is the primary effector of NP physiologic actions (7–9). Thus, it has been suggested that musclin function may be due to modulation of the action of NPs by competition with them for clearance via NPRC binding (8, 9). Indeed, musclin overexpression in osteoblast-lineage cells has been shown to result in elongated bones and marked kyphosis (9), which is similar to the phenotype of mice transgenically overexpressing BNP (10) or CNP (11) or lacking NPRC (12, 13). However, the physiological role of musclin production in skeletal muscles has remained elusive.
In this work, we demonstrate that musclin production by skeletal muscle is stimulated by physical activity and is paralleled by increased systemic musclin levels. Disruption of normal musclin signaling in mice by knockout of the musclin-encoding gene, Ostn (Ostn-KO), results in diminished exercise tolerance coupled with downgraded activity-related atrial NP (ANP)/cyclic guanosine monophosphate (cGMP)/PGC-1α–dependent skeletal muscle mitochondrial biogenesis. Thus, this work identifies a physiological role of musclin in enhancing skeletal muscle oxidative capacity and physical endurance.
Results
Musclin Production and Secretion into the Systemic Circulation Are Stimulated by Exercise.
Normal skeletal muscle function requires tight coordination with the operation of other organs and systems. Such coordination has been attributed in part to the action of myokines. Specifically, “exercise factors,” a subset of myokines whose production and secretion into systemic circulation are stimulated by physical activity, have been shown to modulate skeletal muscle and systemic metabolism, angiogenesis, growth, and inflammation (14).
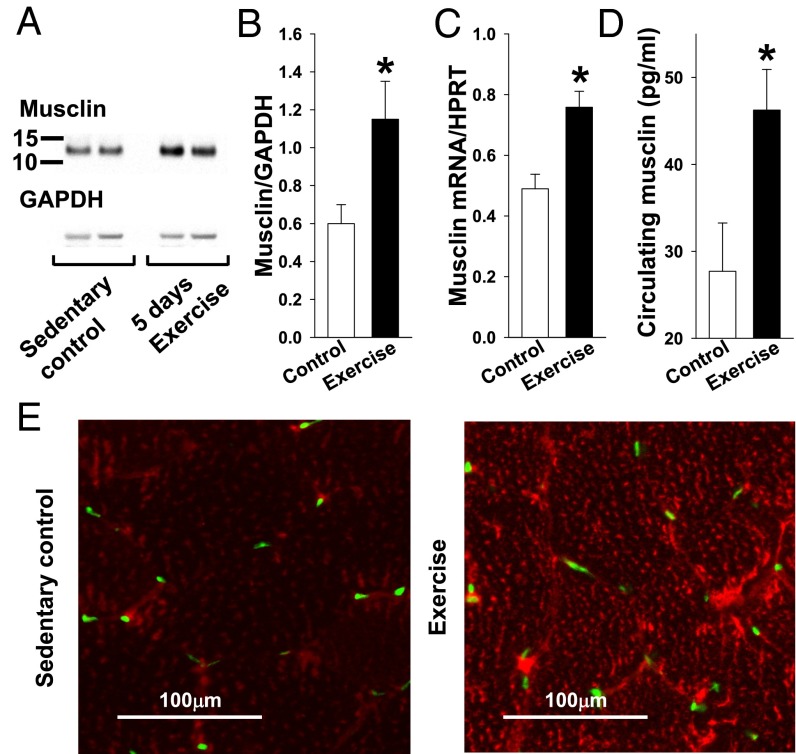
To determine whether musclin is an exercise factor, the level of musclin peptide in skeletal muscle was probed in two groups of wild-type (WT) mice: one group that exercised on a moving treadmill for 45 min daily (exercise) and a sedentary control group. After 5 d of exercise or control treadmill exposure, mice were euthanized, and their tissues were harvested by rapid excision and freeze clamp. Proteins were extracted from gastrocnemius muscles and segregated by Western blot (Fig. 1A), showing that exercise was associated with a nearly 100% increase in skeletal muscle musclin over control conditions [1.15 ± 0.2 vs. 0.60 ± 0.1 arbitrary units (AU), n = 5 each, P < 0.05; Fig. 1B]. A similar increase was demonstrated in skeletal muscle musclin mRNA from tibialis anterior samples (0.75 ± 0.05 vs. 0.49 ± 0.05 AU, n = 5 each, P < 0.05; Fig. 1C), whereas musclin mRNA levels in femur were markedly (96–98%) lower and were unresponsive to exercise [0.02 ± 0.002 vs. 0.02 ± 0.003 AU, respectively, n = 4 each, P = not significant (NS) between exercise and sedentary, P < 0.05 compared with skeletal muscle mRNA]. The increased musclin production by skeletal muscle was paralleled by an increase in the plasma musclin level from 27.71 ± 5.54 pg/mL (n = 3) in sedentary control WT mice to 46.24 ± 4.69 pg/mL in WT mice after exercise (n = 6, P < 0.05; Fig. 1D). Furthermore, immunohistochemistry of gastrocnemius cross-sections demonstrated more intense staining for musclin when mice were after exercise vs. sedentary (Fig. 1E). Thus, musclin production and secretion into the systemic circulation are up-regulated in response to exercise, establishing musclin as an exercise factor.
Fig. 1.
Musclin expression is exercise-responsive. Musclin expression was tested in muscles of WT mice after 5 d of treadmill exercise vs. no exercise (control). (A) Representative Western blots for musclin and GAPDH in protein extracts from gastrocnemius. (B–D) Summary statistics for musclin protein expression normalized to GAPDH by densitometry of Western blots of protein extracts from gastrocnemius (B), tibialis anterior musclin mRNA normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT) by quantitative RT-PCR (qRT-PCR) (C), and musclin peptide expression in plasma by custom ELISA (the y axis range begins at the lower limit for detection for this assay of 20 pg/mL; D). (E) Representative immunohistochemical stains of gastrocnemius cross-sections imaged by confocal microscopy. Red, musclin; green, nuclei. *P < 0.05 vs. control.
Activity-Induced Musclin Production Is Linked to Ca2+-Dependent Activation of Akt.
Regulation of musclin transcription has been linked to Akt activation (4, 6, 15, 16). Akt is a serine/threonine kinase that has emerged as a critical signaling component for the regulation of cellular metabolism, growth, and survival in multiple systems (17). Akt activity is increased in response to numerous stimuli, including a wide variety of growth factors and hormones activating phosphatidylinositol 3-kinase (PI3-kinase) (18–20). Akt can also be activated by mechanisms independent of PI3-kinase—for example, in response to increases in intracellular Ca2+ or cAMP, as occurs with increased muscle contractile activity (21–25).
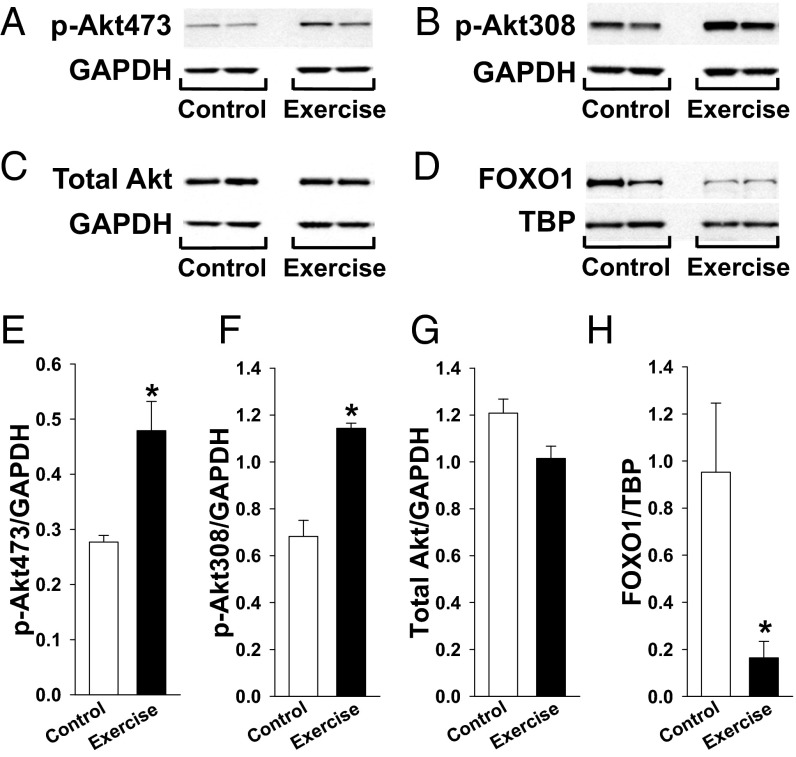
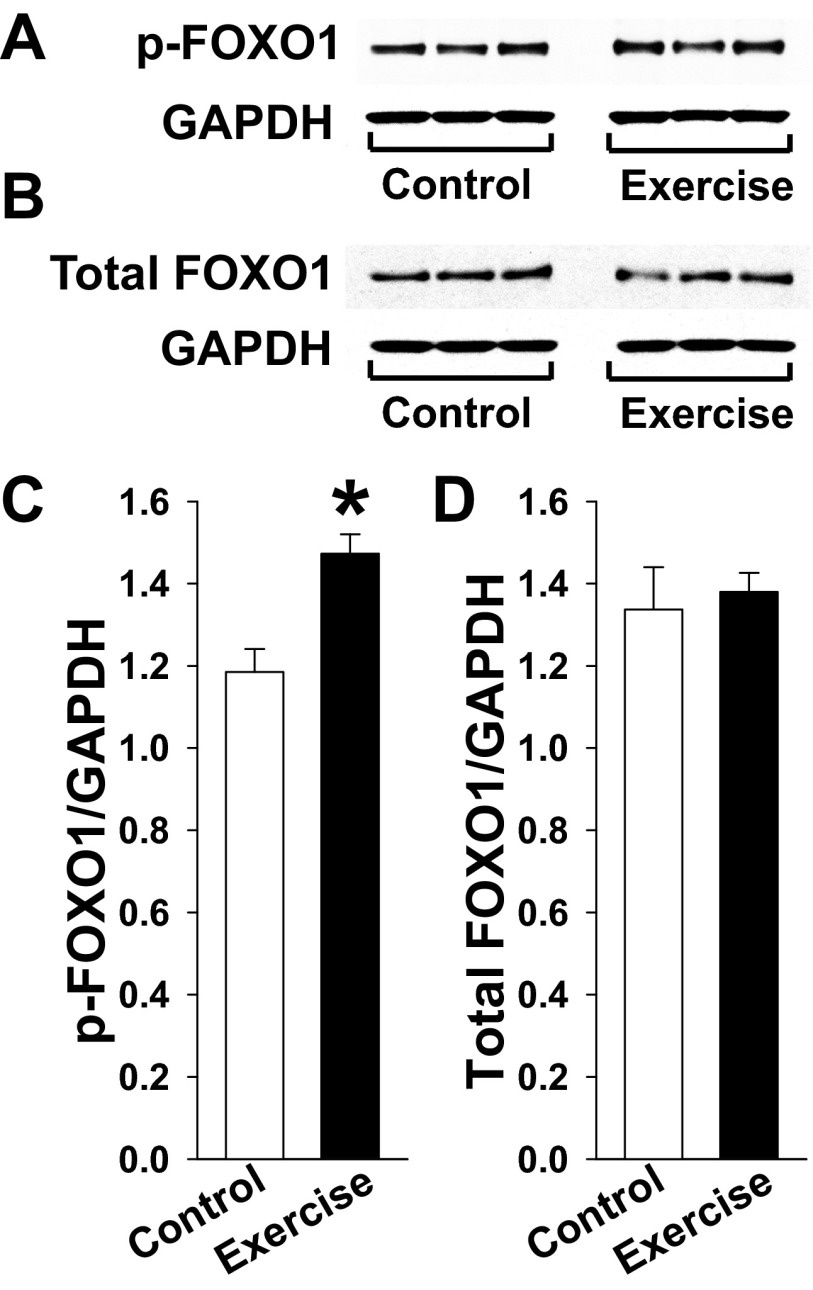
Here we confirm Akt activation in our model of treadmill-exercised mice. Specifically, the levels of phosphorylated Akt (S473 and T308) and total Akt from gastrocnemius of WT mice were compared by Western blot (Fig. 2 A–C), showing a significant increase in phosphorylated Akt (0.48 ± 0.09 vs. 0.28 ± 0.01 AU, n = 5 each, P < 0.05 for S473; Fig. 2E; and 1.14 ± 0.02 vs. 0.68 ± 0.07 AU, n = 5 each, P < 0.05 for T308; Fig. 2F), but not total Akt (1.02 ± 0.05 vs. 1.21 ± 0.06 AU, n = 5 each, P = NS; Fig. 2G), in response to exercise vs. sedentary conditions. Akt is a known regulator of FOXO1 nuclear export (15, 26). Here, we find a significant increase in phosphorylated FOXO1 (1.473 ± 0.047 vs. 1.185 ± 0.056 AU, n = 3 each, P < 0.05), but not total FOXO1 (1.380 ± 0.046 vs. 1.337 ± 0.103 AU, n = 3 each, P = NS) normalized to GAPDH in gastrocnemius muscle from exercised vs. sedentary muscle (Fig. S1). Also, FOXO1 nuclear content quantification by Western blot (Fig. 2D) shows a dramatic reduction in response to exercise (0.16 ± 0.07 vs. 0.95 ± 0.29 AU, n = 5 each, P < 0.05; Fig. 2H). Because FOXO1 is known to inhibit musclin-encoding gene transcription in skeletal muscle (6), this exercise-related reduction in nuclear FOXO1 is consistent with our finding of increased musclin mRNA after exercise. Furthermore, we found no significant exercise-induced changes in musclin mRNA/hypoxanthine guanine phosphoribosyl transferase (HPRT) in Akt1-KO mice (0.47 ± 0.10 AU, n = 5 vs. 0.45 ± 0.06 AU, n = 4, P = NS).
Fig. 2.
Exercise promotes skeletal muscle Akt phosphorylation and FOXO1 nuclear export. Gastrocnemius of WT mice were assayed after 5 d of treadmill exercise vs. no exercise (control). (B–D) Representative Western blots of GAPDH and Akt phosphorylated at residue 473 (A), residue 308 (B), and total Akt (C) in muscle and representative Western blots of TBP and FOXO1 in nuclear extracts from muscle (D). (E–H) Summary statistics for expression of Akt phosphorylated at residue 473 (E), residue 308 (F), and total Akt normalized to GAPDH in muscle (G) and FOXO1 normalized to TBP in nuclear extracts from muscle by densitometry of Western blots (H). TBP, anti-TATA binding protein. *P < 0.05 vs. control.
Fig. S1.
Exercise increases FOXO1 phosphorylation. (A and B) Representative Western blots of phosphorylated FOXO1 (p-FOXO1) (A) and total FOXO1 (B), as well as corresponding GAPDH in gastrocnemius muscle of exercised and nonexercised (control) mice. (C and D) Summary statistics for phosphorylated FOXO1 (C) and total FOXO1 (D) normalized to GAPDH. *P < 0.05 vs. sedentary control.
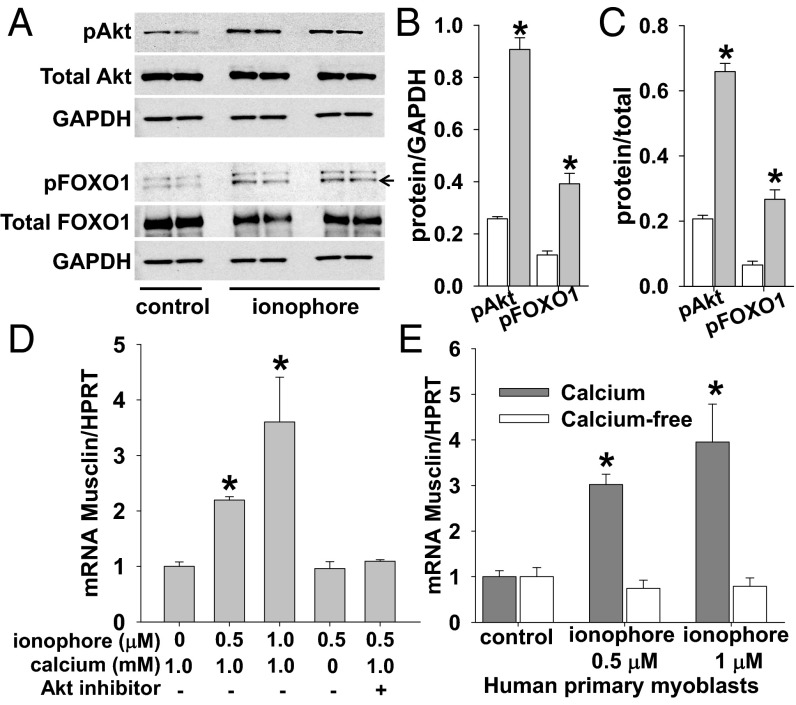
This molecular cascade was verified in a cell culture model of primary skeletal myoblasts isolated from WT mice in which phosphorylation of Akt and FOXO1 were induced by application of a Ca2+ ionophore (A23187; Sigma Aldrich; Fig. 3 A–C; p-Akt/GAPDH 0.26 ± 0.008, n = 2 vs. 0.91 ± 0.05, n = 4, pFOXO1/GAPDH 0.12 ± 0.02, n = 2 vs. 0.39 ± 0.04, n = 4, pAkt/total Akt 0.21 ± 0.01, n = 2 vs. 0.66 ± 0.03, n = 4, pFOXO1/total FOXO1 0.07 ± 0.01, n = 2 vs. 0.27 ± 0.03, n = 4, for control and ionophore conditions, respectively, all in the presence of 1 mM Ca2+; P < 0.05 for all comparisons). This activation of Akt by Ca2+ ionophore was paralleled by augmented musclin production (Fig. 3 D and E). Specifically, an increase in musclin mRNA was induced by Ca2+ ionophore in a dose-dependent manner (1.00 ± 0.08 AU no ionophore vs. 2.20 ± 0.06 AU for 0.5 µM ionophore vs. 3.60 ± 0.81 AU for 1 µM ionophore, all in presence of 1.0 mM Ca2+, n = 3 each, P < 0.05 for each ionophore concentration vs. no ionophore or control; Fig. 3D)—a response that was eliminated when myoblasts were pretreated with Akt inhibitor-viii (Sigma-Aldrich; 1.09 ± 0.03 AU, n = 3, P = NS vs. control; Fig. 3D), or by removal of extracellular Ca2+ from the medium (0.96 ± 0.012 AU, n = 3 each, P = NS vs. control; Fig. 3D). The same musclin response to Ca2+ was observed in a primary culture of myoblasts isolated from healthy human subjects (ZenBio; 1.0 ± 0.03 AU for no ionophore vs. 3.02 ± 0.23 AU for 0.5 µM ionophore vs. 3.95 ± 0.83 AU for 1 µM ionophore, all in presence of 1.0 mM Ca2+, n = 3 each; P < 0.05 for each ionophore concentration vs. no ionophore or control, values in Ca2+-free buffer were 1.0 ± 0.05, 0.74 ± 0.18, and 0.79 ± 0.18 AU, respectively; n = 3 each, P = NS; Fig. 3E), confirming that this mechanism is not specific to mice. These findings all indicate that exercise-related musclin production is driven by the Ca2+–Akt–FOXO1 signaling cascade.
Fig. 3.
Musclin production is stimulated by Ca2+-dependent Akt phosphorylation. (A) Representative Western blots of Akt, FOXO1, and GAPDH from cultured murine primary myoblasts in 1 mM Ca2+ without ionophore (control) vs. with 1 µM ionophore A23187 (Sigma-Aldrich). (B and C) Summary statistics for phosphorylated Akt (pAkt) and phosphorylated FOXO1 (pFOXO1) normalized to GAPDH (B) and total Akt and total FOXO1 (C), respectively, with (gray) and without (white; control) 1 µM ionophore. *P < 0.05 vs. control. (D and E) Summary statistics for musclin mRNA normalized to HPRT in murine cultured primary myoblasts exposed to various concentrations of ionophore, Ca2+ and Akt inhibitor-viii (D) and human cultured primary myoblasts exposed to no Ca2+ vs. 1.0 mM Ca2+ and various doses of ionophore, by densitometry of Western blots (E). *P < 0.05 vs. no ionophore (D) or vs. Ca2+-free (E).
Genetic Disruption of Musclin Production Causes Reduced Physical Endurance.
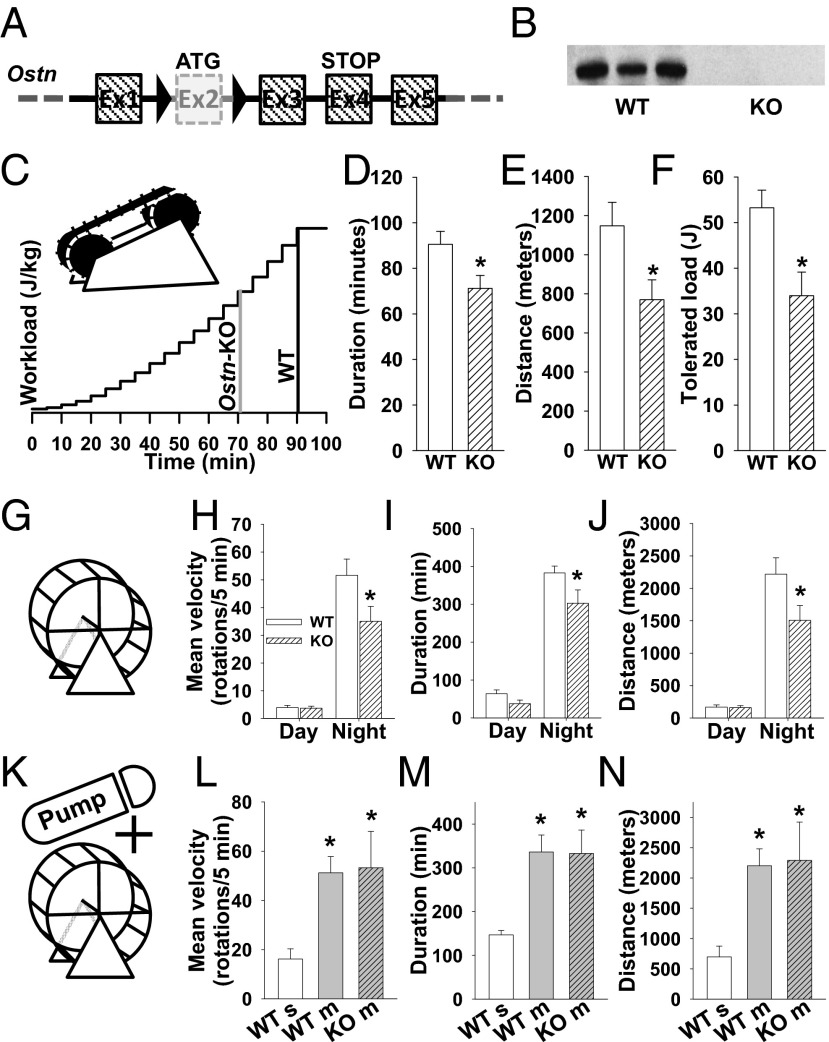
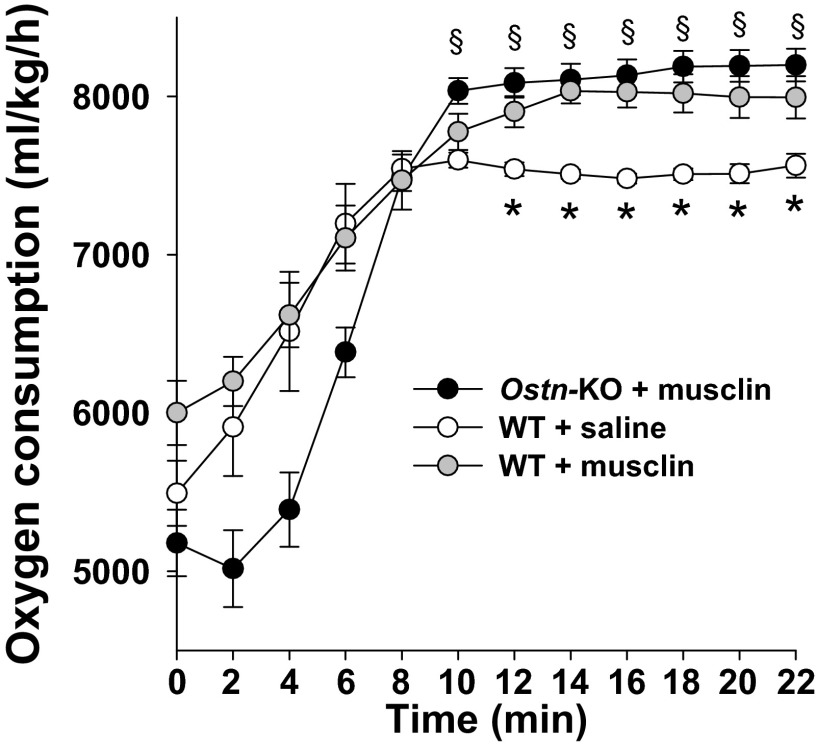
To investigate the physiological significance and function of a physical-activity-induced increase in musclin production, we generated a mouse model with ubiquitous disruption of the musclin-encoding gene, Ostn (genOway) and confirmed the absence of musclin production in skeletal muscle of Ostn-KO mice vs. WT controls by Western blot (Fig. 4 A and B). The Ostn-KO mice, housed normally and fed standard chow, exhibited no skeletal deformities or differences in bone density and no growth abnormalities, blood pressure, or body composition changes (Fig. S2 and Table S1) compared with controls at 7–8 wk of age; however, they do demonstrate lower exercise tolerance than controls. Specifically, when challenged with a program of treadmill exercise with progressive increase in speed and incline (Fig. 4C), Ostn-KO mice demonstrate a significant deficit in exertional tolerance with respect to duration (71 ± 6 vs. 91 ± 6 min., n = 6 each, P < 0.05; Fig. 4D), distance (769 ± 102 vs. 1147 ± 121 m, n = 6 each, P < 0.05; Fig. 4E), and overall workload (34 ± 5 vs. 53 ± 4 J, n = 6 each, P < 0.05; Fig. 4F). Similarly, when mice were offered the opportunity for voluntary exercise on running wheels (Fig. 4G), Ostn-KO mice demonstrated significantly lower mean velocity (35 ± 4 vs. 52 ± 4 rotations per 5 min, n = 6 each, P < 0.05; Fig. 4H), duration (303 ± 38 vs. 383 ± 64 min., n = 6 each, P < 0.05; Fig. 4I), and distance (1,505 ± 231 vs. 2,218 ± 253 m, n = 6 each, P < 0.05; Fig. 4J) during the night when the vast majority of activity was recorded. To confirm that the observed phenotype is related to the absence of musclin in the systemic circulation, mice were implanted with osmotic pumps (Alzet Durect) loaded with saline or 50 µg of musclin. This dose resulted in musclin plasma levels of 69.7 ± 8.8 pg/mL (n = 3), comparable with levels observed in mice following exercise as presented in Fig. 1. Voluntary exercise on running wheels (Fig. 4K) was significantly increased in WT mice treated with musclin (n = 5) compared with WT mice treated with saline (n = 4), with respect to mean velocity (51 ± 7 vs. 16 ± 4 rotations per 5 min, P < 0.05; Fig. 4L), duration (336 ± 39 vs. 147 ± 10 min, P < 0.05; Fig. 4M), and distance (2,202 ± 280 vs. 696 ± 179 m, P < 0.05; Fig. 4N). Furthermore, treatment with musclin “rescued” the Ostn-KO mice (n = 4) because their exercise activity was equalized to that of musclin-treated WT mice (n = 5) in terms of night-time mean velocity (53 ± 15 vs. 51 ± 7 rotations per 5 min, P = NS; Fig. 4L), duration of running (333 ± 53 vs. 336 ± 39 min, P = NS; Fig. 4M), and distance run (2,290 ± 632 vs. 2,202 ± 280 m, P = NS; Fig. 4N). Thus, intact musclin production is critical for optimal exercise performance.
Fig. 4.
Musclin supports physical performance. (A) Schematic of the modified Ostn gene indicating excision of the ATG-containing exon 2 to create the Ostn-KO mouse model. (B) Representative Western blot of musclin from gastrocnemius of WT and Ostn-KO mice. (C) Schematic of the treadmill exercise protocol. Vertical lines indicate mean time points of exercise dropout. (D–F) Summary statistics for treadmill exercise tolerance in terms of duration (D), distance (E), and tolerated workload: Ek + Ep (F). *P < 0.05 Ostn-KO vs. WT. (G) Schematic of a running wheel. (H–J) Summary statistics for voluntary running-wheel exercise performance at day and night in terms of mean velocity (H), duration (I), and distance (J). *P < 0.05 Ostn-KO vs. WT. (K) Schematic of a running wheel and an osmotic pump loaded with musclin (m) or saline (s). (L–N) Summary statistics for voluntary running wheel performance at night in terms of mean velocity (L), duration (M), and distance (N). *P < 0.05 vs. WT saline. KO, Ostn-KO.
Fig. S2.
Skeletal structure is grossly intact in Ostn-KO. Representative projections of high-resolution CT 3D reconstruction of the skeletons of WT (A) and Ostn-KO (B) mice, with enlarged views of the lower extremities of WT (C) and Ostn-KO (D) mice.
Table S1.
Body size, hind-limb bone thickness and length, body composition, and basic hemodynamic properties in mice
| Physiologic parameter | WT | n | Ostn-KO | n | P |
| Muscle mass GCN, mg | 84.3 ± 7.0 | 11 | 93.5 ± 6.1 | 12 | 0.33 |
| Muscle mass QDR, mg | 97.6 ± 9.7 | 11 | 101.0 ± 7.2 | 12 | 0.80 |
| Body length, cm | 9.3 ± 0.1 | 5 | 9.2 ± 0.2 | 5 | 0.41 |
| Fore-limb length, cm | 2.1 ± 0.02 | 5 | 2.2 ± 0.04 | 5 | 0.61 |
| Hind-limb length, cm | 2.7 ± 0.04 | 5 | 2.6 ± 0.06 | 5 | 0.80 |
| Systolic BP, mmHg | 110 ± 2 | 5 | 113 ± 2 | 5 | 0.33 |
| Diastolic BP, mmHg | 63 ± 2 | 5 | 61 ± 2 | 5 | 0.72 |
| Heart rate, beats per min | 516 ± 10 | 5 | 509 ± 14 | 5 | 0.66 |
| Hind-limb CT data | |||||
| Right femur mean cortical thickness, mm | 0.24 ± 0.02 | 3 | 0.24 ± 0.004 | 3 | 0.75 |
| Left femur mean cortical thickness, mm | 0.23 ± 0.004 | 3 | 0.23 ± 0.004 | 3 | 1 |
| Right tibia mean cortical thickness, mm | 0.25 ± 0.007 | 3 | 0.25 ± 0.01 | 3 | 0.87 |
| Left tibia mean cortical thickness, mm | 0.25 ± 0.008 | 2 | 0.24 ± 0.003 | 3 | 0.47 |
| Right femur max cortical thickness, mm | 0.30 ± 0.03 | 3 | 0.31 ± 0.003 | 3 | 0.85 |
| Left femur max cortical thickness, mm | 0.30 ± 0.003 | 3 | 0.31 ± 0.01 | 3 | 0.29 |
| Right tibia max cortical thickness, mm | 0.37 ± 0.009 | 3 | 0.35 ± 0.02 | 3 | 0.4 |
| Left tibia max cortical thickness, mm | 0.36 ± 0.03 | 2 | 0.33 ± 0.005 | 3 | 0.35 |
| Right femur length, mm | 14.46 ± 0.01 | 3 | 14.13 ± 0.20 | 3 | 0.19 |
| Left femur length, mm | 14.46 ± 0.05 | 3 | 15.30 ± 1.06 | 3 | 0.47 |
| Right tibia length, mm | 17.44 ± 0.16 | 3 | 17.06 ± 0.07 | 3 | 0.10 |
| Left tibia length, mm | 17.25 ± 0.15 | 2 | 17.03 ± 0.07 | 3 | 0.23 |
| TD-NMR data | |||||
| Body weight, g | 21.2 ± 0.5 | 15 | 21.9 ± 0.1 | 26 | 0.36 |
| Fat, g | 2.5 ± 0.1 | 15 | 2.6 ± 0.1 | 26 | 0.54 |
| Lean, g | 16.0 ± 0.4 | 15 | 16.2 ± 0.4 | 26 | 0.74 |
| Fluid, g | 2.7 ± 0.1 | 15 | 2.8 ± 0.1 | 26 | 0.62 |
| % fat | 11.9 ± 0.6 | 15 | 12.0 ± 0.5 | 26 | 0.88 |
| % lean | 75.5 ± 0.5 | 15 | 73.9 ± 0.3 | 26 | 0.01 |
| % fluid | 13.0 ± 0.3 | 15 | 12.8 ± 0.3 | 26 | 0.67 |
Left lower leg motion artifact prevented tibial measurements in one WT mouse. BP, blood pressure; GCN, gastrocnemius muscle; QDR, quadriceps femoris muscle.
Musclin Boosts Activity-Related cGMP Production in Skeletal Muscle.
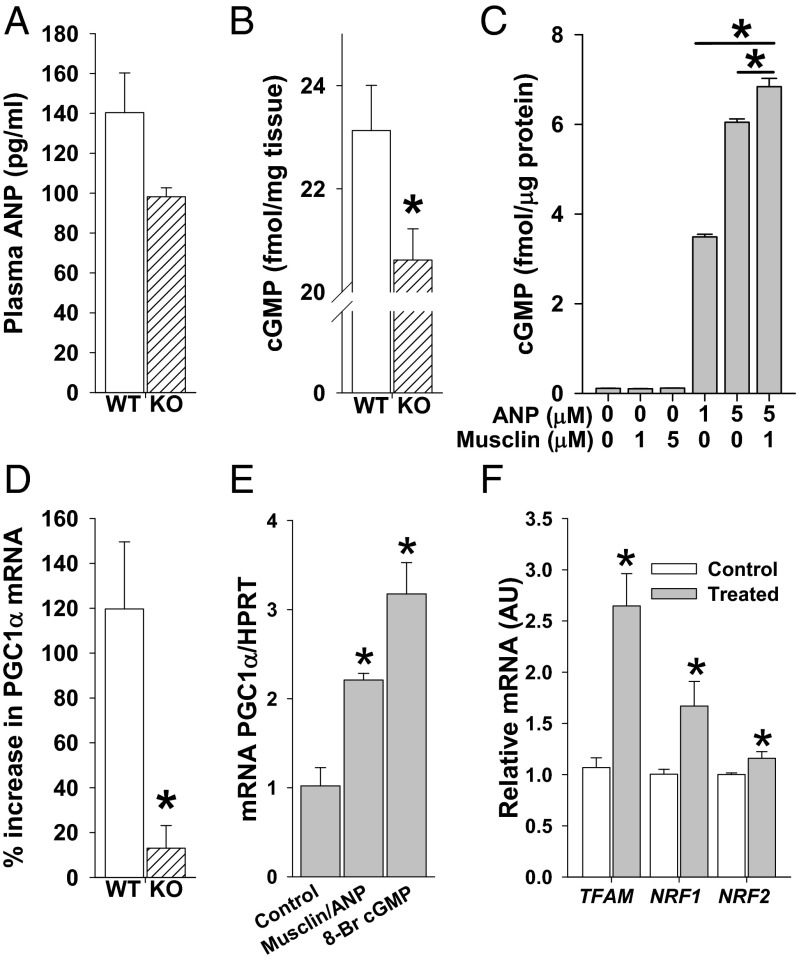
To address the relationship between exercise, musclin, and ANP, we examined WT and Ostn-KO mice after exercise using the same protocol as in Fig. 1, which established a significant exercise-related musclin response in WT mice. We found a trend toward higher-plasma ANP levels in exercised WT (n = 18 mice in six groups) compared with Ostn-KO (n = 15 mice in five groups) mice, although it did not achieve statistical significance (140.4 ± 19.9 pg/mL vs. 98.2 ± 4.5, P = 0.09; Fig. 5A). Furthermore, when gastrocnemius muscles were assayed after exercise, we detected significantly more cGMP in the muscle of WT compared with Ostn-KO mice (23.13 ± 0.88 fmol/mg vs. 20.62 ± 0.60 skeletal muscle tissue, P < 0.05; Fig. 5B). This ANP–musclin interaction with respect to cGMP signaling was further verified in a cell culture model. Specifically, we examined a skeletal myoblast culture exposed to various combinations and concentrations of these two peptides (n = 3 each; Fig. 5C). We found that, as expected, cGMP production was very low when no peptides were added (0.113 ± 0.003 fmol per µg of protein) or when musclin was added without ANP (0.104 ± 0.006 fmol/µg protein for 1 µM musclin and 0.117 ± 0.003 fmol/µg protein for 5 µM musclin; Fig. 5C). Also as expected, exposure to ANP resulted in a vigorous dose-dependent cGMP response (3.491 ± 0.057 fmol per µg of protein for 1 µM ANP and 6.046 ± 0.074 fmol per µg of protein for 5 µM ANP). Importantly, this response was augmented by the addition of musclin (6.840 ± 0.184 fmol per µg of protein for 5 µM ANP + 1 µM musclin, P < 0.05 vs. ANP 5 µM without musclin; Fig. 5C).
Fig. 5.
Musclin augments ANP signaling in skeletal muscle. Mice were exercised on a treadmill for 5 d before undergoing assessment of ANP signaling. (A) Summary statistics for plasma ANP as assessed by ELISA. (B) Summary statistics for cGMP in gastrocnemius by enzyme immunoassay. *P < 0.05 Ostn-KO vs. WT. (C) Summary statistics for cGMP production in a culture of murine primary myoblasts exposed to various concentrations of ANP and musclin. *P < 0.05 vs. columns indicated by bar. (D) Summary statistics for percent increase in peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1-α) mRNA over baseline in response to exercise in tibialis anterior by qRT-PCR. *P < 0.05 vs. WT. (E) Summary statistics for primary myoblast culture mRNA of PGC1-α normalized to HPRT in response to musclin/ANP or to 8-Br-c-GMP. (F) Summary statistics for primary myoblast culture relative mRNA of TFAM, NRF1, and NRF2 with and without musclin/ANP. KO, Ostn-KO.
These data support a synergistic relationship between musclin and ANP and are consistent with the hypothesis that competition for NPRC between musclin and ANP augments local ANP effects due to reduced clearance.
Normal Musclin Signaling Promotes Mitochondrial Biogenesis in Skeletal Muscle.
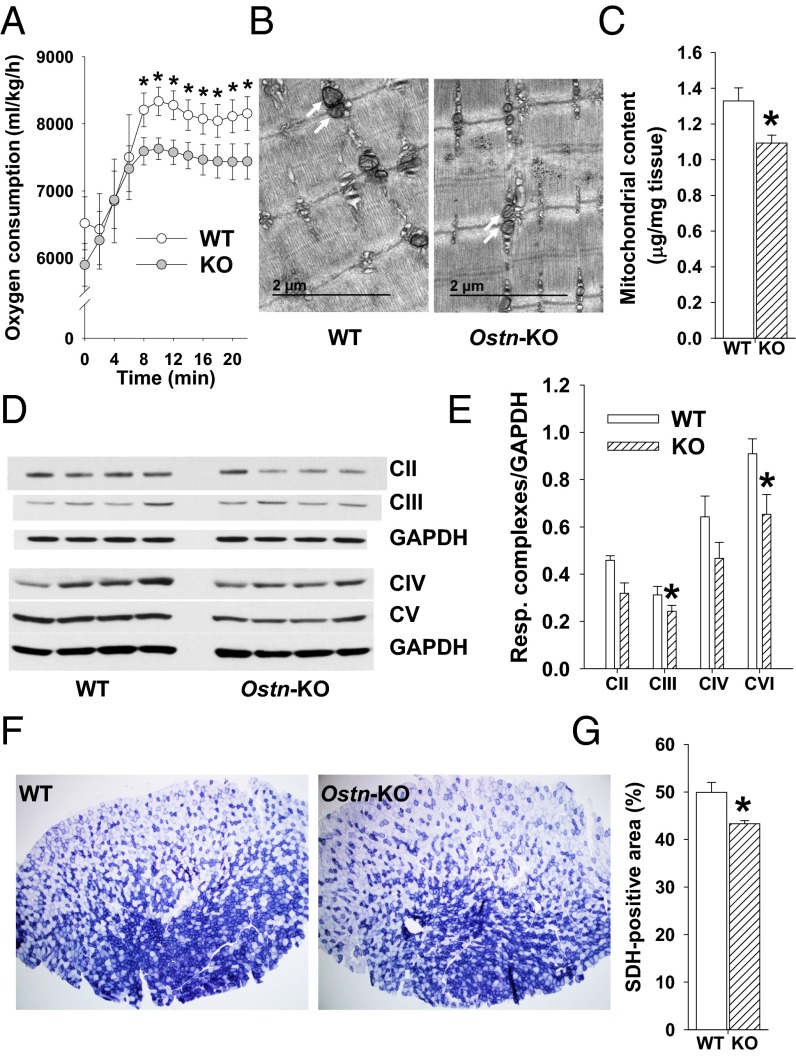
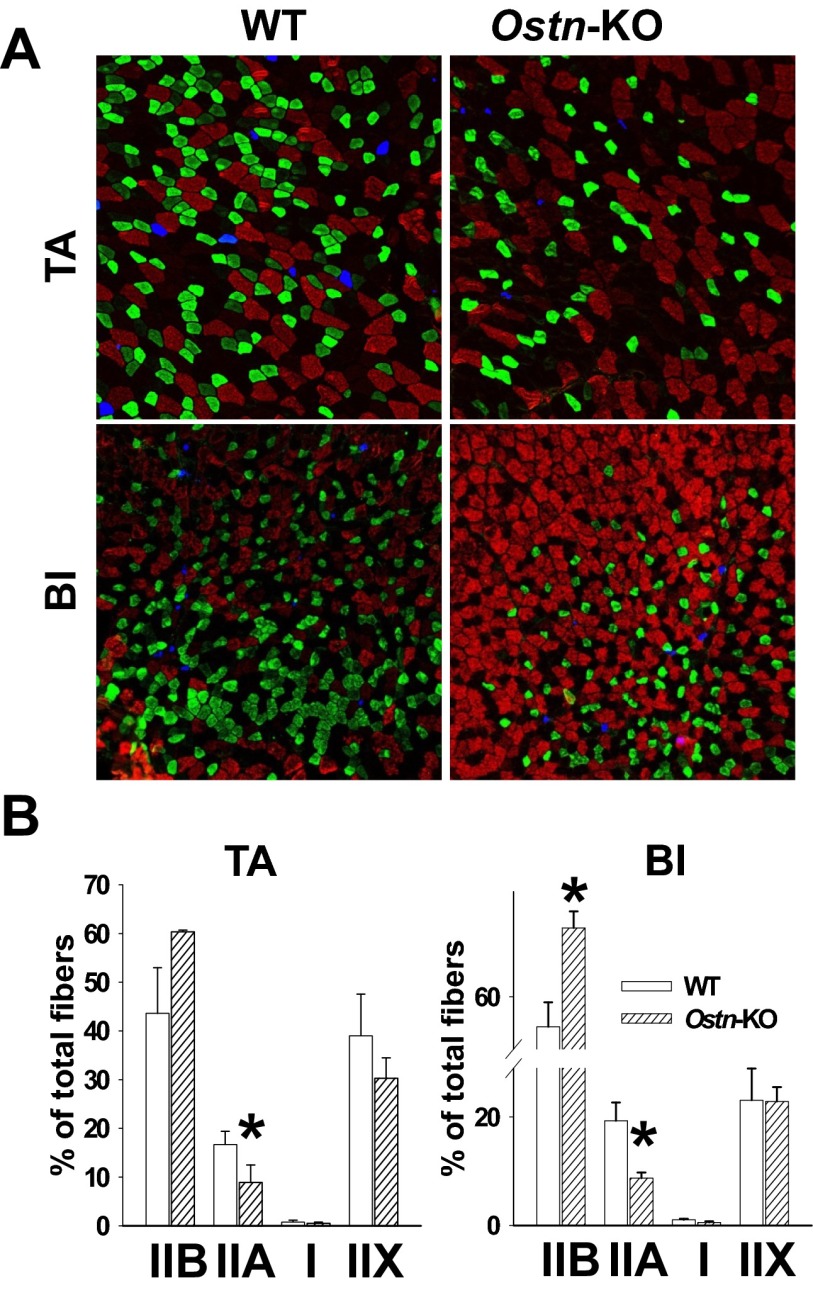
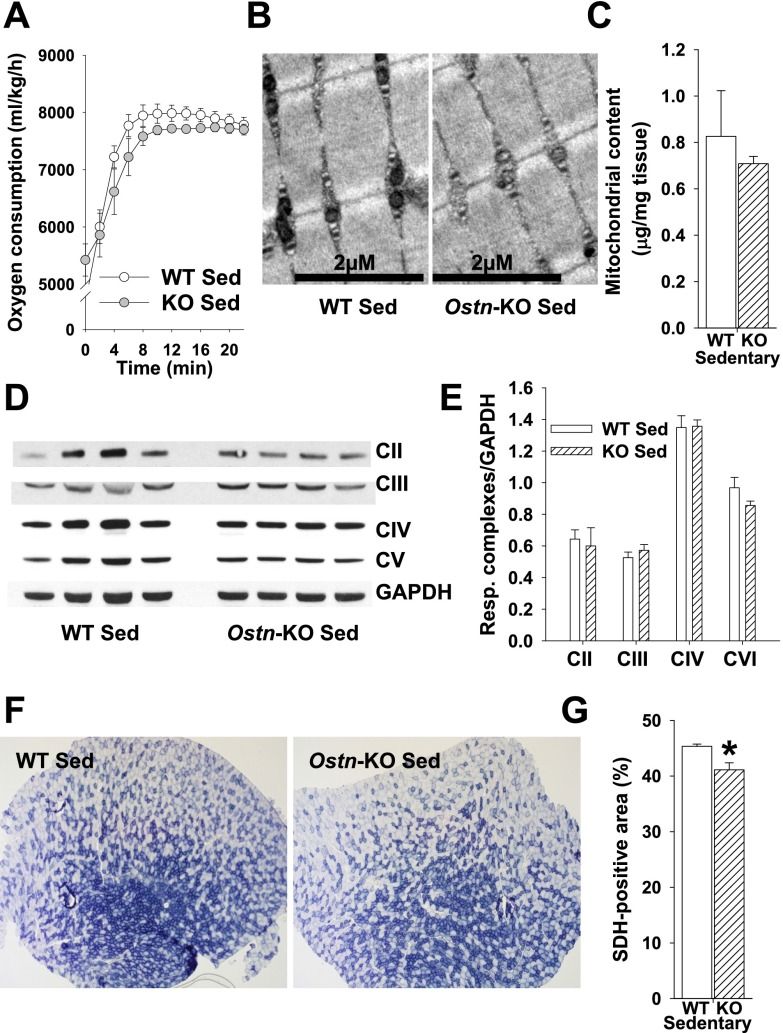
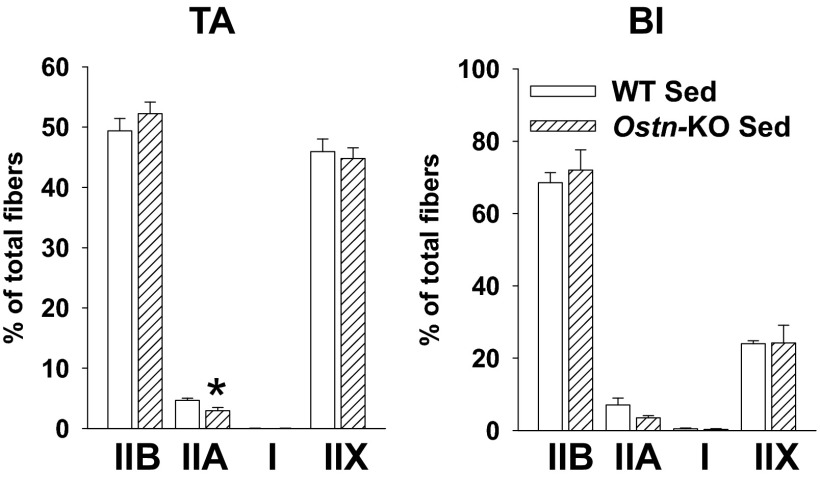
NP/cGMP signaling is increasingly recognized as a key regulator of metabolic homeostasis, including effects on skeletal muscle mitochondrial biogenesis and oxidative phosphorylation potential (27–29). Maximal aerobic capacity (max) is commonly used to estimate overall aerobic fitness based on cardiopulmonary function and oxidative phosphorylation potential (30). To determine whether musclin production impacts aerobic capacity as a potential mechanism underlying differences in exercise tolerance, we monitored exercise-trained Ostn-KO and WT mice on a metabolic treadmill equipped for indirect calorimetry (Columbus instruments). After 5 d of training, mice were placed on the stationary treadmill for 30 min and then were exposed to the protocol of escalating exercise workload to determine their max. The resulting oxygen consumption recorded as a function of time reveals a significantly lower max for Ostn-KO compared with WT (7,629 ± 161 vs. 8,334 ± 212 mL/kg per h, n = 5 and 4, respectively, P < 0.05; Fig. 6A). Interestingly, after 3 wk of musclin infusion delivered via osmotic pumps (Alzet Durect), Ostn-KO and WT mice demonstrated comparable max (8,193 ± 100 vs. 8,034 ± 77 mL/kg per h, n = 3 and 4, respectively, P = NS; Fig. S3). These findings suggest musclin signaling may be tied to oxidative phosphorylation through mitochondrial density, size, or function. To assess this hypothesis, electron micrographs of longitudinal sections through tibialis anterior of exercised mice were examined and appeared to show smaller mitochondrial size in the Ostn-KO compared with WT mice (Fig. 6B). This finding corresponds to a significantly lower mitochondrial protein content when normalized to the wet skeletal muscle weight (1.09 ± 0.04 vs. 1.33 ± 0.07 µg per mg of tissue, n = 6 each, P < 0.05; Fig. 6C), and respiratory complex expression (0.24 ± 0.02 vs. 0.31 ± 0.04 AU for CIII and 0.65 ± 0.08 vs. 0.91 ± 0.06 AU for CVI, n = 3 each, P < 0.05; Fig. 6 D and E) in gastrocnemius muscle homogenates, as well as succinate dehydrogenase (SDH) activity as assessed by percent of immunohistochemical staining in tibialis anterior cross-sections (43.33 ± 0.65 vs. 49.92 ± 2.09%, n = 3 each, P < 0.05; Fig. 6 F and G) from Ostn-KO vs. WT mice. Furthermore, disruption of normal musclin signaling appears to impact fiber type composition. Specifically, histologic examination of skeletal muscles from Ostn-KO vs. WT mice indicates a shift toward more pure glycolytic type IIb fibers in the KO (Fig. S4). Differences between sedentary WT and Ostn-KO mice were less marked (Fig. S5), consistent with the finding that exercise augments musclin signaling in the WT mice. Specifically, differences in oxygen consumption during treadmill testing (n = 4 each), mitochondrial content (0.83 ± 0.20, n = 5 vs. 0.71 ± 0.03 µg/mg tissue, n = 6, P = NS), and respiratory complex expression normalized to GAPDH in gastrocnemius muscle homogenates (0.64 ± 0.06 vs. 0.60 ± 0.02 for CII, 0.53 ± 0.04 vs. 0.57 ± 0.04 for CIII, 1.35 ± 0.08 vs. 1.36 ± 0.04 for CIV, 0.97 ± 0.07 vs. 0.86 ± 0.03 for CVI, n = 4 each, all P = NS) were not significantly different for sedentary WT vs. Ostn-KO mice, respectively, whereas SDH-positive staining of tibialis anterior cross-sections (45.33 ± 0.40 vs. 41.12 ± 1.24%, n = 3 each, *P < 0.05) exhibited a smaller difference than that in exercise-trained WT vs. Ostn-KO mice. The pattern of distribution of fiber types from muscles of sedentary WT and Ostn-KO were similar to those of their exercise-trained counterparts, consistent with a slower adaptation of fiber type to exercise, except that there was a slightly more prominent component of type IIA fibers in exercised mice (Fig. S6; n = 4 each for tibialis anterior and n = 3 each for biceps femoris, *P < 0.05).
Fig. 6.
Musclin signaling improves aerobic capacity and prompts mitochondrial biogenesis. (A) Summary statistics for trend of oxygen consumption over time of exercise-trained mice upon initiation of treadmill exercise at time 0. *P < 0.05 vs. Ostn-KO. (B) Representative electron micrographs of longitudinal tibialis anterior sections from exercised mice. White arrows indicate mitochondria. (C) Summary statistics for mitochondrial content by weight in gastrocnemius isolates of exercised mice. *P < 0.05 vs. WT. (D and E) Representative Western blots of respiratory chain enzymes and GAPDH (D) and summary statistics for respiratory complex expression normalized to GAPDH in gastrocnemius of exercise-trained mice (E). *P < 0.05 vs. WT. (F and G) Representative stains for SDH activity of tibialis anterior cross-sections (F) and summary statistics for percent area of cross-sections stained for SDH activity (G) in exercise-trained mice. *P < 0.05 vs. WT. KO, Ostn-KO.
Fig. S3.
Oxygen consumption of Ostn-KO is rescued by musclin infusion. The oxygen consumption, VO2, over time upon initiation of treadmill exercise was compared in WT and Ostn-KO mice treated with 3 wk of saline or musclin delivered by osmotic pump. Oxygen consumption of WT mice treated with musclin was slightly better for several time points than that of WT mice treated with saline. Oxygen consumption of Ostn-KO mice treated with musclin is equivalent to that of WT mice treated with musclin and was better than WT treated with saline once steady state was achieved. *P < 0.05 for WT + musclin vs. WT + saline; §P < 0.05 for Ostn-KO + musclin vs. WT + saline.
Fig. S4.
Musclin controls skeletal muscle fiber type. (A) Representative immunohistochemical stains for fiber type in cross-sections of biceps femoris (BI) and tibialis anterior (TA) of exercise-trained WT and Ostn-KO mice. Red, IIB; green, IIA; blue, I; black, IIX staining. (B) Summary statistics for fiber type as assessed by counting the number of stained fibers per field. *P < 0.05 vs. WT.
Fig. S5.
Differences in aerobic capacity and markers of mitochondrial biogenesis are minimal in sedentary WT and Ostn-KO mice. (A) Summary statistics for trend of oxygen consumption over time of sedentary WT and Ostn-KO mice upon initiation of treadmill exercise at time 0 (all points NS for WT vs. Ostn-KO). (B) Representative electron micrographs of longitudinal tibialis anterior sections from sedentary mice. (C) Summary statistics for mitochondrial content by weight in gastrocnemius isolates of sedentary mice. *P < 0.05 vs. WT. (D and E) Representative Western blots of respiratory chain enzymes and GAPDH (D) and summary statistics for respiratory complex expression normalized to GAPDH in gastrocnemius (E) of sedentary WT and Ostn-KO mice. (F and G) Representative stains for SDH activity of tibialis anterior cross-sections (F) and summary statistics for percent area of cross-sections (G) stained for SDH activity in sedentary WT and Ostn-KO mice. *P < 0.05 vs. WT. KO, Ostn-KO; Sed, sedentary.
Fig. S6.
Differences in skeletal muscle fiber type are less marked in sedentary WT vs. Ostn-KO mice. Summary statistics for fiber type as assessed by counting number of stained fibers per field in tibialis anterior (TA; Left) and biceps femoris (BI; Right) of sedentary WT and Ostn-KO mice. *P < 0.05 vs. WT.
To address the hypothesis that observed differences in skeletal muscle mitochondrial content of Ostn-KO vs. WT mice are driven by cGMP/peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1-α) signaling, we examined their tibialis anterior and found that, despite similar PGC1-α levels at baseline, (1.729 ± 0.44 vs. 1.125 ±0.1 AU, respectively, n = 5 and 7, P = NS) an exercise-related increase in PGC1-α over this baseline was significantly smaller in Ostn-KO than in WT mice (13.0 ± 0.1% vs. 119.7 ± 29.9% increase respectively, n = 3 each, P < 0.05; Fig. 5D). In a primary myoblast culture model, induction of cGMP by the combination of musclin and ANP (Fig. 5C) was associated with a significant increase in mRNA of PGC1-α (Fig. 5E) and its downstream targets linked to mitochondrial biogenesis, TFAM, NRF1, and NRF2 (Fig. 5F).
These data indicate that exercise tolerance is influenced by musclin via an effect on ANP-dependent PGC1-α regulation of skeletal muscle activity-related mitochondrial biogenesis.
Discussion
This study establishes that production of the peptide musclin is up-regulated in skeletal muscle in response to physical activity and that musclin is secreted into the systemic circulation. Disruption of musclin signaling in Ostn-KO mice is associated with reduced oxidative phosphorylation potential and exercise tolerance that is corrected by musclin-replacement therapy. These findings indicate a previously unrecognized pathway for skeletal muscle metabolic adaptation to exercise.
Although we use forced treadmill exercise to demonstrate the responsiveness of musclin production and secretion to exercise, we found a phenotype of decreased endurance and trends toward lower mitochondrial content and higher presence of IIA glycolytic fibers, even in untrained Ostn-KO mice compared with WT controls. Importantly, these observed trends and changes in sedentary Ostn-KO became much more obvious and significant after exposure to the treadmill exercise protocol. We interpret these findings to indicate that musclin production and secretion, although more easily demonstrated in response to vigorous exercise, is physiologically relevant, even for routine daily activities.
Our data support the hypothesis that musclin modulates effects of cardiac NPs due to its ability to interfere with binding to NPRC (8, 9). First, we demonstrate that musclin itself does not induce cGMP production in primary myoblasts, but, rather, potentiates ANP effects. Further, we confirm the significance of this signaling in vivo. Specifically, we demonstrate that WT mice have significantly higher muscle levels of cGMP after exposure to exercise compared with Ostn-KO mice. The higher level of muscle cGMP in WT vs. Ostn-KO mice is paralleled by a trend toward higher-plasma ANP. Elevation of ANP in plasma after physical activity has traditionally been linked to atrial wall stretch; however, our data suggest that increased production of musclin could also contribute to this phenomenon. Of note, it is possible that our measurement does not reach statistical significance due to significant variability of circulating ANP levels and the inability to use mice as their own controls, because the volume of plasma needed for testing precludes more than a single terminal blood draw per mouse.
We detected an order-of-magnitude lower production of musclin in the bone of our adult mice compared with that from skeletal muscle. This finding is consistent with original reports that indicate that high musclin expression during early bone development sharply declines in a time- and maturity-dependent manner in both mice (5) and humans (31). Furthermore, in contrast to skeletal muscle, no exercise-responsive increase in mRNA levels of musclin was observed in bones. Thus, it seems likely that the elevated systemic circulating musclin levels after exercise are largely supported by augmented production and secretion by skeletal muscle.
Identification of the exercise-responsive nature of musclin signaling, along with its systemic circulation, has important implications for our understanding of exercise-dependent NP signaling. Cardiac NPs are increasingly recognized as hormones with a wide spectrum of targets: In addition to traditional targets of vasculature and kidney, recently their effects on skeletal muscle mitochondrial biogenesis, angiogenesis, lipolysis, and adipose tissue remodeling (browning) have been reported (27–29, 32). Although the present study focuses on one aspect of this signaling network, intramuscular cGMP signaling and mitochondrial biogenesis, it is possible that other cardiac NP signaling targets are similarly affected. For example, musclin may be at least partially responsible for the beneficial effect of exercise on cardiac remodeling. Such targets should be the subject of future investigation.
cGMP signaling has been linked to PGC1-α–dependent mitochondrial biogenesis in many studies in different tissues and organs (33–35). cGMP production in skeletal muscles is typically linked to nitric oxide signaling (34–36), although recently a role for NPs in this process has been established (28). Our data support the importance NP signaling and its regulation by musclin in cGMP/PGC1-α–driven mitochondrial biogenesis: We confirm significantly greater mitochondrial quantity and function by multiple methods and demonstrate the in vivo functional importance by revealing a meaningful increase in the max, a parameter that reflects many factors, including oxidative phosphorylation potential and cardiovascular and pulmonary functions critical for physical endurance, of mice with intact vs. disrupted musclin signaling.
Finally, our demonstration that musclin infusion rescues exercise and oxidative capacity in Ostn-KO mice, as well as enhances exercise and oxidative capacity in untrained WT mice, suggests a potential therapeutic role for musclin. Overexpression of musclin in chondrocytes has been linked to abnormal skeletal growth (9), but such changes may or may not occur with systemically delivered musclin. Thus, to better understand musclin’s therapeutic potential, further studies will be required to determine the extent and durability of beneficial effects and whether there are accompanying long-term deleterious consequences. Such studies may be aided by conditional models.
In summary, this study defines musclin as an exercise-responsive factor promoting skeletal muscle mitochondrial biogenesis and exercise endurance.
Materials and Methods
All animal protocols conform to the Guide for the Care and Use of Laboratory Animals (37) generated by the Institute for Laboratory Animal Research, National Research Council of the National Academies. All animal protocols were approved by the University of Iowa Institutional Animal Care and Use Committee. See SI Materials and Methods for detailed methods. Results are expressed as mean ± SEM.
SI Materials and Methods
Ostn-KO Mouse Model.
Vector construction and targeted knockout strategy were designed together with genOway, where mice were generated based on deletion of a 2.1-kb sequence flanking Ostn exon 2, resulting in inactivation of the ATG and signal peptide. Homology sequences were cloned from murine genomic DNA as three independent fragments by PCR. The first pair of primers (sense: 5′-ATG TTA CAG AAC ATT TGA TCC ATT ACG ACA-3′; antisense: 5′-TGC ACT TCA CAT TAA AAA TTC TTC ACT GC-3′) amplified the 3,341-bp fragment containing the exon 2 upstream sequence. This subclone was used to generate the distal part of the long homology arm of the targeting vector. The second pair of primers (sense: 5′-TAG TAT GCC ATG GTA TTT GTG CTG TGG G-3′; antisense: 5′-TGC TGG TTA CTT TCT CTT CAA GGG CAG-3′) amplified the 2,131-bp fragment containing the exon 2 and neighboring intronic sequences. This subclone was used to generate the proximal part of the long homology arm. The third set of primers (sense: 5′-TTG ATT TGT ACC TAC CTT GGT GCC TGC-3′; antisense: 5′-ACC CAT CAC ATA CAC ACT GCC TTT ACC TAC-3′) amplified the 2,465-bp fragment containing the exon 2 downstream sequence. This subclone was used to generate the short homology arm of the targeting vector and to generate a positive control vector. Amplifications were performed with 15–20 PCR cycles with proofreading thermostable Taq polymerase (Accuprime Taq DNA polymerase high fidelity; Invitrogen) using genomic C57BL/6 embryonic stem (ES) cell DNA. Resulting PCR products were subcloned into the pCR4-TOPO vector (Invitrogen) via TA-cloning. Sequencing of the isolated distal long homology arm region resulted in one clone with only a single mutation in the amplified region that was subsequently corrected before vector construction. Sequencing of the isolated proximal long homology arm region and the isolated short homology arm region identified clones without mutation that were used for vector construction. The target vector and a positive control vector were generated with each individual cloning step validated through restriction analysis and partial sequencing. The target vector contained two inserted loxP sites flanking exon 2, the neomycin-positive selection gene flanked by flippase recognition target sites and the presence of diphtheria toxin A as a negative selection marker. A robust PCR screening strategy and Southern blot for detection of homologous recombination were designed. The targeting vector was linearized by restriction digest with PmeI. The resulting fragment was isolated, purified, and transfected into ES cells according to standard electroporation procedures. Positive selection was started 48 h after electroporation by addition of 200 µg/mL of G418 (Life Technologies). A total of 178 clones were isolated, amplified, and screened by PCR to verify homologous recombination at the 3′ end of the Ostn locus (sense: 5′-GAA CTT CCT GAC TAG GGG AGG AGT AGA AGG-3′; antisense: 5′-CTC TTC TCT GGC TGT GGG TGG AGA C-3′) with the expected amplified product size of 2,159 bp. Eighteen clones recombined at the 3′ end of the Ostn locus were analyzed by a second PCR to test for insertion of the distal loxP site at the 5′ end of the locus. Of the 18 tested ES cell clones, 3 were positive for the presence of the distal loxP site. These 3 recombined clones were further verified by Southern blot analysis. Recombined ES cells were injected into albino C57BL/6 blastocysts, giving rise to two highly chimeric males (>50%) identified by coat color markers. These male mice were bred with C57BL/6 Cre recombinase expressing deleter mice to excise the loxP-flanked sequence and generate heterozygous mice carrying the constitutive knock-out allele. Genotyping by PCR of pups derived from F1 breeding allowed identification of a pup with complete heterozygous Cre-mediated excision of Ostn. A second breeding of this male with C57BL/6 WT females allowed the generation of additional heterozygous mice with complete Cre-mediated excision of Ostn. Knockout and WT alleles were further verified by Southern blot in these animals. Ostn+/− mice were used to generate Ostn−/− (Ostn-KO) and WT controls through further breeding cycles.
Genotyping.
Genotyping was performed on tail tip DNA extracted with the Dneasy Blood and Tissu Kit (Qiagen). PCR was performed by using Platinum Blue PCR Supermix (Invitrogen). To detect the amplification product of the knockout allele of 2,395 bp and the WT allele of 4,420 bp, the following primers were used: forward: 5′-GTG AGG TTA TGA ACA TTC CAA CAG CTA TAT CC-3′; and reverse: 5′-ATG GGG TTA TTT TCC TTG TCC ACC TAC C-3′.
Akt1-KO Mouse Model.
Akt1-KO mice were purchased from Jackson Laboratory.
Animal Experiments.
All animal protocols conform to the Guide for the Care and Use of Laboratory Animals (37) generated by the Institute for Laboratory Animal Research, National Research Council of the National Academies. All animal protocols were approved by the University of Iowa Institutional Animal Care and Use Committee. Ostn-KO and littermate C57BL/6 WT control mice or Akt1-KO (Jackson Laboratory), 7–8 wk old, of either gender, were used for all experiments. For all experiments, mice were anesthetized with inhaled isoflurane (5% induction, 1–1.5% maintenance; Piramal Healthcare) to maintain a respiratory rate of ∼50–60 breaths per minute.
Skeletal reconstruction.
Microcomputed tomography (CT) images of the mice were acquired with the Siemen's Inveon PET/CT scanner. CT parameters for acquisition were as follows: voltage of 80 kV and tube current of 500 µA, 220 rotation degrees with 360 steps, medium-resolution magnification, and a binning of 2. Projections were reconstructed into images by using the manufacturer's software with a downsample factor of 1, beam hardening correction, bilinear interpolation, and Shepp–Logan reconstruction filter (ultimate image pixel size of 36.15 µm). Images were analyzed by using ImageJ (Version 1.50b) with the BoneJ (Version 1.3.12) plugin. Femurs and tibia were cropped and rotated to measure length. Only cortical bone was measured for thickness. For femurs, a 0.54-mm section of bone was measured 3.6 mm from femoral distal growth plate. For tibias, a 0.54-mm section of bone was measured 3.6 mm from the tibial proximal growth plate. The sections were converted to binary images automatically by using the BoneJ plugin and then measured for thickness with the plugin’s Thickness tool. Then, 3D images were created by using the Inveon Research Workplace (Version 4.2) multimodal 3D visualization software.
Physical characteristics.
Calipers were used to measure limb lengths from hip or shoulder joint to the tips of the digits in the fully extended limbs, and body length from nose tip to anus, of anesthetized mice. Blood pressure was measured by tail cuff method in restrained, awake mice acclimated to the apparatus (VisiTech BP Systems) (38).
Body composition.
Whole body composition was obtained by time-domain NMR under isoflurane anesthesia (Bruker Minispec).
Osmotic pumps.
Osmotic pumps (model 1004, 100 µL volume, 0.11 µL/h release rate, 28 d duration; Alzet Durect) loaded with saline vs. 50 µg of mouse musclin peptide (SFSGFGSPLDRLSAG SVEHRGKQRKAVDHSKKR, corresponding to amino acids 80–112; GenBank accession no. AAS87598.1; synthesized by Biosynthesis) were surgically implanted within the peritoneal cavity of mice under sterile conditions and general anesthesia. Mice recovered for 7 d before exercise testing was performed.
Exercise protocols.
A multilane treadmill (Columbus Instruments) was used to simultaneously exercise model mice with their controls. Mice were acclimated on the treadmill daily for 3 d for 20 min/d a velocity of 3.5 m/min and15° inclination. After this acclimation period, mice were exercised daily for 5 consecutive days at a speed of 12 m/min and inclination of 15° for 45 min/day except for the final day when mice were exercised for 20 min immediately before sample (blood, tissue) collection. To test exercise endurance, the exercise protocol consisted of stepwise increases in either incline or velocity at 3-min intervals until mice were no longer able to match the treadmill speed (39–41). For indirect calorimetry, oxygen consumption (VO2), and CO2 generation were measured by using a two-lane modular treadmill connected to the Oxymax indirect calorimetry system (Columbus Instruments) (39, 40). The calorimeter was calibrated before each measurement with a standard span gas (0.501% CO2, 20.53% O2 balanced with N2), and cross-calibrated against room air before each experiment.
Voluntary performance on running wheels.
Mice were housed in individual cages with free access to attached running wheels (Columbus Instruments). Running distance was calculated as 1/6πD⋅ΣRMPi, where D is wheel diameter and ΣRMPi is sum of averaged RPM values.
Blood collection.
Terminal blood collection was performed by direct cardiac puncture.
Molecular Biology.
RNA isolation and qRT-PCR.
Total RNA from mouse tissues and primary myocytes was isolated by using the RNeasy RNA Isolation Kit (Qiagen). A 1-µg primary myocyte or tissue RNA sample was used to synthesize cDNA in 50-µL reactions using Oligo(dT) as primer and SuperScript III Reverse Transcriptase (Invitrogen). Quantitative real-time PCR was performed on Mastercycler epgradient S (Eppendorf) by using SYBR green-based PCRs. For qRT-PCR, 1 µL of reverse transcription reaction was mixed with 10 pmol of each specific primer and 12.5 µL of SYBR PCR Master Mix (Bio-Rad). The reaction was incubated for 40 cycles consisting of denaturation at 95 °C for 10 s and annealing/extension at 59.9 °C for musclin, HPRT, Nrf2, and 56 °C for PGC1-α, Tfam, Nrf1, for 1 min. The quality of the PCR product was routinely checked by a thermal denaturation curve following the qPCR reactions. The threshold cycle (CT) was determined by Realplex2 software (Eppendorf), and quantification of relative mRNA levels was performed by ∆∆CT method or calculation of absolute number of copies based on the standard curve. The primers used in this study are as follows: HPRT forward (F), GGA CCT CTC GAA GTG TTG GAT AC, and HPRT reverse (R), GCT CAT CTT AGG CTT TGT ATT TGG CT; musclin: MuscF, TGT GGA CTT AGC ATC ACA GG, and MuscR, AGC TGA GAG TCT GTC AAG G; PGC1-α: PGC1-αF, TGA TGT GAA TGA CTT GGA TAC AGA CA, and PGC1-αR, GCT CAT TGT TGT ACT GGT TGG ATA TG; TfamF, GGA ATG TGG AGC GTG CTA AAA, and TfamR, TGC TGG AAA AAC ACT TCG GAA TA; Nrf1F, CGC AGC ACC TTT GGA GAA, and Nrf1R, CCC GAC CTG TGG AAT ACT TG; and Nrf2F, CAG CTC AAG GGC ACA GTG C, and Nrf2R,– GTG GCC CAA GTC TTG CTC C.
Western blotting.
Whole-cell protein extracts were prepared by homogenizing skeletal muscle tissue in NaCl 150 mM and Tris⋅HCl 50 mM (pH 7.8), supplemented with 1.5% (wt/vol) Triton X-100, protease, and phosphatase inhibitors (Roche). Nuclear extracts were obtained by using the Subcellular Protein Fractionation Kit for Tissues (Thermo Scientific). Electrophoresis was performed on 3–8% gradient Nu-Page Tris-Acetate or 10% Nu-Page Bis-Tris gels and transferred to 0.2-μm Sequi-Blot PVDF membranes (Bio-Rad). The membranes were blotted with total AKT1 and phospho-AKT1 (S437, T308; Cell Signaling), FOXO1 (Cell Signaling), total OXPHOS (rodent; Abcam), musclin (custom rabbit IgG produced against partial mouse musclin sequence NH2-sfsgfgspldrlsagsvehrgkqrkavdhskkr-COOH, corresponding to amino acids 80–112; GenBank accession no. AAS87598.1; Anaspec), and GAPDH (Santa Cruz Biotechnologies) antibodies. Densitometric analysis of Western blots was performed by using Adobe Photoshop (Adobe Systems).
ELISAs.
The 96-well ELISA Maxisorp plates (Nunc) were coated with custom rabbit antimusclin IgG (Anaspec) at 1 µg per well in BupH carbonate-bicarbonate buffer, pH 9.4 (Thermo Scientific) at 4 °C overnight. On the next day, plates were washed with TBS/0.05% Tween 20 and blocked with 5% (wt/vol) BSA/TBS for 1 h at room temperature. Synthetic musclin peptide was used as a standard (NH2-sfsgfgspldrlsagsvehrgkqrkavdhskkr-COOH, corresponding to amino acids 80–112; GenBank accession no. AAS87598.1; synthesized by Biosynthesis). EDTA–plasma samples and diluted standards were applied to the ELISA plates, and they were incubated overnight at 4 °C. Plates were washed and incubated with biotinylated rat monoclonal antimusclin IgG (clone no. 311417; R&D Systems) for 1.5 h at room temperature. Plates were washed and incubated with SA-HRP conjugate 1 h at room temperature, then washed and incubated with the QuantaBlu Fluorogenic Peroxidase Substrate (Thermo Scientific). Fluorescence was detected at 325/420 nm.
cGMP levels were measured by using the cyclicGMP enzyme immunoassay (EIA) kit (Cayman Chemical). Mice were exercised for 5 d, 12 m/min, 45 min before tissue collection. At the day of the experiment, mice were exercised 12 m/min, 20 min, sedated with isoflurane, and gastrocnemius muscle tissue was collected. Cyclic nucleotides were extracted by using 5% (wt/vol) trichloracetic acid. Samples were acetylated and used for cGMP level measurements according to the manufacturer’s protocol.
ANP levels in plasma were measured by using an ANP (rat, mouse) Fluorescent EIA Kit Ultra-Sensitive (Phoenix Pharmaceuticals). Plasma samples from three mice were combined to collect 1 mL of EDTA-plasma for ANP extraction.
Mitochondrial content.
Mitochondrial protein fraction was isolated from gastrocnemius muscle by using the Mitochondria Isolation Kit (Thermo Scientific) according to manufacturer’s protocol. Mitochondria protein content was determined as amount of mitochondrial protein per mg of wet tissue.
Cell culture.
Mouse primary myoblasts were isolated from gastrocnemius muscle of 4- to 6-wk-old mice. Fresh muscle tissue was digested with 2 mg/mL collagenase II in DMEM-F12 for 1.5 h at 37 °C; then 30 min at 37 °C with 1 mg/mL collagenase II and 0.5 mg/mL dispase in DMEM-F12. Tissue was ground and passed through a 100-µm then 70-µm cell strainer and spun at 180 × g for 10 min. Myoblasts were resuspended in DMEM-F12 supplemented with 20% (vol/vol) FBS, 40 ng/mL basic FGF (bFGF), nonessential amino acids, and 1 mM β-mercaptoethanol. Cells were plated on Matrigel-coated dishes. Cells were maintained in DMEM-F12 supplemented with 20% (vol/vol) FBS, 10 ng/mL bFGF, nonessential amino acids, and 1 mM β-mercaptoethanol until 80% confluent. Then they were differentiated in DMEM-F12 supplemented with 2% (vol/vol) FBS and insulin–transferrin–selenium for 10 d. Human skeletal myoblast primary culture (lot no. SLSK002; Zenbio) was maintained according to the supplier’s manual.
Histology.
Muscles were dissected from anesthetized mice, embedded in OCT compound, and frozen in 2-methylbutane precooled at −165 °C in liquid nitrogen (41). Muscles were stored at −80 °C until used. For histology, 10-µm cross-sections, cut using a Microm cryostat, cooled to −20 °C, were mounted on positively charged slides (Superfrost/Plus; Fisher Scientific) and stored at −80 °C until used.
Fiber type composition.
Serial cross-sections were labeled simultaneously with anti-MHC primary I, IIA, IIB, or IIX antibodies (BA-F8 anti-MHC-I mouse IgG2b, SC-71 anti-MHC IIA mouse IgG, 6H1 anti-MHC IIX mouse IgM, and FB-F3 anti-MHC IIB mouse IgM; University of Iowa Developmental Studies Hybridoma Bank). Slides were then incubated with secondary antibodies (Alexa 647 anti-mouse IgG2b, Alexa 488 anti-mouse anti-IgG, and Alexa 568 anti-mouse IgM; Life Technologies). Slides were stored at −80 °C until imaged. Control sections were also stained without the primary antibody to test for nonspecific secondary antibody binding. Images were obtained by confocal microscopy (LSM 510 Meta; Carl Zeiss).
SDH staining.
Frozen tissue sections were incubated in buffer (50 mM sodium succinate, 50 mM sodium phosphate, and 0.5 mg/mL nitro blue tetrazolium, pH 7.6) for 60 min at 37 °C, followed by washes with H2O, then 30%, 60%, and 90% (vol/vol) acetone solutions to remove unbound nitro blue tetrazolium, and dehydration with sequential 70%, 80%, 90%, and 100% (vol/vol) EtOH and 100% (vol/vol) xylene washes before mounting and imaging by light microscopy (BX-51; Olympus).
Musclin staining.
Tissue cross-sections were labeled with antimusclin primary antibody (Santa Cruz), then incubated with anti-rabbit Alexa 568 antibody (Life Technologies). Nuclei were stained with SYTO 16 green fluorescent nucleic acid stain (Life Technologies). Images were obtained by confocal microscopy (LSM 510 Meta; Carl Zeiss).
Transmission Electron Microscopy.
Muscles were fixed in 2.5% (vol/vol) glutaraldehyde and 0.1 M Na+ cacodylate buffer, pH 7.2, overnight at 4 °C, followed by three 20-min washes in the same, fixed in 4% (wt/vol) OsO4, washed in 0.1 M sodium cacodylate buffer, then dH2O, followed by 2.5% (wt/vol) uranyl acetate. A series of ethanol washes was used to dehydrate the sample, which was then exchanged with an ethanol and Spurr’s mixture series of increasing Spurr’s concentration, culminating in a final solution of 100% Spurr’s resin. Muscles were embedded in Spurr’s resin at 60 °C for 24–48 h. Ultramicrotomy was carried out at 90 nm, and samples were collected on 200 mesh formvard grids for staining with uranyl and lead. Stained sections were examined with a JEOL JEM-1230 transmission electron microscope, and digital images were collected with a Gatan UltraScan 1000 2k × 2k CCD camera.
Statistics.
Results are expressed as mean ± SEM. Comparisons between two groups were made using the two-sided Student's t test and between more than two groups using analysis of variance. A P value < 0.05 was considered statistically significant. Sigma Plot 11 was used for all statistical analyses.
Acknowledgments
We thank Chantal Allamargot, PhD, for her assistance with electron microscopy. This work was supported by National Institutes of Health Grants HL113089 (to D.H-Z.) and HL093368 and DK092412 (to L.V.Z.); Veterans Affairs Merit Review Program 1I0BX000718 (to L.V.Z.); and the Fraternal Order of Eagles Diabetes Research Center.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1514250112/-/DCSupplemental.
References
- 1.Lee IM, et al. Lancet Physical Activity Series Working Group Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen BK, Akerström TC, Nielsen AR, Fischer CP. Role of myokines in exercise and metabolism. J Appl Physiol (1985) 2007;103(3):1093–1098. doi: 10.1152/japplphysiol.00080.2007. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen BK, et al. The metabolic role of IL-6 produced during exercise: Is IL-6 an exercise factor? Proc Nutr Soc. 2004;63(2):263–267. doi: 10.1079/PNS2004338. [DOI] [PubMed] [Google Scholar]
- 4.Nishizawa H, et al. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004;279(19):19391–19395. doi: 10.1074/jbc.C400066200. [DOI] [PubMed] [Google Scholar]
- 5.Thomas G, et al. Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. J Biol Chem. 2003;278(50):50563–50571. doi: 10.1074/jbc.M307310200. [DOI] [PubMed] [Google Scholar]
- 6.Yasui A, et al. Foxo1 represses expression of musclin, a skeletal muscle-derived secretory factor. Biochem Biophys Res Commun. 2007;364(2):358–365. doi: 10.1016/j.bbrc.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Potter LR, Abbey-Hosch S, Dickey DM. Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev. 2006;27(1):47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 8.Kita S, et al. Competitive binding of musclin to natriuretic peptide receptor 3 with atrial natriuretic peptide. J Endocrinol. 2009;201(2):287–295. doi: 10.1677/JOE-08-0551. [DOI] [PubMed] [Google Scholar]
- 9.Moffatt P, et al. Osteocrin is a specific ligand of the natriuretic Peptide clearance receptor that modulates bone growth. J Biol Chem. 2007;282(50):36454–36462. doi: 10.1074/jbc.M708596200. [DOI] [PubMed] [Google Scholar]
- 10.Suda M, et al. Skeletal overgrowth in transgenic mice that overexpress brain natriuretic peptide. Proc Natl Acad Sci USA. 1998;95(5):2337–2342. doi: 10.1073/pnas.95.5.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasoda A, et al. Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10(1):80–86. doi: 10.1038/nm971. [DOI] [PubMed] [Google Scholar]
- 12.Jaubert J, et al. Three new allelic mouse mutations that cause skeletal overgrowth involve the natriuretic peptide receptor C gene (Npr3) Proc Natl Acad Sci USA. 1999;96(18):10278–10283. doi: 10.1073/pnas.96.18.10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsukawa N, et al. The natriuretic peptide clearance receptor locally modulates the physiological effects of the natriuretic peptide system. Proc Natl Acad Sci USA. 1999;96(13):7403–7408. doi: 10.1073/pnas.96.13.7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catoire M, Kersten S. The search for exercise factors in humans. FASEB J. 2015;29(5):1615–1628. doi: 10.1096/fj.14-263699. [DOI] [PubMed] [Google Scholar]
- 15.Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9(3):208–214. doi: 10.1007/s11892-009-0034-5. [DOI] [PubMed] [Google Scholar]
- 16.Stitt TN, et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14(3):395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 17.Wu M, Falasca M, Blough ER. Akt/protein kinase B in skeletal muscle physiology and pathology. J Cell Physiol. 2011;226(1):29–36. doi: 10.1002/jcp.22353. [DOI] [PubMed] [Google Scholar]
- 18.Shaw M, Cohen P, Alessi DR. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide 3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem J. 1998;336(Pt 1):241–246. doi: 10.1042/bj3360241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335(Pt 1):1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: More than just a road to PKB. Biochem J. 2000;346(Pt 3):561–576. [PMC free article] [PubMed] [Google Scholar]
- 21.Sable CL, Filippa N, Hemmings B, Van Obberghen E. cAMP stimulates protein kinase B in a Wortmannin-insensitive manner. FEBS Lett. 1997;409(2):253–257. doi: 10.1016/s0014-5793(97)00518-8. [DOI] [PubMed] [Google Scholar]
- 22.Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature. 1998;396(6711):584–587. doi: 10.1038/25147. [DOI] [PubMed] [Google Scholar]
- 23.Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol (1985) 2001;90(5):1936–1942. doi: 10.1152/jappl.2001.90.5.1936. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ. Contraction regulation of Akt in rat skeletal muscle. J Biol Chem. 2002;277(14):11910–11917. doi: 10.1074/jbc.M112410200. [DOI] [PubMed] [Google Scholar]
- 25.Turinsky J, Damrau-Abney A. Akt kinases and 2-deoxyglucose uptake in rat skeletal muscles in vivo: Study with insulin and exercise. Am J Physiol. 1999;276(1 Pt 2):R277–R282. doi: 10.1152/ajpregu.1999.276.1.R277. [DOI] [PubMed] [Google Scholar]
- 26.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 27.Zois NE, et al. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol. 2014;11(7):403–412. doi: 10.1038/nrcardio.2014.64. [DOI] [PubMed] [Google Scholar]
- 28.Miyashita K, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58(12):2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engeli S, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122(12):4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shephard RJ. Tests of maximum oxygen intake. A critical review. Sports Med. 1984;1(2):99–124. doi: 10.2165/00007256-198401020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Bord S, Ireland DC, Moffatt P, Thomas GP, Compston JE. Characterization of osteocrin expression in human bone. J Histochem Cytochem. 2005;53(10):1181–1187. doi: 10.1369/jhc.4C6561.2005. [DOI] [PubMed] [Google Scholar]
- 32.Gruden G, Landi A, Bruno G. Natriuretic peptides, heart, and adipose tissue: New findings and future developments for diabetes research. Diabetes Care. 2014;37(11):2899–2908. doi: 10.2337/dc14-0669. [DOI] [PubMed] [Google Scholar]
- 33.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 34.Nisoli E, et al. Mitochondrial biogenesis in mammals: The role of endogenous nitric oxide. Science. 2003;299(5608):896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 35.Nisoli E, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proc Natl Acad Sci USA. 2004;101(47):16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haas B, et al. Protein kinase G controls brown fat cell differentiation and mitochondrial biogenesis. Sci Signal. 2009;2(99):ra78. doi: 10.1126/scisignal.2000511. [DOI] [PubMed] [Google Scholar]
- 37.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Natl Inst Health; Bethesda: 1996. DHHS Publ No (NIH) 85-23. [Google Scholar]
- 38.He BJ, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17(12):1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alekseev AE, et al. Sarcolemmal ATP-sensitive K(+) channels control energy expenditure determining body weight. Cell Metab. 2010;11(1):58. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu Z, et al. Sarcolemmal ATP-sensitive potassium channels modulate skeletal muscle function under low-intensity workloads. J Gen Physiol. 2014;143(1):119–134. doi: 10.1085/jgp.201311063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zingman LV, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci USA. 2002;99(20):13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]