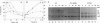Abstract
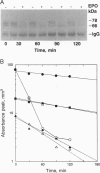
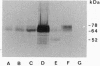
An abundant 70- to 78-kDa form of the erythropoietin receptor (EPOR) was observed in HC-D57 murine erythroleukemia cells deprived of erythropoietin (EPO). In contrast to the 64- and 66-kDa EPOR proteins, these high molecular mass forms of EPOR (hmm-EPOR) correlated well with the number of binding sites and endocytosis of EPO. The hypothesis that hmm-EPOR are more highly glycosylated forms of the EPOR, appear on the cell surface, and represent at least one component of the biologically active EPOR was tested. Consistent findings were as follows. (i) Only hmm-EPOR increased following withdrawal of EPO from HC-D57 cells, correlating with a 10-fold increase in binding of 125I-labeled EPO. In addition, the EPO-dependent downregulation of 125I-EPO binding and disappearance of hmm-EPOR occurred in parallel while the amount of 66-kDa EPOR did not change. (ii) The 78-kDa EPOR was detected in COS cells expressing EPOR cDNA. (iii) Probing of the intact surface of these cells with anti-NH2-terminal antibody recovered only the 78-kDa EPOR. (iv) Enzymatic deglycosylation and dephosphorylation showed that hmm-EPOR apparently resulted from additional N-linked glycosylation of a 62-kDa EPOR. (v) The hmm-EPOR turnover in HC-D57 cells was accelerated 12-fold in the presence of EPO (half-life changed from 3 hr to 15 min). (vi) Anti-phosphotyrosine antiserum detected an EPO-dependent phosphorylation of the 78-kDa EPOR. The kinetics of tyrosine phosphorylation of a 97-kDa protein correlated with the occupancy and internalization of hmm-EPOR. In summary, we suggest that the 78-kDa EPOR is directly involved in the initial biological actions of EPO.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber D. L., D'Andrea A. D. The erythropoietin receptor and the molecular basis of signal transduction. Semin Hematol. 1992 Oct;29(4):293–304. [PubMed] [Google Scholar]
- Carroll M. P., Spivak J. L., McMahon M., Weich N., Rapp U. R., May W. S. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991 Aug 15;266(23):14964–14969. [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- Damen J., Mui A. L., Hughes P., Humphries K., Krystal G. Erythropoietin-induced tyrosine phosphorylations in a high erythropoietin receptor-expressing lymphoid cell line. Blood. 1992 Oct 15;80(8):1923–1932. [PubMed] [Google Scholar]
- Dusanter-Fourt I., Casadevall N., Lacombe C., Muller O., Billat C., Fischer S., Mayeux P. Erythropoietin induces the tyrosine phosphorylation of its own receptor in human erythropoietin-responsive cells. J Biol Chem. 1992 May 25;267(15):10670–10675. [PubMed] [Google Scholar]
- Hosoi T., Sawyer S. T., Krantz S. B. Photoaffinity labeling of the erythropoietin receptor and its identification in a ligand-free form. Biochemistry. 1991 Jan 15;30(2):329–335. doi: 10.1021/bi00216a004. [DOI] [PubMed] [Google Scholar]
- Im J. H., Lee S. J., Kim H. D. Partial purification and characterization of erythropoietin receptors from erythroid progenitor cells. Arch Biochem Biophys. 1990 May 1;278(2):486–491. doi: 10.1016/0003-9861(90)90290-f. [DOI] [PubMed] [Google Scholar]
- Komatsu N., Adamson J. W., Yamamoto K., Altschuler D., Torti M., Marzocchini R., Lapetina E. G. Erythropoietin rapidly induces tyrosine phosphorylation in the human erythropoietin-dependent cell line, UT-7. Blood. 1992 Jul 1;80(1):53–59. [PubMed] [Google Scholar]
- Koury M. J., Bondurant M. C. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science. 1990 Apr 20;248(4953):378–381. doi: 10.1126/science.2326648. [DOI] [PubMed] [Google Scholar]
- Krantz S. B. Erythropoietin. Blood. 1991 Feb 1;77(3):419–434. [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Linnekin D., Evans G. A., D'Andrea A., Farrar W. L. Association of the erythropoietin receptor with protein tyrosine kinase activity. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6237–6241. doi: 10.1073/pnas.89.14.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux P., Lacombe C., Casadevall N., Chretien S., Dusanter I., Gisselbrecht S. Structure of the murine erythropoietin receptor complex. Characterization of the erythropoietin cross-linked proteins. J Biol Chem. 1991 Dec 5;266(34):23380–23385. [PubMed] [Google Scholar]
- Migliaccio A. R., Migliaccio G., D'Andrea A., Baiocchi M., Crotta S., Nicolis S., Ottolenghi S., Adamson J. W. Response to erythropoietin in erythroid subclones of the factor-dependent cell line 32D is determined by translocation of the erythropoietin receptor to the cell surface. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11086–11090. doi: 10.1073/pnas.88.24.11086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle D. E., Quelle F. W., Wojchowski D. M. Mutations in the WSAWSE and cytosolic domains of the erythropoietin receptor affect signal transduction and ligand binding and internalization. Mol Cell Biol. 1992 Oct;12(10):4553–4561. doi: 10.1128/mcb.12.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle F. W., Quelle D. E., Wojchowski D. M. Interleukin 3, granulocyte-macrophage colony-stimulating factor, and transfected erythropoietin receptors mediate tyrosine phosphorylation of a common cytosolic protein (pp100) in FDC-ER cells. J Biol Chem. 1992 Aug 25;267(24):17055–17060. [PubMed] [Google Scholar]
- Quelle F. W., Wojchowski D. M. Proliferative action of erythropoietin is associated with rapid protein tyrosine phosphorylation in responsive B6SUt.EP cells. J Biol Chem. 1991 Jan 5;266(1):609–614. [PubMed] [Google Scholar]
- Ruscetti S. K., Janesch N. J., Chakraborti A., Sawyer S. T., Hankins W. D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990 Mar;64(3):1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Aurigemma R., Yuan C. C., Sawyer S., Blair D. G. Induction of erythropoietin responsiveness in murine hematopoietic cells by the gag-myb-ets-containing ME26 virus. J Virol. 1992 Jan;66(1):20–26. doi: 10.1128/jvi.66.1.20-26.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer S. T., Krantz S. B., Goldwasser E. Binding and receptor-mediated endocytosis of erythropoietin in Friend virus-infected erythroid cells. J Biol Chem. 1987 Apr 25;262(12):5554–5562. [PubMed] [Google Scholar]
- Sawyer S. T. The two proteins of the erythropoietin receptor are structurally similar. J Biol Chem. 1989 Aug 5;264(22):13343–13347. [PubMed] [Google Scholar]
- Spivak J. L., Pham T., Isaacs M., Hankins W. D. Erythropoietin is both a mitogen and a survival factor. Blood. 1991 Mar 15;77(6):1228–1233. [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Lodish H. F. In vitro phosphorylation of the erythropoietin receptor and an associated protein, pp130. Mol Cell Biol. 1992 Feb;12(2):706–715. doi: 10.1128/mcb.12.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]