Significance
De-repressing appendage growth induces development of ectopic wings on the dorsal prothorax (T1) of the neopteran insect Oncopeltus. These T1 wings, albeit fully developed, are small and of primarily dorsal origin. Transcriptome data indicate that incorporation of ventrally originating tissue was a key evolutionary innovation for generating large and useful T2 and T3 wings. Complimentary functional experiments reveal that wings and an adjacent thoracic plate are not developmentally distinct structures, and are coregulated to create tight wing folding that arose during the transition from paleopteran to neopteran insects. Finally, Ultrabithorax regulates the divergence of fore- and hindwing morphology, a culminating but also ancient feature of insect wing diversity. These innovations account for major features of insect wing origin and diversification.
Keywords: wing origins, Sex combs reduced, Ultrabithorax, RNA-seq, vestigial
Abstract
Winged insects underwent an unparalleled evolutionary radiation, but mechanisms underlying the origin and diversification of wings in basal insects are sparsely known compared with more derived holometabolous insects. In the neopteran species Oncopeltus fasciatus, we manipulated wing specification genes and used RNA-seq to obtain both functional and genomic perspectives. Combined with previous studies, our results suggest the following key steps in wing origin and diversification. First, a set of dorsally derived outgrowths evolved along a number of body segments including the first thoracic segment (T1). Homeotic genes were subsequently co-opted to suppress growth of some dorsal flaps in the thorax and abdomen. In T1 this suppression was accomplished by Sex combs reduced, that when experimentally removed, results in an ectopic T1 flap similar to prothoracic winglets present in fossil hemipteroids and other early insects. Global gene-expression differences in ectopic T1 vs. T2/T3 wings suggest that the transition from flaps to wings required ventrally originating cells, homologous with those in ancestral arthropod gill flaps/epipods, to migrate dorsally and fuse with the dorsal flap tissue thereby bringing new functional gene networks; these presumably enabled the T2/T3 wing’s increased size and functionality. Third, “fused” wings became both the wing blade and surrounding regions of the dorsal thorax cuticle, providing tissue for subsequent modifications including wing folding and the fit of folded wings. Finally, Ultrabithorax was co-opted to uncouple the morphology of T2 and T3 wings and to act as a general modifier of hindwings, which in turn governed the subsequent diversification of lineage-specific wing forms.
Some 350 million years ago, the development of insect wings was a seminal event in the evolution of insect body design (1, 2). The ability to fly was critical to insects becoming the most diverse and abundant animal group, and the origin of such novelty has been a focus of intense scientific inquiry for more than a century (3, 4). More recently, through studies of genetic model systems such as Drosophila, the mechanisms of wing morphogenesis have been elucidated (5–12). Still lacking however is a comprehensive understanding of transitional steps connecting the morphology of structures observed in the fossil record with that of the modern-day insects, including wing origins and subsequent diversification.
The initial stages of insect wing evolution are missing from the fossil record and it is therefore necessary to use indirect evidence from fossils that postdate the origin and initial radiation of pterygotes (2). Larvae of many of those taxa featured dorsally positioned outgrowths on each of the thoracic and abdominal segments (2, 13), apparently serial homologs (i.e., similar structures likely arising from a common set of developmental mechanisms). Diverse lineages independently lost those dorsal appendages on the abdomen while undergoing parallel modifications of wing-like structures on thoracic (T1–T3) segments. Specifically, the T1 winglets were always much smaller in fossils and apparently lacked hinge articulation whereas T2 (fore-) and T3 (hind-) wings were fully operational in adults, featuring muscles, venation, and size that rendered them capable of flapping flight (14). T1 winglets were subsequently repressed in multiple lineages (15–18) whereas T2 and T3 wings acquired morphology similar to modern day Paleoptera (mayflies and dragonflies) and other extinct paleopterous orders. The transition from Paleoptera, which rest with wings extended from the body, to Neoptera, which rest with wings folded flat against the body, required changes in the hinge mechanism, with many orders also evolving a precise mechanical fit between wing margins and the adjacent body wall of the dorsal thorax. Finally, the radiation of Neoptera encompassed a further divergence between the fore- and hindwings in terms of their shape, size, and texture (5, 19, 20). Together, this set of transitions accounts for major features of the diversity and lineage-specific wing morphologies among fossil and extant taxa.
To gain insight into genetic mechanisms governing these transitional steps, we used a direct-developing neopteran species, the milkweed bug (Oncopeltus fasciatus; Hemimetabola, Hemiptera). The phylogenetic placement of Oncopeltus, basal to the more derived holometabola (e.g., flies, beetles, bees, butterflies, and so forth that have a pupal stage), is important because holometabolous appendage development occurs from imaginal discs, which are collections of cells that form and commit to appendage identity early in larval development. Oncopeltus and other hemimetabolous insects lack imaginal discs and acquire adult morphology gradually through a series of nymphal stages, similar to early fossil insects. Hence, examination of wing development in a hemimetabolous insect can help resolve the ancestral versus derived status of developmental traits present in holometabola and in general may provide new evolutionary perspectives.
Oncopeltus features brightly colored forewings with a stiff proximal region and a more flexible membranous apex (hemelytra), entirely membranous hind wings, and a well-developed dorsal T2 structure (scutellum) that fits precisely against trailing edges of the folded wings. To examine wing developmental mechanisms underlying these features, we combined a candidate gene approach in which we depleted (via RNA interference, RNAi) the expression of Oncopeltus orthologs of wing specification genes, and a global approach (RNA-seq) (21) that characterized all expressed genes in wild-type T2 and T3 wings, ectopic T1 wings, and wild-type T1 body wall. The results provide independent and expanded insights into the origins and fate of T1 wings, the transition from paleoptera to neoptera, and the eventual diversification of T2 and T3 wing morphology.
Results
Insights About Wing Origins and Functionalization from T1 Ectopic Wings.
Depletion via RNAi of the homeotic gene Sex combs reduced (Scr), which regulates T1 segmental identity (8), results in the formation of a small ectopic wing on T1 (16). Similar small T1 wings are present in diverse early insect fossils (2). Therefore it is possible that repression of Scr releases an ancient wing developmental program. Alternatively, the small T1 ectopic wing (T1 EW) may be a partial conversion to T2 segmental identity; that is, a lower-penetrance phenotype but otherwise analogous to what occurs in T3 when Ultrabithorax (Ubx) is repressed (see below).
To begin to resolve these alternatives, we characterized the transcriptome (RNA-seq) (21) of the developing wing pads of fifth-instar nymphs on all three thoracic segments (including T1 EW in Scr RNAi individuals) and wild-type T1 thoracic body wall (Fig. 1). In the 500 genes most up-regulated in each of the three wing types compared with the T1 body wall, genes involved in imaginal disc morphogenesis were among the most enriched Gene Ontology (GO) categories [Functional group analyses, Database for Annotation, Visualization and Integrated Discovery (DAVID) (22), analyzing Biological Processes level 5: development] (Table S1). Among those was a set of known wing specification genes [vestigial (vg), scalloped (sd), wingless (wg), and apterous (ap)] (Fig. 2A) whose expression did not differ among the three wing types (P = 0.22). However, the wing disc and general appendage specification gene Distal-less (Dll) had reduced expression in T1 EW (more than fourfold lower than T2 wing), as did a number of genes detected only in T2/T3 (Fig. S1 and Table S2).
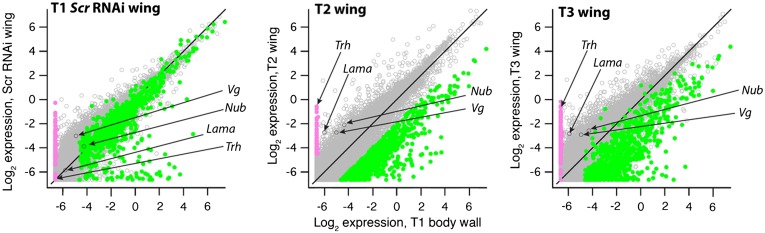
Fig. 1.
Gene expression [mean log2 (normalized read count + 0.01)] in the three wing libraries compared with the same gene in T1 body wall. Genes undetectable or nearly so in T1 body wall but up-regulated >fourfold in T2 wings are colored pink. Genes down-regulated >fourfold in T2 wings are shown in green.
Table S1.
Enrichment analysis of the 500 genes most up-regulated in each of the three wing types compared with T1 body wall
| Rank and wing type | GO | Count | % | P value | Enrichment |
| SCR RNAi ectopic wing | |||||
| 6 | GO:0007444∼imaginal disk development | 38 | 10.2 | 1.30E-05 | 2.1 |
| 9 | GO:0007560∼imaginal disk morphogenesis | 30 | 8.1 | 4.10E-05 | 2.2 |
| 13 | GO:0007444∼wing disk development | 28 | 7.5 | 1.50E-04 | 2.2 |
| 14 | GO:0007472∼wing disk morphogenesis | 24 | 6.5 | 2.10E-04 | 2.3 |
| T2 wing | |||||
| 7 | GO:0007560∼imaginal disk morphogenesis | 23 | 6.3 | 7.40E-04 | 2.1 |
| 9 | GO:0007444∼imaginal disk development | 27 | 7.4 | 1.90E-03 | 1.9 |
| 20 | GO:0007476∼imaginal disk-derived wing morphogenesis | 16 | 4.4 | 1.60E-02 | 1.9 |
| 23 | GO:0007472∼wing disk morphogenesis | 16 | 4.4 | 1.80E-02 | 1.9 |
| T3 wing | |||||
| 3 | GO:0007444∼imaginal disk development | 41 | 10.80 | 1.40E-07 | 2.4 |
| 9 | GO:0007560∼imaginal disk morphogenesis | 31 | 8.10 | 4.60E-06 | 2.4 |
| 12 | GO:0007444∼wing disk development | 30 | 7.90 | 6.90E-06 | 2.4 |
| 13 | GO:0007472∼wing disk morphogenesis | 26 | 6.80 | 9.50E-06 | 2.6 |
| 16 | GO:0007472∼wing disk morphogenesis | 25 | 6.60 | 2.30E-05 | 2.6 |
Shown are the wing development categories within GO Biological Process level 5 (development) that fell in the top 25 functional groups enriched in wings. The background gene set for these analyses was the set of unique Drosophila BLASTx homologs in the filter-passed contigs (n = 6,380). We show the unadjusted P values because enrichment of theses wing development genes was an a priori hypothesis.
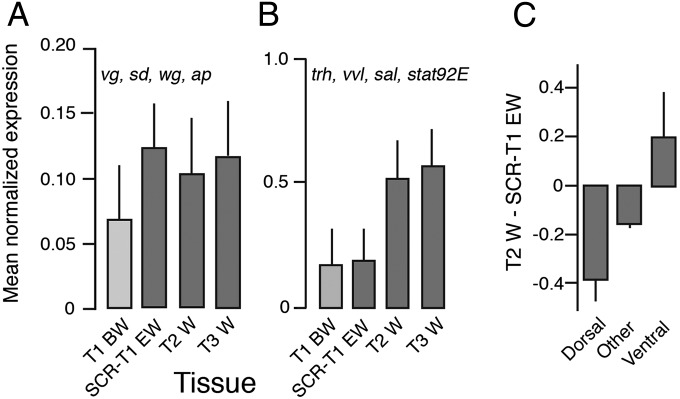
Fig. 2.
Least-square means (+ SE) of normalized read counts of (A) a set of wing specification genes (vg, sd, wg, and ap) equally expressed in all wing types, and (B) the ventral origin genes nub and tracheal genes (trh, vvl, sal, and stat92E) that are missing in the T1 Scr RNAi ectopic wing. These means are from a model that included “gene” and “tissue” as independent variables. (C) Transcriptome-wide mean expression difference between T2 wing and T1 Scr RNAi ectopic wing for genes involved in development of dorsal closure, ventral furrow, or neither.
Fig. S1.
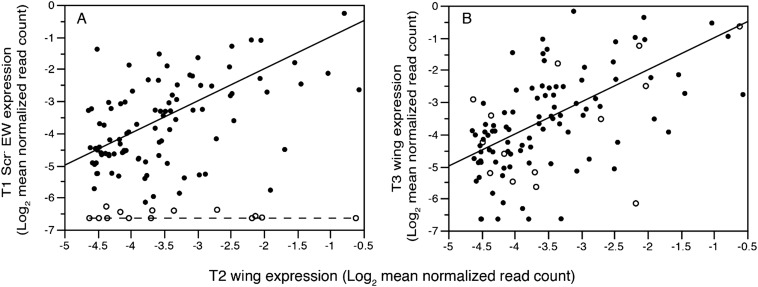
Expression in T1 Scr RNAi ectopic wing (A) and T3 (B) wings compared with T2 wings for 109 wing specific genes (undetectable or nearly so in T1 body wall and at least fourfold higher than minimal expression in T2 wing). Solid line is the line of identity (slope = 1, intercept = 0) and the flat dashed line (A) shows genes (open symbols) missing from the T1 ectopic wing. Note that these same genes, with one exception, are expressed in T3 wing at levels indistinguishable from T2 wing. One gene very highly expressed in all wing types (Moesin) is excluded from this plot because its inclusion necessitates rescaling the axes and reducing clarity of the key result.
Table S2.
Reduced Distal-less expression the T1 ectopic wing and 14 additional T2 wing-specific genes absent in T1 Scr RNAi ectopic wing (points along the flat line in Fig. S1A)
| Transcript | Flybase gene name | Name | Bitscore D mel | Log2 mean norm T1 | Log2 mean norm T1 Scr EW | Log2 mean norm T2 | Log2 mean norm T3 |
| comp12964_c0_seq2 | FBgn0000157 | Distal-less | 168 | −6.64 | −5.27 | −2.88 | −4.78 |
| comp4890_c0_seq1 | FBgn0262139 | Trachealess | 766 | −6.64 | −6.64 | −0.54 | −0.64 |
| comp228_c1_seq14 | FBgn0086906 | Sallimus | 1,322 | −6.63 | −6.58 | −1.85 | −1.25 |
| comp5341_c0_seq6 | FBgn0262111 | Forked | 295 | −6.64 | −6.63 | −1.46 | −2.50 |
| comp11498_c0_seq3 | FBgn0243486 | Reduced ocelli | 246 | −6.64 | −6.41 | −2.82 | −1.80 |
| comp14162_c0_seq1 | FBgn0043792 | CG30427 | 496 | −6.38 | −6.01 | −2.70 | −3.53 |
| comp13582_c0_seq1 | FBgn0052645 | CG32645 | 214 | −6.64 | −6.45 | −3.66 | −4.61 |
| comp8373_c0_seq2 | FBgn0000140 | Abnormal spindle | 369 | −6.64 | −6.64 | −4.48 | −4.25 |
| comp15346_c0_seq1 | FBgn0028370 | Kekkon-3 | 68.9 | −6.64 | −6.64 | −4.19 | −2.92 |
| comp383_c0_seq3 | FBgn0035875 | Cuticular protein 66Cb | 87 | −6.26 | −6.64 | −1.61 | −6.16 |
| comp23245_c0_seq1 | FBgn0038447 | CG14892 | 145 | −6.46 | −6.64 | −3.52 | −5.48 |
| comp13956_c0_seq5 | FBgn0259818 | — | 212 | −6.28 | −6.64 | −4.37 | −5.21 |
| comp9286_c0_seq2 | FBgn0036876 | — | 130 | −6.64 | −6.64 | −3.70 | −5.19 |
| comp7952_c0_seq3 | FBgn0011206 | Boule | 133 | −6.64 | −6.64 | −4.35 | −3.42 |
| comp7767_c0_seq1 | FBgn0036879 | Cuticular protein 76Bb | 92.8 | −6.41 | −6.02 | −3.68 | −5.64 |
A mean normalized expression value of −6.64 represents undetectable expression (see Fig. 1 to relate these expression levels to genes overall).
Most conspicuously absent from T1 EW was trachealess (trh) (Fig. 1), an arthropod ventral appendage gene (23) expressed in the epipods of Artemia (24). During Drosophila embryogenesis trh is expressed in a cell population that migrates from the ventral ectoderm dorsally to become either glands (corpora cardiaca, prothoracic gland) in anterior segments, or tracheae in segments T2 through A8 (25, 26). Also absent from T1 EW (Table S2) was a wing-specific isoform of salimus (sls), a gene involved in muscle development and attachment (27–29) that in Drosophila is coexpressed with trh in ventral-origin cells. Another tracheal morphogenesis gene, forked (f) (30), involved in formation of mechanosensory wing bristles (31), was among the wing-specific genes missing from T1 EW. Additional trh-regulated genes (vvl, spalt, and Stat92E) (26) and the ventral-origin wing specification gene nubbin (nub) (32) were detectable but reduced in T1 EW compared with T2 wing (P = 0.0001; mean difference > twofold) (Fig. 2B). The transcriptome data are therefore robust in showing that T1 EW is developmentally a wing but lacks or has markedly reduced expression of ventral-origin genes such as Dll-, nub-, trh-, and trh-regulated genes that are consistently up-regulated in T2 and T3 wings.
A previous study demonstrated fusion of dorsal and ventral cell populations during the development of wings in basal insects (mayflies: Paleoptera, Ephemeroptera) (33) and hence a strong up-regulation of both dorsal and ventral-origin genes may be a fundamental feature of normal insect wing morphogenesis, with ventral-origin genes specifically absent in T1 wings that were an evolutionary dead end. Alternatively, it is possible that the timing of ectoptic wing development on T1 in Oncopeltus differs from that of T2/T3 wings and therefore the differences in size and gene expression might reflect delayed development of the T1 EW. Hence, we used the transcriptome data to determine if there is evidence for delayed development in T1 EW, and if there is broad support for a reduced ventral contribution to the T1 EW. First, to address developmental timing, we examined expression of lama, a gene that preserves the pluripotency of disc cells and is not expressed after tissue determination (34). lama was more highly expressed in T2 and T3 wings whereas it was nearly undetectable in T1 EW and the T1 body wall (Fig. 1). Absence of lama from T1 EW indicates completion of development rather than delay. In addition, functional studies in Periplaneta americana (cockroach, another neopteran) show that the depletion of Scr also generates small prothoracic wings (17) despite the difference that RNAi can be applied consecutively over five to six nymphal stages (versus only two stages in Oncopeltus). Furthermore, a mutation in another cockroach (Blatella germanica) creates small T1 wings (35). Hence, the small size of T1 ectopic wings in neoptera is highly unlikely to be an artifact resulting from insufficient developmental time to fully grow these appendages. The uniqueness of T1 EW is further supported by the hundreds of genes down-regulated at least fourfold in T2 wings (and generally similar in T3 wings) but not down-regulated in T1 EW (green points in Fig. 1). These independent lines of evidence fully support the notion that the ectopic T1 wing is both a unique and fully differentiated structure rather than being an incompletely developed forewing.
To determine if there is broad transcriptome-wide evidence for underrepresentation of ventral-origin genes in the T1 EW, we determined the expression level of all genes with Drosophila best-hit homologs having GO Biological Processes annotations containing “dorsal closure” or “ventral furrow” (but not both), based on the assumption that these genes are expressed more strongly at opposite ends of the dorso-ventral body axis. T2 wings had reduced expression of dorsal closure genes (n = 149) compared with T1 EW, but greater expression of ventral furrow genes (n = 17; P = 0.03 for the mean T2-T1 EW difference). The expression difference of all other genes (n = 12,257) was intermediate (Fig. 2C). This result provides broad support for reduced expression of ventral-origin genes in the T1 ectopic wing.
Effects of Wing Specification Genes on Neopteran Thoracic Segments.
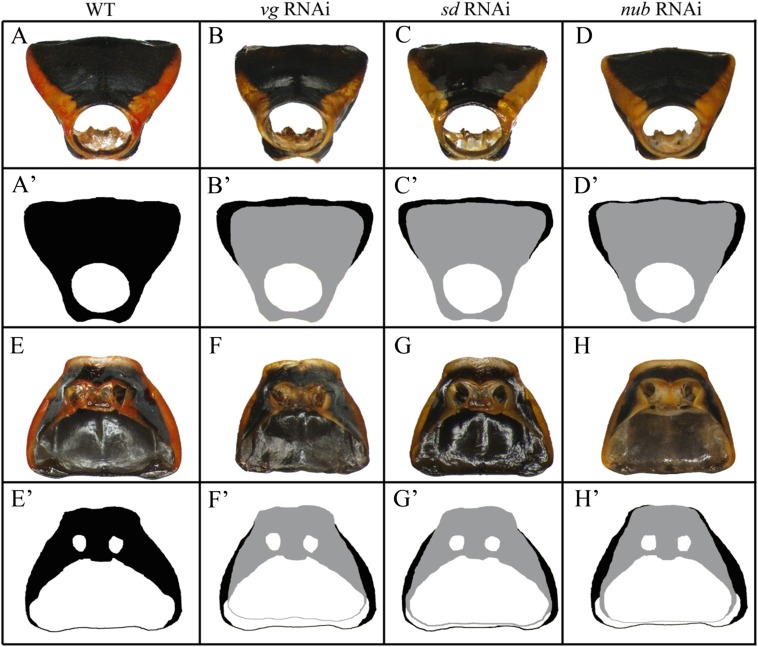
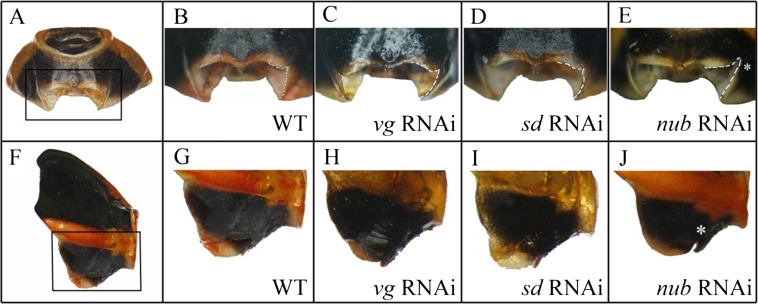
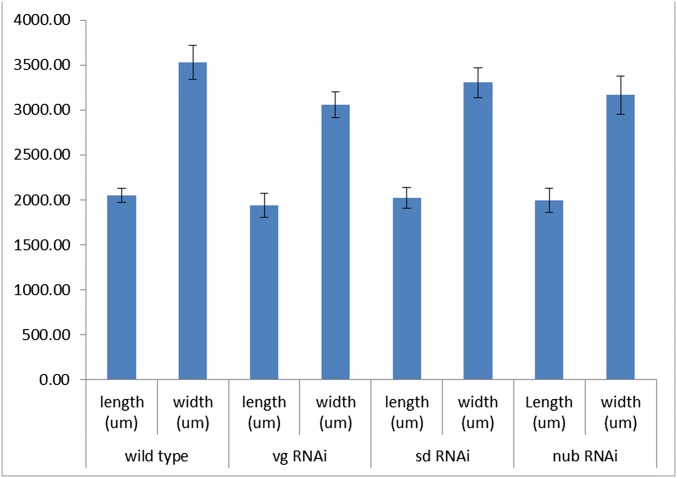
In adult Oncopeltus from nymphs treated with RNAi against key wing genes (vg, sd, and nub), the main effect in T1 was localized in the posterior dorsal pronotum (T1) (Fig. S2). No visible effects were observed on the ventral T1 plates, including the regions corresponding to sternum and epimeron (Fig. S2 E–H and E′–H′). Although these two regions are completely missing in Tribolium vg RNAi adults (18), the only change observed on the ventral prothorax in Oncopeltus is restricted to an area surrounding the leg base (compare Fig. S3B with Fig. S3E). Hence, the functions of wing specification orthologs in Oncopeltus T1 are mainly localized to the dorsal pronotum and have an effect primarily on its overall width (Fig. S4).
Fig. S2.
Effects of vg, sd, and nub RNAi on the prothorax (T1) of Oncopeltus fasciatus. (A–D) The fronto-dorsal view of: wild type (A), vg RNAi (B), sd RNAi (C), and nub RNAi (D). (A′) The outline of the wild-type pronotum. (B′–D′) The overlay of vg RNAi, sd RNAi, and nub RNAi, respectively (gray), onto wild type (black). (E–H) The ventral view of T1 sternum in: wild type (E), vg RNAi (F), sd RNAi (G), and nub RNAi (H). (E′) The outline of wild-type sternum. (F′–H′) The overlay of vg RNAi, sd RNAi, and nub RNAi, respectively (gray), onto wild type (black).
Fig. S3.
(A) Fronto-ventral view of T1 ventral plate. (B–E) close-up of region outlined in A in a wild-type, vg RNAi, sd RNAi, and nub RNAi, respectively. (F) The lateral view of T1 plate. (G–J) The magnified detail of the outlined region in wild-type (G), vg RNAi (H), sd RNAi (I), and nub RNAi (J), respectively. The asterisk in J shows a notch created in the nub RNAi treatment.
Fig. S4.
The effect of wing specification genes on the length and width of the T1 notum (n = 10 for each gene).
Adult T2 morphology in Oncopeltus is comprised of semimembranous wings (hemelytra) that fit tightly against a large, triangularly shaped scutellum on the dorsal side (Fig. 3 A, B, F, and J). Tucked beneath the hemelytra is a pair of membranous T3 hindwings, with distinct shapes and pigmentation (Fig. 3B). Both pairs of wings in vg RNAi adults had alterations in their shape and size, especially the hindwings, which became greatly reduced (Fig. 3C). The sd RNAi wings displayed similar changes, but to a lesser degree (Fig. 3D), consistent with studies in Drosophila where the wings of sd mutants do not result in a complete loss of wings (36). The depletion of nub also caused a reduction in the fore- and hindwing size, and a change in forewing shape (Fig. 3E).
Fig. 3.
The functions of wing genes on the mesothorax (T2) of Oncopeltus. (A) Dorsal morphology of the adult T2 segment; arrowheads point to the clavus of the forewings. (A′) The dissected dorsal T2 plate of a fifth nymph. The dotted line denotes the proximal-most boundary of wing primordia. (B) Wild-type fore- and hindwings. (C–E) The effects of vg RNAi, sd RNAi, and nub RNAi on adult wing morphology, respectively. (F) Dissected dorsal T2 notum of wild-type adult. (G–I) The effect of vg RNAi, sd RNAi, and nub RNAi, respectively. (J–M) The alignment of the scutellum and clavus of the forewing in wild-type (J), vg RNAi (K), sd RNAi (L), and nub RNAi (M) adults, respectively. In K–M, this alignment is disturbed causing scutellum and clavus to lose their close contact to one another (the created open space is artificially colored in green). scu, scutellum.
These genes also affected the scutellum on T2. No such effects were previously observed in beetles (18, 37), possibly because radical reconfiguration of the thorax during the pupal state in holometabolous insects may override any such effects. In Oncopeltus vg RNAi adults, the scutellum lost its tip, and its distinct triangular morphology was changed to a shield-like shape (Fig. 3G). sd RNAi also affected the posterior half of the scutellum, but to a lesser degree (Fig. 3H). In nub RNAi adults, the scutellum was not affected but there was a curving of the forewing clavus [the location of nub expression in Drosophila wings (38)], preventing the wings from lying flat (Fig. 3M).
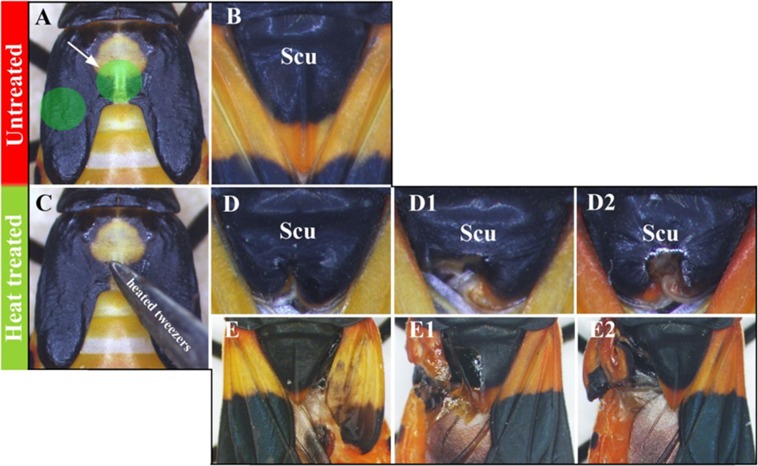
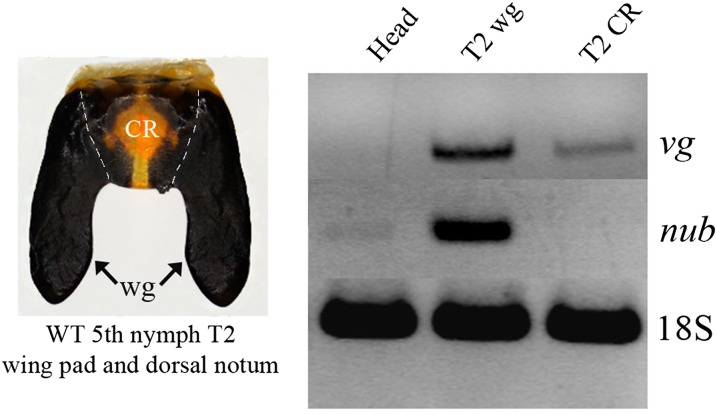
To determine which part of the developing wing pad gives rise to thoracic structures, we performed heat lesions to the central region and lateral extensions (Fig. S5). The former resulted in a misshapen scutellum and unaffected wings whereas the latter had the opposite affect: the wings were malformed and the scutellum remained unchanged. This result suggests that the central region of the developing wing pad gives rise to the scutellum and the lateral extensions form the wing blade. When combined with the RT-PCR results that show vg is expressed in both the central region and lateral extensions, whereas nub is expressed in only the extensions (Fig. S6), these observations corroborate the above RNAi phenotypes, where vg affected both the wing blade and scutellum but nub affected only the wing blade (Fig. 3 C and G vs. Fig. 3 E and I).
Fig. S5.
(A) Dorsal view of a T2 wing pad in wild-type fifth nymph. (B) Final adult T2 morphology with the focus on scutellum. (C) The heat lesions administered to the central region or laterally on the wing primordia of fifth nymphs (green highlighted regions in A) resulted in (respectively) malformation of the scutellum (D–D2) or wings (E–E2) in adults.
Fig. S6.
(Left) The dissected wild-type wing pad in fifth nymph, showing a central region (CR) and wing primordia (wg). (Right) RT-PCR showing expression of vg in the CR and wg regions, whereas nub is only expressed in the wg region.
What Developmental Mechanisms Create a Fully Formed and Functional Wing, Including Fit with Thoracic Plates?
The present insights from Oncopeltus show, to our knowledge for the first time, that vg, which can be thought of as a master wing regulator, also has a function in the T2 notum, namely its central region: the scutellum. Hence, the scutellum may also represent part of the wing program based on the interpretation from previous studies where vg-dependent tissues are considered to be wing serial homologs (18, 37).
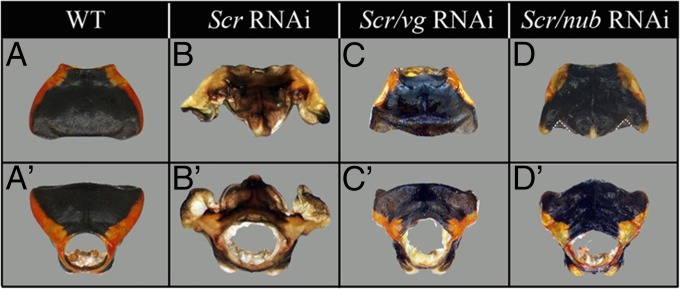
A possible caveat to results depicted in Fig. 3G is that the RNAi was performed at fourth and fifth nymphal stages when wing pads already contained a well-developed scutellum primordium. In other words, the obtained phenotype may be a hypomorph and only partially representative of the full function of vg in the dorsal T2 notum. Ideally, one would perform such experiments at the initial stages of development of the structures under study. Such an opportunity exists in Oncopeltus, where the depletion of Scr at late nymphal stages leads to the development of both ectopic scutellum and wings on the prothorax (Fig. 4B) (16), thus allowing us to examine the functions of vg while these structures are developing de novo. First, we performed the double Scr/vg RNAi. If vg has a key function in wing development with a secondary role in the scutellum, then the T1 wings should not develop but scutellum will, and only its posterior region will be modified. Instead, however, neither structure developed in Scr/vg RNAi individuals (Fig. 4 C and C′). This result reveals that in addition to its function in wings, vg also acts as a key regulator for initialization of the entire scutellum development. In other words, vg functions as both the wing and scutellum master gene. Second, we performed the double Scr/nub RNAi (Fig. 4 D and D′) to determine the degree to which the independent development of the wings and scutellum observed in nub RNAi can be applied to the ectopic T1 structures. Because in T2 nub affects only the wings and not the scutellum (Fig. 3 E and I), we should observe similar effects in T1. Consistent with its conserved role in wing blade formation, the depletion of nub reduces the development of ectopic T1 wings (although not to the same extent as observed in vg knockdowns). In addition, although T1 scutellum was initialized and featured a diagnostic brightly colored posterior tip (Fig. 4 D and D′), it was much smaller and incompletely developed. In summary, these double-RNAi results indicate that initialization of both the wings and scutellum are dependent on vg function, whereas the increase in size of these structures requires nub function.
Fig. 4.
Effects of Scr, Scr/vg, and Scr/nub RNAi on the T1 morphology. (A–D) Dorsal and (A′–D′) frontal view of wild-type T1 plate (A and A′), Scr RNAi T1 plate (B and B′), Scr/vg RNAi T1 plate (C and C′), and Scr/nub RNAi T1 plate (D and D′). To better distinguish the shape of the ectopic scutellum, its outer edges are outlined with a dotted white line in D.
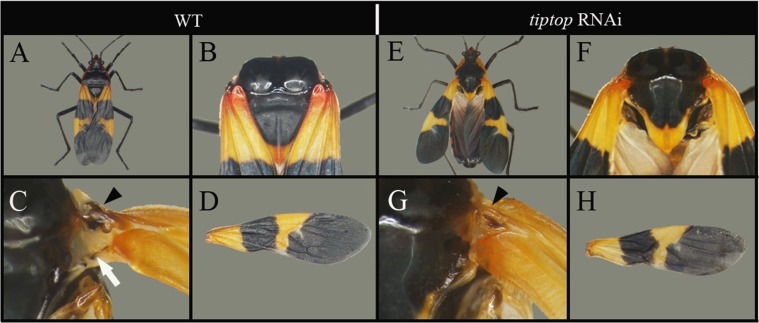
Coordinated development of the scutellum and wing blade likely affects the wing hinge and wing-folding mechanics, the latter of which became highly specialized following the origin in neoptera of the ability to fold the wings flat against the body. To begin to address this we examined the role of tiptop (tio), the Oncopeltus ortholog of teashirt (tsh), which plays a role in hinge development and wing posture in Drosophila (39, 40). In tio RNAi adults we found that the wings failed to fold flat against the body (Fig. S7A vs. Fig. S7E), consistent with previous observations in tsh mutants in Drosophila and similar to the ancestral wing posture still present in Paleoptera. This improper folding of the wings is the result of changes to the shape of the scutellum (Fig. S7F) as well as the development of the hinge (Fig. S7G), but not changes in the wing itself (Fig. S7D vs. Fig. S7H). This result points to tio as a gene that may overcome the otherwise coordinated development of wing blade + scutellum to form the hinge, thereby enabling the transition from paleoptera to neoptera.
Fig. S7.
Effects of tio RNAi on adult morphology. (A) Wild-type adult Oncopeltus showing the wings folded flat on the body. (B) Close-up of wild-type scutellum. (C) Close-up of wild-type T2 hinge showing the sclerite (black arrowhead) and connecting membrane (white arrow) and (D) wild-type forewing. (E) tio RNAi adult showing the misfolding wings. (F) Close-up of tio RNAi scutellum. (G) Close-up of tio RNAi T2 hinge and (H) forewing.
Ultrabithorax Function in T2/T3 Wing Divergence and Evolutionary Diversification.
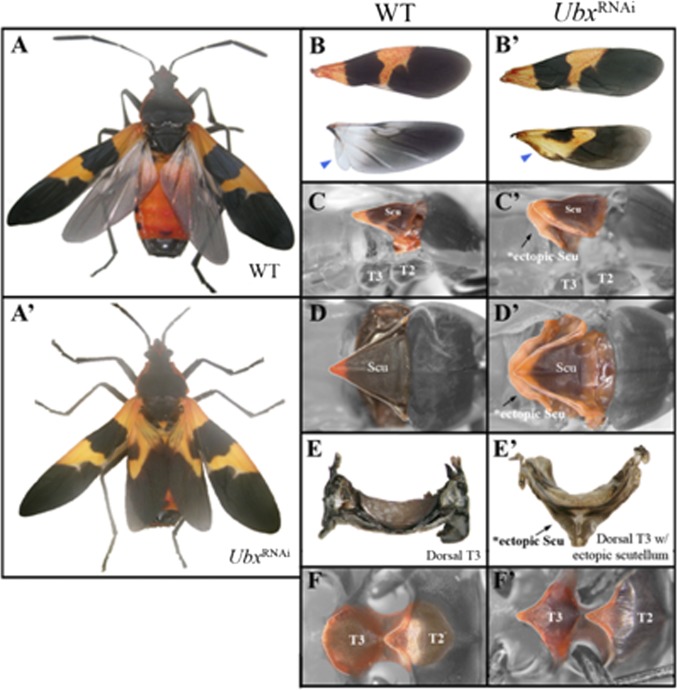
Divergence of T2 and T3 wing shape and texture, along with changes in wing-associated thoracic structures, and diversification of wings and thoraces among insect species, required changes to the default T2 morphology (6). In Drosophila, loss of Ubx converts the T3 segment to a T2 identity, including transformation of halteres to fully developed forewings (19, 20). Similarly, Ubx inhibition in Tribolium transforms the membranous hindwings to forewing-like sclerotized elytra (5). Oncopeltus Ubx RNAi adults had hindwings possessing all key features of forewing morphology, including their distinct shape, texture, and pigmentation (Fig. 5 A′ and B′); this includes the transformation of the proximal anal lobe present in wild-type T3 wing, which assumed a clavus-like T2 morphology (blue arrowheads in Fig. 5 B and B′). Therefore, although the default state of the wing may vary, Ubx functions the same way in each case by always modifying an existing T2 wing to create a divergent hindwing (halteres in Drosophila or membranous wings in both Tribolium and Oncopeltus). In addition to wings, the Ubx RNAi T3 dorsal notum also showed significant alterations and developed an ectopic scutellum (Figs. 5 C and D and C′ and D′). The extent of the change is best visible in dissected plates (Fig. 5 E and E′), enabling a full view of their structure. On the ventral side, the oval-shaped T3 sternum (Fig. 5F) assumed the V-shaped morphology of the T2 sternum (Fig. 5F′). These results show that in a hemimetabolous species Ubx also specifies a unique T3 segment morphology by altering the default T2 segment program. Similar to holometabola, these effects involve both the wings and thoracic plates (5, 8). In terms of its dorsal function, Ubx “makes” hindwings by altering the default forewing program while also repressing the formation of a T2 scutellum. These functions of Ubx most likely arose early in pterygote evolution because divergences in T2/T3 wings occur in certain Paleoptera (Ephemeroptera) as well as Neoptera.
Fig. 5.
The role of Ubx in the Oncopeltus metathorax. (A and A′) Dorsal views of the wild-type and Ubx RNAi adults. (B) Wild-type fore- and hindwing display distinct morphologies with regards to their shape, color, and size. (B′) Although forewings look the same, hindwings are transformed into forewings in Ubx RNAi adults. (C) A side view of the dorsal plates in the wild-type showing that the T2 segment features a well-developed scutellum. (C′) A side view of Ubx RNAi adult indicates a presence of an ectopic scutellum on T3 (black arrow). (D) Close-up view focused on wild-type scutellum. (D′) In Ubx RNAi adults, a second ectopic scutellum forms beneath the T2 scutellum (black arrow). (E) Dissected T3 plate of wild-type adult. (E′) Dissected T3 plate of an Ubx RNAi adult. (F) A close-up view of T2 and T3 ventral plates in wild-type. The ventral T2 has a triangular shape, whereas the T3 is oval. (F′) In Ubx RNAi adults, the ventral T3 is transformed into a ventral T2 sternum. Abbreviations: Scu, scutellum; T2, mesothoracic segment; T3, metathorax thoracic segment.
Discussion
Uniqueness of the T1 Wing and Its Lack of Ventral Genes.
Previous work in beetles revealed that the prothorax (T1) contains vg-dependent tissue, leading to the proposal that the affected T1 regions represent wing serial homologs (18, 37). Our present results show that, in addition to vg, several other genes involved in wing development also have a function in the wingless prothorax of Oncopeltus. By showing that Oncopeltus T1 EW is a unique type of wing and a characteristically T1 structure, rather than a conversion to T2 segmental identity, these results suggest, to our knowledge for the first time, the presence of a T1 wing serial homolog in a contemporary hemimetabolous insect. In contrast to the situation observed in Tribolium, where T1 EW is derived from both dorsal and ventral tissues and is indicative of a conversion of T1 to T2 segmental identity, these wings in Oncopeltus are of strictly dorsal morphological origin and have gene-expression profiles indicating either complete absence or marked reduction of ventral-origin cells.
Implications of the T1 Ectopic Wing for Insect Wing Origins.
Small T1 wings are present in a phylogenetically diverse set of fossil insects, but no insects with T1 wings persist in fossils more recent than about 250 Mya (1). Our results in Oncopeltus, combined with recent developmental insights from Drosophila, provide a hypothesis to explain why T1 wings were likely limited to small size, reduced functionality, and were lost independently in multiple insect orders. Ventrally originating cells in Drosophila embryos migrate dorsally and undergo an epithelial–mesenchymal transition to form the corpora cardiaca and prothoracic glands in the head and T1 segments (25). Serially homologous cells in segments T2 through A8 also migrate dorsally and undergo epithelial–mesenchymal transition to form the main tracheal trunks while also playing a role in T2/T3 wing development. Stunting of the T1 wing may therefore demonstrate the limited potential of notal primarily cuticular cells to form a large and useful wing without the functions contributed by ventral-origin mesenchyme. The gene ventral veins lacking (vvl) acts downstream of trh, suggesting that the ventral component of wing vein formation (critical for wing stiffening) also derives from ventral cells that may have been diverted to gland formation in the T1 segment.
Tracheal placodes and leg primordia arise from a common pool of cells in Drosophila (23) with differences in their fate controlled by the activation state of the wingless signaling pathway. Tracheal cells and spiracles on T2 and T3 arise from locations lateral to the leg bases (23) possibly homologous with the association between gills and appendages in crustaceans, a hypothesis strengthened by the finding that homologs of tracheal inducer genes (trh, vvl) are specifically expressed in the gills of crustaceans (24, 41). Notably, those genes were absent from the small T1 ectopic wings of Oncopeltus. Furthermore, nub affected leg-adjacent morphology of the Oncopeltus T1 ventral thorax and was expressed only in the blade portion of the T2 wing but not the adjacent scutellum, similar to the way nub is expressed only in the posterior compartment of crustacean gills (23). Starting with fossil evidence (42) and continuing with developmental data (18, 23, 25, 32, 33) and present insights from Oncopeltus, genes expressed in crustacean gills appear to mark a cell population that evolved into both tracheae and wings in insects. In wings, the fusion of both dorsal and ventral contributions appears to be required for formation of fully developed and functional wings. Hence, after many decades of debate between proponents of the paranotal lobe vs. wings-from-gills hypotheses for insect wing origins, the answer is becoming increasingly apparent: each side was half correct.
Overview of the Origin and Divergence of Insect Wings.
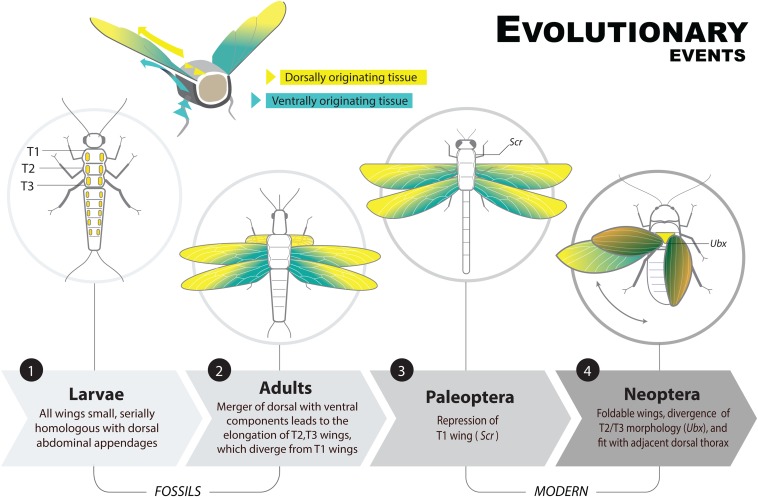
The origin and divergence of wings involved a multistep process outlined in Fig. 6. First, we propose that dorsally derived outgrowths evolved along a number of body segments, most notably T1 and some abdominal segments. Fossils containing these features (including larvae) gave rise to the paranotal theory of wing origins (1, 43). Dorsal origins are evident in the position and the expression of primarily dorsally derived genes in the ectopic T1 wings of Oncopeltus. Next, Hox genes were co-opted to suppress a number of these dorsal flaps (7). Either before or after this step, ventrally derived cells possibly homologous to ancestral arthropod epipods migrated dorsally and fused with the cells that give rise to the dorsal flaps (32). This fusion brought gene networks that likely enabled an increase in size of the outgrowths (shown empirically here by the effect of nub on the size of the T1 EW) as well as features necessary for articulation. Elongation of these dorsal flaps also featured regionalization, with a discrete lateral blade (the wing) connected by a hinge to a central region (the scutellum); these structures coevolved and became independently regulated, allowing formation of a hinge region without affecting wing blade development, which ultimately permitted wing folding. Finally, Ubx was co-opted to uncouple the wing morphologies of T2 and T3, facilitating the divergence of wing forms within species and diversification among species. These results represent a broad outline of key mechanisms underlying the origin and diversification of insect wings, which brought about the first flying animals and triggered the largest evolutionary radiation of multicellular life.
Fig. 6.
Major events in the divergence and segmental diversification of insect wings. Note the presence of a T1 winglet in fossil insects, which persisted until after T2 and T3 wings elongated. Additionally, the divergence of T2/T3 wing morphology occurs also in some Paleoptera (Ephemeroptera), which if mediated by Ubx would place this feature at a more basal location than depicted here. Illustration by Daorong Fang.
Materials and Methods
Gene Cloning, dsRNA Synthesis, and RNAi.
Injections and dsRNA synthesis were performed as previously described (16). Detailed information including primer sequences and RT-PCR controls, are in SI Material and Methods and Fig. S8.
Fig. S8.
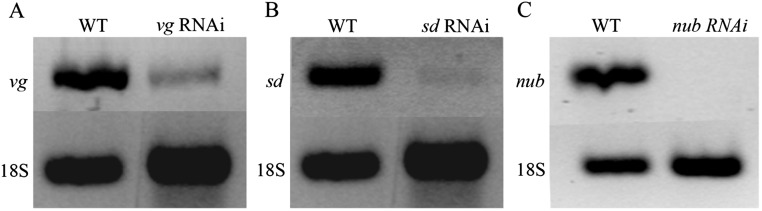
RT-PCR analysis of vg,sd and nub mRNA expression levels. Lane 1 shows high levels of vg (A), sd (B), and nub (C) in wild-type Oncopeltus fifth nymphs; lane 2 shows greatly reduced levels of expression in the corresponding RNAi nymphs. 18S mRNA levels were used for controls for all three genes.
Library Preparation, Transcript Assembly, and Blast Annotation.
Bar coded libraries (n = 12, one for each combination of treatment and replicate; NCBI SRA accession SRP066252) were prepared using the TruSeq RNA Sample Preparation Kit (Illumina). Contigs from the assembly were searched using BLASTx against the following sets of proteins: Uniprot, Drosophila melanogaster, Tribolium casteneum, and Daphnia pulex. Detailed information including RNA isolation, high-throughput sequencing, read processing, assembly and functional group analysis are in SI Material and Methods.
SI Materials and Methods
Cloning and Sequence Analysis of Genes.
Mixed-stage Oncopeltus faciatus embryos were used for total RNA extraction. The production of cDNA, RT-PCR, and cloning were performed according to Li and Popadić (44). To amplify the Of vg fragment, nested PCR was performed using the following primers: outer primers FW 5′GAGGACAGCGGTACTGAAGC, REV 5′TAGTAGGACGCAGGGTCGAG and inner FW 5′ACACTGACGAGGACGAGGAC, REV 5′TACTGCATATTCGCCTGGTG, with the final set of primers yielding a 377-bp fragment. To amplify Of sd, known sequences of sd were aligned and the following degenerate primers were designed for nested PCR: outer FW 5′GAYGARGGIAARATGTAYGGIMG, REV 5′IGTICGDAYIGCICKICCYTCC and inner FW 5′ATHTAYCCICCITGYGGIMGIMG and REV 5′IACYTTYTCIACIACAYAGYTTICC, with the final primer set resulting in a 531-bp fragment. The PCR conditions for both vg and sd was 94 °C for 2 min; 35 cycles of 94 °C for 30 s, 50 °C for 30 s, 72 °C for 30 s; one cycle of 72 °C for 7 min. To amplify the Of tio fragment, primers were designed based on a known sequence (GenBank accession no. AF533539.1) with PCR conditions 94 °C for 2 min; 35 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s; one cycle of 72 °C for 7 min. Ten clones from each fragment were isolated and sequenced. The resulting sequences were compared with each other and known sequences from GenBank and were identified as orthologous genes. Previously described fragments of Of nub (45), Of Scr (16), and Of Ubx (46) were used.
RNAi.
Double-stranded RNA (dsRNA) was prepared and injected into the abdomens of Oncopeltus fourth and fifth nympal stages consecutively, as described by Chesebro et al. (16). Approximately 2 µL of dsRNA was injected at a concentration of 2–3 µg/µL into each individual. For vg RNAi, a total of 45 individuals were injected of which 35 survived to adulthood. For sd RNAi, a total of 75 individuals were injected, of which 22 survived to adulthood. For nub RNAi, a total of 25 individuals were injected, of which a total of 19 survived. For tio RNAi, a total of 15 individuals were injected, of which a total of 9 survived. For Scr RNAi, a total of 15 individuals were injected, 10 of which survived to adulthood. For Ubx RNAi, a total of 105 adults were examined. For the double RNAi experiments, 25 individuals were injected for each condition, of which 15 survived for Scr/vg RNAi and 19 survived for Scr/nub RNAi. To address nonspecific effects, we injected a previously cloned 710-bp fragment of the jellyfish GFP, as described by Hrycaj et al. (17). To determine the efficiency of our RNAi approach, we performed independent RT-PCR analyses on vg, sd, and nub controls (shown in Fig. S8). Oncopeltus fifth nymphs were injected with 2 µL of dsRNA of vg, sd, and nub dsRNA at 2.5 µg/μL and allowed to develop for 3–4 d. Next, the wing pads of two individuals were used for total RNA extraction and cDNA generation, as described by Chesebro et al. (16). This same procedure was carried out using two wild-type individuals. Equal amounts of cDNA from wild-type and RNAi fifth nymphs were used as templates in individual PCR reactions to assess the amount of relative transcripts that were abolished in injected individuals. As positive controls, primers designed to the Oncopeltus 18S ribosomal subunit were used in all cases (17).
Treatments and Sample Pooling for RNA-Seq Analyses.
A previously characterized fragment of Of Scr (16) was used to inject fourth and fifth nymphs consecutively with 2 µL of 2–3 µg/µL of dsRNA. Approximately 3 d after the last injection the nymphs were killed and the wild type T1 dorso-lateral body wall, ecotopic Scr-RNAi T1 wing pad, and the wild type T2 and T3 wing pads were dissected from the bodies in PBT and placed immediately in TRIzol (Life Technologies-Invitrogen). Three replicates for each thoracic segment were prepared and each replicate contained four independent samples. Similarly staged wild-type nymphs were prepared identically for controls.
RNA Isolation, Library Preparation, and High-Throughput Sequencing.
Dissected wing buds were placed immediately into TRIzol (Life Technologies-Invitrogen) in 1.5-mL screw-top tubes with five 2.3-mm–diameter stainless steel beads and shaken on a mixer mill for 3 min at 20 Hz to disrupt cells. Total RNA was isolated from the homogenate following the manufacturer’s protocol except for an additional wash with acid phenol:chloroform:isoamyl alcohol [25:24:1 (vol:vol:vol)] to reduce DNA contamination and the addition of glycogen (10 µg) during RNA precipitation with isopropanol. Isolates were DNased and further purified using the RNeasy Micro Kit (Qiagen). Total RNA was evaluated using a Bioanalyzer (RNA Nano Chip, Agilent Technologies), which revealed no detectable DNA contamination and RNA integrity numbers ranging from 8.1 to 9.0. Equal amounts of total RNA (4.5 µg quantified by measuring UV absorbance) from each replicate (n = 3 replicates × 4 treatments = 12 pools) were submitted for sequencing.
The Michigan State University Research Technology Support Facility Genomics Core prepared bar coded libraries (n = 12, one for each combination of treatment and replicate) using the TruSeq RNA Sample Preparation Kit (Illumina). Two equimolar pools of six libraries were run on two flowcells of an Illumina Genome Analyzer IIx to yield 2 × 75-bp paired-end reads. An average of about 8.8 M raw clusters with an average insert size of 151 bp were obtained per library (∼106 million total read pairs).
Read Preprocessing, Transcript Assembly, and Abundance Estimation.
Artificial duplicate read pairs (from PCR-amplified library fragments generated during library preparation) were filtered out of the raw read set using CLC Assembly Cell v3.22.55705 with the “remove_duplicates” tool, using sequence information from both paired-end reads. Reads were then quality trimmed using the CLC Assembly Cell “quality_trim” tool, retaining high-quality regions longer than half the read length and containing at least 90% of the nucleotides with a quality score greater than 20. An average of ∼85% of reads passed these quality-filtering parameters. The filtered and quality-trimmed reads from all 12 libraries were combined and assembled together using the Trinity RNA-Seq pipeline release 2011-10-29 (47) using the default parameter settings and keeping all transcripts longer than 200 bp.
To verify the quality of the assembly, we coassembled our contigs >250-bases long with those of a previous 454 transcriptome from Oncopeltus embryos (48). A majority of the coassembled gene models (61% of the total) contained only one contig from our assembly and one from the Ewen-Campen et al. (48) assembly; this indicated highly concordant results. Visual inspection of haphazardly selected coassembled contigs confirmed this conclusion; in most cases, the coassembled sequences showed end-to-end or nearly so agreement and only minor variation (i.e., SNPs). We proceeded using only our own reads and assembly because the RNA-seq analysis depends on read counts mapped to a specific assembly, including alternative splice forms. The coassembly is available from J.H.M. upon request.
Transcript- and component-level gene expression were quantified for each library by mapping the original read set back to the assembly using BWA (49) and RSEM (50) distributed and implemented with the Trinity pipeline using default parameters. Read count data were normalized using the trimmed mean of the M values (TMM) method (51) as follows: a scaling factor was computed for each replicate library within a treatment via the normFact routine in edgeR using filtered read count data and one replicate of the T1 wing bud treatment as a reference. Counts for each gene within a library were divided by the product of the scaling factor multiplied by the library size.
Blast Annotation and Functional Group Analysis.
Contigs from the assembly were searched using BLASTx against the following sets of proteins: Uniprot, Drosophila melanogaster, Tribolium casteneum, and Daphnia pulex. All blast hits with a bitscore > 45 (generally e-value < 10−5) were considered significant hits. Subsequent analyses used only contigs that had a significant blast hit against at least one of those protein collections.
We used normalized read counts from the blast-annotated contigs to examine differential gene expression (RNA-seq) across the four tissue types (T1 body wall; Scr RNAi wing, T2 wing, T3 wing). After filtering out contigs with low expression (<20 total reads, or a zero reading in at least one library of each tissue type), remaining contigs (n = 12,429) had expression levels that tended to be highly correlated (R generally >0.82) across the replicate tissue libraries [using log2 (normalized read count + 0.01)]. This was not true for libraries T1_1 and T3_3, which had correlation coefficients all <0.83 with replicates of the same tissue type and as low as <0.57 across tissue types. For that reason, those two libraries (T1_1, T3_5) were judged to be of reduced quality and were excluded from subsequent analyses.
Functional group analyses, to determine overrepresentation of functionally related genes in ranked lists of tissue-specific up- or down-regulated genes, was performed with the GO annotations of the Drosophila BLASTx homologs (associated with the Flybase gene IDs) of our Oncopeltus contigs and DAVID (22). DAVID compares submitted lists of GO annotations against a background list, for which we used the list of all filter-passed contigs with Drosophila blast hits (bitscore > 45). By using the observed group of all expressed genes as the background set, overrepresentation of, for example, appendage-specific genes, is not bias resulting from working with wing tissue, but rather an indication that appendage-specific genes were differentially expressed among the set of genes expressed in wings.
Acknowledgments
We thank Thomas R. Lemonds, Dr. Mark VanBerkum, and two anonymous reviewers for helpful comments that greatly improved this manuscript; Venessa Verstraete for help with imaging; and Daorong Fang for her work on the illustration in Fig. 6. This work was partially supported by National Institutes of Health Grant GM071927 (to A.P.), Wayne State University Graduate Enhancement Funds (to V.M. and J.L.), funds from the Huck Institutes for Life Sciences and Grants NSF IOS-0950416 and IOS-1354667 (to J.H.M.), and National Science Foundation Grant PGRR- 0922742.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509517112/-/DCSupplemental.
Data deposition: The sequences reported in this paper have been deposited in the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA), www.ncbi.nlm.nih.gov/sra (accession no. SRP066252).
References
- 1.Grimaldi D, Engel MS. Evolution of the Insects. Cambridge Univ Press; New York: 2005. Insects take to the skies; pp. 155–187. [Google Scholar]
- 2.Wootton RJ, Kukalová-Peck J. Flight adaptations in Palaeozoic Palaeoptera (Insecta) Biol Rev Camb Philos Soc. 2000;75(1):129–167. doi: 10.1017/s0006323199005459. [DOI] [PubMed] [Google Scholar]
- 3.Dudley R. The Biomechanics of Insect Flight: Form, Function, and Evolution. Princeton Univ Press; Princeton, NJ: 2000. [Google Scholar]
- 4.Jockusch EL, Nagy LM. Insect evolution: How did insect wings originate? Curr Biol. 1997;7(6):R358–R361. doi: 10.1016/s0960-9822(06)00174-6. [DOI] [PubMed] [Google Scholar]
- 5.Tomoyasu Y, Wheeler SR, Denell RE. Ultrabithorax is required for membranous wing identity in the beetle Tribolium castaneum. Nature. 2005;433(7026):643–647. doi: 10.1038/nature03272. [DOI] [PubMed] [Google Scholar]
- 6.Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- 7.Carroll SB, Weatherbee SD, Langeland JA. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375(6526):58–61. doi: 10.1038/375058a0. [DOI] [PubMed] [Google Scholar]
- 8.Struhl G. Genes controlling segmental specification in the Drosophila thorax. Proc Natl Acad Sci USA. 1982;79(23):7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini DR, Kaufman TC. Insect appendages and comparative ontogenetics. Dev Biol. 2005;286(1):57–77. doi: 10.1016/j.ydbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Jockusch EL, Ober KA. Hypothesis testing in evolutionary developmental biology: A case study from insect wings. J Hered. 2004;95(5):382–396. doi: 10.1093/jhered/esh064. [DOI] [PubMed] [Google Scholar]
- 11.Shubin N, Tabin C, Carroll S. Fossils, genes and the evolution of animal limbs. Nature. 1997;388(6643):639–648. doi: 10.1038/41710. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell BC, Jockusch EL. The expression of wingless and Engrailed in developing embryos of the mayfly Ephoron leukon (Ephemeroptera: Polymitarcyidae) Dev Genes Evol. 2010;220(1-2):11–24. doi: 10.1007/s00427-010-0324-6. [DOI] [PubMed] [Google Scholar]
- 13.Staniczek A, Bechly G, Godunko R. Coxoplectoptera, a new fossil order of Palaeoptera (Arthropoda: Insecta), with comments on the phylogeny of the stem group of mayflies (Ephemeroptera) Insect Syst Evol. 2011;42(2):101–138. [Google Scholar]
- 14.Wootton RJ, Kukalova-Peck J, Newman DJS, Muzon J. Smart engineering in the mid-carboniferous: How well could Palaeozoic dragonflies Fly? Science. 1998;282(5389):749–751. doi: 10.1126/science.282.5389.749. [DOI] [PubMed] [Google Scholar]
- 15.Rogers BT, Peterson MD, Kaufman TC. Evolution of the insect body plan as revealed by the Sex combs reduced expression pattern. Development. 1997;124(1):149–157. doi: 10.1242/dev.124.1.149. [DOI] [PubMed] [Google Scholar]
- 16.Chesebro J, Hrycaj S, Mahfooz N, Popadić A. Diverging functions of Scr between embryonic and post-embryonic development in a hemimetabolous insect, Oncopeltus fasciatus. Dev Biol. 2009;329(1):142–151. doi: 10.1016/j.ydbio.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hrycaj S, Chesebro J, Popadić A. Functional analysis of Scr during embryonic and post-embryonic development in the cockroach, Periplaneta americana. Dev Biol. 2010;341(1):324–334. doi: 10.1016/j.ydbio.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clark-Hachtel CM, Linz DM, Tomoyasu Y. Insights into insect wing origin provided by functional analysis of vestigial in the red flour beetle, Tribolium castaneum. Proc Natl Acad Sci USA. 2013;110(42):16951–16956. doi: 10.1073/pnas.1304332110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlopoulos A, Akam M. Hox gene Ultrabithorax regulates distinct sets of target genes at successive stages of Drosophila haltere morphogenesis. Proc Natl Acad Sci USA. 2011;108(7):2855–2860. doi: 10.1073/pnas.1015077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bender W, et al. Molecular genetics of the Bithorax complex in Drosophila melanogaster. Science. 1983;221(4605):23–29. doi: 10.1126/science.221.4605.23. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Gerstein M, Snyder M. RNA-seq: A revolutionary tool for transcriptomics. Nat Rev Genet. 2009;10(1):57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 23.Franch-Marro X, Martín N, Averof M, Casanova J. Association of tracheal placodes with leg primordia in Drosophila and implications for the origin of insect tracheal systems. Development. 2006;133(5):785–790. doi: 10.1242/dev.02260. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell B, Crews ST. Expression of the Artemia trachealess gene in the salt gland and epipod. Evol Dev. 2002;4(5):344–353. doi: 10.1046/j.1525-142x.2002.02023.x. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Higueras C, Sotillos S, Castelli-Gair Hombría J. Common origin of insect trachea and endocrine organs from a segmentally repeated precursor. Curr Biol. 2014;24(1):76–81. doi: 10.1016/j.cub.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Chung S, Chavez C, Andrew DJ. Trachealess (Trh) regulates all tracheal genes during Drosophila embryogenesis. Dev Biol. 2011;360(1):160–172. doi: 10.1016/j.ydbio.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furlong EEM, Andersen EC, Null B, White KP, Scott MP. Patterns of gene expression during Drosophila mesoderm development. Science. 2001;293(5535):1629–1633. doi: 10.1126/science.1062660. [DOI] [PubMed] [Google Scholar]
- 28.Hakeda S, Endo S, Saigo K. Requirements of Kettin, a giant muscle protein highly conserved in overall structure in evolution, for normal muscle function, viability, and flight activity of Drosophila. J Cell Biol. 2000;148(1):101–114. doi: 10.1083/jcb.148.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Featherstone D, Davis W, Rushton E, Broadie K. Drosophila D-titin is required for myoblast fusion and skeletal muscle striation. J Cell Sci. 2000;113(Pt 17):3103–3115. doi: 10.1242/jcs.113.17.3103. [DOI] [PubMed] [Google Scholar]
- 30.Okenve-Ramos P, Llimargas M. Fascin links Btl/FGFR signalling to the actin cytoskeleton during Drosophila tracheal morphogenesis. Development. 2014;141(4):929–939. doi: 10.1242/dev.103218. [DOI] [PubMed] [Google Scholar]
- 31.Guild GM, Connelly PS, Ruggiero L, Vranich KA, Tilney LG. Actin filament bundles in Drosophila wing hairs: Hairs and bristles use different strategies for assembly. Mol Biol Cell. 2005;16(8):3620–3631. doi: 10.1091/mbc.E05-03-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Averof M, Cohen SM. Evolutionary origin of insect wings from ancestral gills. Nature. 1997;385(6617):627–630. doi: 10.1038/385627a0. [DOI] [PubMed] [Google Scholar]
- 33.Niwa N, et al. Evolutionary origin of the insect wing via integration of two developmental modules. Evol Dev. 2010;12(2):168–176. doi: 10.1111/j.1525-142X.2010.00402.x. [DOI] [PubMed] [Google Scholar]
- 34.Berger C, et al. FACS purification and transcriptome analysis of Drosophila neural stem cells reveals a role for Klumpfuss in self-renewal. Cell Reports. 2012;2(2):407–418. doi: 10.1016/j.celrep.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ross MH. Pronotal wings in Blattella germanica (L.) and their possible evolutionary significance. Am Midl Nat. 1964;71(1):161–180. [Google Scholar]
- 36.Campbell S, et al. The scalloped gene encodes a novel, evolutionarily conserved transcription factor required for sensory organ differentiation in Drosophila. Genes Dev. 1992;6(3):367–379. doi: 10.1101/gad.6.3.367. [DOI] [PubMed] [Google Scholar]
- 37.Ohde T, Yaginuma T, Niimi T. Insect morphological diversification through the modification of wing serial homologs. Science. 2013;340(6131):495–498. doi: 10.1126/science.1234219. [DOI] [PubMed] [Google Scholar]
- 38.Zirin JD, Mann RS. Differing strategies for the establishment and maintenance of teashirt and homothorax repression in the Drosophila wing. Development. 2004;131(22):5683–5693. doi: 10.1242/dev.01450. [DOI] [PubMed] [Google Scholar]
- 39.Soanes KH, Bell JB. Rediscovery and further characterization of the aeroplane (ae) wing posture mutation in Drosophila melanogaster. Genome. 1999;42(3):403–411. doi: 10.1139/g98-143. [DOI] [PubMed] [Google Scholar]
- 40.Soanes KH, et al. Identification of a regulatory allele of teashirt (tsh) in Drosophila melanogaster that affects wing hinge development. An adult-specific tsh enhancer in Drosophila. Mech Dev. 2001;105(1-2):145–151. doi: 10.1016/s0925-4773(01)00397-5. [DOI] [PubMed] [Google Scholar]
- 41.Chavez M, et al. APH-1, a POU homeobox gene expressed in the salt gland of the crustacean Artemia franciscana. Mech Dev. 1999;87(1-2):207–212. doi: 10.1016/s0925-4773(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 42.Kukalova-Peck J. Origin and evolution of insect wings and their relation to metamorphosis, as documented by the fossil record. J Morphol. 1978;156(1):53–125. doi: 10.1002/jmor.1051560104. [DOI] [PubMed] [Google Scholar]
- 43.Rasnitsyn AP. A modified paranotal theory of insect wing origin. J Morphol. 1981;168(3):331–338. doi: 10.1002/jmor.1051680309. [DOI] [PubMed] [Google Scholar]
- 44.Li H, Popadić A. Analysis of nubbin expression patterns in insects. Evol Dev. 2004;6(5):310–324. doi: 10.1111/j.1525-142X.2004.04039.x. [DOI] [PubMed] [Google Scholar]
- 45.Hrycaj S, Mihajlovic M, Mahfooz N, Couso JP, Popadić A. RNAi analysis of nubbin embryonic functions in a hemimetabolous insect, Oncopeltus fasciatus. Evol Dev. 2008;10(6):705–716. doi: 10.1111/j.1525-142X.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 46.Mahfooz N, Turchyn N, Mihajlovic M, Hrycaj S, Popadić A. Ubx regulates differential enlargement and diversification of insect hind legs. PLoS One. 2007;2(9):e866. doi: 10.1371/journal.pone.0000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grabherr MG, et al. Full-length transcriptome assembly from RNA-seq data without a reference genome. Nat Biotechnol. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ewen-Campen B, et al. The maternal and early embryonic transcriptome of the milkweed bug Oncopeltus fasciatus. BMC Genomics. 2011;12:61. doi: 10.1186/1471-2164-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson MD, Oshlack A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010;11(3):R25. doi: 10.1186/gb-2010-11-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]