Significance
Estrogens are known to rapidly affect learning and memory, but how they do so is not well understood. Here we identify the hippocampus as a brain structure responsible for rapid estradiol-induced improvement of general discrimination learning in female mice, which coincides with a substantial increase in hippocampal dendritic spines, the postsynaptic sites of excitatory synapses. Until now, it had been assumed that estradiol-induced increases in hippocampal synapses would result in increased excitatory input into the neuron. Surprisingly, we find the opposite: increases in hippocampal spines are associated with a decrease in the excitatory glutamatergic input. This finding suggests that estradiol improves learning through inducing the formation of silent or immature synapses, which provide the substrate for storing new memories.
Keywords: immature synapse, short-term memory, structural plasticity, synaptic plasticity, signal-to-noise ratio
Abstract
Dramatic increases in hippocampal spine synapse density are known to occur within minutes of estrogen exposure. Until now, it has been assumed that enhanced spinogenesis increased excitatory input received by the CA1 pyramidal neurons, but how this facilitated learning and memory was unclear. Delivery of 17β-estradiol or an estrogen receptor (ER)-α (but not ER-β) agonist into the dorsal hippocampus rapidly improved general discrimination learning in female mice. The same treatments increased CA1 dendritic spines in hippocampal sections over a time course consistent with the learning acquisition phase. Surprisingly, estrogen-activated spinogenesis was associated with a decrease in CA1 hippocampal excitatory input, rapidly and transiently reducing CA1 AMPA activity via a mechanism likely reflecting AMPA receptor internalization and creation of silent or immature synapses. We propose that estrogens promote hippocampally mediated learning via a mechanism resembling some of the broad features of normal development, an initial overproduction of functionally immature connections being subsequently “pruned” by experience.
Estradiol rapidly and dramatically increases hippocampal dendritic spine and synapse density within minutes of application (1–4). There is a strong correlative association between estrogen-induced spinogenesis and improvements in cognition (5); however, the relationship of these structural changes to estrogen-induced alterations in hippocampal function is unclear. Our laboratory recently reported that the density of hippocampal CA1 pyramidal dendritic spines increases very rapidly after systemic treatment with 17β-estradiol or estrogen receptor (ER) -selective agonists in ovariectomized female mice, changes that are paralleled by learning enhancements (2, 3). Estrogen-induced rapid structural changes are substantial, increasing spine density by 30–50% within 15–40 min of hormone application (1–3, 6). As a result, adult rodents can experience the addition of thousands of CA1 synapses within a span of minutes after exposure to estradiol. These effects of estrogens reproduce the changes occurring during the 4-d estrous cycle of female rodents, which include the induction of CA1 spines (7).
How these processes contribute to the behavioral changes observed after estradiol treatment is not understood. Estradiol enhances excitatory neurotransmission throughout the hippocampus (8–10), and activates BDNF signaling in the mossy fiber system (11). Dendritic spines turn over more rapidly in the hippocampus than in the neocortex (12), particularly in the case of estradiol-induced spines (13). Such rapid, transient, and apparently indiscriminate increases in excitatory synapse formation would seem, at first sight, to be more likely to interfere with preexisting brain circuits and impair normal information processing than to enhance cognitive function.
How then, does enhancement of spine formation lead to improved cognitive function? To address this question, we focused specifically on the effects of estradiol in the dorsal CA1 hippocampus, as a site mediating the rapid improving effects of estrogens on learning. Estradiol application, via a mechanism involving ERα, rapidly increases dendritic spine density in the CA1 pyramidal stratum oriens and stratum radiatum subregions. However, contrary to our initial assumptions based on previous work in this field, increased spinogenesis did not result in increased CA1 excitatory input. Rather, estrogen-induced formation of hippocampal spines was associated with a decrease in the ability of CA1 neurons to respond to AMPA receptor (AMPAR) activation, resulting in decreased excitatory input to CA1 neurons. This appears to be the result of AMPAR internalization from the synaptic membrane (6). Taken together, our data suggest that estrogens induce formation of “silent” or immature synapses that act as a substrate for the storage of new memories (14, 15). This finding explains estrogens’ ability to rapidly improve learning without precipitating uncontrolled activation of the hippocampal circuitry. These effects of estradiol on CA1 pyramidal neurons phenotypically resemble the structural and functional properties of neurons during development, when neurons with higher levels of spine density and higher numbers of silent/immature synapses are present before the activity dependent refinement of neural circuitry.
Results
Intrahippocampal 17β-Estradiol Rapidly Improves Learning.
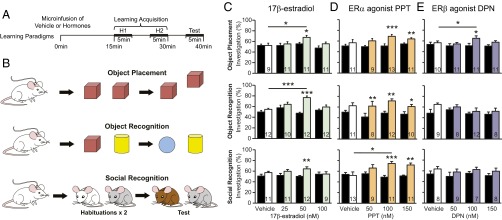
To determine the role of the hippocampus in mediating estrogens’ rapid effects on learning (2), ovariectomized CD1 mice were fitted with bilateral guide cannulae aimed at the dorsal anterior hippocampus. Mice were microinjected with 17β-estradiol (25, 50, or 100 nM) or the vehicle control [artificial cerebrospinal fluid (aCSF) + 0.02% ethanol] in a between-subjects design. After 15 min, animals were trained on learning tasks modified to examine estrogens’ rapid effects on different types of memory (3): spatial learning (object placement), item recognition (object recognition), and conspecific recognition (social recognition) (Fig. 1B). These tasks rely on the natural tendency of mice to sniff novel or displaced stimuli more than familiar stimuli. Because systemic estrogen administration previously induced learning and memory enhancements, we used tasks with reduced learning opportunities (Fig. 1A) such that vehicle controls do not display discrimination of the novel stimulus at test (3). Effects of treatment on investigation durations and other behavioral measures were recorded and are discussed below.
Fig. 1.
Intrahippocampal 17β-estradiol and ERα agonist PPT (but not ERβ agonist DPN) rapidly improve general learning. (A) Learning paradigm timeline. “H” indicates habituation. (B) Behavioral paradigms, each performed with experimentally naïve mice. (C–E) Percent investigation (PI) scores of mice during object placement (Top), object recognition (Middle), or social recognition (Bottom) learning paradigms. Black bars are PI at habituation (average of H1 and H2), white/colored bars the PI at test and contain group numbers, mean ± SEM. (C) Object placement and recognition: Microinfusion of 50 nM 17β-estradiol into the hippocampus improves object placement and object recognition learning within 40 min of administration (F3, 38 = 3.14, P < 0.05 and F3, 42 = 9.56, P < 0.001, post hoc q = 3.87, df = 18, P < 0.05 and q = 7.13, df = 22, P < 0.001, respectively). Groups treated with 50 nM of 17β-estradiol also demonstrate recognition of the displaced or novel object during test (PI at test is significantly higher than habituation; t = −2.55, df = 10, P < 0.05 and t = −8.28, df = 11, P < 0.05). Social recognition: Mice receiving 50 nM of 17β-estradiol discriminate the novel individual during the social recognition task (t = 3.38, df = 11, P < 0.01). (D) Object placement: Mice receiving 100 nM and 150 nM of ERα agonist PPT distinguish the displaced object (t = 5.79, df = 12, P < 0.001 and t = 3.30, df = 10, P < 0.01). Object recognition: Administering 50 nM, 100 nM, or 150 nM PPT result in successful discrimination of the novel object (50 nM, t = 3.88, df = 7, P < 0.01; 100 nM, t = 4.16, df = 11, P < 0.01; 150 nM, t = 2.90, df = 9, P < 0.05). Social recognition: 100 nM of PPT improves social recognition learning (F3, 40 = 3.13, P < 0.05, post hoc q = 3.92, df = 22, P < 0.05). Groups administered 100 nM and 150 nM PPT demonstrate discrimination of the novel individual at test (t = 5.18, df = 10, P < 0.001 and t = 4.39, df = 10, P < 0.01). (E) Object placement: 100 nM of DPN significantly improves object placement learning (F3, 38 = 4.01, P < 0.05, post hoc q = 3.66, df = 21, P < 0.05). This group also distinguishes the displaced object at test (t = 2.67, df = 9, P < 0.05). Object and social recognition: DPN does not improve object or social recognition learning. *P < 0.05, **P < 0.01, ***P < 0.001.
Object placement, object recognition, and social recognition performances were all improved within 40 min of intrahippocampal 17β-estradiol administration. Fifty nanomolars of 17β-estradiol improved object placement and object recognition above vehicle controls (Fig. 1C). In addition, mice receiving 50 nM 17β-estradiol were capable of distinguishing the displaced and novel stimulus in all three learning paradigms (investigation percent at test was higher than at habituation) (Fig. 1C). Twenty-five nanomolars and 100 nM 17β-estradiol did not affect learning and memory performance, demonstrating that 17β-estradiol has an inverse U-shaped dose–response curve, consistent with literature on estrogenic effects (3). Therefore, infusing 50 nM of 17β-estradiol into the hippocampus enhances general learning and memory.
Learning Enhancements Are Mediated by ERα.
To identify the estrogen receptor responsible for these learning enhancements, we microinfused 50, 100, or 150 nM of ERα-selective agonist PPT [1,3,5-Tris(4-hydroxyphenyl)-4-propyl-1H- pyrazole] or ERβ-selective agonist DPN [2,3-bis(4-hydroxyphenyl)-propionitrile; or vehicle (aCSF + 0.02% ethanol) into the hippocampus and tested mice on the learning paradigms described above. Higher doses were used for these selective agonists compared with 17β-estradiol because PPT and DPN have a ∼50% binding affinity to ERα and ERβ, respectively, relative to estradiol (16, 17). One-hundred nanomolars PPT improved social recognition above vehicle controls (Fig. 1D), and groups administered 100 nM or 150 nM PPT were able to successfully identify the novel and displaced stimuli in all three learning tests (Fig. 1D). In contrast, the ERβ agonist DPN improved performance only on the object placement task (100 nM DPN) (Fig. 1E), and did not improve object or social recognition (Fig. 1E). Therefore, the learning and memory effects of 17β-estradiol are replicated by intrahippocampal delivery of the ERα agonist PPT.
Effects Are Not Secondary to Changes in Other Behaviors.
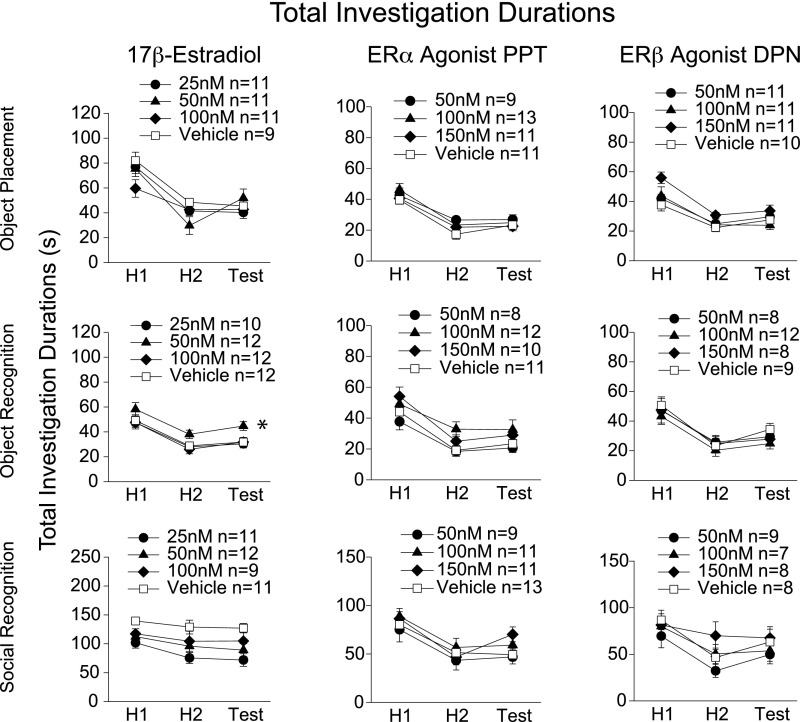
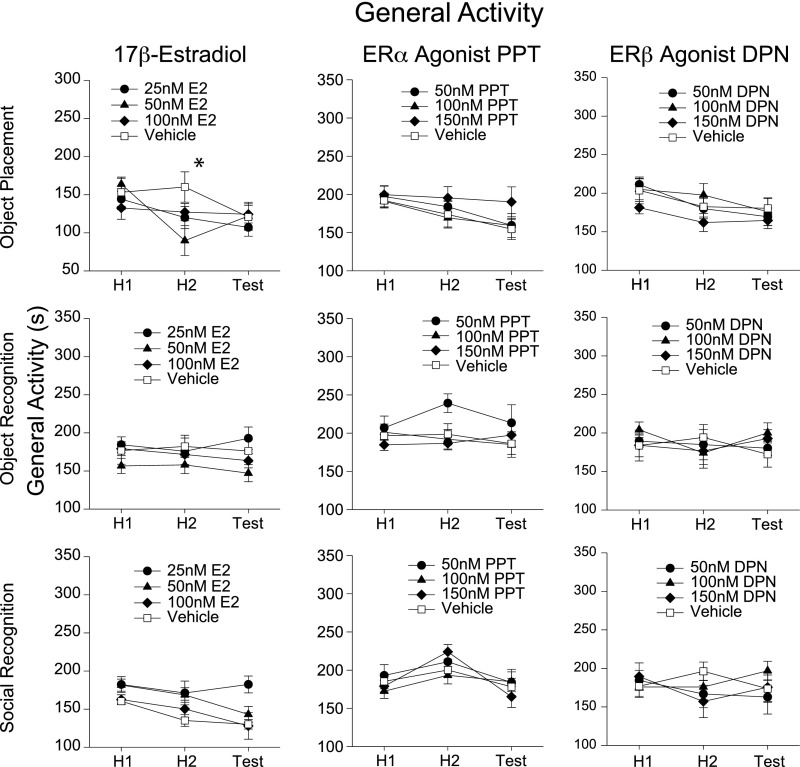
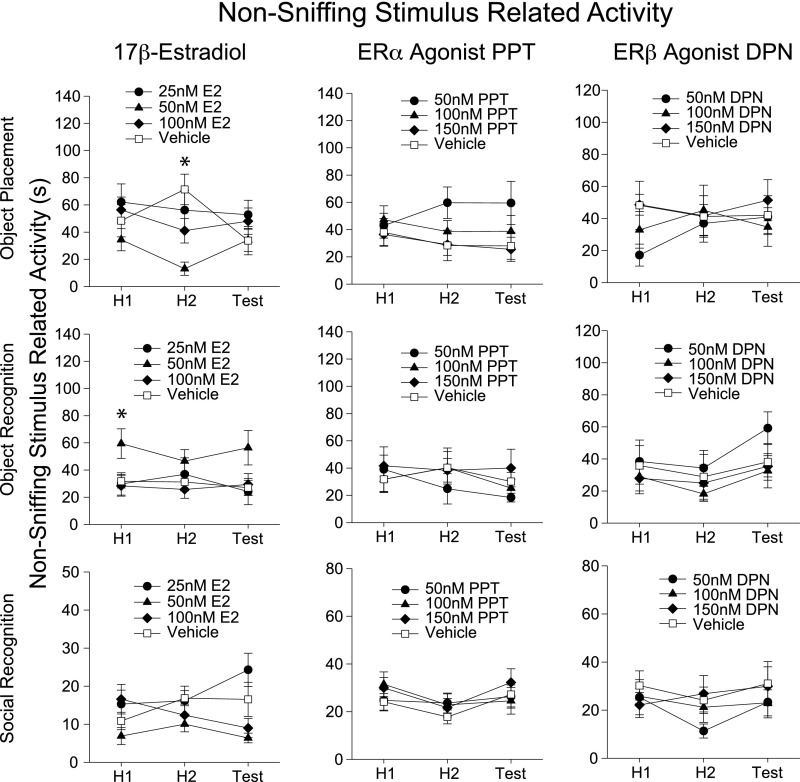
We performed an ethological analysis on the mice during the learning paradigms, recording behaviors listed in Table S1. Intrahippocampal delivery of 17β-estradiol or ER agonists did not generally affect other behaviors recorded during the task [Fig. S1, total investigation durations (novel + familiar stimulus investigation durations) and Figs. S2 and S3]. Hence, the learning enhancements reported cannot be explained by changes such as increased interest in stimuli or enhanced activity. The one exception is the higher total investigation duration detected in the 17β-estradiol 50-nM group during the object recognition paradigm (Fig. S1). However, it is unlikely that this higher investigation duration indicates an enhanced interest in objects that then leads to a facilitation of object recognition learning, because this effect was not found in the object placement paradigm, in PPT experiments, or during systemic experiments (2, 3). Therefore, we conclude that intrahippocampal delivery of 17β-estradiol, via ERα, rapidly and specifically enhances learning and memory.
Table S1.
List and description of the behaviors collected and analyzed for learning paradigms [modified from Phan et al. (3)]
| Behavior | Description |
| Sniff stimulus | Sniff/Investigation directed at object or mouse stimulus. Nose twitching and within 1–2 mm of stimuli. |
| Bite stimulus | Biting of object stimuli or mouse stimulus cylinders. |
| Sit/climb on stimulus | Sitting/climbing on object stimulus. All four paws off the cage floor. |
| Dig | Using forepaws to propel bedding in posterior direction. |
| Bury | Using forepaws to push bedding away from body in anterior direction. |
| Horizontal exploration | Walking, and exploration of cage, includes nonstimulus sniffing. |
| Rearing | Two forepaws off the cage floor. |
| Self-groom | Grooming, forepaws moving over face and body. |
| Inactivity | Includes behaviors sitting, laying down, freezing and sleeping. |
| Stereotypy | Strange repetitive behaviors (>three repetitions), including jumps, head shakes, or lid chews, and so forth. |
Fig. S1.
17β-Estradiol, PPT, and DPN do not consistently affect total investigation durations of stimuli during the learning paradigms. Total investigation durations (novel + familiar stimulus investigations), mean ± SEM. For brevity, only meaningful differences are discussed here and in the main text. Groups demonstrating enhanced learning did not have an increased investigation duration compared with vehicle controls in the learning experiments, except during the 17β-estradiol object recognition experiment (F3, 84 = 3.697, P < 0.05, post hoc q = 3.537, df = 22, P < 0.05). However, it is unlikely that this higher investigation duration indicates an enhanced interest in objects that then leads to a facilitation of object recognition learning, because this effect was not found during the object placement paradigm, in PPT experiments, nor during systemic experiments (2, 3). *P < 0.05.
Fig. S2.
Analysis of general activity behaviors in mice during learning paradigms. For graphical simplicity, relevant recorded behaviors have been summed into a general activity measure. Analyses of individual behaviors and data interpretation do not differ from the summaries reported here. General activity includes horizontal exploration, rearing, nonobject-related digging, and burying behavior. For brevity, we note behaviors that differ between vehicle and treatment groups demonstrating learning effects. (No treatment dose consistently produced effects on any behavior recorded.) Mice given 50 nM of 17β-estradiol had significantly lower general activity during habituation 2 compared with vehicle during object placement task (F6, 74 = 3.31, P < 0.01, post hoc q = 4.45, df = 18, P < 0.05). These observations cannot account for the enhanced learning observed in this group via increased arousal or interest in objects, for example. *P < 0.05.
Fig. S3.
Analysis of nonsniffing stimulus-related behaviors in mice during learning paradigms. For graphical simplicity, relevant recorded behaviors have been summed into a nonsniffing stimulus-related activity measure. Analyses on individual behaviors and data interpretation do not differ from the summaries reported here. Nonsniffing stimulus-related behavior includes biting objects, sitting/climbing on stimulus, digging and burying stimuli. For brevity, we note behaviors that differ between vehicle and treatment groups demonstrating learning effects. (No treatment dose consistently produced effects on any behavior recorded.) Mice given 50 nM of 17β-estradiol had significantly lower nonsniffing-related activity during habituation 2 compared with vehicle during object placement task (F6, 74 = 3.10, P < 0.01, post hoc q = 5.33, df = 18, P < 0.01). These observations cannot account for the enhanced learning observed in these groups via increased arousal or interest in objects, for example. Mice receiving 50 nM 17β-estradiol displayed significantly higher nonsniffing stimulus-related activity during habituation 1 of the object recognition task. (Two-way repeated measures ANOVA failed the test for equal variance; therefore, a one-way ANOVA was used to analyze differences between treatment at each test. F3, 41 = 3.31, P < 0.05, post hoc q = 3.36, df = 20, P < 0.05). This may be interpreted as an enhanced interest in objects. However, this was not repeated in the object placement experiments, and is not replicated in subsequent experiments on object recognition. Therefore, the differences here are likely caused by random variations in behavior, and not a result of treatment effects on behavior. *P < 0.05.
17β-Estradiol Rapidly Increases CA1 Spine Density.
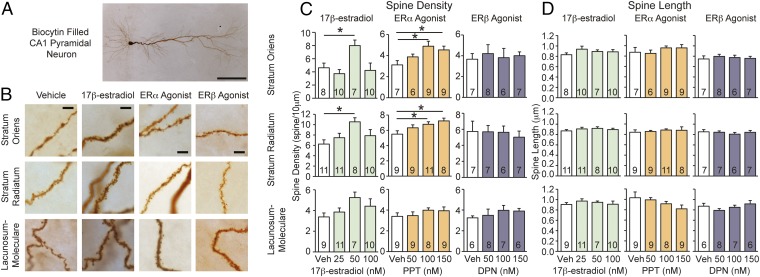
To determine the cellular effects of estradiol on CA1 pyramidal neurons, the same doses of 17β-estradiol used in the learning tasks were bath-applied to hippocampal sections from experimentally naïve mice. CA1 pyramidal neurons from regions corresponding to our cannula placements (Fig. S4) were whole-cell patch-clamped and electrophysiological measures were obtained (data below), filled with biocytin, then visualized with diaminobenzidine (DAB) staining to reveal their structure. Dendritic spine density increased within the stratum oriens and stratum radiatum after 20–30 min of treatment with 50 nM of 17β-estradiol (Fig. 2 B and C). This timeframe coincides with the training sessions of the learning experiments. We did not detect changes in dendritic spine length (Fig. 2D); however, this may be because our analyses were completed on 2D images. The lack of significance in the stratum lacunosum-moleculare may be because of the greater experimental variance observed in this stratum: previous reports have detected estrogen-induced spine changes within this hippocampal subregion (4).
Fig. S4.
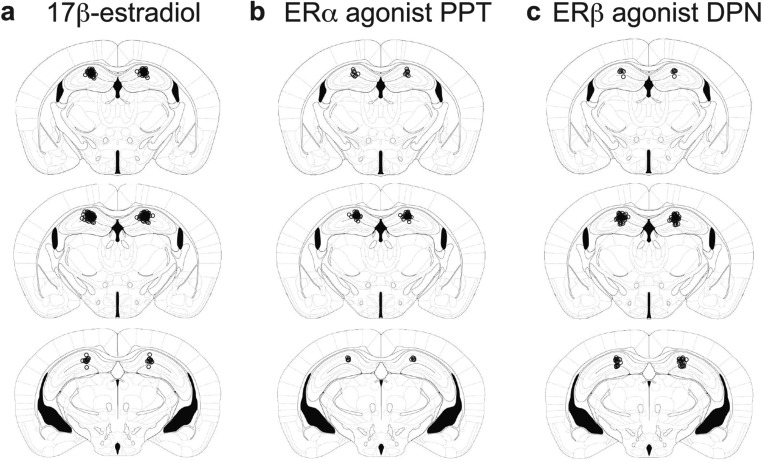
Injection cannula placements in experimental mouse brains. Images adapted from ref. 34: −1.70, −1.82, and −2.46 mm from bregma (Top to Bottom). Open circles mark the ends of microinfusion injectors in all mice brains used for (A) 17β-estradiol, (B) PPT, and (C) DPN learning experiments.
Fig. 2.
17β-Estradiol and ERα agonist PPT (but not ERβ agonist DPN) rapidly increase CA1 dendritic spines. (A) Biocytin filled CA1 pyramidal neurons. (Scale bar, 100 μm.) (B) Images of CA1 dendrites from slices treated with 20–30 min of vehicle, 50 nM 17β-estradiol, 100 nM ERα agonist PPT, or 100 nM ERβ agonist DPN. (Scale bars, 5 μm.) (C) Measures of spine density. 17β-Estradiol: 50 nM 17β-estradiol increases spine density in the stratum oriens and stratum radiatum (stratum oriens: F3, 31 = 4.35, P < 0.05; post hoc q = 3.59, df = 13 P < 0.05 and stratum radiatum, F3, 36 = 3.36, P < 0.05; post hoc q = 4.43, df = 17, P < 0.05). Spine density in the lacunosum-moleculare is not statistically significant (F3, 33 = 2.00, P = 0.13). ERα agonist PPT: 100 nM or 150 nM of ERα agonist PPT increases spine density in the stratum oriens and stratum radiatum (stratum oriens, F3,27 = 3.68, P < 0.05; post hoc 100 nM: q = 4.39, df = 14, P < 0.05, 150 nM: q = 3.52, df = 14, P < 0.05 and stratum radiatum, F3, 34 = 3.13, P < 0.05; post hoc 100 nM q = 3.55, df = 18, P < 0.05, 150 nM: q = 4.10, df = 15, P < 0.05). ERβ agonist DPN: Did not affect spine density. (D) None of the treatments affect average spine length; mean ± SEM. *P < 0.05.
Rapid Increases in CA1 Spine Density Are Mediated by ERα.
We bath-applied the same doses of ERα agonist PPT or ERβ agonist DPN (50, 100, or 150 nM) used in the learning tasks, or vehicle (aCSF + 0.02% ethanol), onto hippocampal sections. Twenty to 30 min of exposure to 100 nM or 150 nM of ERα agonist PPT increased stratum oriens and stratum radiatum spine density (Fig. 2 B and C). Again, changes within the lacunosum-moleculare did not reach statistical significance. In contrast, there were no rapid effects of ERβ agonist DPN on spine density within any CA1 hippocampal subregion examined (Fig. 2 B and C). Consistent with the effects of 17β-estradiol, neither PPT nor DPN resulted in a discernable change in dendritic spine length (Fig. 2D).
17β-Estradiol Decreases CA1 AMPA Miniature Excitatory Postsynaptic Current Frequency.
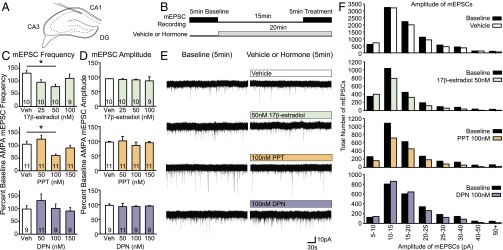
What are the consequences of rapid increases in hippocampal dendritic spine density for CA1 pyramidal neuron connectivity? Estrogens rapidly enhance hippocampal excitability, in part via presynaptic changes in CA3 Schaffer collateral terminals, which synapse onto CA1 (8–10). However, because we were interested in CA1 pyramidal neuron changes that accompany CA1 dendritic spine changes, we investigated estradiol effects on miniature excitatory postsynaptic currents (mEPSCs) resulting from spontaneous vesicular release of neurotransmitters. The same doses of 17β-estradiol used in learning and dendritic spine experiments were bath applied onto hippocampal sections for 15–20 min (Fig. 3B), coinciding with the onset of learning acquisition during our behavioral experiments (Fig. 1A).
Fig. 3.
Rapid effects of 17β-estradiol and estrogen receptor agonists on CA1 pyramidal neuron mEPSCs. (A) Location of patched neurons corresponds to location of bilateral cannulas in behavioral experiments. (B) Schematic of treatment protocols. (C and D) Percent baseline of mEPSC frequency and amplitude; mean ± SEM. (C) Fifteen to 20 min of 50 nM 17β-estradiol or 100 nM ERα agonist PPT reduces CA1 mEPSC frequency (Estradiol: F3, 35 = 3.18, P < 0.05; post hoc q = 4.20, df = 18, P < 0.05; PPT: F3, 40 = 5.72, P < 0.01; post hoc q = 3.93, df = 20, P < 0.05). ERβ agonist DPN does not affect mEPSC frequency. (D) Treatments do not affect mEPSC amplitude. (E) Five-minute mEPSC traces of baseline (Left) and traces after 15–20 min of vehicle, 50 nM 17β-estradiol, 100 nM PPT, or 100nM DPN application (Right). White and colored boxes indicate the presence of vehicle or hormones during the recording. (F) Total number of mEPSCs for all neurons recorded within a treatment group. 17β-Estradiol and PPT appear to decrease mEPSCs across all amplitudes fairly equally. *P < 0.05.
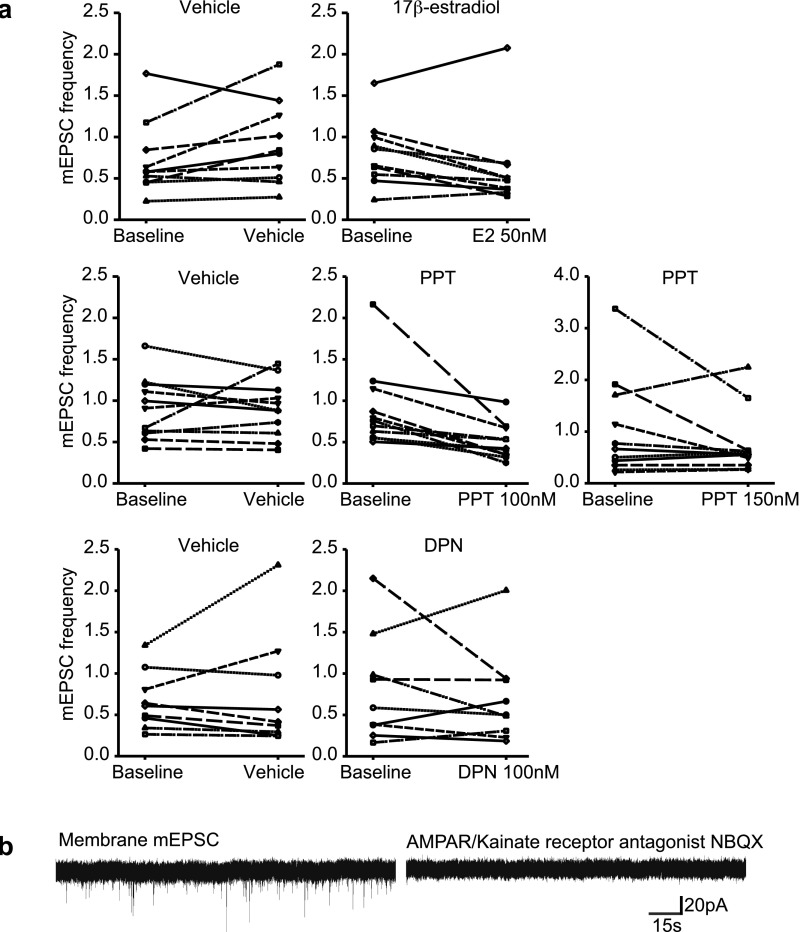
Surprisingly, 17β-estradiol decreased CA1 pyramidal mEPSC frequency in neurons with increased spine density. Fifteen to 20 min of treatment with 50 nM 17β-estradiol significantly reduced mEPSC frequency in CA1 neurons, whereas 25 nM or 100 nM 17β-estradiol did not have an effect (Fig. 3 C and E and Fig. S5A). Because our recordings were obtained at −70 mV, these mEPSC responses are not NMDA receptor (NMDAR) mediated. Addition of the AMPA/kainate receptor antagonist NBQX consistently abolished recorded mEPSCs (Fig. S5B). 17β-Estradiol affected mEPSC frequency but not amplitude (Fig. 3 D and F).
Fig. S5.
17β-Estradiol, PPT, and DPN effects on hippocampal mEPSCs. (A) CA1 hippocampal mEPSC frequencies before (baseline) and after 15–20 min of 17β-estradiol, ERα agonist PPT, or ERβ agonist DPN. Depicted are select doses of interest. (B) mEPSCs observed are AMPA-mediated currents. The application of AMPA antagonist NBQX eliminates mEPSCs observed.
17β-Estradiol Decreases CA1 Responses to AMPA.
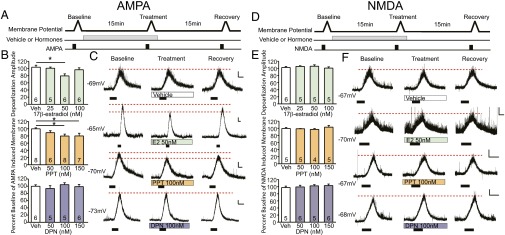
If the estradiol-induced decrease in mEPSC frequency resulted from a postsynaptic mechanism, this may be because of the internalization of AMPARs (6, 18) or the movement of synaptic AMPARs to perisynaptic sites. To investigate these possibilities, we examined membrane potential responses of CA1 pyramidal neurons to bath application of (S)-AMPA (Fig. 4 A–C and Fig. S6). This approach allowed us to examine the effects of estradiol on the ability of a CA1 pyramidal neuron to depolarize upon AMPAR activation. Internalization of AMPAR should result in a decreased response to the AMPA agonist, whereas the movement of receptors from postsynaptic sites to perisynaptic sites should not affect CA1 responses to bath-applied AMPA. Because 17β-estradiol is reported to affect CA1 pyramidal NMDAR subunit composition and function over longer periods of exposure (24–48 h), we also examined whether estradiol might alter CA1 responses to NMDA in a separate group of neurons (Fig. 4 D–F) (19, 20). Membrane potentials were recorded from CA1 pyramidal neurons during bath application of subthreshold concentrations (i.e., concentrations that did not produce action potential firing) of (S)-AMPA or NMDA to elicit a 5- to 7-mV amplitude depolarization on average. Consistent with our mEPSC results, 15–20 min of 50 nM 17β-estradiol (Fig. 4 B and C) decreased the amplitude of AMPAR-mediated depolarization. In contrast, 17β-estradiol did not affect CA1 responses to NMDA (Fig. 4 E and F).
Fig. 4.
Rapid effects of estrogens and receptor agonists on CA1 AMPA- or NMDA-induced membrane depolarization. (A and D) Treatment protocol for (S)-AMPA or NMDA induced depolarization measures. (B and E) Percent baseline of membrane depolarization amplitude elicited by (S)-AMPA or NMDA; mean ± SEM. (B) Fifteen to 20 min of 50 nM 17β-estradiol decreases AMPA-mediated membrane potential amplitudes (F3, 19 = 4.05, P < 0.05, post hoc q = 4.45, df = 8, P < 0.05). One-hundred nanomolars or 150 nM of PPT also decreases AMPA-mediated membrane depolarization (F3, 25 = 3.33, P < 0.05, post hoc for 100 nM q = 3.91, df = 14, P < 0.05, and for 150 nM q = 3.73, df = 13, P < 0.05). DPN does not affect AMPA membrane depolarization amplitude. (E) Hormone treatments do not affect NMDA induced membrane depolarization. (C and F) Membrane potential traces. Black boxes indicate length and duration of (S)-AMPA or NMDA agonist application. [Scale bars, 2 mV (vertical axis) and 1 min (horizontal axis).] *P < 0.05.
Fig. S6.
(S)-AMPA mediated membrane depolarization amplitudes before (baseline) and after 15–20 min of treatment with 17β-estradiol, ERα agonist PPT, or ERβ agonist DPN. Results from doses of interest are shown.
ERα Agonist PPT Decreases CA1 AMPAR Responses.
All of 17β-estradiol’s effects on the electrophysiological properties of CA1 pyramidal neurons were replicated by ERα agonist PPT. Bath application of 100 nM PPT increased CA1 dendritic spine density (Fig. 2C), and decreased mEPSC frequency after 15–20 min of treatment (Fig. 3C and Fig. S5A). Similarly, PPT at 100 nM and 150 nM also reduced the membrane depolarization amplitude elicited by (S)-AMPA agonist (Fig. 4 B and C and Fig. S6). In contrast, the ERβ agonist DPN did not affect mEPSC frequency (Fig. 3C and Fig. S5A), nor did it affect AMPA-induced membrane depolarizations (Fig. 4 B and C and Fig. S6). None of the treatments affected NMDAR-mediated membrane depolarizations (Fig. 4 E and F).
Decreased AMPAR Function Is Time-Dependent and Transient.
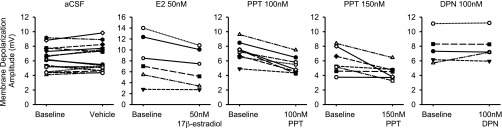
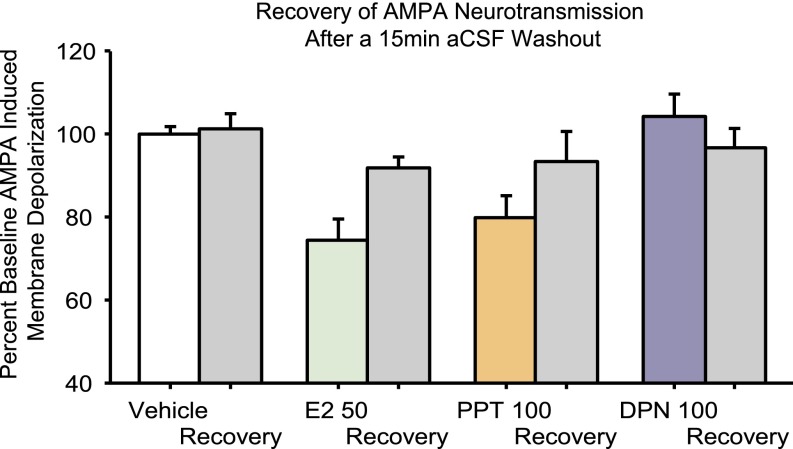
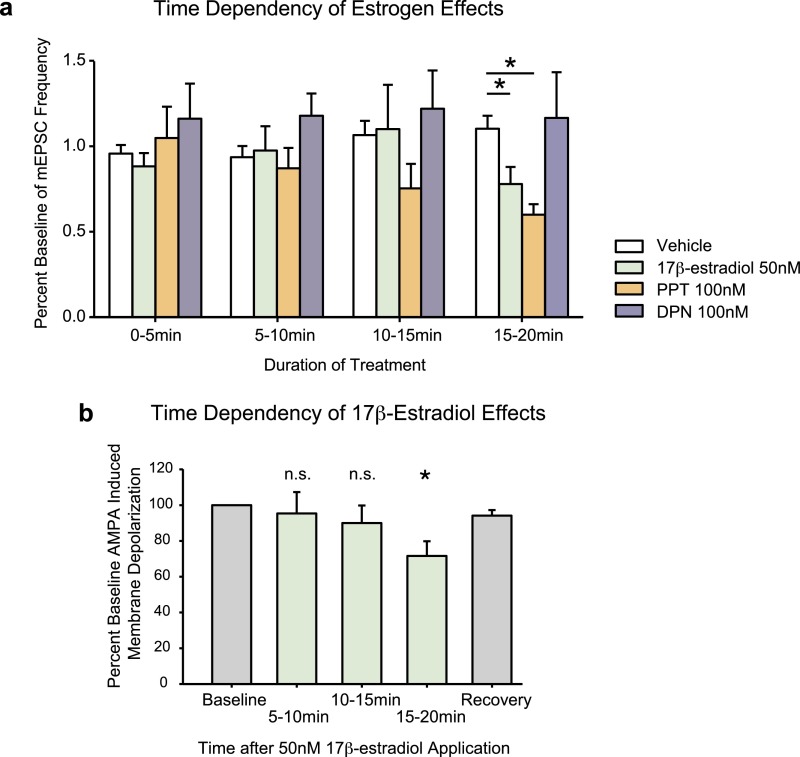
In a subset of neurons, a 15-min aCSF washout was applied after treatment with 17β-estradiol or PPT. This process resulted in a partial recovery of AMPA-induced membrane depolarizations. The average recovery for effective doses of 17β-estradiol and PPT was 89–93% of baseline (average effect of 17β-estradiol or PPT was 60–79% of baseline). The equivalent of a washout measure for vehicle controls was 99–105% of baseline (Fig. S7). Interestingly, estrogen effects on AMPA neurotransmission are first detected after 15 min of treatment (Fig. S8). When 5- to 10-min time points were examined during pilot studies, no effect of 17β-estradiol, PPT, or DPN could be detected.
Fig. S7.
A 15-min aCSF washout recovery measure taken from a subset of neurons reveals partial recovery of AMPA membrane depolarization amplitude after treatment with 50 nM 17β-estradiol and 100 nM PPT. This graph includes a combination of measures taken from experimental neurons as well as data from pilot studies.
Fig. S8.
(A) Time dependency of rapid estrogen effects on mEPSC frequency. Vehicle group includes data from all three mEPSC experiments. Fifty nanomolars of 17β-estradiol and 100 nM of PPT decrease percent baseline mEPSC frequency only after 15–20 min of hormone treatment, but not at earlier time points. (Kruskal–Wallis H = 17.512, df = 3, P < 0.001, Dunn’s post hoc 50 nM 17β-estradiol: q = 2.396, df = 36, P < 0.05, 100 nM PPT: q = 3.944, df = 37, P < 0.05). (B) Time dependency of 17β-estradiol effects. This graph includes a combination of both experimental and pilot data examining estradiol effects on AMPA-elicited membrane depolarization amplitudes. Treatment of neurons with 50 nM of 17β-estradiol for greater than 15 min decreases AMPA neurotransmission. Significances indicate differences from baseline amplitudes using paired t tests (5–10 min: P = 0.9, 10–15 min: P = 0.8, 15–20 min: t = 2.54, df = 21, P < 0.05). Each treatment group represents an independent set of neurons, and the recovery measure is taken from a subset of neurons from the 15- to 20-min treatment group, following a 15-min washout with aCSF. *P < 0.05. n.s., nonsignificant.
Discussion
Estrogens, via ERα, Enhance Learning and Affect Hippocampal Connectivity.
17β-Estradiol delivered directly to the dorsal CA1 hippocampus facilitates object placement, object recognition, and social recognition learning within 40 min of administration (Fig. 1). Experiments using estrogen receptor agonists reveal these effects are mimicked by ERα activation. Conversely, ERβ activation enhances performance only in the object placement task. Thus, 17β-estradiol, via an ERα mechanism, rapidly facilitates general learning on discrimination tasks, whereas ERβ effects appear limited to spatial tasks. The same doses of 17β-estradiol and ERα agonist PPT that enhanced learning also increased dendritic spine density within the stratum oriens and stratum radiatum of CA1 pyramidal neurons after 20–30 min (Fig. 2). Interestingly, the ERβ-mediated spatial learning enhancements are not accompanied by rapid, large-scale changes in CA1 spine density. Although 17β-estradiol and PPT structurally increased the density of hippocampal spines, functionally the CA1 hippocampal excitatory input was decreased in those same neurons. After 15–20 min of application, 17β-estradiol and PPT attenuated both AMPA mEPSC frequency and AMPA induced membrane depolarization in CA1 pyramidal neurons (Figs. 3 and 4 and Figs. S5 and S6). Thus, surprisingly, estrogen-mediated increases in hippocampal spine density do not result in increased hippocampal excitatory input.
Estrogen-Induced Immature Synapses and Learning and Memory.
17β-Estradiol and ERα agonist PPT increased spine density while decreasing AMPAR responses. Dendritic spines with no or low numbers of synaptic AMPARs are silent or immature spines, and are considered “learning spines”; structures capable of long-term potentiation through the rapid insertion of AMPARs (14). Thus, they are thought to be the sites where new memories can be stored. In contrast, mature synapses or “memory spines” with high levels of AMPARs, are not capable of long-term potentiation (14), and are thought to be the sites where previously stored memories are maintained. 17β-Estradiol and ERα agonist PPT thus appear to induce the formation of learning spines in dorsal hippocampal CA1 pyramidal neurons, which likely have corresponding presynaptic inputs (6). Although the occurrence of silent/immature excitatory synapses is low in adult hippocampal and cortical neurons, they are found at high frequencies during development, correspond with the opening of critical periods, and are thought to allow a dramatic degree of plasticity for the activity dependent refinement of neural circuits (15). Thus, it seems as though estradiol, through ERα activation, rapidly promotes a hippocampal neuronal phenotype that is structurally and functionally similar to developmental neurons.
Based on our findings, we propose the following mechanism of action for estrogens’ rapid enhancement of hippocampal learning. During behavioral training, mice intrahippocampally treated with 17β-estradiol or PPT experience a rapid increase in hippocampal “silent/immature” synapses containing relatively few AMPARs. We speculate that upon neural activity induced by learning, a subset of these spines would be potentiated and stabilized through AMPAR insertion into the synapse (14, 21), followed by pruning of unused synapses. Behaviorally, this hypothesis could be tested using transgenic mice that allow the labeling of neurons involved in a specific learning event.
This proposed mechanism of estrogen-enhanced learning is supported by in vitro findings in cultured cortical neurons and the two-step estrogen plasticity model, which reported estrogen-treated neurons have an enhanced AMPA response following activation of NMDARs (6). Although in contrast to the present findings, estradiol-induction of dendritic spines in cortical neurons appears to be ERβ-dependent (22). We demonstrate for the first time (to our knowledge) that the rapid effects of estrogens, which include increased dendritic spines and AMPAR internalization in the hippocampus, occur in parallel with behavioral learning enhancements.
Different Effects of ERα and ERβ Activation.
We report different roles for ERα and ERβ in rapid estrogen-mediated learning enhancement. ERα activation replicated all of the effects of 17β-estradiol on learning, dendritic spines, and CA1 responses to AMPAR activation. ERβ activation however, did not increase spine density, nor did it affect CA1 pyramidal neuron responses to AMPA or NMDA, and enhanced only object placement learning. Our data suggest the existence of distinct nongenomic mechanisms of action for ERα and ERβ. Interestingly, we also recently demonstrated that the rapid learning and hippocampal dendritic spine effects of G protein-coupled estrogen receptor (GPER) activation are more comparable to those of ERα than ERβ (23).
In female rat hippocampal sections, ERβ activation has been shown to increase presynaptic vesicular glutamate release from the Schaffer collaterals onto CA1 neurons, whereas ERα or GPER activation had no effect (9). Hence the intrahippocampal delivery of ERβ agonist DPN may enhance object placement performance via presynaptic mechanisms. This finding would suggest that ERβ activation rapidly facilitates memory formation through increasing the activity-dependent stimulation received by CA1 pyramidal neurons that is required for synapse strengthening. Therefore, ERα and ERβ may rapidly affect hippocampal learning through separate pre- and postsynaptic mechanisms, respectively.
Estrogens Rapidly Affect CA1 AMPAR but Not NMDAR Responses.
Although 17β-estradiol and the ERα agonist PPT attenuated AMPAR responses, none of the estrogen treatments affected NMDAR-mediated membrane depolarizations. This finding is in agreement with experiments demonstrating rapid AMPAR cycling into and out of the synapse (within minutes), while NMDARs do not display the same rapid turnover (24). Because these changes occur after ∼15 min of estrogen application, they likely result from changes in cell-signaling cascades (25), rather than a direct effect of estrogens on AMPARs (Fig. S8). Our findings are consistent with our learning effects, and with other data demonstrating estrogens begin to affect sexual behavior and aggression ∼15 min after administration (26, 27).
In the CA3 region of the hippocampus, metabotropic glutamate receptor activation combined with GPER activation was recently shown to reduce AMPAR subunit GluA1 and induce long-term depression (18). Although there are differences in AMPAR plasticity between the CA3 and CA1 (18), similar mechanisms may underlie the effects we find with ERα in CA1.
AMPAR Internalization and Learning and Memory.
Curiously, our findings demonstrate that estrogens not only induce the formation of new CA1 hippocampal dendritic spines, but they also appear to cause the internalization of surface AMPAR at preexisting synapses. There are two, nonmutually exclusive reasons why this could lead to enhanced learning and memory. First, our data suggest a conversion of some mature contacts to immature or silent type synapses, promoting circuit rewiring that facilitates learning by providing increased synaptic sites for information storage. As a consequence, the rapid effects of estrogens via ERα may cause weakening of some previously established memories (28) and facilitate their overwriting or updating if activated by a stimulus or a learning event (29). Second, the decrease in AMPA responses we observe can lead to an increase in the signal-to-noise ratio that improves learning and memory. Noise within the nervous system presents challenges for information processing, and is suggested to be a major source of behavioral variability (30). Because the transmission of neuronal signals occurs nonlinearly, small fluctuations, such as spontaneous vesicular release, can significantly influence neuronal function (30, 31). As we have shown, estrogens rapidly decrease hippocampal mEPSC frequency, and thus may reduce hippocampal noise to aid information processing. This interpretation suggests that the rapid effects of estrogens on learning are not dependent on increases in spine density per se, and would be replicated by decreased spontaneous neurotransmitter release. We consider the former hypothesis more likely given the strong correlation between estrogen-induced spine changes and behavior (5), the importance of silent/immature synapses for plasticity and neural circuit establishment (15), and the ability of silent/immature spines to undergo synaptic potentiation to store and maintain memories (14, 21).
Conclusions
To our knowledge, these results demonstrate for the first time that estrogen action within the hippocampus rapidly improves general discrimination learning, which is associated with a decrease in AMPA-mediated CA1 pyramidal excitatory input, despite the addition of new dendritic spines. Our data support the idea that estrogens rapidly and nongenomically increase the formation of silent or immature synapses within the CA1 hippocampus, via ERα activation. The formation and existence of immature or silent synapses has been proposed to be important for the establishment and modification of neural circuits (15). We propose that rapid ERα-mediated increases in immature or silent synapses phenotypically resemble the structural and functional aspects of developmental hippocampal neurons, promoting plasticity and facilitating learning.
Methods
Subjects.
Young adult female CD1 mice (Mus musculus) were ovariectomized and implanted with bilateral cannulae in the anterior dorsal hippocampus for learning experiments. Pregnant female CD1 mice were purchased, and female pups 19- to 32-d-old were used for dendritic spine and electrophysiology experiments. Experiments were conducted in accordance with the Canadian Council on Animal Care, approved by University of Guelph's Animal Care and Use Committee. See SI Methods for details.
Dendritic Spine and Electrophysiology Analysis.
Electrophysiological recordings were obtained from CA1 pyramidal neurons, a subset of which were filled with biocytin, then visualized using DAB. See SI Methods for details.
SI Methods
Subjects.
Young adult female CD1 mice (Mus musculus) were ovariectomized, implanted with bilateral cannulae, and used for learning experiments (2.5-mo-old; Charles River). Pregnant female CD1 mice were purchased (14- to 16-d gestation; Charles River), and female pups 19- to 32-d-old were used for dendritic spine and electrophysiology experiments. ERα and ERβ mRNA has been detected in mice during this time (32). All animals used for these experiments were housed in the Central Animal Facility, University of Guelph, on a reversed light/dark cycle (12:12-h, lights on at 2,000 h) at 21 ± 1 °C. Mice were held in clear polyethylene cages (26 cm × 16 cm × 12 cm) with corncob bedding, environmental enrichment, and tap water and rodent chow ad libitum (14% Protein Rodent Maintenance Diet or 18% Rodent Diet for pregnant females, Harlan Teklad). Litters of pups remained with their mother until being killed for electrophysiology experiments. Mice tested in learning paradigms were individually housed after surgery. Cages were not changed for at least 3 d before learning experiments (for establishment of a home cage territory), whereas ovariectomized stimulus mice (used for social recognition experiments) were group housed. The evening before behavioral testing, mice were moved into the testing room to acclimate. All test mice were used only in one learning experiment. Experiments were conducted in accordance with the Canadian Council on Animal Care, approved by University of Guelph’s Animal Care and Use Committee.
Ovariectomy and Cannulation Implantation Surgeries.
Adult mice were ovariectomized as previously described (2). Briefly, mice were anesthetized (isoflurane), placed in a stereotaxic frame using atraumatic ear bars (David Kopf instruments), subcutaneously injected with an analgesic and anti-inflammatory (carprofen 50 mg/kg; Rimadyl, Pfizer Canada). Ovaries were removed from the dorsal side, local anesthetic was dripped onto the incision (mixture of bupivacaine 0.17%; Hospira; and lidocaine 0.67%; Alveda Pharmaceuticals), then closed with a surgical clip (MikRon Autoclip 9-mm wound clips, MikRon Precision). Immediately following the ovariectomy, the skin on the dorsal surface of the mouse skull was excised, and local anesthetic was dripped onto the incision site, as described above. Two holes were drilled for 26-G bilateral guide cannulae aimed at the anterior dorsal hippocampus (Plastics One, HRS Scientific), at stereotaxic coordinates 1.7-mm posterior to bregma and 1.5-mm lateral to midline, 1.3 mm below skull surface. Injectors extended 1 mm beyond the end of the guide cannula, for a final dorsal-ventral position of 2.3 mm below the skull surface. Three holes were drilled into the skull, into which three jeweler’s screws were placed. Dental cement (Central Dental) was used to form a headcap around the screws and guide cannulae. Mice received an intraperitoneal injection of 0.5 mL of saline for rehydration postsurgery. Learning experiments were performed 10–15 d after surgery.
After behavioral testing, mice were intrahippocampally infused with Chicago blue dye (1% in PBS) to check cannula placements. Whole-brain tissue was extracted 40 min after dye infusion and fixed in 4% (wt/vol) paraformaldehyde for 2 wk, placed in 30% (wt/vol) sucrose PBS for 3–5 d, frozen at −80 °C, then sectioned coronally at 30-μm thickness using a cryostat (Leica CM 1850, Leica Microsystems). Site of injectors in the hippocampus for all experiments have been provided in Fig. S4. Estradiol appears to remain localized to sites of microinfusion (33). Animals with off target cannula were excluded from analysis (one mouse). Vaginal smears were taken and stained with Giemsa. Cell morphology was examined to ensure effectiveness of ovariectomy, as previously described (2, 3).
Rapid Learning Paradigms.
Each experiment and dose group consisted of a unique set of experimentally naïve mice. All drugs were purchased from Sigma-Aldrich unless otherwise stated. Animals were gently restrained by hand and microinfused with 17β-estradiol (25, 50, or 100 nM), PPT (50, 100, or 150 nM), DPN (50, 100, or 150 nM) or aCSF vehicle (126 mM NaCl, 5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM d-glucose, 1.25 mM NaH2PO4, 26 mM NaHCO3, 0.02% ethanol). 17β-Estradiol doses were tested during object placement pilot studies and chosen to replicate our systemic effects with 17β-estradiol (2). PPT and DPN doses were then chosen to account for ∼50% binding affinity to estrogen receptors compared with 17β-estradiol (16, 17). For behavioral paradigms, 0.5 μL was infused per hemisphere at a rate of 0.2 μL per minute using a microinfusion pump (PHD 2000, Harvard Apparatus), and injectors were left in for 1 min after infusions before removal. Mice received drug infusions 15 min before testing (Fig. 1A). Each learning paradigm was completed within 40 min of drug administration, thus targeting the rapid effects of 17β-estradiol, PPT, and DPN. Learning paradigms consisted of two habituation sessions and a test, each 5 min in duration, separated by 5-min intervals (3). The learning paradigms were designed so that vehicle controls do not demonstrate learning, to test for improving effects of treatments. When given greater numbers of habituations, vehicle-treated ovariectomized control mice can perform these tasks (3).
All behavioral paradigms were conducted in home cage under red light, during the dark phase of the light cycle. Habituation and test sessions were recorded under infrared light (8 mm Handycam Nightshot, Sony) for ethological analysis. During all intertest intervals, objects and the cylinders used to present stimulus mice (described below) were washed to remove odor cues using an odorless detergent and baking soda. Objects were held in place using Velcro, and were previously tested to ensure mice did not have an initial preference for any one object. Objects consisted of a glass cube, stainless steel drain catcher, and plastic hairclip.
Ten behaviors listed in Table S1 were collected from each learning experiment using The Observer Video Analysis software (Noldus Information Technology) by one of five observers blind to drug treatment. Active sniffing of stimuli (nose twitching within ∼1–2 mm of the stimulus) was considered investigation behavior. Mice naturally investigate (sniff) novel or displaced stimuli more than familiar ones. Therefore, we calculated percent investigation = N/(N+F) × 100, where N is the time spent investigating a novel or displaced stimulus (or during habituations, the stimulus that will be replaced/displaced) and F is the time spent investigating the familiar stimulus. Investigation percent during habituations is ∼50% (chance); if the experimental mice recognize the novel or displaced stimulus during test, investigation percent is statistically greater than at habituation (2, 3). Animals with total investigation durations of less than 5 s during test (4% of animals) as well as outliers (>2 SDs ± mean; 2% of animals) were excluded.
Object placement learning paradigm.
Two identical objects were placed in two consistent locations against one side of the cage during habituations. During the test, one of the objects was moved to a novel location on the opposite side of the cage (Fig. 1B, Top). The object moved was counterbalanced.
Object recognition learning paradigm.
Two different objects were presented to the test mouse during habituations, and one of the two objects was replaced with a novel third object during test (Fig. 1B, Middle). The object that was replaced was counterbalanced and the two positions of the objects remained consistent throughout the paradigm.
Social recognition learning paradigm.
Group-housed 2.5- to 4-mo-old ovariectomized mice were randomly chosen as stimulus animals for social recognition experiments. They were presented to experimental mice in clear Plexiglas cylinders with perforations at the bottom (to which stimulus animals had previously been habituated), allowing passage of olfactory cues. During habituations, a test mouse was presented with the same two stimulus mice (e.g., A and B) in consistent positions. During the test, one of the two stimulus mice was replaced with a novel mouse (e.g., A and C) (Fig. 1B, Bottom). The individual replaced was counterbalanced.
Slice Preparation.
Female mice were anesthetized with isoflurane, decapitated, and brain tissue was extracted in oxygenated (95% O2, 5% CO2) ice-cold sucrose aCSF (342 mM sucrose, 180 mM d-glucose, 84 mM NaHCO3, 111 mM CaCl2, 120 mM MgSO4, 75 mM KCl, 120 mM NaH2PO4), where they were coronally sectioned using a vibrating microtome (Leica VT1000s, Leica Microsystems) at 200- to 300-μm thickness. Sections of anterior dorsal hippocampus were placed in oxygenated aCSF (58 mM NaCl, 180 mM d-glucose, 84 mM NaHCO3, 111 mM CaCl2, 120 mM MgSO4, 75 mM KCl, 120 mM NaH2PO4) at 32 °C to recover for at least 1 h before whole-cell patch-clamp recording. This region of the hippocampus coincided with cannula placements in the learning experiments.
Dendritic Spine Analysis.
Borosilicate glass recording electrodes (4–7 MΩ) were pulled using a micropipette puller (P-97, Sutter Instrument Company) and filled with intracellular solution (140 mM K-gluconate, 5 mM EGTA, 2 mM MgCl2, 1 mM CaCl2, 0.6 mM NaHCO3, 10 mM Hepes, 2 mM Mg-ATP, 2 mM Na2-ATP, 0.3 mM Na-GTP, 8.3 mM sucrose, 1% biocytin). CA1 hippocampal neurons were visualized using an infrared filter (Carl Zeiss) and patched using a Multiclamp 700B amplifier and Clampex software (v10, Molecular Devices). Electrophysiological recordings were obtained from these neurons (details below), then sections were added to 4% (wt/vol) paraformaldehyde for 1–3 wk. In some neurons, a 15- to 20-min aCSF wash was applied after hormone or vehicle treatment to obtain a recovery measure. For these neurons, hormone or vehicle was reapplied for 15–20 min before sections were taken for staining. Hippocampal sections were incubated at room temperature in Vectastain Elite ABC kit at a dilution of 1:400 (Vector Labs) with 0.3% TritonX for 24 h, then stained with diaminobenzidine tetrahydrochloride (DAB-4HCl; Polysciences) with 0.001% H2O2 for 15 min. Images were taken from one to two dendrites each in the stratum oriens, stratum radiatum, and stratum lacunosum-moleculare per neuron (Fig. 2A) (63× oil objective, Axio Imager D1, captured with AxioCam MRc5 digital camera, Carl Zeiss). Sampling region was from secondary dendrite ∼30–50% (radiatum) and 80–100% (lacunosum-moleculare) the length of the apical dendrite, as well as 40–60% the length of basal dendrite (oriens). Measures were taken from >10-μm length of the dendrite, averaged per subregion. Numbers per group are depicted in graphs (Fig. 2). Spine analyses were completed by one of two observers blind to treatment.
Whole-Cell Patch-Clamp Recordings.
Recordings of current (voltage-clamp mode) and membrane potential (current-clamp mode) were taken from separate hippocampal CA1 pyramidal neurons (one neuron per brain section) (Fig. 3A). All recordings were obtained at room temperature. A gravity-driven flow rate of 1.5–2 mL/min was used during recordings. mEPSC recordings were done in the presence of TTX (1 μM) and picrotoxin (100 μM), and voltage was clamped at −70mV. Five to 10 min after obtaining a patch, 5 min of mEPSCs were recorded (baseline measure) (Fig. 3B). For membrane depolarization experiments, 5–10 min after patching, 0.2–0.75 μM of (S)-AMPA or 10–15 μM of NMDA (Tocris Bioscience) was bath-applied for 15 s to 1.5 min to elicit, on average, 6–7 mV (AMPA) or 5 mV (NMDA) amplitude depolarization (baseline measure) (Fig. 4 A and D). Depolarizations were maintained at subthreshold levels. Following this baseline measure, 17β-estradiol (25, 50, or 100 nM), ERα agonist PPT (50, 100, or 150 nM), ERβ agonist DPN (50, 100, or 150 nM), or vehicle (aCSF+0.02% ethanol) was bath-applied for 15 min. Another 5 min of mEPSCs were recorded, or for membrane depolarizations (S)-AMPA or NMDA was reapplied, in the presence of 17β-estradiol, PPT, DPN, or vehicle control. For membrane potential recordings, a third measure for AMPA or NMDA depolarization was taken after 10–15 min of washout with aCSF (recovery measure). mEPSC recordings were analyzed using MiniAnalysis software (Synaptosoft); membrane depolarization amplitudes were analyzed using Clampfit (Molecular Devices). Neurons that sensitized or desensitized (increasing or decreasing responses obvious to the naked eye) to repeated applications of either AMPA or NMDA in the presence of aCSF during current-clamp recordings were excluded from the data set (12% of neurons).
Data Analysis.
For behavioral experiments, specific numbers of mice used for learning paradigms are detailed in bars and figure legends for investigation durations (Fig. S1, line graphs). Two-way repeated measures ANOVAs were used to analyze behavioral data, with factors habituations and test (repeated measure) and treatment (between groups). Investigation percent for statistical analysis was expressed as a ratio and were arcsin-transformed. Preference scores for habituations 1 and 2 were averaged. To reduce type I errors, specific a priori binary mean comparisons were planned in the statistical model to assess changes in preference score at test (the experimental condition). Specifically, within the ANOVA model paired t tests were used to assess differences in preference scores between habituation and test within each treatment group, and one-way ANOVAs and Student-Neuman–Keuls post hocs were used to assess differences in preference scores at test between doses. Nonsignificant results are not reported unless otherwise meaningful.
For dendritic spine and electrophysiology experiments, one-way ANOVAs and Student-Neuman–Keuls post hocs, or ANOVA on Ranks with Dunn’s post hocs were used for analysis (as appropriate). Specific numbers of neurons in each experiment are indicated on the bar graphs (Figs. 2–4).
Acknowledgments
Thanks to Lauren Hishon, Jasmine Aulak, Enaam Chleilat, Angela Meersseman, and Paul A. S. Sheppard for their help in behavioral data collection and brain tissue sectioning. This work was supported in part by Natural Sciences and Engineering Research Council Grant 400212 (to E.C.) and Canada Foundation for Innovation Infrastructure Grant 046468 (to E.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1522150112/-/DCSupplemental.
References
- 1.MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146(1):287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- 2.Phan A, et al. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37(10):2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152(4):1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- 4.Murakami G, et al. Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun. 2006;351(2):553–558. doi: 10.1016/j.bbrc.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 5.Spencer JL, et al. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29(2):219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava DP, et al. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA. 2008;105(38):14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10(12):4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scharfman HE, Mercurio TC, Goodman JH, Wilson MA, MacLusky NJ. Hippocampal excitability increases during the estrous cycle in the rat: A potential role for brain-derived neurotrophic factor. J Neurosci. 2003;23(37):11641–11652. doi: 10.1523/JNEUROSCI.23-37-11641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30(48):16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harte-Hargrove LC, Maclusky NJ, Scharfman HE. Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: Implications for normal brain function and disease. Neuroscience. 2013;239:46–66. doi: 10.1016/j.neuroscience.2012.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attardo A, Fitzgerald JE, Schnitzer MJ. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 2015;523(7562):592–596. doi: 10.1038/nature14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendez P, Garcia-Segura LM, Muller D. Estradiol promotes spine growth and synapse formation without affecting pre-established networks. Hippocampus. 2011;21(12):1263–1267. doi: 10.1002/hipo.20875. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429(6993):761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanse E, Seth H, Riebe I. AMPA-silent synapses in brain development and pathology. Nat Rev Neurosci. 2013;14(12):839–850. doi: 10.1038/nrn3642. [DOI] [PubMed] [Google Scholar]
- 16.Stauffer SR, et al. Pyrazole ligands: Structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43(26):4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 17.Meyers MJ, et al. Estrogen receptor-beta potency-selective ligands: Structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 18.Briz V, Liu Y, Zhu G, Bi X, Baudry M. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release. J Cell Biol. 2015;210(7):1225–1237. doi: 10.1083/jcb.201504092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26(33):8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122(3):716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462(7275):920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava DP, Woolfrey KM, Liu F, Brandon NJ, Penzes P. Estrogen receptor β activity modulates synaptic signaling and structure. J Neurosci. 2010;30(40):13454–13460. doi: 10.1523/JNEUROSCI.3264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabor C, Lymer J, Phan A, Choleris E. Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol Behav. 2015;149:53–60. doi: 10.1016/j.physbeh.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Lüscher C, et al. Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron. 1999;24(3):649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 25.Sellers K, Raval P, Srivastava DP. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front Neuroendocrinol. 2015;36:72–89. doi: 10.1016/j.yfrne.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behav Brain Res. 2006;166(1):110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Trainor BC, Finy MS, Nelson RJ. Rapid effects of estradiol on male aggression depend on photoperiod in reproductively non-responsive mice. Horm Behav. 2008;53(1):192–199. doi: 10.1016/j.yhbeh.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, et al. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci USA. 2007;104(52):20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong Z, et al. Hippocampal long-term depression mediates spatial reversal learning in the Morris water maze. Neuropharmacology. 2013;64:65–73. doi: 10.1016/j.neuropharm.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Faisal AA, Selen LP, Wolpert DM. Noise in the nervous system. Nat Rev Neurosci. 2008;9(4):292–303. doi: 10.1038/nrn2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fatt P, Katz B. Some observations on biological noise. Nature. 1950;166(4223):597–598. doi: 10.1038/166597a0. [DOI] [PubMed] [Google Scholar]
- 32.Sugiyama N, et al. Spatiotemporal dynamics of the expression of estrogen receptors in the postnatal mouse brain. Mol Psychiatry. 2009;14(2):223–232, 117. doi: 10.1038/mp.2008.118. [DOI] [PubMed] [Google Scholar]
- 33.Zurkovsky L, Brown SL, Boyd SE, Fell JA, Korol DL. Estrogen modulates learning in female rats by acting directly at distinct memory systems. Neuroscience. 2007;144(1):26–37. doi: 10.1016/j.neuroscience.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G, Franklin KBJ, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2nd Ed Academic; San Diego: 2001. [Google Scholar]