Significance
Epstein–Barr virus (EBV) normally establishes a lytic infection in differentiated epithelial cells. However, in the abnormal context of nasopharyngeal carcinoma (NPC), EBV latently infects undifferentiated epithelial cells. Here we demonstrate that the EBV genome can become 5-hydroxymethylated and that this DNA modification affects EBV lytic reactivation. We find that 5-hydroxymethylcytosine accumulates during differentiation of normal epithelial cells but not in EBV+ NPCs. Furthermore, we show that ten–eleven translocation (TET) enzymes dysregulate lytic viral reactivation by altering the 5-methylcytosine and 5-hydroxymethylcytosine state of lytic promoters. These data suggest that loss of TET activity may promote cellular hypermethylation and alter EBV gene regulation in NPC tumors.
Keywords: EBV, 5hmC, lytic reactivation, NPC
Abstract
Latent Epstein–Barr virus (EBV) infection and cellular hypermethylation are hallmarks of undifferentiated nasopharyngeal carcinoma (NPC). However, EBV infection of normal oral epithelial cells is confined to differentiated cells and is lytic. Here we demonstrate that the EBV genome can become 5-hydroxymethylated and that this DNA modification affects EBV lytic reactivation. We show that global 5-hydroxymethylcytosine (5hmC)-modified DNA accumulates during normal epithelial-cell differentiation, whereas EBV+ NPCs have little if any 5hmC-modified DNA. Furthermore, we find that increasing cellular ten–eleven translocation (TET) activity [which converts methylated cytosine (5mC) to 5hmC] decreases methylation, and increases 5hmC modification, of lytic EBV promoters in EBV-infected cell lines containing highly methylated viral genomes. Conversely, inhibition of endogenous TET activity increases lytic EBV promoter methylation in an EBV-infected telomerase-immortalized normal oral keratinocyte (NOKs) cell line where lytic viral promoters are largely unmethylated. We demonstrate that these cytosine modifications differentially affect the ability of the two EBV immediate-early proteins, BZLF1 (Z) and BRLF1 (R), to induce the lytic form of viral infection. Although methylation of lytic EBV promoters increases Z-mediated and inhibits R-mediated lytic reactivation, 5hmC modification of lytic EBV promoters has the opposite effect. We also identify a specific CpG-containing Z-binding site on the BRLF1 promoter that must be methylated for Z-mediated viral reactivation and show that TET-mediated 5hmC modification of this site in NOKs prevents Z-mediated viral reactivation. Decreased 5-hydroxymethylation of cellular and viral genes may contribute to NPC formation.
Epstein–Barr virus (EBV) is a gamma-herpesvirus that is the causative agent of infectious mononucleosis. It also contributes to the development of epithelial- and B-cell malignancies such as nasopharyngeal carcinoma (NPC) and Burkitt lymphoma (1, 2). Like all herpesviruses, EBV has both latent and lytic forms of infection. Latent EBV infection occurs in normal B lymphocytes, as well as in EBV-associated B-cell and epithelial-cell malignancies, and promotes transformation of EBV-infected tumor cells (1, 2). Lytic EBV infection, which is required for horizontal spread of the virus from host to host, occurs in differentiated oropharyngeal epithelial cells, B-cell receptor-activated B cells, and plasma cells (3–8).
The EBV genome becomes highly methylated following infection of normal B cells and within B-cell and epithelial-cell tumors (9). CpG methylation of the EBV genome is detectable within 2 wk postinfection in B cells (10) and plays a critical role in promoting the most stringent (and least immunogenic) form of viral latency (reviewed in refs. 11, 12). In addition, methylation of the viral genome is required for the ability of the EBV BZLF1 (Z) immediate-early protein to induce the latent to lytic switch, because Z preferentially binds to and activates the methylated forms of lytic EBV promoters (reviewed in refs. 11, 13). Z (also known as EB1, ZEBRA, and Zta) is a bZip protein homologous to AP-1 and binds to AP-1–like sites (Z-responsive elements, ZREs) that often contain CpG motifs (11, 13–15). Once established, EBV genome methylation is maintained during latent viral genome replication (licensed and mediated by host cell replication machinery) via the enzymatic activity of DNA methyltransferases (1, 2). However, the virally encoded replication machinery mediating the lytic form of viral DNA replication does not preserve viral genome methylation (9, 11), and therefore, packaged EBV genomes are always unmethylated.
EBV infection of normal differentiated epithelial cells is completely lytic, and the viral genome does not become methylated in these cells (1–5, 7, 9, 10). Lytic viral gene expression following EBV infection of normal epithelial cells likely reflects the ability of the other EBV immediate-early (IE) protein, BRLF1 (R), to efficiently activate unmethylated lytic viral promoters. We recently showed that overexpression of R, but not Z, induces lytic EBV gene expression and replication in a latently infected telomerase-immortalized normal oral keratinocyte (NOKs) line, where the lytic viral promoters remain largely unmethylated (16, 17). R activates lytic gene expression by binding to R-response elements (RREs) with a consensus sequence of GNCCN9GGNG (N9 is a nine-nucleotide spacer region that can be any sequence) (18) or indirectly by interacting with cellular transcription factors (13). Z and R activate one another’s promoters, and once both IE proteins are expressed, they cooperate to induce fully lytic infection regardless of whether the viral genome is methylated or unmethylated (13, 16).
Given the profound effect that cytosine methylation plays on the ability of Z versus R to activate lytic EBV gene expression, we have now explored whether another more recently described cytosine modification, 5-hydroxymethylcytosine (5hmC), also affects the ability of Z and/or R to regulate lytic EBV gene promoters. The 5hmC modification occurs as an intermediate during active demethylation of cytosines in vivo, especially during early zygote development (reviewed in ref. 19). Removal of CpG methylation begins when one of the three ten–eleven translocation (TET) enzymes (TET1, TET2, or TET3) hydroxylates 5-methylcytosine (5mC), producing 5hmC (20, 21). 5hmC can then be lost passively through multiple rounds of DNA replication, as the mark is not recognized by DNMT1 (19). Alternatively, 5hmC and further oxidized forms of this modification can be actively removed through multiple pathways, often involving base excision repair (reviewed in refs. 19, 22). Although 5hmC is rare in most cells, it is relatively abundant in certain cell types such as Purkinje neurons, embryonic stem cells, and others (19, 22). Additionally, 5hmC accumulates during differentiation of many cell types and is extremely low in undifferentiated cancer cells (23, 24). When 5hmC is maintained on the cellular genome, it is usually associated with gene activation (19, 25).
Global reduction of 5-hydroxymethylation via various mechanisms is commonly found in myeloid cancers, glioblastoma, and melanoma, and recently 9% of NPC tumors were found to have a mutation in TET1, TET2, or TET3 (22, 26–29). In addition to mutations in the TET family members, mutations that disrupt α-ketoglutarate production, which is required for the activity of all TET enzymes, can also decrease 5hmC accumulation. Such mutations are found in over 70% of secondary glioblastomas and are common in acute myeloid leukemia (22, 26). In particular, the isocitrate dehydrogenase (IDH) 1 and 2 enzymes, which normally convert isocitrate into α-ketoglutarate, are mutated to forms [including IDH1(R132H) and IDH2(R172K)] that instead convert isocitrate into the oncometabolite 2-hydroxyglutarate, which inhibits the function of all three TET enzymes (29).
Here we show that TET-mediated 5hmC modification of lytic EBV promoters inhibits Z binding and activation of these promoters, while promoting R activation. Furthermore, we identify a CpG-containing Z-binding site in the R promoter that is 5hmC-modified in EBV-infected NOKs and demonstrate that inhibition of endogenous TET activity converts the 5hmC mark into a 5mC mark and restores the ability of Z to induce lytic EBV reactivation in this cell line. Finally, using 5hmC-specific immunohistochemistry (IHC), we confirm that global 5hmC-modified DNA is very low or undetectable in EBV+ NPCs but accumulates during differentiation of normal tonsil epithelium. These results reveal that TET-mediated 5hmC modification of lytic EBV promoters regulates lytic viral reactivation and suggest that decreased 5hmC modification of both cellular and viral genes may contribute to NPC tumors.
Results
5hmC-Modified DNA Accumulates in Differentiated Normal Tonsil Epithelium and in Both Differentiated and Undifferentiated NOKs but Not in NPC Tumors.
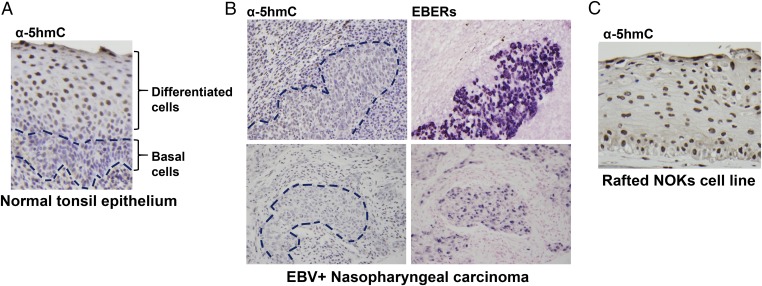
Given the finding that the methylation status of the EBV genome determines which viral-immediate early protein can induce lytic reactivation, we examined the level of global 5hmC-modified DNA using IHC. 5hmC-modifed DNA was not detectable in undifferentiated normal tonsillar epithelium but was easily detected in the more differentiated layers of the tonsillar epithelium (Fig. 1A). In contrast, 5hmC-modified DNA was either very low or not detected in nine undifferentiated NPC tumor specimens examined (Fig. 1B and Fig. S1), although staining was visible in the normal surrounding cells. Interestingly, NOKs (telomerase-immortalized NOKs), which support an unusually low level of lytic EBV promoter methylation in comparison with other EBV-infected epithelial cell lines (16, 17), had easily detectable 5hmC-modified DNA, even in the less differentiated basal cells (Fig. 1C). These results suggest that high TET activity may explain the low level of lytic EBV promoter methylation in stably EBV-infected NOKs. Furthermore, because global 5hmC-modified cellular DNA is strongly increased by differentiation of normal tonsillar epithelial cells, the EBV genome may likewise be more likely to become 5hmC-modified in differentiated (versus undifferentiated) normal epithelial cells. Conversely, the low level of global 5hmC-modified DNA in NPC tumor specimens, consistent with the undifferentiated state of these tumors as well as the possible presence of TET gene mutations, suggests that 5hmC modification of the EBV genome would be unlikely to occur in these tumors.
Fig. 1.
5hmC accumulates in differentiated tonsil epithelium but not in NPCs. (A) 5hmC level was assessed by IHC in normal tonsil epithelium; the undifferentiated basal cell layer is outlined. (B) 5hmC IHC (Left) and EBER in situ hybridization (Right) staining of two representative EBV+ NPCs. EBV-positive cells are outlined on Left. (C) 5hmC staining of telomerase-immortalized NOKs cells differentiated in air-interface cultures. Original magnification of all figures, 40×.
Fig. S1.
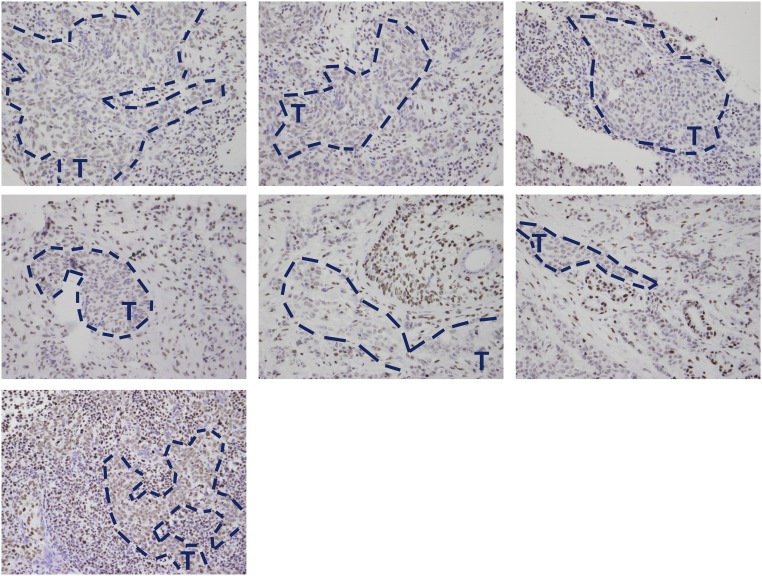
EBV-positive NPCs have decreased levels of 5hmC. 5hmC modification was assessed by IHC staining of seven EBV+ NPCs. Representative areas of EBV-positive cells (confirmed by EBER staining of tumor slides) are outlined in blue and indicated by a “T.” Original magnification of all figures, 40×.
Z Binding to ZREs in Vitro Is Inhibited by 5hmC, but R Binding to RREs Is Unaffected.
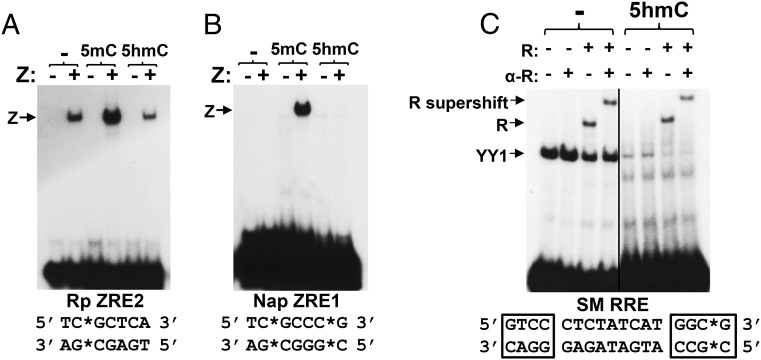
Because cytosine methylation greatly enhances the ability of Z to bind to certain CpG-containing ZREs, we next examined how 5hmC modification of two different CpG-containing ZREs affects Z binding in vitro. As shown in Fig. 2, oligonucleotide probes containing ZRE motifs from either the ZRE2 site in the EBV R promoter or the ZRE1 site in the EBV BRRFl (Na) promoter were commercially synthesized to contain an unmodified cytosine, 5mC, or 5hmC at the nucleotide positions indicated by an asterisk. Binding was assayed using reticulocyte lysate-synthesized Z protein, compared with lysate as a control. As previously described (30), Z bound to the unmethylated form of the R promoter (Rp) ZRE2 motif, although binding was clearly increased by methylation (Fig. 2A). When the methylation mark on the Rp ZRE2 motif was converted to a 5hmC mark, Z binding was reduced to a level similar to that of the unmethylated probe. In the case of the Na promoter ZRE1 motif, which we previously showed is almost totally methylation-dependent in regard to Z binding (31), conversion of the methylated cytosine into a 5hmC-modified cytosine abrogated Z binding. These data suggest that Z “reads” 5hmC-modified ZREs similarly as unmodified ZREs and indicate that TET-mediated 5hmC modification of methylated ZREs in vivo, should it occur, would reverse the ability of Z to bind to these promoters.
Fig. 2.
5hmC modification of CpG-containing binding motifs inhibits Z but not R binding in vitro. Z binding to unmodified, 5mC-modified, or 5hmC-modified CpG-containing ZREs (A and B) or R binding to an unmodified or 5hmC-modified RRE (C) was examined using EMSA as described in Materials and Methods. Z–DNA and R–DNA complexes are indicated with arrows, and binding of the cellular protein YY1 to the RRE is also denoted. ZRE and RRE sequences are shown below each EMSA, with the modified cytosines indicated by asterisks and nucleotides where R directly contacts DNA boxed. R-binding studies were performed with or without a supershifting anti-R antibody as indicated. (A) ZRE2 site from the BRLF1 (R) promoter. (B) ZRE1 site from the BRRF1 (Na) promoter. (C) RRE motif from the SM promoter.
We next explored how 5hmC modification affects R DNA binding, as many R-binding sites also contain CpG motifs. We previously showed that CpG cytosine methylation does not affect the ability of R to bind to lytic EBV promoters in vitro or in vivo, although methylation of lytic promoters decreases R-mediated transcriptional activation (16). A probe encoding an SM promoter R-binding motif [RRE sequence shown below electrophoretic mobility shift assay (EMSA) image] was commercially synthesized to contain 5hmC or unmodified cytosine (denoted with an asterisk) within one of the core regions (boxed) required for R binding to DNA (18). As demonstrated in Fig. 2C, R bound similarly to both the unmodified and 5-hydroxymethylated probes (indicated with arrows), although binding of cellular protein YY1 to the motif (18) was decreased by 5hmC modification. These results suggest that R binds equally well to unmodified and 5-hydroxymethylated CpG-containing binding motifs, even when they are located in the core part of the motif where R makes direct contact with DNA. These results, along with our previous findings (16), suggest that neither 5mC nor 5hmC modification of R-binding motifs affects R binding.
TET2 Reduces Z- but Not R-Mediated Activation of Methylated CpG-Containing Lytic EBV Promoters.
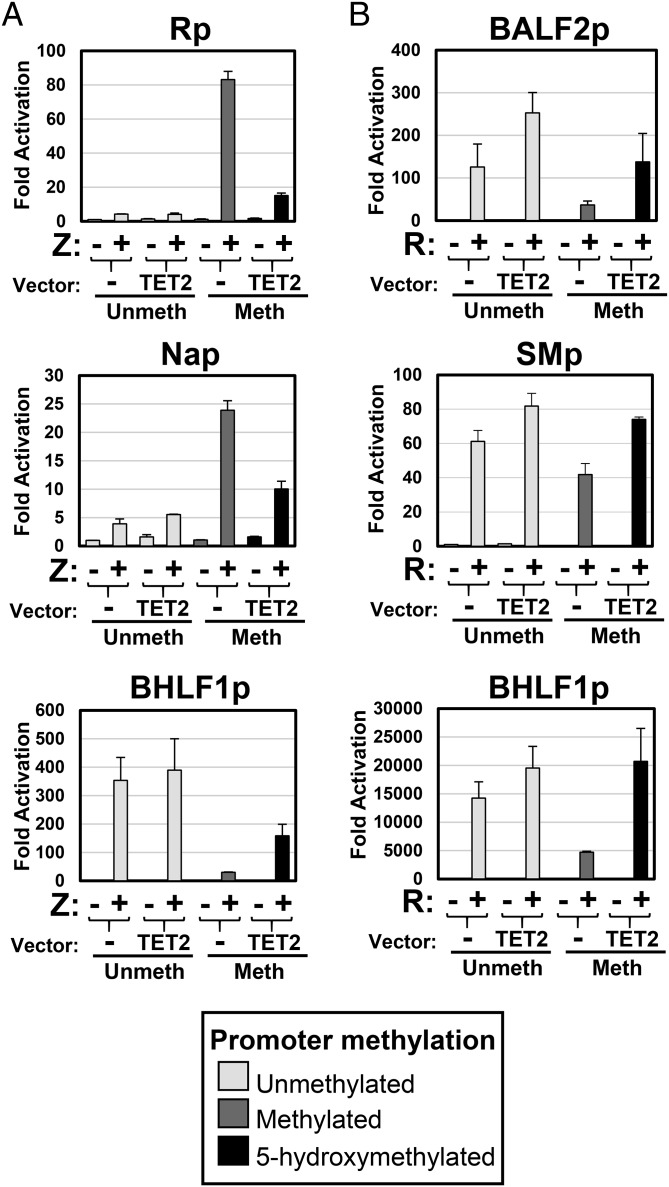
Next we performed luciferase assays to determine if cotransfection with a highly active TET2 expression vector (containing the catalytic domain of mouse Tet2) inhibits the ability of Z to activate lytic EBV promoters with methylated CpG-containing ZREs. TET2 converts methylated CpGs (but not unmodified CpGs) into 5hmC-modified CpGs (20, 21). Three different lytic EBV promoters [BRLF1 (R), BRRF1 (Na), and BHLF1] were cloned upstream of the luciferase gene in the pCpGL-basic vector, which lacks CpG motifs in the vector backbone (32). The promoter vectors were methylated or mock-treated in vitro using CpG methyltransferase (M.SssI) and transfected with and without the TET2 vector into EBV-negative HONE.1 cells. As we and others previously reported (16, 30, 31, 33), methylation greatly enhances Z activation of the R and Na promoters, both of which contain ZREs with CpGs (Fig. 3A). Of note, consistent with the finding that Z does not bind well to 5hmC-modifed ZREs (Fig. 2), cotransfected TET2 inhibited Z activation of the methylated R and Na promoters (Fig. 3A), presumably by converting the methylated CpG motifs into 5hmC-modified CpG motifs. Consistent with this interpretation, TET2 did not decrease (and in fact enhanced) Z activation of the methylated BHLF1 promoter construct, in which all promoter ZREs are CpG-free and hence cannot be 5mC- or 5hmC-modified. We have previously shown that the BHLF1 promoter is exceptional in that methylation of the promoter at CpG sites outside of ZREs inhibits Z-mediated transactivation (16, 33). Immunoblot analysis confirmed that similar levels of Z were expressed in the presence and absence of TET2 (Fig. S2).
Fig. 3.
TET2 differentially affects Z versus R transactivation of lytic EBV promoters. The ability of Z and R to induce expression of unmethylated, methylated, and 5-hydroxymethylated lytic viral promoters was assessed by luciferase assays. EBV promoter-luciferase constructs (containing various lytic EBV promoters inserted upstream of the luciferase gene in a CpG-free vector) were methylated or mock-treated in vitro using M.SssI as indicated and transfected into EBV-negative HONE.1 cells with either vector controls, a Z expression vector (with or without a TET2 expression vector) (A), or an R expression vector (with or without a TET2 expression vector) (B). Luciferase assays were performed 2 d posttransfection; the fold luciferase activation is shown relative to the activity of the unmethylated promoter transfected with control vectors (set at 1). The error bars indicate +1 SD calculated from three replicate experiments.
Fig. S2.
Equal levels of Z and R are expressed in the presence and absence of TET2 in the reporter gene assays. The effect of 5hmC on Z versus R transactivation of lytic promoters was measured with luciferase assays (Fig. 3). EBV-negative HONE.1 cells were transfected with vector controls and Z (A) or R (B) ± TET2. Representative immunoblots of the luciferase assay lysates show the levels of transfected Z, R, and TET2 (A and B); β-actin served as a loading control.
We likewise examined the effect of cotransfected TET2 on the ability of R to activate three different lytic promoters (BALF2, SM, and BHLF1) that have CpG-containing R-binding motifs. We previously showed that although R binds equally well to the unmethylated and methylated forms of these promoters, CpG methylation greatly reduces the ability of R to activate these promoters (16). As previously reported, we found that methylation substantially inhibited R activation of each promoter (Fig. 3B). Importantly, cotransfected TET2 restored the ability of R to activate methylated lytic promoters (Fig. 3B). Immunoblot analysis confirmed that the level of R expression was similar in the presence and absence of TET2 (Fig. S2).
Z Binding to the Endogenous Viral Genome Is Inhibited by TET-Mediated 5-Hydroxymethylation.
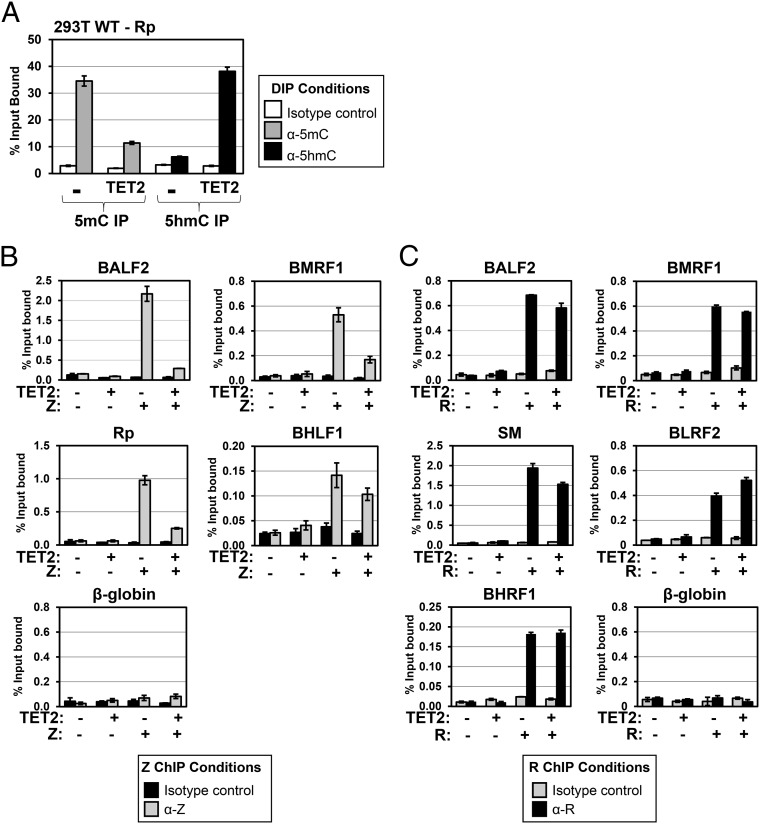
To determine if 5-hydroxymethylation of the intact EBV genome affects the ability of Z to bind to lytic promoters in vivo, we transfected latently infected cells with the highly active TET2 expression vector, which was previously shown to greatly increase the global level of 5hmC-modified cellular DNA when transfected into cells (21). Using DNA immunoprecipitation (DIP) assays, we confirmed that the transfected TET2 vector greatly increases the amount of 5hmC-modified and greatly decreases the amount of 5mC-modified EBV BRLF1 (R) promoter DNA in latently infected EBV-293T cells (Fig. 4A). We next examined the effect of cotransfected TET2 on the ability of Z to bind to various different lytic EBV promoters in a 293 cell line stably infected with an R-deleted EBV mutant; this line was chosen so that Z binding to lytic EBV promoters could be examined in the absence of any TET2 effect on R function. Importantly, in vivo ChIP assays demonstrated that cotransfected TET2 greatly decreased Z binding to three different lytic EBV promoters [BRLF1 (Rp), BALF2, and BMRF1] with CpG-containing ZREs that are preferentially bound by Z in the methylated form but did not substantially affect Z binding to an EBV promoter (BHLF1) that has only CpG-free ZREs (Fig. 4B) (16, 33). Consistent with the EMSA result shown in Fig. 2C, cotransfected TET2 had little if any effect on R binding to five different lytic viral promoters on the endogenous EBV genome in 293 cells infected with a Z-deleted EBV mutant (Fig. 4C). Immunoblot analysis confirmed that similar levels of Z or R were expressed in the presence and absence of TET2 (Fig. S3).
Fig. 4.
Z binding to lytic promoters in vivo is inhibited by 5hmC modication of the EBV genome, whereas R binding is unaffected. (A) DIP assays were performed in EBV-infected 293T cells transfected with pcDNA control vector (–) or a TET2 catalytic domain expression vector, using IgG isotype control ab (white bars), anti-5mC ab (gray bars, Left), or anti-5hmC ab (black bars, Right), and then quantitative PCR (qPCR) amplification was done using primers spanning the EBV R promoter (as described in Materials and Methods and listed in Table S1) to determine the effect of TET2 on the amount of 5mC-modified versus 5hmC-modified Rp. The percent immunoprecipitated DNA (compared with input DNA) is shown for each condition. (B) The 293 cells infected with an R-deleted EBV mutant were transfected with control vector, Z alone, TET2 alone, or Z + TET2 as indicated, and ChIP assays were performed using mouse IgG isotype control (black bars) or anti-Z ab (gray bars). (C) The 293 cells infected with a Z-deleted EBV mutant were transfected with control vector, R alone, TET2 alone, or R + TET2 as indicated, and ChIP assays were performed using rabbit IgG isotype control (gray bars) or anti-R ab (black bars). (B and C) The percent input DNA bound by Z and R was determined with qPCR using primers spanning various EBV promoters as well as the negative control β-globin cellular promoter. (A–C) The error bars indicate +1 SD calculated from three replicate experiments.
Fig. S3.
Similar levels of Z and R are transfected with and without TET2 in the ChIP assays. The ability of Z and R to bind to EBV lytic promoters in the presence and absence of TET2 was assayed using ChIP. (A) 293–Rstop cells (HEK 293 cells infected with a mutant EBV virus that cannot express R) were transfected with vector control, TET2, Z, and Z+ TET2. (B) 293-ZKO cells (HEK 239 cells infected with a mutant EBV virus that cannot express Z) were transfected with vector control, TET2, R, and R+ TET2. (A and B) Cells were harvested 48 h later, and a portion of the cells used in the ChIP assays shown in Fig. 4 were examined by immunoblot to determine the levels of transfected Z or R, and TET2; β-actin or tubulin served as a loading control.
TET2 Differentially Affects Z- Versus R-Induced Lytic Reactivation in EBV-Infected Cell Lines.
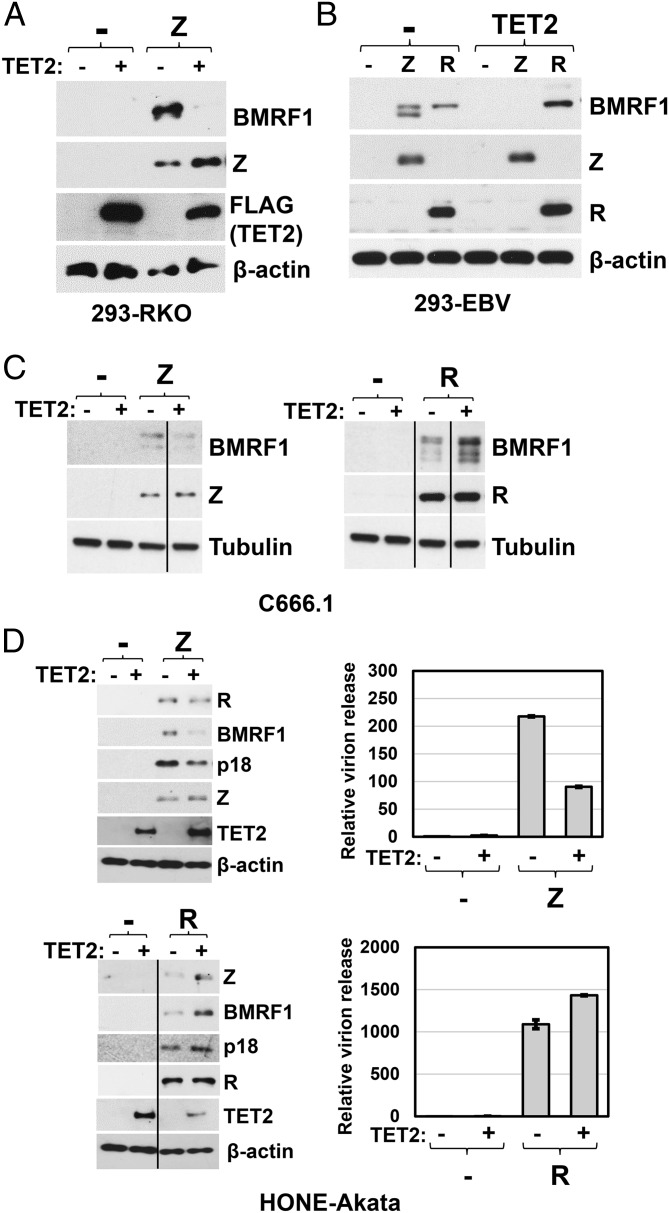
To determine if TET2 expression alters the ability of the Z or R proteins to activate early lytic viral protein expression in latently infected cell lines, we transfected cell lines with either Z or R expression vectors, in the presence or absence of cotransfected TET2, and performed immunoblots to examine the level of various lytic viral proteins. Consistent with the ability of TET2 to decrease Z binding to lytic viral promoters (Fig. 4B), TET2 inhibited the ability of Z to induce lytic viral protein expression in EBV-infected 293 cells, C666.1 cells, and HONE-Akata cells (Fig. 5 A–D). In contrast, cotransfected TET2 enhanced the ability of R to induce lytic viral protein expression from the endogenous viral genome in the same three cell lines (Fig. 5 B–D). Cotransfected TET2 also differentially affected the ability of Z versus R to induce virion release in HONE-Akata cells (Fig. 5D). These results are similar to those observed in the reporter gene assays (Fig. 3) and suggest that 5hmC modification of lytic EBV promoters, like CpG methylation, has different effects on the ability of Z versus R to activate lytic EBV gene expression.
Fig. 5.
5hmC modification of the EBV genome inhibits Z-mediated but not R-mediated lytic reactivation. The effect of TET2 overexpression on Z- and R-induced lytic gene expression from the endogenous viral genome was analyzed using immunoblots. EBV+ (A) 293-RKO cells were transfected with control vector, Z alone, TET2 alone, or Z and TET2 together. (B) 293-EBV cells (B95.8 strain), (C) C666.1 cells, and (D, Left) HONE-Akata cells were transfected with Z or R expression vectors in the presence or absence of cotransfected TET2. Immunoblot analysis was performed 2 d posttransfection to compare the levels of Z- or R-induced EBV lytic expression of the following proteins: BMRF1 (A–D), R (D), Z (D), and late viral protein p18 (D), as well as transfected Z or R and TET2 (A–D). β-actin or tubulin served as a loading control. (D, Right) HONE-Akata cells were transfected with Z or R expression vectors in the presence and absence of cotransfected TET2. Media was harvested 3 d posttransfection, and released EBV virions were quantified by qPCR as described in Materials and Methods. The error bars indicate SE calculated from three replicate experiments.
Inhibition of Endogenous TET Activity via Expression of the Mutant IDH1(R132H) Protein Allows Z to Initiate Lytic Reactivation in EBV-Infected NOKs.
To date, the only EBV-infected cell line known to be lytically reactivated by overexpression of R, but not Z, is the NOKs line (16). We and others previously showed that a number of lytic viral promoters, particularly the BRLF1 (Rp) promoter, have relatively low methylation levels in EBV-positive NOKs compared with other EBV-infected cell lines (16, 17). This suggests that the inability of Z to activate lytic EBV promoters (particularly the BRLF1 promoter) in NOKs may be due to inadequate methylation of CpG-containing ZREs. Given our finding that NOKs have a high level of global 5hmC-modified DNA (Fig. 1C), we hypothesized that TET enzymes may inhibit Z binding to the EBV genome in this cell type by converting 5mC-modified cytosines in the viral genome into 5hmC-modified cytosines, which might then be converted into unmodified cytosines following cellular replication.
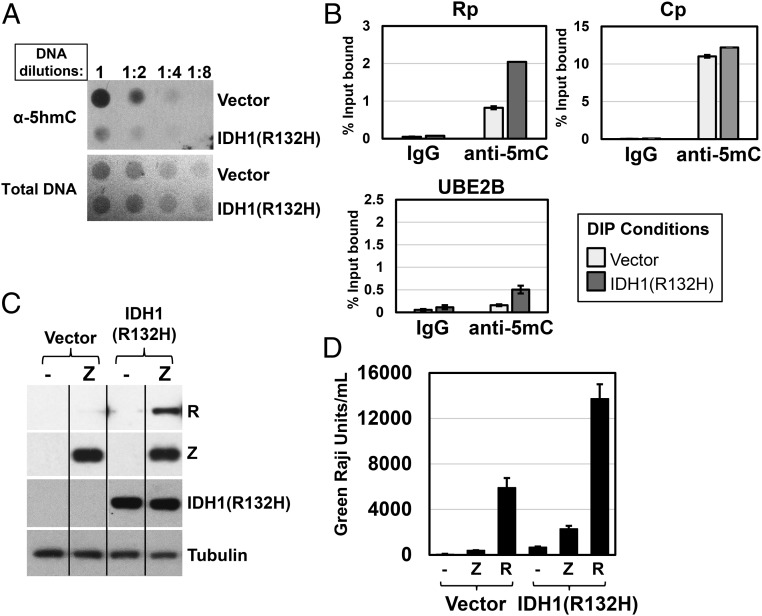
To determine if constitutive TET activity contributes to the low level of lytic EBV promoter methylation in EBV-infected NOKs (which express both TET2 and TET1), we infected EBV-positive NOKs with a retroviral vector expressing a mutant form of IDH1 [IDH1(R132H)], which inhibits the activity of all three cellular TET enzymes, or a control retroviral vector and selected the cells in puromycin for at least 1 mo. A DNA dot blot, using antibodies that specifically recognize only 5hmC-modified DNA, confirmed that by 1 mo after infection with the IDH1 mutant protein, the level of 5hmC-modified DNA was clearly decreased (Fig. 6A).
Fig. 6.
Inhibition of TET activity enhances methylation of the EBV R promoter and restores Z-mediated activation of R expression in EBV-infected NOKs. EBV-positive NOKs were infected with control or IDH1(R132H)-expressing retrovirus vectors and selected with puromycin for 1 mo. (A) A dot blot was performed to measure global levels of 5hmC-modified DNA in control versus IDH1(R132H)-expressing cells as described in Materials and Methods. (B) DIP assays were performed on DNA isolated from EBV-positive NOKs infected with retrovirus control vector (white bars) or the IDH1(R132H)-expressing retrovirus (gray bars) using mouse IgG isotype control or anti-5mC antibody, and then qPCR was performed using primers spanning the EBV R or C promoters, or the cellular UBE2B promoter. The error bars indicate +1 SD calculated from three replicate experiments. (C) EBV+ NOKs infected with control retrovirus or IDH1(R132H)-expressing retrovirus were transfected with a Z expression vector (or vector control), and immunoblot analysis was performed to examine the level of transfected Z, induced R expression (from the latent EBV genome), mutant IDH1(R132H) expression, and tubulin (loading control). (D) EBV+ NOKs infected with control retrovirus or IDH1(R132H)-expressing retrovirus were transfected with vector control, a Z expression vector, or an R expression vector. Media was harvested 3 d posttransfection, and production of infectious virions was quantified using a green Raji assay as described in Materials and Methods. The error bars indicate SE calculated from three replicate experiments.
To determine if loss of TET activity results in enhanced methylation of the EBV Rp, we performed a DIP assay to compare the levels of 5mC at various promoters in the control vector versus IDH1(R132H)-expressing lines. These assays revealed that the EBV R promoter had increased methylation when TET activity was inhibited (Fig. 6B). In contrast, the methylation status of the EBV Cp promoter (a viral latency promoter that drives EBNA gene expression in B cells but is not used in EBV-infected NOKs) (17) was not affected, suggesting that this promoter is not a major target for TET-mediated demethylation in this cell line. The cellular UBE2B promoter, which contains an unmethylated CpG island in many cell types, served as a negative control for methylation and as expected was not significantly methylated in the vector control cells, although its methylation status was increased by expression of the IDH1(R132H) mutant. Thus, the UBE2B promoter may also be a target for TET activity in NOKs, consistent with a previous report showing 5hmC on the UBE2B promoter in human embryonic stem cells (34).
To examine whether inhibition of TET activity restores the ability of Z to induce lytic EBV gene expression in NOKs, cells were transfected with a Z expression vector or a control vector, and the level of R expression induced by Z was examined by immunoblot. As previously reported by our group, transfected Z did not activate R expression in EBV-positive NOKs infected with the control retroviral vector (Fig. 6C). However, transfected Z activated R expression in NOKs when TET activity was inhibited for over 1 mo using the mutant IDH1(R132H) protein (Fig. 6C). In addition, inhibition of TET function restored the ability of transfected Z to produce infectious virions in NOKs (Fig. 6D). Similar results were obtained in three separate independently generated lines. Together, these results suggest that TET activity plays an important role in preventing methylation of the lytic EBV R promoter in EBV-infected NOKs and thereby inhibits Z-mediated lytic viral reactivation. Interestingly, inhibition of TET2 function in EBV-infected NOKs also increased the amount of constitutive virion production as well as the amount of virions produced following R transfection (Fig. 6D). This latter result can be explained by the fact that the EBV genomes were still largely unmethylated after 1 mo of TET inhibition (thus still providing a viral template for R-initiated lytic expression), whereas R-induced Z protein was now able to induce lytic gene expression from the newly methylated EBV genomes.
TET Activity Enhances 5hmC Modification and Decreases 5mC Modification at a Specific ZRE CpG Motif in the EBV R Promoter.
To determine if TET2 globally modifies 5hmC and 5mC levels on CpG-containing ZREs in Rp or only affects specific CpG motifs, CpG methylation was quantified using bisulfite treatment of DNA followed by pyrosequencing (Fig. 7A). To distinguish between 5mC- versus 5hmC-modified sites, the DNA was pretreated with an oxidizing agent, KRuO4 (Oxi), or mock-treated (Mock), as oxidation of 5hmC, but not 5mC, results in conversion of the base into uracil such that 5-hydroxymethylated sites are read like unmodified cytosines after this treatment (35). Therefore, comparison of the reference DNA sequence to the mock and oxidized samples reveals which sites are methylated versus 5-hydroxymethylated.
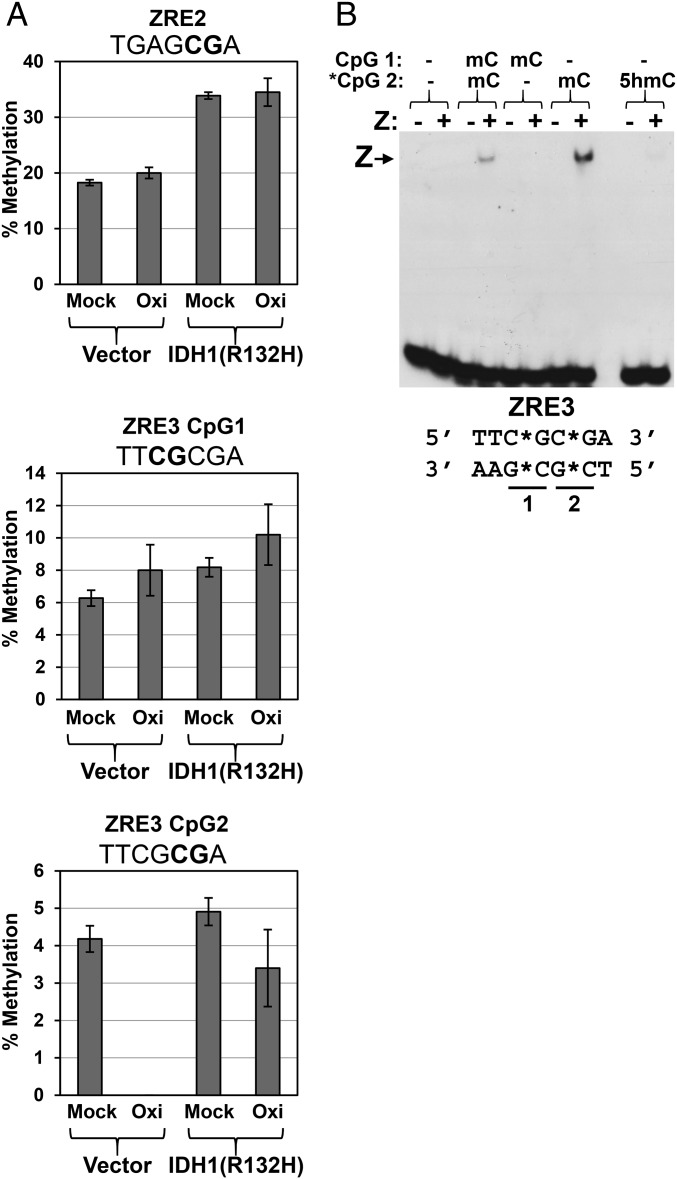
Fig. 7.
The R promoter ZRE3 site is 5-hydroxymethylated in EBV-infected NOKs via a TET-dependent mechanism. (A) DNA was isolated from EBV-positive NOKs infected with either the retrovirus control vector or the IDH1(R132H)-expressing retrovirus, and then the DNA was either mock-treated (Mock) or oxidized with KRuO4 (Oxi), followed by bisulfite conversion and pyrosequencing as described in Materials and Methods. The sequences of each ZRE motif are shown above the respective graph, with the CpG sites bolded; the two adjacent CpGs in ZRE3 are numbered 1 and 2. The error bars indicate SE calculated from two replicate experiments where each sample was pyrosequenced in triplicate. (B) The ability of Z to bind to the Rp ZRE3 site containing various patterns of methylation and 5-hydroxymethylation was examined using EMSA; the cytosine modification status in each lane is shown above the EMSA image. Z–DNA complexes are indicated with an arrow.
We previously showed that the Rp contains two different CpG-containing ZREs (ZRE2 and ZRE3), both of which are required for efficient Z activation of the methylated form of the promoter (30). Pyrosequencing results revealed that methylation of the single CpG site within the Rp ZRE2 motif was increased approximately twofold in the presence of the mutant IDH1(R132H) protein; however, 5hmC modification of this site could not be detected, suggesting that this modification is very transient at this site, and presumably rapidly removed by cellular DNA replication and/or via the base excision repair pathway (19, 22) (Fig. 7A). Nevertheless, the increased methylation at this site that occurs following inhibition of TET activity suggests that the 5hmC pathway plays a role in preventing 5mC modification at this site.
Interestingly, in the case of the Rp ZRE3 site (TCGCGA), which contains two adjacent CpG motifs, low-level constitutive 5hmC modification was detectable on the second CpG motif (shown in bold), but not the first, in EBV-infected NOKs, and this modification was lost when TET activity was inhibited by the IDH1 mutant protein. Furthermore, loss of TET function increased the level of 5mC modification at the second CpG (which can be 5hmC-modified), but not significantly affecting the amount of 5mC modification at the adjacent CpG motif (Fig. 7A). Of note, in silico modeling of Z binding to the Rp ZRE3 motif predicts that Z interacts directly with the second (potentially 5hmC-modified) CpG motif but not the adjacent first CpG motif (31). To confirm that methylation of the second CpG motif in the Rp ZRE3 site is specifically required for Z binding, we performed EMSA assays using oligonucleotide probes containing different combinations of methylated and unmethylated CpG motifs in the Rp ZRE3 sequence (Fig. 7B). These results confirmed that methylation of the second (bolded) CpG motif in RpZRE3 (TCGCGA), but not the first CpG motif, is specifically required for Z binding to this motif. In addition, 5hmC modification of the Rp ZRE3 probe abrogated Z binding. Together, these results suggest that TET-mediated 5hmC modification of the second (but not first) CpG motif in the Rp ZRE3 site inhibits Z binding to this motif and prevents Z-mediated activation of R expression. These results also show that the effect of TET loss on the EBV genome CpG methylation state is extremely context-dependent.
Discussion
The undifferentiated form of NPC is almost universally associated with EBV infection, and a recently published genomic landscape of NPC demonstrated that mutations in the cellular TET1, TET2, or TET3 genes occur in 9% of NPC tumors (28). TET enzymes convert methylated cytosines into 5-hydroxymethylated cytosines, which can then lead to cytosine demethylation following DNA replication. Here we show that 5hmC modification of the EBV genome differentially affects the ability of the two EBV IE proteins to activate lytic gene expression and demonstrate that endogenous TET activity regulates EBV lytic reactivation in EBV-infected NOKs. In addition, we find that differentiation of normal epithelial cells leads to increased global 5hmC. These results are the first, to our knowledge, to show that constitutive 5hmC modification of a viral genome substantially alters viral gene regulation and furthermore suggest that loss of 5hmC in NPC tumors (via TET gene mutations or other mechanisms) not only affects cellular gene expression but also alters EBV gene regulation.
We previously showed that although cytosine methylation is commonly required for the ability of Z to activate lytic EBV promoters, it has the opposite effect on the ability of R to activate the same promoters (16). As a “pioneer” factor, once Z is bound to DNA, it can activate promoters even in the presence of inhibitory chromatin modifications normally associated with 5mC modification (36). In the case of R, CpG methylation does not affect R binding to RREs on lytic promoters but decreases R-mediated acetylation of histone 3 lysine 9 (16). Therefore, R, but not Z, appears to require open chromatin to induce lytic gene expression.
Here we show that 5hmC modification also produces different effects on Z- versus R-mediated activation of lytic EBV promoters. Although 5hmC modification of CpG-containing Z-binding motifs prevents efficient Z binding to, and activation of, lytic EBV promoters, the 5hmC modification does not inhibit R binding to RREs and in fact enhances R-mediated activation of promoters. 5hmC modification may increase R-mediated activation of methylated lytic promoters both by removing the inhibitory cytosine methylation mark and by inducing an open chromatin conformation. In glioblastoma cells, 5hmC recruits a complex that methylates arginine 3 of histone 4, thus activating the expression of genes involved in glioblastomagenesis (37). Another recent report by Mendonca et al. showed that 5hmC-modified DNA may convert chromatin to a more open and active state by weakening the DNA–H2A–H2B dimer interaction (38).
EBV-infected NOKs are so far unique among stably EBV-infected cell lines with regard to their dependence upon R, but not Z, expression to convert to a lytic form of infection, and our results here suggest that this may reflect the unusually high level of constitutive 5hmC modification and TET activity in this cell line. This phenotype may reflect the relatively “normal” state of the NOKs line, which can differentiate in air–interface cultures (Fig. 1) and in response to calcium/serum. We previously suggested that insufficient methylation of the R promoter in NOKs inhibits Z-mediated EBV reactivation (16, 17). Both Z and R transcriptional function are required to activate many lytic EBV promoters, and thus activation of R expression is the essential first step in Z-mediated lytic reactivation. We demonstrate here that global inhibition of TET activity in NOKs [using the IDH1(R132H) mutant] reverses low-level constitutive 5hmC modification of a specific CpG motif (CpG2) within an essential Z-binding motif (ZRE3) in the R promoter (Fig. 7). Furthermore, we show that this specific CpG motif switches from having no detectable methylation to having detectable methylation (5%) when TET activity is inhibited (Fig. 7). Additionally, we can detect the “footprint” of transient 5hmC modification at the Rp ZRE2 site, as inhibition of TET activity also results in a twofold gain of 5mC at this site, although 5hmC was not detected at this site by pyrosequencing. Even though the level of Rp methylation at the ZRE3 CpG2 motif remains relatively low (5%) following 1 mo of TET inhibition, the fact that EBV-infected cells generally have many copies of the genome per cell, combined with an absolute necessity for ZRE3 CpG2 methylation for Z binding (Fig. 7), presumably allows even this low level of methylation to substantially increase Z-mediated viral reactivation in NOKs, where this site normally has no detectable methylation.
At this point, it is not clear how TET proteins direct 5hmC to a specific CpG within the Rp ZRE3 motif, while sparing the adjacent CpG motif. Increasing evidence suggests that cellular transcription factors can interact with TET proteins and tether them to promoters (reviewed in ref. 22), and we suspect this is likewise the case for the EBV Rp, although the exact transcription factor playing this role remains unknown. Importantly, we show that the ZRE3 CpG2 motif targeted by 5hmC must be methylated for Z to bind to ZRE3, whereas methylation of the adjacent CpG1 motif (not modified by 5hmC) is not required.
Our results here suggest the following model in regard to how loss of TET activity in EBV-infected epithelial cells, or the presence of other modifications that decrease the amount of global 5hmC, might promote NPC. First, we predict that absence of TET activity in EBV-infected normal differentiated epithelial cells initially enhances EBV latency by increasing 5mC modification of lytic viral promoters. In normal differentiated epithelial cells, R, rather than Z, is likely to drive lytic gene expression, as the incoming viral genome is unmethylated, and viral latency does not normally occur in these cells. As shown here, 5mC modification of lytic EBV promoters inhibits the ability of R to activate lytic gene expression (Figs. 3 and 5), and TET loss promotes 5mC modification of lytic EBV promoters (Figs. 6 and 7). Given our finding that normal undifferentiated tonsillar epithelial cells have very little 5hmC modification even in the absence of TET mutations (Fig. 1), TET mutations in NPC may initially occur in the differentiated epithelium, perhaps even before EBV infection. Loss of TET function would likely result in a selective growth advantage in newly EBV-infected differentiated epithelial cells not only by inducing methylation of cellular tumor suppressor genes but also by reducing R-mediated lytic EBV infection (which eventually results in cell death) and decreasing viral immunogenicity. Whether loss of TET function and/or the establishment of latent EBV infection also promotes dedifferentiation of epithelial cells is an important unanswered question.
Subsequently, as the viral genome becomes progressively more methylated in TET-deficient epithelial cells, we predict EBV acquires susceptibility to Z-mediated lytic reactivation, as methylation of lytic viral promoters greatly increases Z binding to, and activation of, lytic EBV promoters (Figs. 2–7). Furthermore, as shown here (Figs. 2 and 3), TET-induced 5hmC modification of lytic viral promoters inhibits Z binding to, and activation of, these promoters, and the 5hmC modification would no longer occur when TET activity is lost. Consistent with this part of the model, the EBV genome is highly methylated in NPC tumors, and NPC patients characteristically have unusually high IgA antibody titers to lytic EBV antigens even before the tumors become clinically apparent (39).
Finally, later on during NPC evolution, we propose that Z expression in tumors must be largely turned off to reduce the immunogenicity associated with lytic viral protein expression and to prevent virally induced lytic cell death. Consistent with this part of the model, NPC tumors commonly have only rare cells expressing lytic viral proteins, despite the high level of antibody titers to lytic viral proteins in NPC patients. Loss of Z expression in NPCs may reflect the absence of differentiation-dependent cellular transcription factors (BLIMP1 and KLF4) that synergistically activate the Z and R promoters (40). In conclusion, our results here suggest that loss of TET function in EBV-infected epithelial cells, or the presence of other mutations that globally inhibit 5hmC, may initiate a series of events that promote EBV-induced NPC not only by enhancing methylation of cellular tumor suppressor genes but also by altering 5mC and 5hmC modification of lytic EBV promoters.
Materials and Methods
IHC and EBER studies were performed with formalin-fixed, paraffin-embedded tissue sections and cells. Samples were deparaffinized, hydrated, and treated with 10 mM citrate buffer (0.05% Tween 20, pH 6.0) for 20 min in a water bath at 98 °C. To detect 5hmC, slides were treated with 2N HCl for 30 min after antigen retrieval, rinsed in distilled water, and then treated with 100 mM Tris·HCl (pH 8.5) for 10 min. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxidase solution, and nonspecific labeling was blocked in a 2.5% (vol/vol) normal horse serum blocking solution (Vector Labs). Sections were incubated with the primary antibody for 1 h at room temperature. The anti-5hmC polyclonal primary antibody (Active Motif, 39769; 1:500–1:3,000) was used. An ImmPRESS Anti-Rabbit Ig (Peroxidase) Polymer Detection Kit (Vector Labs) was used by following the manufacturer’s instructions. Colors were developed with the diaminobenzidine tetrachloride substrate kit (Vector Laboratories Inc.) by following the manufacturer’s instructions. EBER in situ hybridization studies were conducted using the Peptide Nucleic Acid (PNA) Probe/Fluorescein Detection Kit (DakoCytomation) according to the manufacturer’s protocol as previously described (41). Human normal tonsil tissue slides (IHC World TS-H5024) and NPC panel slides (US Biomax NPC111 and NPC481) were commercially purchased. NOKs slides were prepared and sectioned as described in SI Materials and Methods (kindly provided by Paul Lambert and Dennis Lee, University of Wisconsin-Madison, Madison, Wisconsin).
A detailed description of all experimental methods is provided in SI Materials and Methods.
SI Materials and Methods
Cell Lines and Culture.
All cell lines were maintained in growth medium (Invitrogen) supplemented with 10% FBS and 1% penicillin–streptomycin (Invitrogen) at 37 °C with 5% CO2 and 100% humidity, except where indicated.
HONE.1 (a gift from Ron Glaser, Ohio State University, Columbus, OH), an EBV-negative carcinoma cell line, was cultured in Roswell Park Memorial Institute 1640 media (RPMI). HONE-Akata cells (a gift from Lawrence Young, Warwick Medical School, University of Warwick, Coventry, United Kingdom) were derived from HONE.1 cells by superinfection with the Akata strain of EBV and were maintained in RPMI 1640 with G418 (400 μg/mL). HEK 293 cells are an epithelial cell line derived from human embryonic kidney tissue and were maintained in Dulbecco’s modified Eagle medium (DMEM). The 293–EBV cells were derived by transfecting HEK 293 cells with wild-type EBV B95.8 strain 2089 Bacmid DNA as previously described (42) and were a gift from Wolfgang Hammerschmidt, Research Unit Gene Vectors, Helmholtz Zentrum München, German Research Center for Environmental Health, and German Centre for Infection Research (DZIF), Munich, Germany, and Henri-Jacques Delecluse, German Cancer Research Centre (DKFZ), Heidelberg, Germany; these cells were maintained in DMEM with hygromycin (100 µg/mL). The 293–Rstop cells were created by transfecting HEK 293 cells with a mutated 2089 Bacmid containing stop codon mutations early in the BRLF1 (R) ORF as previously described (43) and were maintained in DMEM with hygromycin (100 µg/mL). The 293 Z knockout (KO) and 293-RKO cells [a gift from Wolfgang Hammerschmidt, Research Unit Gene Vectors, Helmholtz Zentrum München, German Research Center for Environmental Health, and German Centre for Infection Research (DZIF), Munich, Germany, and Henri-Jacques Delecluse, German Cancer Research Centre (DKFZ), Heidelberg, Germany] were derived by infecting HEK 293 cells with the respective Z-deleted or R-deleted 2089 bacmids as previously described (42) and were maintained in DMEM with hygromycin (100 µg/mL). HEK 293T cells contain the simian virus 40 (SV40) T antigen and were acquired from the ATCC. The 293T–EBV cells were derived by transfecting HEK 293T cells with wild-type EBV B95.8 Bacmind DNA and were maintained with DMEM plus hygromycin (100 µg/mL). HeLa cells (obtained from the ATCC), an epithelial cervical cancer line, were maintained in DMEM. C666.1 cells are the only authentic EBV-positive NPC cell line in culture (44, 45) and were maintained in RPMI [a gift from Dolly Huang, The Chinese University of Hong Kong, Shatin, Hong Kong (via Bill Sugden, University of Wisconsin-Madison, Madison, WI)]. Raji cells are an EBV-positive Burkitt lymphoma cell line and were maintained in RPMI (obtained from the ATCC). NOKs-Akata cells were derived from NOKs cells [a gift from Karl Munger, Harvard University, Boston (via Paul Lambert, University of Wisconsin-Madison, Madison, WI)], a telomerase-immortalized normal oral keratinocyte cell line (46), by coculturing EBV-negative NOKs with Akata-GFP Burkitt lymphoma cells as previously described (16) and were maintained in keratinocyte-SFM (K-SFM) media supplemented with epidermal growth factor, bovine pituitary extract, 0.22% penicillin–streptomycin, and G418 (50 μg/mL).
Plasmids and Cloning.
Plasmid DNA was purified through columns using the Qiagen Midi/Maxiprep kit according to the manufacturer's instructions. pSG5 was acquired from Stratagene. SG5-Z (a gift from Diane Hayward, Johns Hopkins University, Baltimore, MD) contains the Z genomic sequence under the control of the SV40 early promoter (47). The SG5-BRLF1 expression vector (a gift from Diane Hayward, Johns Hopkins University, Baltimore, MD) encodes the R ORF from the EBV strain M-ABA under the control of SV40 promoter and was constructed as previously described (48). SG5-BRLF1 aa1–550 (R550) was created using the Stratagene QuikChange II XL site-directed mutagenesis kit as previously described (16). pcDNA3.1(+) was obtained from Invitrogen. pcDNA3–FLAG–TET2 (a gift from Yi Zhang, Boston Children's Hospital, Harvard Medical School, Boston) contains a FLAG-tagged catalytic domain portion of the mouse Tet2 coding sequence under the control of the cytomegalovirus promoter (21).
The CpG-free, promoterless luciferase reporter gene construct, pCpGL-basic (a gift from Micheal Rehli, Universitätsklinikum Regensburg, Regensburg, Germany), was constructed as previously described (32). Several EBV promoters were PCR-amplified using the EBV B95.8 genome as template and cloned upstream of the luciferase gene in pCpGL-basic using the SpeI and BglII restriction sites. The following promoters were cloned into pCpGL-basic (the position in the EBV genome GenBank AJ507799.2 is in parentheses): BALF2p (164,318–164,917), BRLF1p (93,856–94,961), BRRF1p (92,159–92,872), and SMp (72,023–72,633). The BHLF1p-luciferase reporter gene construct was created by PCR-amplifying the divergent BHLF1 and BHRF1 promoter sequences (40,492–41,509) within the oriLyt region of EBV B95.8 genomic DNA with the primer set 5′-CCCCAGATCTCGACGCTGGCGAGCCGGGCC-3′ and 5′-CCCCAGATCTGTGATGAAACAGGCAACTCC-3′ and was cloned upstream of the luciferase gene in pCpGL-basic using the BglII restriction site.
The IDH1(R132H) retrovirus was created by mutating wild-type IDH1-pCMV6-AC (Origene SC322129) from arginine at position 132 into histidine by site-directed mutagenesis (Stratagene QuikChange) according to the manufacturer’s instructions. IDH1(R132H) was then amplified by PCR and cloned into pCMMP-MCS-IRES-Puro (a gift from Bill Sugden, University of Wisconsin-Madison, Madison, WI).
Transient Transfection.
HONE.1, HONE-Akata, C666.1, all HEK 293 lines, and NOKs-Akata cells were transfected using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s instructions. HeLa cells were transfected with FuGENE6 transfection reagent (Roche) according to the manufacturer’s instructions.
Organotypic Raft Cultures.
NOKs cells were grown in raft cultures to induce 3D growth with layers of differentiated normal stratified epithelium as previously described (49). We used 1 mL of a collagen mix (3.0 mg/mL; Wako Chemicals) containing F-12 medium, 10% FBS, and 1% penicillin–streptomycin to coat Transwell inserts (24 mm diameter, 0.4 µm pore size; Costar). The remaining collagen mix was seeded with 600 μL of human foreskin fibroblasts at 7.5 × 105 cells/mL, and 2.5 mL of cells plus collagen was plated on the collagen-coated Transwell inserts per sample. After 4 d of incubation of the Transwell inserts embedded with fibroblasts in F-12 medium containing 10% FBS and 1% penicillin–streptomycin in a 5% CO2 in an incubator at 37 °C, 150 µL of keratinocytes (1.4 × 106 cells/mL) in keratinocyte plating medium [F medium (1.88 mM Ca2+)] containing 0.5% FBS, adenine (24 μg/mL), cholera toxin (8.4 ng/mL), hydrocortisone (2.4 μg/mL), and insulin (5 μg/mL) were plated onto the collagen dermal equivalent. The keratinocytes were allowed to incubate 4 d and then were separated onto three 1-in2 cotton pads (Bio-Rad) in a six-well tray (BD Biosciences). The rafts were fed from below the Transwell insert with cornification medium (keratinocyte plating medium containing 5% FBS and 10 μM C8:0) that was replaced every other day. Finally, 11 d after being raised to the liquid–air interface, the rafts were harvested, embedded in 2% agar–1% formalin, fixed in 4% formalin at 4 °C overnight, embedded in paraffin, and cut into 4-μm-thick cross-sections.
Electrophoretic Mobility Shift Assays.
EMSAs were performed as previously described (18, 31). Oligonucleotide probes were commercially synthesized [University of Wisconsin-Madison Biotechnology Center and Integrated DNA Technologies (IDT)] to contain either unmodified, methylated, or hydroxymethylated CpG sites. The following probes were synthesized: Rp ZRE 2 (5′-TAAAATCGCTCATAAGCTTA-3′), Nap ZRE 1 (5′-CATTCTCGCCCGTGGGCC-3′), Rp ZRE3 (5′-AAAATTCGCGATGCTATA-3′), and SM RRE (5′-GGCCCAGATGTCCCTCTATCATGGCGCAGACATTCTC-3′). The underlined portion of the probes is the location where Z or R directly contacts DNA, and the bold nucleotides are CpGs that were modified or not. The oligonucleotides were annealed by heating to 95 °C for 10 min followed by a gradual cooling to room temperature. The probes were purified by extracting double-stranded oligonucleotides from a 15% polyacrylamide gel and were labeled using T4 polynucleotide kinase (NEB) with [γ-32P]ATP (Perkin-Elmer). Labeled probes were desalted with G-25 Sephadex columns (GE Healthcare), and cpm was quantified using scintillation.
The Z protein was translated in vitro using the TNT T7 Quick Coupled Transcription/Translation System (Promega) according to the manufacturer’s instructions. EMSAs to assess Z binding were performed with Z-binding buffer [100 mM KCl, 20 mM Hepes (pH 7.3), 10% glycerol, 0.2 mM EDTA, and 4 mM DTT with 2 µg of poly(dI/dC) (Pharmacia)], 3 μL of in vitro translated protein, and 16 µg BSA. The protein and binding buffer mixture was allowed to incubate for 5 min, and then 20,000 cpm of labeled probe were added. The mixture containing labeled probes was allowed to incubate for an additional 20 min.
EMSAs to test R binding were performed using whole-cell extracts containing the truncated R protein (amino acids 1–550) that is missing the inhibitory carboxy-teminal domain. Whole-cell extracts were produced by overexpressing SG5-R550 or vector control in HeLa cells. Cells were harvested 48 h posttransfection in lysis buffer [0.42 M NaCl, 20 mM Hepes (pH 7.5), 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, and 1× proteasome inhibitor mixture (Roche)], spun down at 9,000 × g at 4 °C for 15 min, and quantified using a Bradford assay (Bio-Rad) as previously described (18). R EMSAs were performed in R-binding buffer [10 mM Hepes (pH 7.5), 50 mM NaCl, 2 mM MgCl2, 2.5 μM ZnSO4, 0.5 M EDTA, 1 mM DTT, 15% glycerol, and 0.5 μg poly(dI-dC)]. Briefly, 1 μg of whole-cell extract was incubated in R-binding buffer and 16 µg BSA at room temperature. After 5 min, 30,000 cpm of labeled probe were added and allowed to incubate for 20 min at room temperature. We added 1 μL of anti-R antibody (Argene) to the supershift conditions, and reactions were allowed to incubate for an additional 20 min at room temperature. The reaction mixtures of both R and Z EMSAs were resolved on a 4% polyacrylamide gel in 0.5× Tris–borate–EDTA buffer and electrophoresed at 35 mA for 1 h. Gels were dried on Whatman paper under a vacuum and exposed to autoradiography film for 12–40 h at −80 °C.
In Vitro Methylation of Reporter Gene Constructs.
EBV promoters inserted into the pCpGL-basic reporter gene construct were methylated or mock-treated in vitro using CpG methyltransferase M.SssI (NEB) according to the manufacturer’s instructions. The DNA was cleaned by phenol chloroform extraction and salt precipitation. Viral promoter CpG methylation was verified by enzymatic digestion with two restriction enzymes (NEB) that share the same cut site: HpaII (methylation inhibits digestion) and MspI (cuts regardless of methylation status).
Reporter Gene Assays.
HONE.1 cells were transfected in 12-well dishes. Z studies were performed with 50 ng of pCpGL-basic promoter constructs, 10 ng of Z alone, 400 ng of TET2 alone, 5 ng of Z plus 400 ng of TET2, and up to 500 ng of SG5 control expression vectors. R transactivation was measured using 50 ng of pCpGL-basic promoter constructs, 10 ng of R alone, 250 ng of TET2 alone, 3.5 ng of R plus 250 ng of TET2, and up to 500 ng of SG5 control expression vectors. At 48 h posttransfection, cells were rinsed with PBS and harvested in 1× reporter lysis buffer (Promega). Lysates were frozen using dry ice and then thawed, and relative luciferase units were quantified with Promega luciferase assay reagent using a BD Monolight 3010 luminometer (BD Biosciences). At least three independent experiments were performed in triplicate for each condition.
DNA Immunoprecipitation Assays.
DNA was isolated from HEK 293T–EBV and NOKs-Akata cells using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma) according to the manufacturer’s instructions. DNA was sonicated to an average size of 300 bp and quantified using a Nanodrop (Thermo Scientific). We resuspended 8 µg of DNA in up to 500 µL of Tris–EDTA (TE) and denatured it by heating at 95 °C for 10 min followed by rapidly chilling on ice. We removed 10% (50 µL) to be used as input, and 50 µL of 10× immunoprecipitation (IP) buffer [100 mM sodium phosphate buffer (pH 7.0), 1.4 M NaCl, 0.5% Triton X-100] was added. The samples were precleared with protein A/G beads for 2 h and separated into 125 µL aliquots (one for each antibody and isotype control). Samples were immunoprecipitated overnight with the following antibodies or isotype controls: 1 µL of anti-5hmC (Active Motif, 39769), 1 µg rabbit isotype anti-IgG control (Cell Signaling, 39769), 1 µg of anti-5mC 33D3 (Active Motif, C15200081), and 1 µg mouse isotype anti-IgG control (Santa Cruz, sc-2025). The antibody-bound DNA was captured with protein A/G beads for 2 h at 4 °C. The beads were washed 5 times with 1× IP buffer, eluted overnight at 55 °C in elution buffer [50 mM Tris∙HCl (pH 8.0), 10 mM EDTA, 0.5% SDS, 0.25 mg/mL proteinase K], and cleaned using the Qiagen gel extraction kit. qPCR was used to determine the presence and relative amount of specific DNA fragments containing 5hmC and 5mC.
Chromatin Immunoprecipitation Assays.
To analyze how 5hmC affects Z binding, 293–Rstop cells were transfected in 10-cm dishes with vector control, 5 µg of TET2 alone, 750 ng of Z with and without 5 µg TET2, and up to 6 µg of pcDNA3-FLAG. To analyze how 5hmC affects R binding, 293 ZKO cells were transfected in 10-cm dishes with vector control, 1,000 ng of R alone, 5 µg of TET2 alone, 600 ng of R plus 5 µg TET2, and up to 6 µg of SG5. After 4 h, the media was replaced with fresh DMEM containing 100 µg/mL acyclovir (Selleckchem.com). Cells were harvested at 48 h posttransfection, cross-linked for 10 min at room temperature with 1% paraformaldehyde, and quenched using 125 mM glycine. Nuclei were isolated, lysed, and subjected to sonication to produce ∼500-bp DNA fragments. We used 2 µg of the following antibodies to immunoprecipitate DNA–protein complexes: anti-BZLF1 (Santa Cruz, sc-53904), mouse isotype anti-IgG control (Santa Cruz, sc-2025), anti-BRLF1 (produced by Pierce against the peptide EDPDEETSQAVKALREMAD as previously described) (50), and rabbit isotype anti-IgG control (Cell Signaling, 39769). After IP, DNA–protein complexes were washed with low-salt, high-salt, lithium chloride, and TE wash buffers. The protein–DNA cross-links were reversed at 55 °C for 2 h using 0.25 mg/mL of proteinase K and 0.2 M NaCl. Immunoprecipitated DNA was purified using the Qiagen gel extraction kit. qPCR was used to determine the presence and relative amount of specific DNA fragments that were bound by Z or R.
Quantitative PCR.
The relative amount of immunoprecipitated DNA fragments from ChIP and DIP assays was measured using qPCR analysis with SYBR green (Bio-Rad) according to the manufacturer’s instructions. Samples were analyzed using an ABI Prism 7900 real-time PCR system (Applied Biosystems). Promoters were amplified with the primers listed in Table S1. A dilution series of each input sample was used to create a standard curve of the threshold cycle (CT) value for each dilution. Percent input bound of each sample was calculated with the determined standard curve. Each condition and input dilution was loaded in triplicate.
Table S1.
Primers used for qPCR analysis
| Promoter (ref.) | Forward | Reverse |
| BALF2 | TGTGGTCATCCAGGTAGTTTCGCA | CAGTGTACAACGACCACTACGACT |
| BHLF1 (17) | CTCTTTTTGGGGTCTCTGTG | CCTCCTCCTCTCGTTATCC |
| BHRF1 | GTCACCTTTGCACATTTGGTCAGC | AAAGTGATGCATCCCAAAGGCAGC |
| BLRF2 | ACTGAAGCCCAGGACCAGTTCTA | TAAGACAAGCGTCAGAAGTGCCCA |
| BMRF1 (17) | CACTGCGGTGGAGGTAGAG | GGTGGTGTGCCATACAAGG |
| BRLF1 | GCATGGGCGGGACAATCGCAATATAA | CCAGCCAGATGTTCAGGAACCAAA |
| Cp | CCTAGGCCAGCCAGAGATAAT | AGATAGCACTCGACGCACTG |
| β-globin | GAGGCTCTGACCATAACCAAA | GACAAGGCTGCAAGCTATACTA |
| SM | CGGTTTGCTCAAACGTGACATGGA | AATGTCTGCGCCATGATAGAGGGA |
| UBE2B (18) | CTCAGGGGTGGATTGTTGAC | TGTGGATTCAAAGACCACGA |
All primers are listed 5′ to 3′. The reference (ref.) is provided if the primer pair was taken from another source.
Immunoblotting.
Immunoblotting was performed as previously described (30). Cells were harvested in Sumo lysis buffer containing 1× proteasome inhibitor mixture (Roche). Protein concentration of the lysates was quantified using the Sumo protein assay (Bio-Rad). Equal amounts of protein were loaded on 6%, 10%, or 4–20% gradient (Bio-Rad) SDS–polyacrylamide gels, resolved, and transferred to nitrocellulose membranes. Membranes were blocked in a PBS solution with 5% milk and 0.1% Tween 20 and then incubated with primary antibody diluted in the blocking solution. The following antibodies were used: anti–β-actin (Sigma, A5441; 1:5,000), anti-BMRF1 (Millipore, MAB8186; 1:3,000), anti-BRLF1 [produced by Pierce against the peptide EDPDEETSQAVKALREMAD as previously described (50); 1:2,500], anti-BZLF1 (Santa Cruz, sc-53904; 1:250), anti-FLAG (Sigma, F7425; 1:800), anti-IDH1 R132H (Dianova, DIA-H09; 1:500), anti-p18 (Thermo, PA1-73003; 1:1,000), anti-TET2 C2 (Abiocode, R1086-6b; 1:1,000), and anti-Tubulin (Sigma, T5168; 1:4,000). The secondary antibodies used were horseradish peroxidase (HRP)–goat anti-mouse (Pierce, 31430; 1:5,000) and HRP–goat anti-rabbit (Pierce, 31460; 1:10,000).
Quantification of Released Virions.
HONE-Akata cells in six-well dishes were transfected with 5 ng of Z or 25 ng of R, with and without 300 ng of TET2, and up to 1 µg of SG5. Fresh media was added to cells 1 d posttransfection and collected 3 d posttransfection. Cell lysates were also harvested in Sumo buffer for immunoblot analysis. Media was centrifuged at 13,400 × g to pellet cellular debris, and supernatant was treated with 10 µg DNase for 30 min at 37 °C to digest any naked DNA. DNase enzymatic activity was stopped by adding EDTA pH 8 to a final concentration of 4 mM. Virions were disrupted by adding SDS to a concentration of 0.1% and proteinase K to a concentration of 100 µg/mL and incubated at 37 °C for 2 h. DNA was cleaned by phenol/chloroform extraction, followed by salt precipitation and resuspension in 50 µL of TE. Virion production relative to the vector control condition was assessed using qPCR with the BRLF1 primers (Table S1).
Infectious virions released from NOKs-Akata were quantified using a green Raji assay as previously described (51). NOKs-Akata pCMMP and IDH1(R132H) retroviral cell lines were plated in six-well dishes and transfected with control SG5 vector, 800 ng of Z, 200 ng of R, and up to 1 µg of SG5. Media was replaced 1 d posttransfection and harvested 3 d posttransfection. Cell lysates were also harvested in Sumo buffer for immunoblot analysis. Media was filtered through a 0.8 µm pore size filter and incubated with 2 × 105 Raji cells in 10-fold dilutions (100, 10, and 1 µL of supernatant) in a 24-well dish. Phorbol-12-myristate-13-acetate (20 ng/mL) and sodium butyrate (3 mM final concentration) were added 24 h after infection. GFP-positive Raji cells were counted 3 d postinfection to determine viral titer.
Packaging of Retrovirus and Selecting Stable Lines.
The 293T cells were transfected with 10 µg of pCMMP vector control or IDH1(R132H) plus 1 µg of VSV-G and 3 µg of Gag/Pol in 10-cm dishes. The media was replaced with fresh K-SFM 16 h posttransfection. Media containing retrovirus was harvested at 48 h and 72 h posttransfection, filtered through 0.8 µm pore-sized filters, and added directly to target cells (NOKs-Akata) in 12-well dishes. Stable cell lines were selected 72 h postinfection with 0.4 µg/mL puromycin. Cells were selected for at least 1 mo before performing other studies.
Dot Blots.
Dot blots were performed with DNA isolated from various cell lines using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma) according to the manufacturer’s instructions. NaOH was added to 2 µg of DNA at a final concentration of 0.2 M. Single-stranded DNA was produced by incubating the samples at 95 °C for 5 min followed by immediate incubation on ice. To neutralize the NaOH, ammonium acetate was added to a final concentration of 0.8 M. DNA was serially diluted 1:2, four times, yielding dilutions with 1,000, 500, 250, and 125 ng of DNA. DNA was spotted on a 0.22-µm nitrocellulose membrane that had been presoaked in 2× SSC (0.3 M NaCl, 30 mM sodium citrate, pH 7.0) through a dot blot apparatus and cross-linked with UV irradiation. The membrane was blocked with a Tris-buffered saline solution with 5% milk and 0.1% Tween 20 and then incubated with primary antibody diluted in the blocking solution. The primary antibody was anti-5hmC (Active Motif, 39769; 1:10,000), and the secondary antibody was HRP–goat anti-rabbit (Pierce, 31460; 1:2,000).
DNA Bisulfite Conversion.
NOKs-Akata cells stably infected for 1–2 mo with the control retroviral vector or IDH1(R132H) retrovirus were treated with 100 µg/mL of acyclovir (Selleckchem.com) every other day for 4 d. Cells were also concurrently treated with 50 µg/mL of vitamin C (an essential cofactor for TET enzymes). Cells were harvested and DNA was isolated using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma) according to the manufacturer’s instructions. We resuspended 10 µg of DNA (concentrated using salt precipitation) in H2O. Single-stranded DNA was produced by adding NaOH to a concentration of 50 mM and heating at 37 °C for 30 min, followed by immediate incubation on ice. To differentiate 5hmC from 5mC after bisulfite conversion, 500 ng of DNA was oxidized, or not, for 1 h on ice with 1 µL of a 15 mM solution of KRuO4 in 50 mM NaOH (KRuO4 final concentration of 0.6 mM) according to the protocol established by Booth et al. (35). Oxidation of 5hmC results in conversion to uracil during bisulfite treatment, whereas 5mC is unchanged. Samples were cleaned with DNA Clean & Concentrator-5 Kit (Zymo Research) using 7× the volume of DNA-binding buffer according to the manufacturer’s protocol. We bisulfate-converted 250 ng of DNA and cleaned it using the Epitect Bisulfite Kit (Qiagen) according to the manufacturer’s instructions.
Pyrosequencing.
Using bisulfite-converted DNA as a template, the R promoter was amplified for 50 cycles with the flanking primer set upstream 5′-TTTTGAATATTTGGTTGGGGTATTA-3′ and downstream (biotinylated) 5′-AAAAAAACATTCCATAAAACAAACAA-3′ with Amplitaq Gold DNA Polymerase with Buffer II and MgCl2 (Applied Biosystems). MgCl2 was titrated to find the optimal concentration of 2.25 mM. Additionally, annealing temperature during thermocycling was optimized at 60 °C to correct for PCR bias of unmethylated bisulfate-treated DNA. Pyrosequencing was performed using the following sequencing primers: ZRE2 5′-GGTATTAATTAAGTTTATGAG-3′ and ZRE3 5′-TTGGAGGAAATATTGTAGT-3′ on a PyroMark MD. DNA methylation was quantified using the PyroMark CpG software.
Acknowledgments
We thank Karl Munger (Harvard University), Ron Glaser (Ohio State University), Lawrence Young (Warwick Medical School, University of Warwick), Henri-Jacques Delecluse [German Cancer Research Centre (DKFZ)], Wolfgang Hammerschmidt [Research Unit Gene Vectors, Helmholtz Zentrum München, German Research Center for Environmental Health, and German Centre for Infection Research (DZIF)], and Bill Sugden (University of Wisconsin-Madison) for cell lines; Yi Zhang (Boston Children's Hospital, Harvard Medical School) for the TET2 expression vector; Michael Rehli (Universitätsklinikum Regensburg) for the pCpGL-basic luciferase vector; Bill Sugden (University of Wisconsin-Madison) for the pCMMP retrovirus; Harlene Edwards (University of Wisconsin-Madison) for histochemical assistance; Yonga Xing (University of Wisconsin-Madison) for analysis of Z binding to the BRLF1 ZRE3 site; and members of the E.C.J., S.C.K., P.L., and Mertz laboratories (University of Wisconsin-Madison) for suggestions and discussions. This work was supported by NIH Grants 5-PO1-CA022443-38 and 5-R01-DE023939-02.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513432112/-/DCSupplemental.
References
- 1.Rickinson AB, Kieff E. Epstein-Barr virus. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2655–2700. [Google Scholar]
- 2.Kieff E, Rickinson AB. Epstein-Barr virus and its replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th Ed. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 2603–2654. [Google Scholar]
- 3.Li QX, et al. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992;356(6367):347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- 4.Temple RM, et al. Efficient replication of Epstein-Barr virus in stratified epithelium in vitro. Proc Natl Acad Sci USA. 2014;111(46):16544–16549. doi: 10.1073/pnas.1400818111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenspan JS, et al. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N Engl J Med. 1985;313(25):1564–1571. doi: 10.1056/NEJM198512193132502. [DOI] [PubMed] [Google Scholar]
- 6.Young LS, et al. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J Virol. 1991;65(6):2868–2874. doi: 10.1128/jvi.65.6.2868-2874.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niedobitek G, et al. Epstein-Barr virus infection in oral hairy leukoplakia: Virus replication in the absence of a detectable latent phase. J Gen Virol. 1991;72(Pt 12):3035–3046. doi: 10.1099/0022-1317-72-12-3035. [DOI] [PubMed] [Google Scholar]
- 8.Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79(2):1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez AF, et al. The dynamic DNA methylomes of double-stranded DNA viruses associated with human cancer. Genome Res. 2009;19(3):438–451. doi: 10.1101/gr.083550.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalla M, Schmeinck A, Bergbauer M, Pich D, Hammerschmidt W. AP-1 homolog BZLF1 of Epstein-Barr virus has two essential functions dependent on the epigenetic state of the viral genome. Proc Natl Acad Sci USA. 2010;107(2):850–855. doi: 10.1073/pnas.0911948107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woellmer A, Hammerschmidt W. Epstein-Barr virus and host cell methylation: regulation of latency, replication and virus reactivation. Curr Opin Virol. 2013;3(3):260–265. doi: 10.1016/j.coviro.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tempera I, Lieberman PM. Epigenetic regulation of EBV persistence and oncogenesis. Semin Cancer Biol. 2014;26:22–29. doi: 10.1016/j.semcancer.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenney SC, Mertz JE. Regulation of the latent-lytic switch in Epstein-Barr virus. Semin Cancer Biol. 2014;26:60–68. doi: 10.1016/j.semcancer.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flower K, et al. Epigenetic control of viral life-cycle by a DNA-methylation dependent transcription factor. PLoS One. 2011;6(10):e25922. doi: 10.1371/journal.pone.0025922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu K-P, et al. Latency of Epstein-Barr virus is disrupted by gain-of-function mutant cellular AP-1 proteins that preferentially bind methylated DNA. Proc Natl Acad Sci USA. 2013;110(20):8176–8181. doi: 10.1073/pnas.1301577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wille CK, et al. Viral genome methylation differentially affects the ability of BZLF1 versus BRLF1 to activate Epstein-Barr virus lytic gene expression and viral replication. J Virol. 2013;87(2):935–950. doi: 10.1128/JVI.01790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birdwell CE, et al. Genome-wide DNA methylation as an epigenetic consequence of Epstein-Barr virus infection of immortalized keratinocytes. J Virol. 2014;88(19):11442–11458. doi: 10.1128/JVI.00972-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L-W, Chang P-J, Delecluse H-J, Miller G. Marked variation in response of consensus binding elements for the Rta protein of Epstein-Barr virus. J Virol. 2005;79(15):9635–9650. doi: 10.1128/JVI.79.15.9635-9650.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastor WA, Aravind L, Rao A. TETonic shift: Biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol. 2013;14(6):341–356. doi: 10.1038/nrm3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahiliani M, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324(5929):930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito S, et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466(7310):1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delatte B, Deplus R, Fuks F. Playing TETris with DNA modifications. EMBO J. 2014;33(11):1198–1211. doi: 10.15252/embj.201488290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene. 2013;32(5):663–669. doi: 10.1038/onc.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R, et al. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation. 2013;128(18):2047–2057. doi: 10.1161/CIRCULATIONAHA.113.002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestor CE, et al. Tissue type is a major modifier of the 5-hydroxymethylcytosine content of human genes. Genome Res. 2012;22(3):467–477. doi: 10.1101/gr.126417.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mohr F, Döhner K, Buske C, Rawat VPS. TET genes: New players in DNA demethylation and important determinants for stemness. Exp Hematol. 2011;39(3):272–281. doi: 10.1016/j.exphem.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Lian CG, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150(6):1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin D-C, et al. The genomic landscape of nasopharyngeal carcinoma. Nat Genet. 2014;46(8):866–871. doi: 10.1038/ng.3006. [DOI] [PubMed] [Google Scholar]
- 29.Figueroa ME, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18(6):553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhende PM, Seaman WT, Delecluse H-J, Kenney SC. The EBV lytic switch protein, Z, preferentially binds to and activates the methylated viral genome. Nat Genet. 2004;36(10):1099–1104. doi: 10.1038/ng1424. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson SJ, et al. Methylation-dependent binding of the Epstein-Barr virus BZLF1 protein to viral promoters. PLoS Pathog. 2009;5(3):e1000356. doi: 10.1371/journal.ppat.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klug M, Rehli M. Functional analysis of promoter CpG methylation using a CpG-free luciferase reporter vector. Epigenetics. 2006;1(3):127–130. doi: 10.4161/epi.1.3.3327. [DOI] [PubMed] [Google Scholar]
- 33.Bergbauer M, et al. CpG-methylation regulates a class of Epstein-Barr virus promoters. PLoS Pathog. 2010;6(9):e1001114. doi: 10.1371/journal.ppat.1001114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson AB, et al. A novel method for the efficient and selective identification of 5-hydroxymethylcytosine in genomic DNA. Nucleic Acids Res. 2011;39(8):e55. doi: 10.1093/nar/gkr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Booth MJ, et al. Quantitative sequencing of 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution. Science. 2012;336(6083):934–937. doi: 10.1126/science.1220671. [DOI] [PubMed] [Google Scholar]
- 36.Woellmer A, Arteaga-Salas JM, Hammerschmidt W. BZLF1 governs CpG-methylated chromatin of Epstein-Barr Virus reversing epigenetic repression. PLoS Pathog. 2012;8(9):e1002902. doi: 10.1371/journal.ppat.1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takai H, et al. 5-Hydroxymethylcytosine plays a critical role in glioblastomagenesis by recruiting the CHTOP-methylosome complex. Cell Reports. 2014;9(1):48–60. doi: 10.1016/j.celrep.2014.08.071. [DOI] [PubMed] [Google Scholar]
- 38.Mendonca A, Chang EH, Liu W, Yuan C. Hydroxymethylation of DNA influences nucleosomal conformation and stability in vitro. Biochim Biophys Acta. 2014;1839(11):1323–1329. doi: 10.1016/j.bbagrm.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Chan KH, et al. EBV specific antibody-based and DNA-based assays in serologic diagnosis of nasopharyngeal carcinoma. Int J Cancer. 2003;105(5):706–709. doi: 10.1002/ijc.11130. [DOI] [PubMed] [Google Scholar]
- 40.Nawandar DM, et al. Differentiation-dependent KLF4 expression promotes lytic Epstein-Barr Virus infection in epithelial cells. PLoS Pathog. 2015;11(10):e1005195. doi: 10.1371/journal.ppat.1005195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma S-D, et al. An Epstein-Barr Virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J Virol. 2012;86(15):7976–7987. doi: 10.1128/JVI.00770-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feederle R, et al. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 2000;19(12):3080–3089. doi: 10.1093/emboj/19.12.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robinson AR, Kwek SS, Hagemeier SR, Wille CK, Kenney SC. Cellular transcription factor Oct-1 interacts with the Epstein-Barr virus BRLF1 protein to promote disruption of viral latency. J Virol. 2011;85(17):8940–8953. doi: 10.1128/JVI.00569-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheung ST, et al. Nasopharyngeal carcinoma cell line (C666-1) consistently harbouring Epstein-Barr virus. Int J Cancer. 1999;83(1):121–126. doi: 10.1002/(sici)1097-0215(19990924)83:1<121::aid-ijc21>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 45.Strong MJ, et al. Comprehensive high-throughput RNA sequencing analysis reveals contamination of multiple nasopharyngeal carcinoma cell lines with HeLa cell genomes. J Virol. 2014;88(18):10696–10704. doi: 10.1128/JVI.01457-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piboonniyom SO, Timmermann S, Hinds P, Münger K. Aberrations in the MTS1 tumor suppressor locus in oral squamous cell carcinoma lines preferentially affect the INK4A gene and result in increased cdk6 activity. Oral Oncol. 2002;38(2):179–186. doi: 10.1016/s1368-8375(01)00042-2. [DOI] [PubMed] [Google Scholar]
- 47.Sarisky RT, et al. A replication function associated with the activation domain of the Epstein-Barr virus Zta transactivator. J Virol. 1996;70(12):8340–8347. doi: 10.1128/jvi.70.12.8340-8347.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardwick JM, Lieberman PM, Hayward SD. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lambert PF, et al. Using an immortalized cell line to study the HPV life cycle in organotypic “raft” cultures. Methods Mol Med. 2005;119:141–155. doi: 10.1385/1-59259-982-6:141. [DOI] [PubMed] [Google Scholar]
- 50.Reusch JA, Nawandar DM, Wright KL, Kenney SC, Mertz JE. Cellular differentiation regulator BLIMP1 induces Epstein-Barr virus lytic reactivation in epithelial and B cells by activating transcription from both the R and Z promoters. J Virol. 2015;89(3):1731–1743. doi: 10.1128/JVI.02781-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hong GK, et al. The BRRF1 early gene of Epstein-Barr virus encodes a transcription factor that enhances induction of lytic infection by BRLF1. J Virol. 2004;78(10):4983–4992. doi: 10.1128/JVI.78.10.4983-4992.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]