Significance
Lungs are highly susceptible to inflammation. However, molecular insights into how external inflammation enhances metastatic outgrowth in the lungs remain lacking. Clinically, approaches are required to block tumor outgrowth in secondary organs for effective treatment of metastatic cancers. We demonstrate a previously unidentified mechanism of thrombospondin-1 (Tsp-1) regulation by inflammatory neutrophil proteases in the metastatic organ. Our findings suggest the potential of using neutrophil protease inhibitors as antimetastatic therapies.
Keywords: metastasis, inflammation, neutrophils, proteases, thrombospondin-1
Abstract
Inflammation is inextricably associated with primary tumor progression. However, the contribution of inflammation to tumor outgrowth in metastatic organs has remained underexplored. Here, we show that extrinsic inflammation in the lungs leads to the recruitment of bone marrow-derived neutrophils, which degranulate azurophilic granules to release the Ser proteases, elastase and cathepsin G, resulting in the proteolytic destruction of the antitumorigenic factor thrombospondin-1 (Tsp-1). Genetic ablation of these neutrophil proteases protected Tsp-1 from degradation and suppressed lung metastasis. These results provide mechanistic insights into the contribution of inflammatory neutrophils to metastasis and highlight the unique neutrophil protease–Tsp-1 axis as a potential antimetastatic therapeutic target.
The contribution of inflammation to primary tumor progression is well documented (1); however, little is known about its role in metastatic outgrowth in distant organs. The lung, which is a frequent site of metastasis from extrapulmonary neoplasms, is susceptible to inflammatory insults. Bacterial infection-induced, metastasis-conducive environments in the lung (2, 3) and cigarette smoke-induced inflammation were associated with pulmonary metastasis from breast cancer (2, 4).
Bacterial lipopolysaccharide (LPS) is a well-characterized inducer of inflammation because its binding to toll-like receptor 4 (TLR4) results in nuclear factor kappa B (NF-κB) activation and expression of proinflammatory cytokines, including interleukin-1 beta (IL-1β), tumor necrosis factor alpha (TNF-α), and IL-6 (5). LPS-induced acute lung injury is marked by increased neutrophil influx and up-regulation of proinflammatory cytokines. Similar phenotypes are observed in other lung inflammatory conditions, including asthma (6), chronic obstructive pulmonary disease (7), and pneumonia (8, 9). LPS-mediated lung inflammation is associated with breast and colon cancer metastasis to the lungs (10–12).
The mechanisms by which inflammation contributes to metastatic outgrowth in distant organs have remained underexplored. From a clinical perspective, although blocking primary tumor invasion and blocking dissemination are considered effective approaches in suppressing metastasis, an important question is how best to treat patients whose tumor has already metastasized. Thus, approaches are required to block tumor outgrowth in secondary organs for effective treatment of metastatic cancers. In this study, using two independent models of lung inflammation, we show enhanced recruitment of neutrophils, which degranulate to release the Ser proteases, neutrophil elastase (NE) and cathepsin G (CG), to degrade thrombospondin-1 (Tsp-1) in the lung microenvironment, enhancing metastatic outgrowth. Protease deficiency protected Tsp-1 from proteolysis and suppressed metastasis, providing a previously unidentified mechanism of Tsp-1 regulation in the metastatic organ.
Results
Neutrophil-Mediated Lung Inflammation Enhances Metastatic Outgrowth.
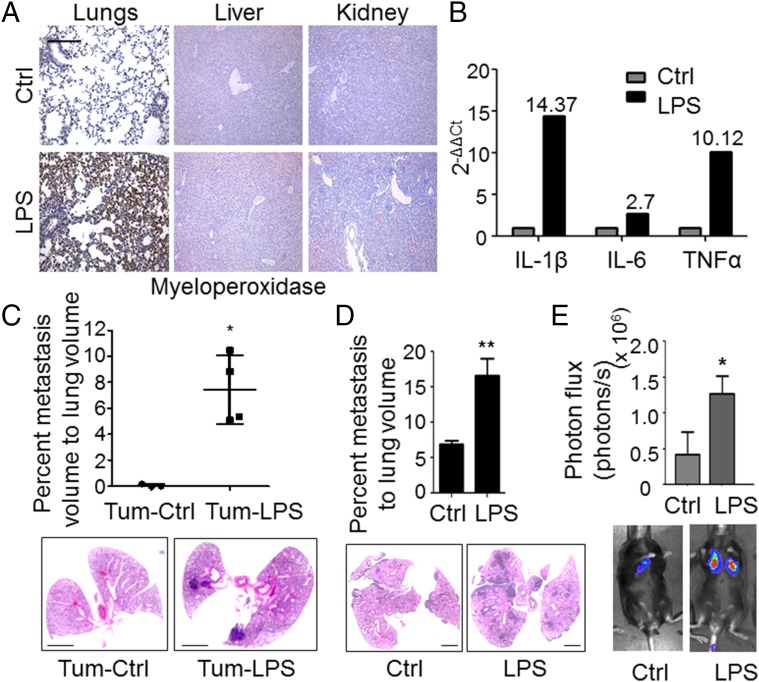
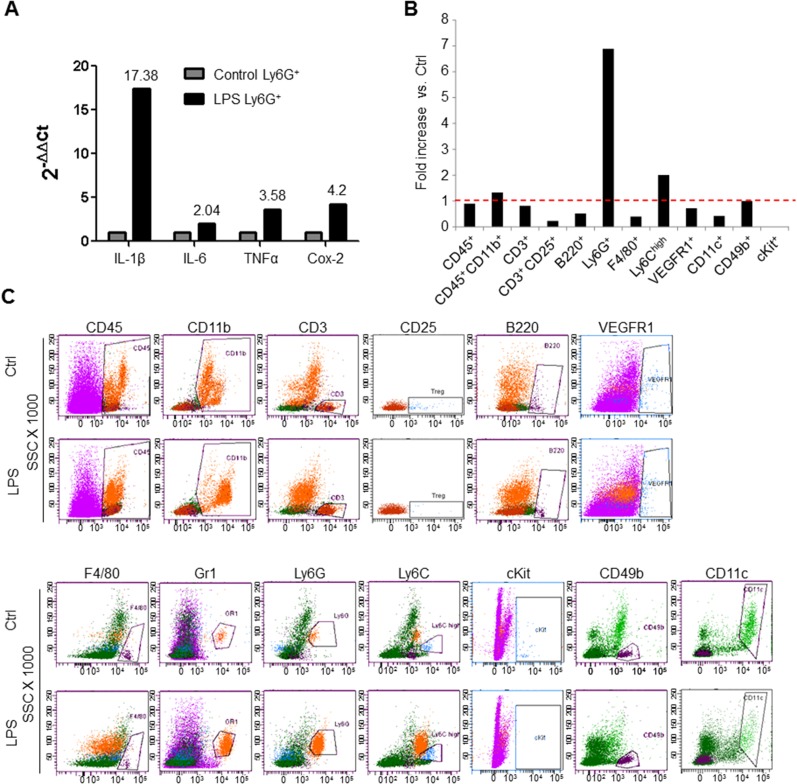
To determine the contribution of inflammation in the lungs to the outgrowth of metastasis, we generated lung-specific inflammation through intranasal instillation of LPS. As expected, intranasal LPS administration confined the recruitment of neutrophils to the lungs and not to other organs, ruling out systemic inflammation (Fig. 1A). These lungs and the recruited Ly6G+ neutrophils expressed potent inflammatory mediators, including IL-1β, TNF-α, IL-6, and cyclooxygenase-2 (Cox-2) (Fig. 1B and Fig. S1A). Further characterization by flow cytometry showed increased recruitment (>sevenfold) of bone marrow (BM)-derived CD45+ CD11b+ Ly6G+ neutrophils (Fig. S1 B and C). Taken together, these data suggest that intranasal LPS administration generates an inflammatory microenvironment in the lungs characterized by an influx of neutrophils and the induction of inflammatory mediators.
Fig. 1.
Intranasal LPS administration up-regulates neutrophil recruitment and enhances metastasis to the lung. (A) Representative immunohistochemistry of myeloperoxidase (brown) in PBS-treated control (Ctrl) or LPS-treated mouse lung, liver, and kidney (n = 5 mice per group, four sections per mouse, 7–10 fields per section). (Scale bar: 100 μm.) (B) Representative quantitative RT-PCR depicting the normalized fold expression of the proinflammatory cytokines IL-1β, IL-6, and TNF-α in LPS-challenged lungs compared with control PBS-challenged lungs. (C, Top) Stereological quantitation of metastasis in lungs of PBS-challenged (Tum-Ctrl) or LPS-challenged (Tum-LPS) mice 6 wk after primary tumor inoculation in the skin. Data are represented as the mean ± SD (n = 3 Ctrl mice and n = 4 LPS mice, five to eight sections per lung). *P = 0.025 (one-tailed Mann–Whitney U test). (C, Bottom) Representative H&E stains of lungs. (Scale bars: 2 mm.) (D, Top) Stereological quantitation of B16-BL6 melanoma lung metastases in Ctrl or LPS-challenged mice 18 d following tail vein injection of 2 × 105 B16-BL6 cells. Data are plotted as the mean ± SEM (n = 10 mice per group). **P = 0.002 (one-tailed t test with Welch’s correction). (D, Bottom) Representative H&E stains. (Scale bars: 2 mm.) (E, Top) Quantitation using bioluminescence imaging (BLI) of LLC pulmonary metastases in Ctrl or LPS-challenged mice 15 d following tail vein injection of 1 × 105 LLC cells. Data are plotted as the mean ± SEM (n = 5 mice per group, similar data in two repeat experiments). *P = 0.048 (one-tailed Mann–Whitney U test). (E, Bottom) Representative BLI images.
Fig. S1.
Up-regulation of proinflammatory mediators and recruitment of BM-derived cells to lungs after intranasal instillation of LPS. (A) Representative quantitative RT-PCR depicting the normalized fold expression of the proinflammatory mediators IL-1β, IL-6, TNF-α, and Cox-2 in Ly6G+ cells sorted from LPS-challenged lungs compared with control PBS-challenged lungs. Control expression levels are set to 1. Numbers signify fold expression relative to controls (n = 3 mice per group). Experiments were repeated three times with comparable results. (B) Representative flow cytometry-based quantitation of recruited BM-derived cells in the lungs of PBS-treated mice (Ctrl) and LPS-challenged mice 3 d after the last PBS/LPS dose. Fold increase is depicted as the percentage of each cell population in LPS-treated lungs compared with the percentage of each cell population in control lungs. The red dotted line represents a fold increase of 1 (n = 3 mice per group). (C) Representative flow cytometry scatter plots of BM-derived cells in the lungs of PBS-treated mice and LPS-treated mice. B220, B cells; CD11b, myeloid cells; CD11c, dendritic cells; CD25, lymphocytes; CD3, T cells; CD45, hematopoietic cells; CD49b, NK cells; cKit, hematopoietic progenitor cells; F4/80, macrophages; Gr1, myeloid cells; Ly6C, monocytes; Ly6G, granulocytes (neutrophils); VEGFR1, hematopoietic progenitor cells. Experiments were repeated three times with similar results.
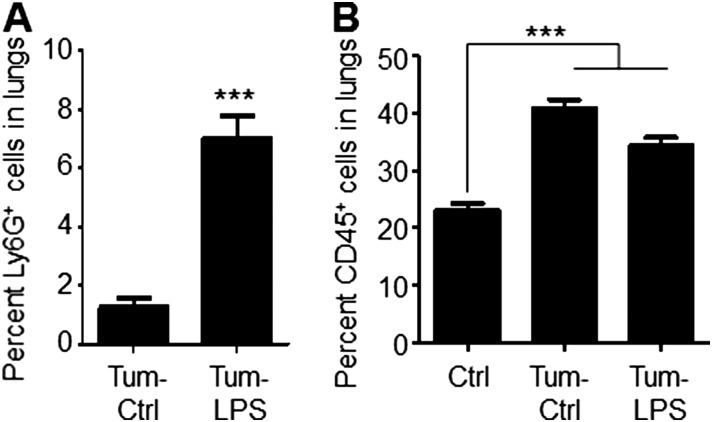
To determine the consequence of lung inflammation on metastasis, we used orthotopic B16-BL6 melanoma, which metastasizes to the lungs from the skin (13). Enhanced melanoma metastasis was observed in LPS-inflamed lungs (Fig. 1C), associated with a marked increase in Ly6G+ neutrophils (Tum-LPS) compared to controls (Tum-Ctrl) (Fig. S2A). These data are consistent with the findings of LPS-challenged, tumor-free mice (Fig. 1A and Fig. S1 B and C), and suggest an association between neutrophil recruitment and metastasis.
Fig. S2.
LPS challenge enhances neutrophil recruitment to the lung in an orthotopic model of melanoma. (A) Immunofluorescence-based quantitation of Ly6G+ neutrophil recruitment to lungs in tumor-bearing mice 3 d after the last intranasal administration of PBS (Tum-Ctrl) or LPS (Tum-LPS). Data are represented as the mean ± SEM (n = 3 mice per group, two sections per lung, nine fields per section). ***P < 0.0001 (one-tailed Mann–Whitney U test). (B) Immunofluorescence-based quantitation of CD45+ hematopoietic cell recruitment to lungs in non–tumor-bearing mice (Ctrl) and tumor-bearing mice 3 d after the last intranasal administration of PBS or LPS. Data are represented as the mean ± SEM (n = 3 mice per group, two sections per lung, nine fields per section). ***P < 0.0001 (one-tailed t test) for comparisons between Ctrl and Tum-Ctrl, as well as between Ctrl and Tum-LPS.
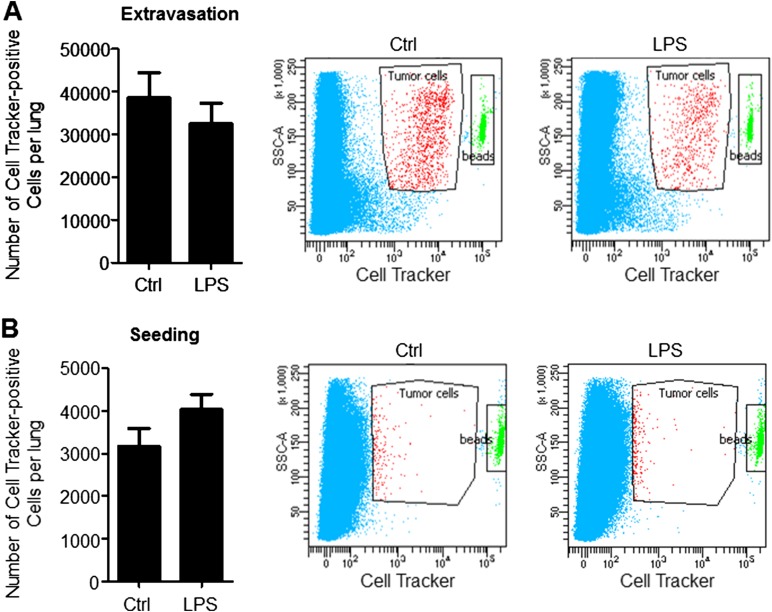
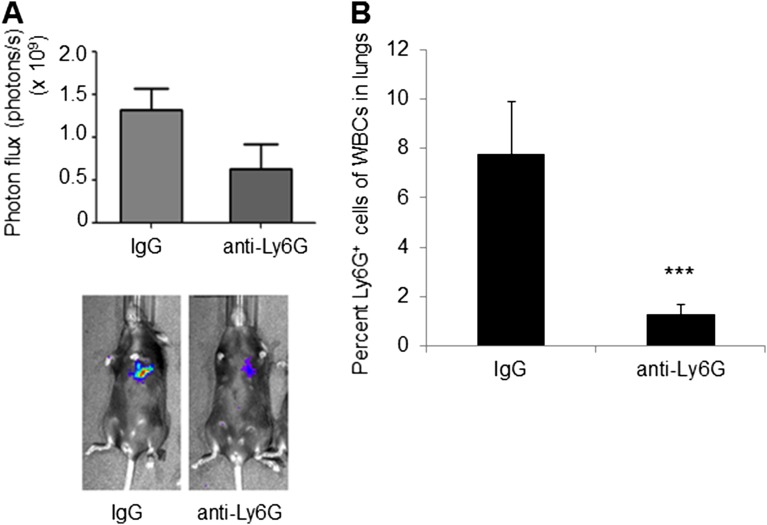
Having demonstrated that inflammation confined to the lung microenvironment can promote metastasis of orthotopic primary tumors, we next interrogated the molecular mechanisms by which neutrophil-mediated lung inflammation contributes to metastatic outgrowth. However, the orthotopic tumor model is not optimal to interrogate mechanisms, since primary tumors systemically generate BM-derived premetastatic niches with inflammatory characteristics in metastatic organs (14–16). Consistent with this notion, lungs of tumor-bearing mice (Tum-Ctrl) showed increased CD45+ cell content (Fig. S2B), suggesting that an experimental metastasis model is necessary to circumvent the confounding effects of primary tumor-generated lung inflammation. Importantly, LPS-inflamed lungs showed an increased metastatic burden following i.v. administration of melanoma cells (Fig. 1D). Similarly, Lewis lung carcinoma (LLC) cells, widely used in metastasis studies (17, 18), also showed increased lung metastasis following LPS challenge (Fig. 1E), suggesting that lung inflammation has an impact on metastasis across tumor types. Importantly, there was no significant difference in the number of cells that extravasate or seed in LPS-conditioned lungs compared with controls (Fig. S3), suggesting that the differences in lung tumor burden observed are due to enhanced tumor initiation or outgrowth after initial seeding. Importantly, neutrophil depletion with anti-Ly6G neutralizing Ab (19) reduced lung metastasis (Fig. S4), confirming the specific and dominant contribution of neutrophils to the inflammation-mediated metastatic phenotype.
Fig. S3.
LPS does not increase extravasation or seeding of LLC cells tail vein injected 3 d after the last LPS dose. (A, Left) Flow cytometry-based quantitation of the number of Cell Tracker dye-positive cells in lungs 2 h after cell injection. Data are represented as the mean ± SEM (n = 3 mice per group). P = 0.629 (two-tailed Mann–Whitney U test). (A, Right) Representative flow cytometry scatter plots of Cell Tracker dye-positive LLC cells in lungs. (B, Left) Flow cytometry-based quantitation of the number of Cell Tracker dye-positive cells in lungs 48 h after cell injection. Data are represented as the mean ± SEM (n = 3 mice per group). P = 0.2 (two-tailed Mann–Whitney U test. (B, Right) Representative flow cytometry scatter plots of Cell Tracker dye-positive LLC cells in lungs.
Fig. S4.
Depletion of Ly6G+ cells suppresses metastasis. (A, Top) Quantification of pulmonary metastases following LLC tail vein injection using BLI in mice administered IgG isotype Ab or anti-Ly6G neutralizing Ab (n = 5 per group). (A, Bottom) Representative BLI images. (B) Flow cytometry-based quantification of Ly6G+ cells in lungs of isotype-treated and anti–Ly6G-treated mice (n = 3 per group). ***P = 0.00016.
Inflamed Lungs Show Loss of Tsp-1 and Up-Regulation of Neutrophil Ser Protease Activity.
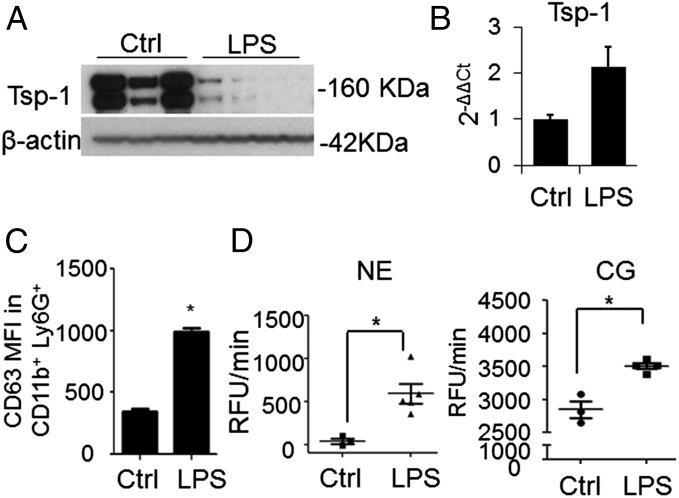
To elucidate the mechanisms by which neutrophils in the inflamed lungs promote metastasis, we focused on Tsp-1, a secreted ECM protein critical for lung homeostasis and inflammation (20, 21). Tsp-1 deficiency extends inflammation and confers worse lung fibrosis in response to bleomycin treatment (22). Furthermore, we had previously demonstrated that myeloid cell-derived Tsp-1 contributes to lung metastasis suppression (23). Analysis of inflamed lungs showed loss of Tsp-1 protein compared with controls (Fig. 2A). Given that Tsp-1 is a potent inhibitor of tumor angiogenesis and growth (24, 25), we posited that decreased Tsp-1 protein may enhance metastasis. The loss of Tsp-1 protein was not due to a reduction in neutrophil Tsp-1 mRNA levels (Fig. 2B), suggesting posttranslational regulation of Tsp-1, and potentially, proteolytic degradation by neutrophil proteases. Of note, the trend of increased neutrophil Tsp-1 mRNA in LPS-inflamed lungs can be attributed to the activation of neutrophil transcription by inflammation (26).
Fig. 2.
Neutrophil recruitment in LPS-inflamed lungs is associated with Tsp-1 degradation. (A) Western blot analysis of Tsp-1 levels in the lungs of WT mice challenged with PBS (Ctrl, n = 3 mice) or LPS (n = 4 mice). β-Actin was used as a loading control. The experiment was reproduced three times with comparable results. (B) Quantitative RT-PCR analysis of Tsp-1 in flow cytometry-sorted Ly6G+ cells from the lungs of PBS-challenged (Ctrl) or LPS-challenged mice. Samples were normalized based on equal Ly6G+ cell numbers, and normalized to GAPDH mRNA. Data are plotted as the mean ± SEM (n = 3 mice per group, in duplicates). The PBS group was used as a control for relative expression (2−ΔΔCt). (C) Flow cytometry analysis of CD63 median fluorescence intensity (MFI) in the CD11b+ Ly6G+ population in lungs of control and LPS-challenged mice. Data are plotted as the mean ± SEM (n = 4 mice per group). *P = 0.029 (one-tailed Mann–Whitney U test). The experiment was reproduced twice with comparable results. (D) NE activity (Left) and CG activity (Right) in lungs of PBS-treated (Ctrl) and LPS-treated mice. Activity is presented as relative fluorescence units per minute (RFU/min) and plotted as the mean ± SEM (n = 4 mice per group). *P = 0.018 and *P = 0.029 for NE activity and CG activity, respectively (one-tailed Mann–Whitney U test).
Neutrophils carry Ser proteases, among which NE and CG have binding affinity for Tsp-1 (27). Given that NE and CG are stored in specialized azurophilic granules, and inflammation is a major trigger of neutrophil degranulation (28), we speculated that degranulating neutrophils in the inflamed lungs, by virtue of secreting NE and CG, may mediate Tsp-1 proteolysis. Indeed, increased neutrophil degranulation was detected in inflamed lungs, as determined by cell surface presentation of the azurophilic granule membrane molecule CD63 (29) (Fig. 2C). Increased neutrophil degranulation was associated with enhanced activity of both NE and CG in inflamed lungs (Fig. 2D).
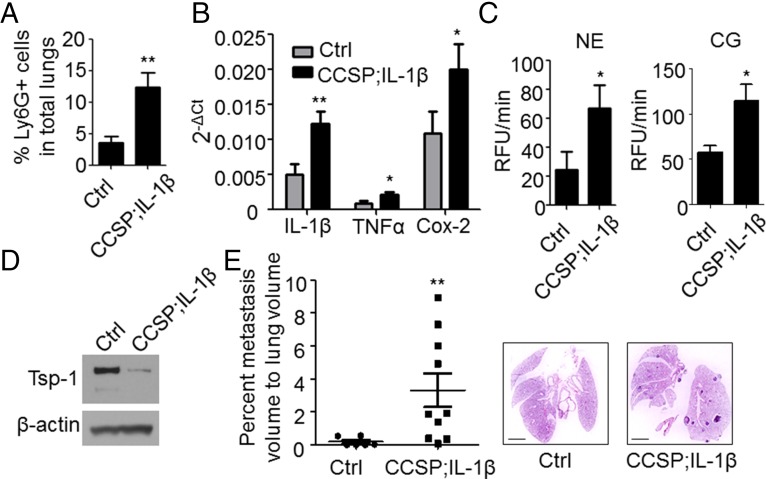
To determine that the mechanisms identified above are not confined to the LPS model of inflammation, we evaluated a genetic model of inflammation, Clara cell secretory protein (CCSP)–tetracycline-controlled transactivator (rtTA); tetO–IL-1β, where the potent inflammatory cytokine IL-1β is conditionally expressed in the lung epithelium, under the CCSP promoter (30). Consistent with the LPS model, conditional expression of IL-1β increased the recruitment of Ly6G+ neutrophils, elevated the levels of proinflammatory mediators, and induced a significant increase in NE and CG activities associated with degradation of Tsp-1 protein (Fig. 3 A–D and Fig. S5A). This phenotype translated to an enhanced metastatic burden in lungs following tail vein administration of tumor cells (Fig. 3E and Fig. S5B).
Fig. 3.
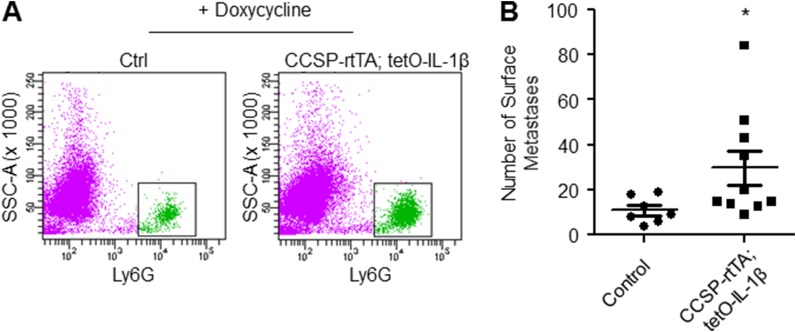
Conditional expression of IL-1β in lungs promotes neutrophil recruitment, Tsp-1 degradation, and enhanced metastatic outgrowth. (A) Flow cytometry-based quantification of Ly6G+ cells in lungs of CCSP–tetracycline-controlled transactivator (rtTA); tetO–IL-1β or age-matched control littermates (WT or CCSP-rtTA single-transgenic) administered doxycycline for 10 d (n = 5 controls and n = 7 CCSP-rtTA; tetO–IL-1β). **P = 0.0064 (one-tailed t test). Data are represented as the mean ± SEM. (B) Representative quantitative RT-PCR depicting the normalized expression of the proinflammatory mediators IL-1β, TNF-α, and Cox-2 in lungs of CCSP-rtTA; tetO–IL-1β mice and controls administered doxycycline for 10 d (n = 5 controls and n = 7 CCSP-rtTA; tetO-IL-1β). **P = 0.005 and *P = 0.025 for IL-1β and TNF-α, respectively (one-tailed t test). *P = 0.014 for Cox-2 (one-tailed Mann–Whitney U test). Data are represented as the mean ± SEM. (C) NE activity (Left) and CG activity (Right) in lungs of CCSP-rtTA; tetO–IL-1β mice and controls administered doxycycline for 10 d (n = 5 controls and n = 7 CCSP-rtTA; tetO–IL-1β). Activity is represented as RFU/min. *P = 0.038 and *P = 0.013 for NE and CG activity, respectively (one-tailed t test). Data are represented as the mean ± SEM. (D) Western blot analysis of Tsp-1 in pooled lungs of bitransgenic mice or littermates administered doxycycline for 10 d (n = 5 controls and n = 7 CCSP-rtTA; tetO–IL-1β). β-Actin is used as a loading control. (E, Left) Stereological measurements of lung metastases 28 d after tail vein injection of 1 × 106 mouse mammary tumor virus-polyoma middle T antigen (MMTV-PyMT) (FVB/N strain)–derived breast cancer single-cell suspensions into syngeneic CCSP-rtTA; tetO–IL-1β or age-matched control littermates (FVB/N strain) that were administered doxycycline for 8 d. Data are plotted as the mean ± SEM (n = 6 controls and n = 10 CCSP-rtTA; tetO–IL-1β). **P = 0.005 (one-tailed Mann–Whitney U test). (E, Right) Representative H&E stains. (Scale bars: 2 mm.)
Fig. S5.
Conditional expression of IL-1β in lungs promotes neutrophil recruitment, Tsp-1 degradation, and enhanced metastatic outgrowth. (A) Representative scatter plots depicting flow cytometry analysis of Ly6G+ cells in lungs of CCSP-rtTA; tetO–IL-1β or age-matched control littermates (WT or CCSP-rtTA single-transgenic) administered doxycycline for 10 d. (B) Quantitation of number of surface metastases in lungs 28 d after tail vein injection of 1 × 106 mouse mammary tumor virus-polyoma middle T antigen (MMTV-PyMT) (FVB/N strain)–derived breast cancer single-cell suspensions into syngeneic CCSP-rtTA; tetO–IL-1β or age-matched control littermates (FVB/N strain) that were administered doxycycline for 8 d. Data are plotted as the mean ± SEM (n = 7 controls and n = 10 CCSP-rtTA; tetO–IL-1β). *P = 0.018 (one-tailed t test with Welch’s correction).
NE and CG Degrade Tsp-1 in Vitro.
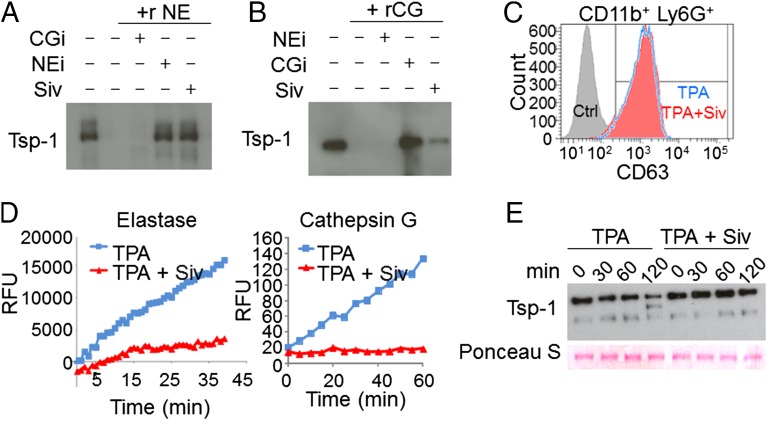
To show that NE and CG possess Tsp-1 proteolysis activity, we performed in vitro assays. Both NE and CG degraded recombinant Tsp-1, which was blocked in the presence of protease-specific inhibitors (Fig. 4 A and B). We also tested sivelestat (ONO-5046), a pharmacological inhibitor of NE (IC50 = 44 nM, Ki = 0.2 μM), used for treatment of acute lung injury in animals (31, 32), and humans (33). As expected, sivelestat inhibited NE-mediated Tsp-1 proteolysis (Fig. 4A). Strikingly, we found that sivelestat also reduced CG-mediated Tsp-1 proteolysis (Fig. 4B), thereby exhibiting dual protease inhibitor activity. This dual activity can be explained by structural homology and a conserved catalytic triad in these proteases (34). To expand upon the role of neutrophils in Tsp-1 proteolysis through degranulation of proteases, we isolated neutrophils and induced degranulation with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) (35). TPA-induced neutrophil degranulation was confirmed by the membrane presentation of CD63 (Fig. 4C). Conditioned media (CM) from TPA-treated neutrophils showed an increase in NE and CG protease activity (Fig. 4D) and induced Tsp-1 proteolysis (Fig. 4E). Notably, sivelestat concurrently blocked NE and CG protease activity in the CM and protected Tsp-1 from proteolysis (Fig. 4 D and E) without inhibiting neutrophil degranulation (Fig. 4C).
Fig. 4.
Neutrophil-derived Ser proteases mediate proteolysis of Tsp-1. (A and B) In vitro degradation assays of recombinant Tsp-1 with recombinant NE and CG proteases, alone or in combination with the specific inhibitors of NE or CG, and sivelestat. Recombinant (r) protein incubations were followed by Western blot analysis for Tsp-1 levels. Experiments were repeated three times with similar results. (C) Representative flow cytometry analysis of degranulation marker CD63 in CD11b+ Ly6G+ cells cultured in vitro with 0.01% DMSO (Ctrl, solid gray histogram), 20 nM TPA (TPA, empty blue histogram), or 20 nM TPA + 0.05 μg/μL sivelestat (Siv, solid red histogram). Experiments were repeated three times with similar results. (D) Representative protease activity assays for NE (Left) and CG (Right) in CM of Ly6G+ cells cultured in vitro with 20 nM TPA or 20 nM TPA + 0.05 μg/μL Siv. Activities are represented as RFUs as a function of time. Experiments were repeated twice with similar results. (E) Western blot analysis of Tsp-1 in CM of Ly6G+ cells cultured in the presence of TPA or TPA + Siv at t = 0, 30, 60, and 120 min. Ponceau S staining shows equal protein loading.
NE and CG Degrade Tsp-1 in Vivo.
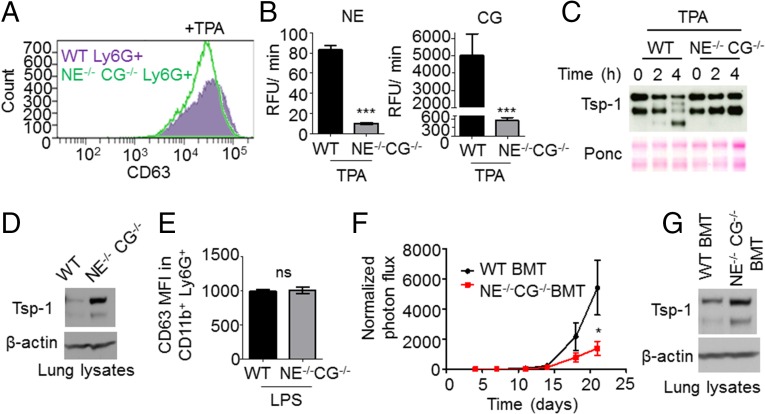
To demonstrate the causal relationship between NE and CG release from neutrophils and the degradation of Tsp-1, we used Ly6G+ neutrophils harvested from the BM of NE−/− CG−/− mice. NE−/− CG−/− neutrophils showed similar degrees of degranulation as WT neutrophils following TPA treatment (Fig. 5A). As expected, NE−/− CG−/− neutrophils lacking CG and NE activity (Fig. 5B) failed to induce Tsp-1 degradation (Fig. 5C). In vivo, LPS-induced lung inflammation showed Tsp-1 degradation in WT mice, as expected; however, strikingly, lungs of NE−/− CG−/− mice showed protection of Tsp-1 from proteolysis (Fig. 5D), a phenotype not attributed to defects in degranulation (Fig. 5E).
Fig. 5.
Neutrophil Ser proteases NE and CG work in concert to degrade Tsp-1 and enhance metastasis. (A) Representative flow cytometry analysis of the degranulation marker CD63, in CD11b+ Ly6G+ cells isolated from WT mouse lungs (solid purple histogram) and NE−/− CG−/− mouse lungs (empty green histogram) and cultured in vitro with 20 nM TPA. Experiments were repeated three times with similar results. (B) NE activity (Left) and CG activity (Right) in CM of Ly6G+ cells isolated from WT and NE−/− CG−/− mouse BM and cultured with 20 nM TPA for 30 min. Data are plotted as the mean ± SEM (n = 3 per group). ***P < 0.001 for NE activity and for CG activity [analysis of covariance (ANCOVA), comparing activity curves]. (C) Representative Western blot analysis of Tsp-1 in CM from Ly6G+ cells isolated from WT and NE−/− CG−/− mouse BM and cultured with 20 nM TPA for t = 0, 2, or 4 h. Ponceau S (Ponc) staining is used as a loading control. Experiments were repeated twice with similar results. (D) Western blot analysis of Tsp-1 protein in lungs of WT and NE−/− CG−/− mice treated with LPS. Protein samples are pooled from four mice per group. β-Actin serves as a loading control. (E) Flow cytometry analysis of the MFI of the degranulation marker CD63 in CD11b+ Ly6G+ cells in lungs of WT and NE−/− CG−/− mice treated with LPS. Data are plotted as the mean ± SEM (n = 4 lungs per group). ns, not significant (two-tailed Mann–Whitney U test). (F) Quantification of metastatic burden via BLI in WT BMT and NE−/− CG−/− BMT mice treated with LPS and tail vein injected with LLC tumor cells. Signals were normalized to the readings obtained at day 4 for each individual animal. Data at each time point are plotted as the mean ± SEM (n = 8 mice per group). *P = 0.038 at day 21 (one-tailed t test with Welch’s correction). (G) Western blot analysis of Tsp-1 in lung samples from WT BMT and NE−/− CG−/− BMT mice treated with LPS. Samples are pooled from three mice per group. β-Actin serves as a loading control.
Taken together, these data suggest that neutrophil proteases NE and CG mediate Tsp-1 proteolysis both in vitro and in vivo, highlighting a previously unidentified mechanism of Tsp-1 regulation by the combined proteolytic activity of NE and CG.
NE and CG Activities Are Required for Inflammation-Enhanced Metastasis.
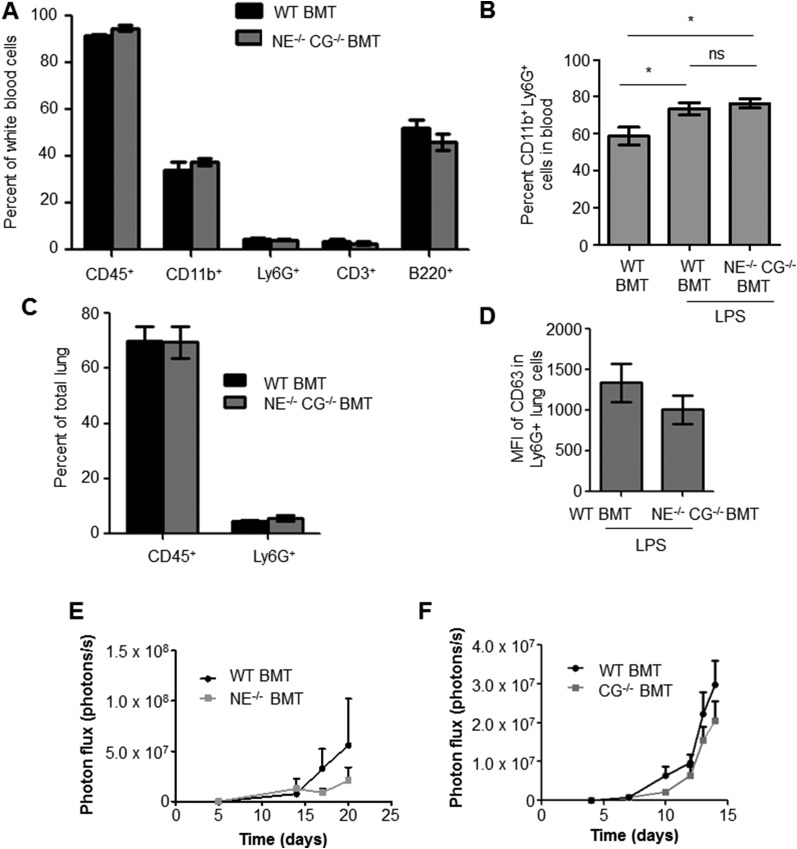
To determine the contribution of the NE/CG–Tsp-1 axis in promoting metastasis, we generated NE and CG deficiency. To ensure that the protease deficiency is confined specifically to the BM cells in the inflamed lungs, a bone marrow transplantation (BMT) approach was used. Specifically, BM cells harvested from NE−/− CG−/− mice were transplanted into irradiated syngeneic WT mice to generate a cohort of NE−/− CG−/− BMT mice. As controls, BM from WT mice was transplanted into irradiated WT mice, generating WT BMT mice. Importantly, NE and CG deficiency in BM cells in NE−/− CG−/− BMT mice did not perturb the mobilization, recruitment, or degranulation of neutrophils after LPS treatment (Fig. S6 B–D). Moreover, deficiency of NE and CG did not have an impact on hematopoietic cell subsets (Fig. S6A). Strikingly, impaired lung metastasis was observed after LPS challenge in NE−/− CG−/− BMT mice compared with WT BMT mice (Fig. 5F). Western blot analysis of the total lung lysates showed protection of Tsp-1 from proteolysis in NE−/− CG−/− BMT mice compared with WT BMT controls (Fig. 5G). Deficiency of either NE or CG only partially suppressed metastasis (Fig. S6 E and F), consistent with the ability of both of these proteases to degrade Tsp-1. Taken together, these results suggest the contribution of the NE/CG–Tsp-1 axis to inflammation-driven metastasis.
Fig. S6.
Double KO of NE and CG in BM does not affect hematopoietic cell composition, Ly6G+ cell circulation in blood, recruitment to lungs, or degranulation after LPS challenge. (A) Flow cytometry-based quantitation of various hematopoietic cell subsets in blood of WT BMT and NE−/− CG−/− BMT mice (P = 0.1 for CD45+ cells, P = 0.4 for CD11b+ cells, P = 0.4 for Ly6G+ cells, P = 0.475 for CD3+ cells, P = 0.628 for B220+ cells). Statistical analysis used is the two-tailed Mann–Whitney U test. (B) Quantitation of CD11b+ Ly6G+ cells in blood of PBS-treated WT BMT and LPS-treated WT BMT or NE−/− CG−/− BMT mice (n = 4 mice per group). *P = 0.044 between WT BMT and WT BMT + LPS (two-tailed t test); *P = 0.016 between WT BMT and NE−/− CG−/− BMT + LPS (two-tailed t test). ns, not significant. (C) Flow cytometry-based quantitation of CD45+ hematopoietic cells and Ly6G+ neutrophils recruited to lungs of WT BMT and NE−/− CG−/− BMT mice treated with LPS (n = 4 mice per group). Differences are not statistically significant, as determined by the two-tailed Mann–Whitney U test. Lungs were collected 2 d after LPS treatment. (D) Flow cytometry-based quantitation of CD63 median fluorescence intensity (MFI) of Ly6G+ cells in lungs of WT BMT and NE−/− CG−/− BMT mice treated with LPS (n = 4 per group). P = 0.305 (two-tailed unpaired t test). (E) Quantitation of BLI showing luciferase-labeled LLC cell outgrowth in lungs of WT BMT and NE−/− BMT mice (n = 8 mice per group). P = 0.4796 (one-tailed Mann–Whitney U test). (F) Quantitation of BLI showing luciferase-labeled LLC cell outgrowth in lungs of WT BMT and CG−/− BMT mice (n = 8 mice per group).
Discussion
Primary tumors systemically induce metastasis-promoting microenvironments referred to as the “premetastatic niche” in distant organs (36). These microenvironments are composed of a variety of BM-derived cellular populations and exhibit inflammatory features (14, 15). Identifying mechanisms by which tumor-derived premetastatic niches contribute to tumor outgrowth is an area of active investigation; however, little is known about the mechanisms by which extrinsic inflammation in the lungs can influence metastasis of extrapulmonary neoplasms. We have described a previously unidentified mechanism, whereby neutrophils recruited in large numbers to the inflamed lungs degranulate and release stored proteases. These proteases specifically target a potent antitumorigenic factor, Tsp-1, resulting in its degradation. This mechanism appears to be responsible for the enhanced metastatic phenotype observed in inflamed lungs (Fig. 6), since deleting both NE and CG in the BM compartment suppresses metastasis. However, future studies using NE−/− CG−/− Tsp-1−/− triple-KO mice are required to establish firmly the causal link between the NE/CG–Tsp-1 axis and metastasis.
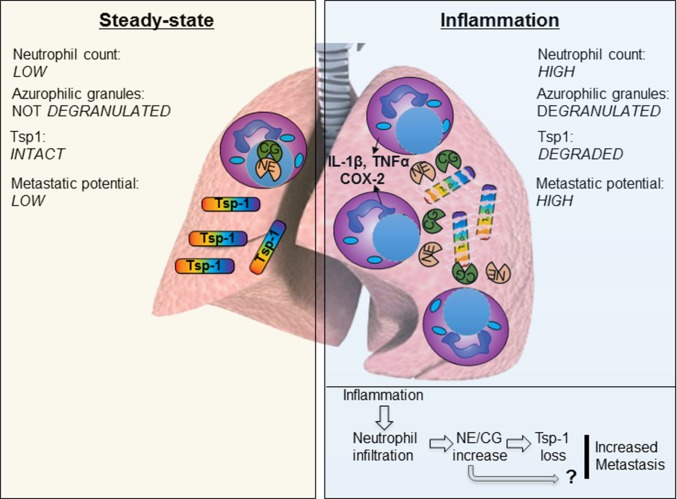
Fig. 6.
Proposed model of inflammation-enhanced metastasis. Under normal physiological conditions (steady state), lungs exhibit sparse neutrophil counts and abundant intact and functional Tsp-1. Inflammation in the lungs causes enhanced influx of BM-derived neutrophils, which are the first responders at the infection site. Activated neutrophils produce potent inflammatory mediators, including IL-1β, TNF-α, IL-6, and Cox-2, and they degranulate their azurophilic granules, releasing the Ser proteases NE and CG. NE and CG degrade Tsp-1, rendering the lung microenvironment conducive to increased metastatic outgrowth. Whether CG and NE also have a non–Tsp-1–mediated role in metastasis is not known and is depicted by a “?”.
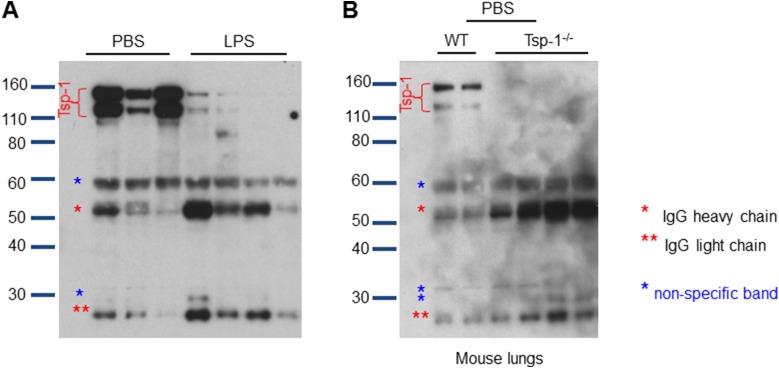
The finding that NE and CG can degrade Tsp-1 and have an impact on its antitumorigenic function is unique, since previous studies have demonstrated that specific Tsp-1 cleavage contributes to Tsp-1 function. A disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAM-TS1)–mediated cleavage of Tsp-1 was shown to release a C-terminal 110-kDa antiangiogenic fragment from matrix-bound Tsp-1 (37). In another study, thrombin was shown to cleave Tsp-1 to release a 25-kDa N-terminal heparin-binding fragment (38). Furthermore, a 140-kDa C-terminal fragment of Tsp-1 released by thrombin was shown to sequester basic FGF, hence inhibiting endothelial cell proliferation (39). In our study, none of these products appeared in the inflamed lungs of mice (Fig. S7), suggesting a novel mechanism of Tsp-1 regulation by the combined proteolytic activity of NE and CG.
Fig. S7.
Proteases in vivo mediate complete degradation of Tsp-1. (A) Full blot of PBS-challenged and LPS-challenged mouse lungs probed for Tsp-1. Full-length Tsp-1 runs as a double band between 110 kDa and 170 kDa. (B) Tsp-1 blot from Tsp-1−/− mice is used to determine nonspecific bands, which appear as a result of using a mouse-derived Tsp-1 Ab on mouse lungs and having to use an anti-mouse secondary Ab for detection. Of note, the Tsp-1 Ab clone we use is the Ab-11 clone, which has the ability to recognize the N-terminal heparin-binding domain, the calcium-binding domain, and the collagen type V-binding domain of Tsp-1.
LPS-inflamed lungs also showed changes in the recruitment of other cell populations (Fig. S1 B and C), which may also contribute to metastasis. However, our focus was to evaluate the most significant increase in neutrophil recruitment, since these cells are critical players in inflammation. Importantly, neutrophil depletion conferred a dramatic antimetastatic phenotype (Fig. S4), suggesting that in the context of inflammation, the neutrophils exert a specific and dominant effect on metastasis. Furthermore, knocking out both neutrophil-restricted proteases, NE and CG, significantly impaired metastasis (Fig. 5F).
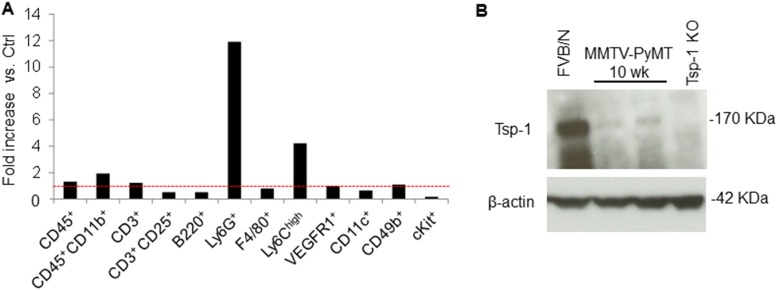
Our study also provides insights into the exquisite functional plasticity of the Ly6G+ cells, because they are the main source of both the proteases and the substrate, Tsp-1, in the lungs (23). Under normal physiological conditions, these neutrophils produce intact functional Tsp-1 that confers a tumor-inhibitory microenvironment. However, under inflammatory conditions, the same neutrophils antagonize the antimetastatic effect of Tsp-1 by degranulating and releasing NE and CG proteases. Such plasticity of neutrophils has been previously described in primary tumors, where TGF-β can drive neutrophils toward a protumorigenic “N2” phenotype (40, 41). Paradoxically, tumor-entrained neutrophils that are recruited to the premetastatic lungs have been shown to block metastatic seeding (42). In our study, the inflammatory neutrophils have not been entrained by tumor cells but merely respond to external inflammation-promoting insults. Tsp-1 degradation is a consequence of this response, which inevitably renders the lung microenvironment more conducive to tumor outgrowth. Strikingly, mouse mammary tumor virus-polyoma middle T antigen (MMTV-PyMT) mice with primary breast tumors generated a premetastatic niche in the lungs with abundant neutrophil infiltration associated with a conspicuous loss of Tsp-1 protein (Fig. S8), suggesting that primary tumors can generate an inflammation-like, Tsp-1–deficient microenvironment in the lungs.
Fig. S8.
MMTV-PyMT lungs show recruitment of neutrophils and Tsp-1 degradation. (A) Representative flow cytometry-based quantitation of recruited BM-derived cells in the lungs of 10-wk-old FVB/N mice (Ctrl) and 10-wk-old MMTV-PyMT mice. Fold increase is depicted as the percentage of each cell population in LPS-treated lungs compared with the percentage of each cell population in control lungs. The red dotted line represents a fold increase of 1 (n = 3 mice per group). (B) Western blot analysis of Tsp-1 protein in the lungs of FVB/N mice, 10-wk-old MMTV-PyMT mice, and Tsp-1−/− mice. β-Actin is used as a loading control.
From the perspective of targeting metastases, it has been emphasized that therapy should be targeted not only against tumor cells but also against the host microenvironment, which contributes to, and supports, the progressive growth and survival of metastatic cancer cells (43). Insight from our studies suggests that targeting neutrophils may not be a viable strategy because it may neutralize the antimetastatic effects of neutrophil-derived Tsp-1 in the microenvironment. However, we believe that targeting the neutrophil NE/CG–Tsp-1 axis may have clinical value in the prevention of metastasis or inhibition of its outgrowth.
Materials and Methods
A detailed description of materials and methods can be found in SI Materials and Methods. All animal work was conducted in accordance with a protocol approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College.
BM Isolation and BMT.
BMT was performed by injecting 1 × 107 total BM cells retroorbitally into lethally irradiated (900 rad), 8-wk-old C57BL/6 female mice.
LPS Treatments.
LPS derived from Escherichia coli strain 0111:B4 was administered intranasally in a 50-μL volume at a concentration of 0.25 mg/mL on days 0, 3, and 7.
Metastasis Assay, Bioluminescence Imaging, and Analysis.
For experimental metastasis, 8-wk-old C57BL/6 mice were injected via the tail vein with 5 × 105 luciferase-labeled LLC cells or 2 × 105 B16-BL6 unlabeled melanoma cells. LLC pulmonary metastases were monitored by live animal bioluminescence imaging, where mice were anesthetized and injected retroorbitally with 75 mg/kg of D-luciferin in 50 μL total volume.
Neutrophil Isolation, Culture, and Degranulation.
BM cells were subjected to magnetic bead isolation using an anti-Ly6G microbead kit (MACS; Miltenyi Biotec). Ly6G+ cells were cultured for 30 min at 37 °C in DMSO vehicle (final concentration of 0.01% DMSO) or 20 nM TPA.
NE and CG Activity Assays.
NE activity was measured using an EnzChek Elastase assay kit (Molecular Probes) according to the manufacturer’s protocol, and CG was measured using a Sensolyte 520 Cathepsin G assay kit (AnaSpec) according to the manufacturer’s protocol.
SI Materials and Methods
Mice and Cell Lines.
WT C57BL/6J and NE KO mice were obtained from The Jackson Laboratory. CG KO mice in the C57BL/6J strain were a gift from Christine Pham (Washington University, St. Louis, MO). NE and CG mice in the C57BL/6J background were bred and genotyped according to standard protocols. Eight-week-old female mice of the above-mentioned genotypes were used for experiments.
CCSP–tetracycline-controlled transactivator (rtTA) mice (FBV/N; The Jackson Laboratory) were bred with tetO–IL-1β mice (FBV/N; a kind gift from Timothy Wang, Columbia University, New York) to yield the bitransgenic CCSP-rtTA; tetO–IL-1β mice.
Murine LLC cells stably expressing RFP and firefly luciferase were cultured in DMEM supplemented with 10% (vol/vol) FBS (18, 23).
Murine B16-BL6 melanoma cells (a gift from Randolph Watnick, Boston’s Children Hospital, Boston) were cultured in DMEM supplemented with 10% (vol/vol) FBS (13).
BM Isolation and BMT.
BM harvesting and BMT were performed using our published methods. BMT was performed by injecting 1 × 107 total BM cells retroorbitally into lethally irradiated (900 rad), 8-wk-old C57BL/6 female mice. BM cells were harvested by flushing femurs and tibias of donor animals, including WT and KO for NE and CG. After 4 wk of BM engraftment, reconstitution efficiency was monitored by a PCR assay of genomic DNA from peripheral blood for the absence of NE and CG.
Metastasis Assay, Bioluminescence Imaging, and Analysis.
For experimental metastasis, 8-wk-old C57BL/6 mice were injected via the tail vein with 5 × 105 luciferase-labeled LLC cells or 2 × 105 B16-BL6 unlabeled melanoma cells. LLC pulmonary metastases were monitored by live animal bioluminescence imaging (BLI; Xenogen) once per week. Briefly, mice were anesthetized and injected in the retroorbital plexus with 75 mg/kg of D-luciferin in 50 μL total volume. For BLI plots, photon flux was calculated for each mouse by using the same circular region of interest encompassing the thorax of the mouse. B16-BL6 pulmonary metastases were assessed by stereology following H&E staining of the lungs 18 d after tumor cell i.v. injection.
To study metastasis from orthotopic tumors, 8-wk-old C57BL/6 mice were injected s.c. in the flank with 105 B16-BL6 unlabeled melanoma cells in a total volume of 50 μL of PBS. After 3 wk of primary tumor growth, LPS was administered intranasally on days 0, 3, and 7. Primary tumors were then resected on day 10, and a few lungs were harvested for analysis of neutrophil recruitment. Metastasis was allowed to progress for an additional 3 wk, at which time all lungs were harvested for H&E staining and stereology.
Ly6G Depletion.
Eight-week-old C57BL/6 mice were injected via the tail vein with 5 × 105 luciferase-labeled LLC cells. At day 5, mice were injected via the retroorbital route with anti-Ly6G mAb (1A8) or IgG control (2.5 mg/kg, purified rat anti-mouse Ly6G and rat IgG2a κ-isotype control; BD Pharmingen).
LPS Treatments.
LPS derived from E. coli strain 0111:B4 was purchased from Sigma Chemical Co. Mice were anesthetized using isoflurane, and LPS was administered intranasally in a 50-μL volume at a concentration of 0.25 mg/mL on days 0, 3, and 7. In the case of tumor-free experiments, lungs were collected at day 10 (3 d after the last LPS treatment) and analyzed.
In experiments assessing metastatic growth following LPS treatment, 5 × 105 LLC cells or 2 × 105 B16-BL6 melanoma cells were injected i.v. via the tail vein 3 d after the end of LPS treatment.
Neutrophil Isolation, Culture, and Degranulation.
BM cells were harvested from femurs and tibias of WT C57/BL6 mice or NE−/− CG−/− mice by flushing the bones with 1× PBS. Total BM cells were then subjected to magnetic bead isolation using an anti-Ly6G microbead kit (MACS; Miltenyi Biotec). Ly6G+ cell population purity was then assessed by flow cytometric analysis.
Approximately 3 million cells were seeded in a total volume of 200 μL of serum-free RPMI in wells of a 48-well plate. Cells were cultured for 30 min at 37 °C in DMSO vehicle (final concentration of 0.01% DMSO) or 20 nM 12-O-tetradecanoylphorbol-13-acetate (TPA; Cell Signaling Technology). Neutrophil cell conditioned medium was collected by centrifuging the cells (300 × g for 10 min at room temperature). The conditioned medium was incubated at 37 °C for the indicated time points, with or without the addition of sivelestat (ONO-5046; TOCRIS Bioscience) at a final concentration of 0.05 μg/μL. Degranulation was measured by flow cytometric analysis using the marker CD63.
NE and CG Activity Assays.
After in vitro degranulation of Ly6G+ cells, protease activity was measured in CM. NE activity was measured using an EnzChek Elastase assay kit (Molecular Probes) according to the manufacturer’s protocol, and CG was measured using a Sensolyte 520 Cathepsin G assay kit (AnaSpec) according to the manufacturer’s protocol.
For protease activity analysis from lung samples, protein was extracted in 1× radioimmunoprecipitation assay (RIPA) buffer without protease inhibitors. For NE activity, analysis was conducted with 40 μg of protein. For CG activity, analysis was conducted with 5 μg of protein.
Microscopy.
Animals were euthanized at the end of experiments, and lungs were quickly perfused by injecting 5 mL of cold PBS through the right ventricle of the heart. One part of the lung from each animal was fixed in 3.7% formalin, and the other part was treated with collagenase-A to create a homogeneous cellular solution and analyzed by flow cytometry, or it was snap-frozen for RNA and protein extraction.
For microscopy, following formalin fixation, tissues were cryoembedded in Tissue-Tek O.C.T. embedding compound (Electron Microscopy Sciences). Lungs were sectioned at a thickness of 7 μm. Lung sections were stained with H&E for morphometric determinations using stereology.
Immunohistochemistry for myeloperoxidase was performed using Pierce myeloperoxidase polyclonal rabbit Ab solution (Thermo Scientific) and EnVision+ System HRP-labeled antirabbit (DAB+) Ab (Dako).
Tissue Sampling and Stereology.
Lungs were perfused by injecting 5 mL of cold PBS through the right ventricle of the heart. Lungs were then fixed in 3.7% formaldehyde overnight, followed by 2 d in 30% sucrose at 4 °C. To prevent the tissue shrinkage that occurs during paraffin embedding, lungs were cryoembedded in Tissue-Tek O.C.T. embedding compound, snap-frozen on dry ice, and stored at −80 °C until further sampling.
For systematic uniform and random sampling (44), the frozen lungs were sectioned along the coronal plane, with the first cut starting at a random position and the entire lung cut into 1.0-mm-thick parallel slabs, resulting in six to seven slabs per lung. Cryostat sections from each slab were 7 μm thick. Sections were stained with H&E.
The stereological measurements of lung and metastasis volumes were performed using the Axiovision 4.8 software (Carl Zeiss, Inc.) area measurement function and following the Cavalieri principle:
where V is volume of lung or volume of metastasis, T is the slab thickness, or intersectional distance (1.0 mm as indicated above), and Areas are measured from one section per slab and added up for the entire six to seven slabs.
Finally, data were presented as the percentage of metastasis volume per lung volume.
Flow Cytometry and Cell Sorting.
To obtain single-cell suspensions, lungs were minced and then digested in collagenase/DNase mix (Roche) for 30 min at 37 °C. Cells were washed, strained, and resuspended in fluorescence-activated cell sorting (FACS) staining buffer (PBS + 2 mM EDTA, 1% BSA). For analysis of peripheral blood, blood was collected from the tails of mice in anticoagulant buffer (PBS with 5 mM EDTA). RBCs were eliminated by incubation in RBC Lysis Buffer (5 Prime) for 10 min on ice. Cell suspensions were preblocked in FACS buffer plus Fc block (CD16/CD32, 1:30; BD Biosciences Pharmingen) and then incubated with the following primary Abs: rat IgG2ακ-and IgG2αβ-isotype control (BD Pharmingen), Ly6G (clone 1A8; BD Pharmingen), Ly6C (clone HK1.4; Biolegend), Gr1 (clone RB6-8C5; Biolegend), CD11b (clone M1/70; BD Pharmingen), CD45 (clone 30-F1; Biolegend), CD3 (clone 17A2; Biolegend), B220 (clone RA3-6B2; Biolegend), CD25 (clone PC61; Biolegend), CD49b (clone DX5; BD Pharmingen), cKit (clone 2B8; BD Pharmingen), VEGFR1 (BD Pharmingen), CD11c (clone N418; Biolegend), and CD63 (clone NVG-2; Biolegend). SYTOX blue (Invitrogen) was added and incubated for 5 min at room temperature in each cell staining tube to facilitate the elimination of dead cells by flow cytometry.
Labeled cell populations were measured by an LSRII flow cytometer coupled with FACSDiva software (BD Bioscience). Flow cytometry analysis was performed using a variety of controls, including isotype Abs and unstained samples for determining appropriate gates, voltages, and compensations required in multivariate flow cytometry. For sorting, targeted cell populations were gated within FACSDiva software and sorted by an Aria II sorter (BD Bioscience). For sorting, SYTOX blue-negative was used to remove dead cells. The CD11b+ Ly6G+ subset was isolated and processed for RNA isolation using a PicoPure RNA isolation kit (Arcturus).
For Cell Tracker dye experiments monitoring extravasation and seeding, LLC cells were incubated with 10 μM CellTracker Deep Red dye (Life Technologies) for 45 min. Then, 5 × 105 cells were injected via the tail vein of mice 3 d after the third LPS or PBS treatment (Materials and Methods, LPS Treatments). Extravasation and seeding were assessed by collecting lungs 2 h and 48 h after tumor cell injection, respectively. Lungs were minced and prepared for flow cytometric detection of CellTracker-positive tumor cells.
Quantitative RT-PCR Analysis.
Total RNA from flow cytometry-sorted cells was extracted using the PicoPure RNA extraction kit following the manufacturer’s protocol. RNA was converted to cDNA using qScript cDNA Supermix (Quanta Biosciences). Quantitative PCR was performed with primers and iQTM SYBER Green Master Mix (Biorad). Each sample was duplicated to minimize pipetting error. A standard protocol of initial denaturing at 95 °C for 10 min and 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 30, followed by final extension at 72 °C for 5 min and melt curve analysis, was applied on a BioRad CFX96 Real Time System (BioRad) coupled with Bio-Rad-CFX Manager software. The relative abundance of each transcript compared with the control was calculated using the Δ-Ct (cycle threshold) method.
The primer sequences used for RT-PCR were as follows:
Mus-GAPDH forward: CATGGCCTTCCGTGTTCCTA
Mus-GAPDH reverse: GCGGCACGTCAGATCCA
Mus–Tsp-1 forward: CTTGAGGCAGATGAAGAAGACC
Mus–Tsp-1 reverse: ACTGACACCACTTGTTGCTTCC
Mus–IL-6 forward: AGACAAAGCCAGAGTCCTTCAG
Mus–IL-6 reverse: TTAGGAGAGCATTGGAAATTGG
Mus–IL-1β forward: TGTGTAATGAAAGACGGCACAC
Mus–IL-1β reverse: TCAAACTCCACTTTGCTCTTGA
Mus–TNF-α forward: GTCTACTGAACTTCGGGGTGAT
Mus–TNF-α reverse: TTGAGAAGATGATCTGAGTGTGAG
Mus–Cox-2 forward: CCATTGACCAGAGCAGAGAGAT
Mus–Cox-2 reverse: CTTTCAATTCTGCAGCCATTTC
Western Blot Analysis.
Cells were homogenized in lysis buffer (BioRad) containing protease inhibitors (Roche Applied Science). Samples were boiled in 1× SDS sampling buffer and loaded onto 4–12% gradient Bis-Tris NuPAGE gels (Invitrogen). Western blotting was performed using Abs specific for Tsp-1 (Clone Ab-11; Labvision) and β-actin (Sigma–Aldrich).
Recombinant Tsp-1 (R&D Systems) was incubated with recombinant NE (R&D Systems) or recombinant CG (Genway), in the presence of the NE inhibitor N-(Methoxysuccinyl)-Ala-Ala-Pro-Val-chloromethyl ketone (Sigma) or the CG inhibitor Z-Gly-Leu-Phe-CMK (MP Biomedicals).
Statistical Analysis.
Results, expressed as the mean ± SEM, are shown by error bars, unless otherwise stated. Analyses of different treatment groups were performed using the Mann–Whitney U or Student t test, utilizing GraphPad Prism statistics software. P values <0.05 were considered significant.
Acknowledgments
We thank Dr. Hyejin Choi and Dr. Kari Fischer for comments. Support from the Cornell Center on the Microenvironment and Metastasis (Grant U54CA14387 to V.M. and C.F.) and the National Lung Cancer Partnership (R.C. and D.G.) is recognized. R.C. was supported by fellowships from the “Government of Navarra” and the “Camara Navarra de Comercio” (Spain).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.M.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507294112/-/DCSupplemental.
References
- 1.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12(8):715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 2.Murin S, Pinkerton KE, Hubbard NE, Erickson K. The effect of cigarette smoke exposure on pulmonary metastatic disease in a murine model of metastatic breast cancer. Chest. 2004;125(4):1467–1471. doi: 10.1378/chest.125.4.1467. [DOI] [PubMed] [Google Scholar]
- 3.Smith HA, Kang Y. Acute infection induces a metastatic niche: A double menace for cancer patients. Clin Cancer Res. 2013;19(17):4547–4549. doi: 10.1158/1078-0432.CCR-13-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murin S, Inciardi J. Cigarette smoking and the risk of pulmonary metastasis from breast cancer. Chest. 2001;119(6):1635–1640. doi: 10.1378/chest.119.6.1635. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: Insights from clinical studies. Proc Am Thorac Soc. 2009;6(3):256–259. doi: 10.1513/pats.200808-087RM. [DOI] [PubMed] [Google Scholar]
- 7.Jeffery PK. Structural and inflammatory changes in COPD: A comparison with asthma. Thorax. 1998;53(2):129–136. doi: 10.1136/thx.53.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77(2):568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamamoto K, et al. Roles of lung epithelium in neutrophil recruitment during pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2014;50(2):253–262. doi: 10.1165/rcmb.2013-0114OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an important role in acute lung infection-enhanced lung tumor metastasis. Clin Cancer Res. 2013;19(17):4706–4716. doi: 10.1158/1078-0432.CCR-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J-L, Maeda S, Hsu L-C, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation-induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell. 2004;6(3):297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Jiang M, et al. Systemic inflammation promotes lung metastasis via E-selectin upregulation in mouse breast cancer model. Cancer Biol Ther. 2014;15(6):789–796. doi: 10.4161/cbt.28552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura K, et al. Characterization of mouse melanoma cell lines by their mortal malignancy using an experimental metastatic model. Life Sci. 2002;70(7):791–798. doi: 10.1016/s0024-3205(01)01454-0. [DOI] [PubMed] [Google Scholar]
- 14.Psaila B, Lyden D. The metastatic niche: Adapting the foreign soil. Nat Rev Cancer. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiratsuka S, et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat Cell Biol. 2008;10(11):1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 16.Gao D, et al. Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 2012;72(6):1384–1394. doi: 10.1158/0008-5472.CAN-11-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S, et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457(7225):102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao D, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319(5860):195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 19.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83(1):64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 20.Lawler J, et al. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101(5):982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez-Dee Z, Pidcock K, Gutierrez LS. Thrombospondin-1: Multiple paths to inflammation. Mediators Inflamm. 2011;2011:296069. doi: 10.1155/2011/296069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ezzie ME, et al. Thrombospondin-1-deficient mice are not protected from bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;44(4):556–561. doi: 10.1165/rcmb.2009-0019OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catena R, et al. Bone marrow-derived Gr1+ cells can generate a metastasis-resistant microenvironment via induced secretion of thrombospondin-1. Cancer Discov. 2013;3(5):578–589. doi: 10.1158/2159-8290.CD-12-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Good DJ, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA. 1990;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawler J. Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med. 2002;6(1):1–12. doi: 10.1111/j.1582-4934.2002.tb00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes PJ, Karin M. Nuclear factor-kappaB: A pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336(15):1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 27.Hogg PJ, Jiménez BM, Chesterman CN. Identification of possible inhibitory reactive centers in thrombospondin 1 that may bind cathepsin G and neutrophil elastase. Biochemistry. 1994;33(21):6531–6537. doi: 10.1021/bi00187a021. [DOI] [PubMed] [Google Scholar]
- 28.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5(14):1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Kuijpers TW, et al. Membrane surface antigen expression on neutrophils: A reappraisal of the use of surface markers for neutrophil activation. Blood. 1991;78(4):1105–1111. [PubMed] [Google Scholar]
- 30.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1β causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32(4):311–318. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 31.Hagio T, et al. Elastase inhibition reduced death associated with acid aspiration-induced lung injury in hamsters. Eur J Pharmacol. 2004;488(1-3):173–180. doi: 10.1016/j.ejphar.2004.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Hagio T, Matsumoto S, Nakao S, Matsuoka S, Kawabata K. Sivelestat, a specific neutrophil elastase inhibitor, prevented phorbol myristate acetate-induced acute lung injury in conscious rabbits. Pulm Pharmacol Ther. 2005;18(4):285–290. doi: 10.1016/j.pupt.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Iwata K, et al. Effect of neutrophil elastase inhibitor (sivelestat sodium) in the treatment of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): A systematic review and meta-analysis. Intern Med. 2010;49(22):2423–2432. doi: 10.2169/internalmedicine.49.4010. [DOI] [PubMed] [Google Scholar]
- 34.Averhoff P, Kolbe M, Zychlinsky A, Weinrauch Y. Single residue determines the specificity of neutrophil elastase for Shigella virulence factors. J Mol Biol. 2008;377(4):1053–1066. doi: 10.1016/j.jmb.2007.12.034. [DOI] [PubMed] [Google Scholar]
- 35.Grinstein S, Furuya W. Cytoplasmic pH regulation in activated human neutrophils: effects of adenosine and pertussis toxin on Na+/H+ exchange and metabolic acidification. Biochim Biophys Acta. 1986;889(3):301–309. doi: 10.1016/0167-4889(86)90192-8. [DOI] [PubMed] [Google Scholar]
- 36.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9(4):239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee NV, et al. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006;25(22):5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lawler J, Connolly JE, Ferro P, Derick LH. Thrombin and chymotrypsin interactions with thrombospondin. Ann N Y Acad Sci. 1986;485:273–287. doi: 10.1111/j.1749-6632.1986.tb34589.x. [DOI] [PubMed] [Google Scholar]
- 39.Taraboletti G, et al. The 140-kilodalton antiangiogenic fragment of thrombospondin-1 binds to basic fibroblast growth factor. Cell Growth Differ. 1997;8(4):471–479. [PubMed] [Google Scholar]
- 40.Fridlender ZG, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: Friend or foe? Carcinogenesis. 2012;33(5):949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 42.Granot Z, et al. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20(3):300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fidler IJ. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 44.Nielsen BS, et al. A precise and efficient stereological method for determining murine lung metastasis volumes. Am J Pathol. 2001;158(6):1997–2003. doi: 10.1016/S0002-9440(10)64671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]