Abstract
Angiogenesis is a key feature of central nervous system injury. A neovessel-derived signal mediated by prostacyclin triggers axonal sprouting and functional recovery in a mouse model of inflammatory spinal cord injury (pages 1658–1664). Are such angiocrine signals relevant to neurovascular remodeling and recovery in other neurological contexts?
Acute central nervous system (CNS) injury induces damage but also a process of delayed tissue repair. Neural repair involves remodeling the boundaries of the lesion, forming new blood vessels, elaborating new neuronal connections and generating new populations of neurons and glia. The formation of new blood vessels through sprouting from preexisting vessels1, a phenomenon called angiogenesis, occurs in stroke, traumatic brain and spinal cord injury, and multiple sclerosis during the repair phase of the disease process2,3. Angiogenesis has been correlated with improved functional outcome in both animal models and human stroke2; however, causative roles for angiogenesis in neural repair and recovery after CNS injury remain poorly defined.
Neovessels are formed in perilesional tissue in both inflammatory and traumatic spinal cord injury3,4. In this issue of Nature Medicine, Muramatsu et al.5 use a highly targeted inflammatory injury to the thoracic spinal cord that damages descending corticospinal tract (CST) projections from the brain to model some aspects of human multiple sclerosis. The neovessels formed around the injured tissue secrete a factor called prostacyclin that binds damaged CST neurons, enhancing axonal growth and motor recovery in mice with spinal cord injury. The findings unveil a link between angiogenesis and axonal sprouting and identify a potential pathway to target in CNS disease.
A previous study using a similar model of spinal cord injury has shown that these CST axons sprout in response to injury to form new connections with propriospinal or intersegmental circuits, the local projections within the spinal cord that can bypass the lesion6. Although the formation of these new CST connections mediated elements of functional recovery6,7, what initiated their formation and how they are developed in topographic relationship to the injury and to the cell bodies of the intersegmental neurons are still unclear.
The link between angiogenesis and axonal growth has been well documented in the peripheral nervous system (PNS)—common signaling systems mediate molecular interactions between the developing vasculature and the PNS1. In ischemic stroke or in brain or spinal cord trauma, angiogenesis occurs too late to facilitate recovery through an effect on blood flow. During tissue remodeling after stroke, however, neuronal dendrites reshape along vascular profiles8, and in the case of spinal cord injury, axonal sprouting occurs in close association to blood vessels4,9,10. Certain drugs and cell-based therapies such as statins, a modified form of activated protein C and progenitor cell transplants stimulate both angiogenesis and axonal sprouting11,12. These data have established the physical proximity of neuronal processes and axonal sprouting with blood vessels in the regenerating CNS but have not revealed the vessel-derived molecular signals that mediate their interaction.
Muramatsu et al.5 showed that new blood vessels form in the spinal cord proximal to the injury site and the descending projections from the motor cortex sprout collaterals in this same region in injured mice. In the circuitry of the injured spinal cord, amounts of PGI2 (prostaglan-din I2 or prostacyclin) synthase (PGIS), the final enzyme in prostacyclin production, and of the prostacyclin metabolite 6-keto-PGF1α are elevated during the peak of post-injury angiogenesis. The prostacyclin receptor, IP receptor, which was expressed in motor cortical neurons, was involved in functional recovery, as siRNA knock-down of this receptor well away from the site of injury blocked axonal sprouting and delayed motor recovery5. Selective knockdown of PGIS in the blood vessels of the injured spinal cord also had the same blocking effect. Direct delivery of IP receptor agonists such as iloprost promoted axonal sprouting, enhanced axonal signaling through the lesion site and improved functional recovery, whereas delivery of antagonists such as PGIS or siRNA-mediated knockdown of IP receptor blocked these processes. Interestingly, deletion of PGIS in blood vessels near the injury site did not block angiogenesis, suggesting that angiogenesis and neovessel formation per se are not responsible for axonal sprouting and recovery; instead, the eicosanoid signaling pathway through prostacyclin from these vessels to nearby axons is the key element.
The evidence begins to describe the molecular links between angiogenesis, axonal sprouting and CNS recovery from injury; however, there are also important limitations. In development and in preclinical studies of disease models of axonal sprouting, axons and blood vessels are physically associated. Yet no such physical link was demonstrated by Muramatsu et al.5. Is this vessel-derived prostacyclin signal trophic or tropic? Prostacyclin activation of its IP receptor on neurons elevates intracellular cyclic AMP levels, which in most axonal outgrowth systems promotes axonal sprouting as well as directional growth cone movement toward the source of the molecule that promotes elevated cyclic AMP12. It is surprising that vessel-derived prostacyclin, as a local signal for cyclic AMP induction in a growing axon, does not localize this axon to the source (along or in close proximity to the neovessels). The targeted inflammatory injury model used by Muramatsu et al.5 has the advantage of providing for focal delivery of experimental manipulations. However, it is further afield from the human multiple sclerosis condition and involves a complicated delivery of three inflammatory stimuli (systemic myelin basic protein in Freund’s adjuvant, focal tumor necrosis factor-α and γ-interferon, and systemic delivery of pertussis toxin). For clinical relevance, it will be important to confirm that this prostacyclin signaling system is active in other multiple sclerosis models, such as the traditional experimental autoimmune encephalitis models. If the finding that neovessel-derived prostacyclin induces local axonal sprouting and functional recovery in other CNS injury and regeneration models, then this might be clinically targeted with focal delivery of prostacyclin agonists into recovering tissue.
Tissue repair after acute CNS injury occurs in a unique cellular environment adjacent to the injury site that involves cellular events not normally active in most brain areas— angiogenesis is linked to neurogenesis in an area of active axonal sprouting and tissue remodeling. The new study from Muramatsu et al.5 adds to a body of evidence demonstrating a unique regenerative neurovascular niche in perilesional tissue that mediates many processes in recovery of function (Fig. 1). For example, angiogenic blood vessels signal to newly born neurons after stroke to localize to peri-infarct tissue13. In this neurovascular niche after stroke, neurogenesis is causally regulated by angiogenesis and dependent on angiogenic vessel–derived, or angiocrine, factors13. As with the relationship of axons and blood vessels in the developing PNS, common molecular factors—ephrins, vascular endothelial growth factor, semaphorins and others—participate in axon and vascular signaling in the regenerative neurovascular niche14. Common cellular signaling events, or molecular control points, crucial for the regenerative neurovascular niche may therefore be identified that can serve as targets to promote tissue repair at multiple levels after acute CNS injury.
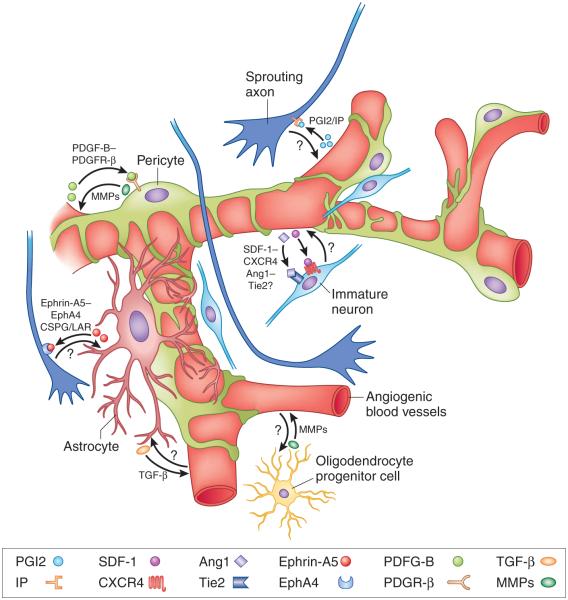
Figure 1.
The regenerative neurovascular niche. Acute CNS injury induces new blood vessels (angiogenesis), new neurons (neurogenesis) and new connections (axonal sprouting). These regenerative processes occur in tissue adjacent to the primary injury site in perilesional tissue. The cellular elements of this regenerative environment are the angiogenic blood vessels, pericytes, astrocytes, immature neurons and sprouting axons. Muramatsu et al.5 show in a mouse spinal cord injury model that prostacyclin is secreted by angiogenic blood vessel endothelial cells and signals through its IP receptor on corticospinal tract neurons to induce axonal sprouting into perilesional tissue. Other studies have identified molecular signals that communicate among the other cellular elements of the regenerative neurovascular niche. Angiocrine factors, such as stromal-derived factor 1 (SDF-1) and angiopoietin 1 (Ang1) from angiogenic blood vessels attract migrating immature neurons that express cognate receptors, such as CXCR4 and Tie2, respectively; the localization of these into injured tissue is associated with recovery13. Platelet-derived growth factor B (PDGF-B) secreted by endothelial cells is trophic for pericytes that express its cognate receptor, platelet-derived growth factor receptor β (PDGFR-β). PDGFR-β that is expressed by neurons and astrocytes within this niche mediates neuroprotection and glial scar formation after injury. Astrocyte-derived ephrin-A5 (a vascular signaling molecule) binds EphA4 receptor and blocks growth of sprouting axons. Reactive astrocytes alter endothelial cell production of tissue plasminogen factor via transforming growth factor β (TGF-β). Reactive astrocytes produce large amounts of chondroitin sulfate proteoglycans (CSPGs), which form a strong barrier for regenerating axons that express CSPG receptors, such as the leukocyte common antigen-related phosphatase receptor (LAR). Both pericytes and oligodendrocyte precursor cells are a source of matrix metalloproteinases (MMPs), which regulate blood vessel remodeling.
Well outside of their traditional conception as passive blood flow conduits, angiogenic blood vessels are a rich tissue source for secreted and membrane-bound signaling molecules that have been shown to form instructive vascular niches in cancer and peripheral injury environments. The paracrine signaling role described by Muramatsu et al.5 for spinal cord endothelial cells may be part of an emerging wavefront in the study of angiogenesis and neural repair in acute CNS injury.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Andrew J Brumm, Department of Neurology, Geffen School of Medicine at the University of California–Los Angeles, Los Angeles, California, USA.
S Thomas Carmichael, Department of Neurology, Geffen School of Medicine at the University of California–Los Angeles, Los Angeles, California, USA, scarmichael@mednet.ucla.edu.
References
- 1.Quaegebeur A, Lange C, Carmeliet P. Neuron. 2011;71:406–424. doi: 10.1016/j.neuron.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Ergul A, Alhusban A, Fagan SC. Stroke. 2012;43:2270–2274. doi: 10.1161/STROKEAHA.111.642710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oudega M. Cell Tissue Res. 2012;349:269–288. doi: 10.1007/s00441-012-1440-6. [DOI] [PubMed] [Google Scholar]
- 4.Loy DN, et al. J. Comp. Neurol. 2002;445:308–324. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- 5.Muramatsu R, et al. Nat. Med. 2012;18:1658–1664. doi: 10.1038/nm.2943. [DOI] [PubMed] [Google Scholar]
- 6.Kerschensteiner M, et al. J. Exp. Med. 2004;200:1027–1038. doi: 10.1084/jem.20040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bareyre FM, et al. Nat. Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 8.Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. J. Neurosci. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dray C, Rougon G, Debarbieux F. Proc. Natl. Acad. Sci. USA. 2009;106:9459–9464. doi: 10.1073/pnas.0900222106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, et al. Ann. Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 11.Thiyagarajan M, Fernández JA, Lane SM, Griffin JH, Zlokovic BV. J. Neurosci. 2008;28:12788–12797. doi: 10.1523/JNEUROSCI.3485-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng JQ, Poo MM. Annu. Rev. Cell Dev. Biol. 2007;23:375–404. doi: 10.1146/annurev.cellbio.23.090506.123221. [DOI] [PubMed] [Google Scholar]
- 13.Ohab JJ, Fleming S, Blesch A, Carmichael ST. J. Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overman JJ, et al. Proc. Natl. Acad. Sci. USA. 2012;109:E2230–E2239. doi: 10.1073/pnas.1204386109. [DOI] [PMC free article] [PubMed] [Google Scholar]