Abstract
Desmoplastic melanoma (DM) is a variant of spindle cell melanoma typically found on chronically sun-damaged skin of older individuals. Early diagnosis can be challenging because it is often amelanotic and has a predominantly dermal component. DM can be difficult to diagnose not only clinically but also histologically, and can be mistaken for a variety of benign and malignant non-melanocytic spindle cell tumors when viewed on prepared histopathology slides. Pathologists have observed that DMs can manifest significant variation with respect to the extent of intratumoral cellularity, fibrosis and/or perineural invasion. Furthermore, some tumors present with a pure desmoplastic invasive component (>90%) while other tumors display mixed features of desmoplastic and non-desmoplastic melanoma. This has lead to the separation of DM into two histologic subtypes, pure and mixed. With a focus on the distinction between pure and mixed DM, this review will detail what is currently known about the diagnostic features of DM, discuss risk and prognostic factors and examine the current literature on disease progression and management.
INTRODUCTION
Desmoplastic melanoma (DM) is a relatively uncommon variant of melanoma, accounting for less than 4% of primary cutaneous melanomas.1 The overall incidence rate is 2.0 per million with annual percentage increase of 4.6% per year.2 Its clinical behavior differs from other subtypes of melanoma, by having a higher tendency for persistent local growth and less frequent nodal metastases. DM can arise de novo or in association with other melanoma subtypes, most often of the lentigo maligna type. Based on clinical behavior and histological presentation, DM has been proposed as a sarcomatoid variant of melanoma.3-7 The term DM initially referred to the association of invasive tumor cells with abundant stromal collagen but has been further classified into two histopathological subtypes: pure DM (pDM) and mixed DM (mDM) based on the degree of desmoplasia present in the tumor.8 Patients with pDM have less frequent lymph node involvement and tend to have a less aggressive clinical course than patients with mDM.
RISK FACTORS
The male to female ratio for DM is approximately 2:1 and the mean age at diagnosis is 66 years.2 In contrast, non-desmoplastic melanoma has a male to female ratio of 1.3:1 and the median age at diagnosis is 60 years.9, 10 Chronic UV exposure has been linked to DM and this may account for distribution pattern with approximately half of DM found on the head and neck (51%), extremities (30%), and trunk (17%).2 In contrast to non-desmoplastic melanomas, the association with other risk factors such as intermittent UV exposure, dysplastic nevus syndrome, and family history of melanoma has not been fully elucidated for DM.
DIAGNOSTIC CONSIDERATIONS
Clinical and Dermoscopic Features
The diagnosis of DM is challenging since its clinical presentation is often non-specific. As such, the differential diagnosis is broad and includes: scar, dermatofibroma, neurofibroma or malignant lesions such as basal cell carcinoma, squamous cell carcinoma and amelanotic melanoma.11 Clinically, DM often presents as amelanotic nodules or plaques, or as ill-defined scar-like lesions (Figures 1-2). Acral and mucosal DMs have also been reported.12-14 Given its association with lentigo maligna melanoma (LMM), it is advisable to palpate the skin suspected of LMM to detect any firm subcutaneous nodule that may point to DM. The clinical features according to the histopathological subtype have not been well-described. From our observations, we speculate that mDM may be easier to recognize clinically, since this subtype is more often associated with LMM or superficial spreading melanoma (SSM).15, 16 In other words, the recognition of a LMM or SSM component is what leads to biopsy. In contrast, pDM may be relatively inconspicuous since these tend to appear as dermal nodules or plaques that usually lack an epidermal component and pigmentation.
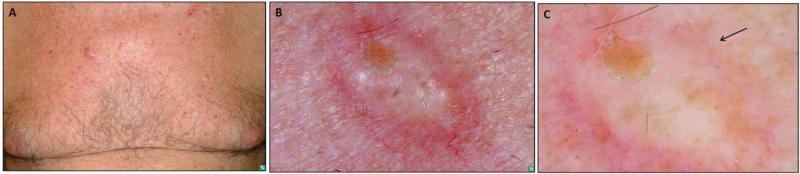
Figure 1.
Clinical and dermoscopic features of desmoplastic melanoma, pure type. (A) overview (B) close-up shows a firm cystic to scar-like nodule on the chest of 67 year-old male with a history of multiple primary melanomas. Biopsy revealed a 6.1mm DM, pure subtype, Clark's level V, Mitotic index 1, with perineural invasion. (C) Under dermoscopy, atypical dotted vessels were observed (arrow)
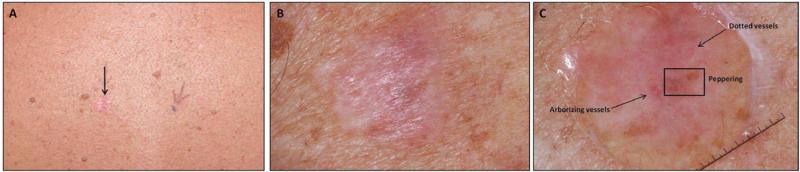
Figure 2.
Clinical and dermoscopic features of desmoplastic melanoma, mixed type. (A) overview (B) close-up shows an erythematous plaque on the mid-back of 71 year old male (arrow). This proved to be a 0.95mm superficial spreading melanoma with focal invasive desmoplastic melanoma, mixed subtype. (C) Under dermoscopy, regression structures, vascular blush and polymorphous vessels were observed.
Dermoscopy is a useful aid that improves diagnostic accuracy for the non-desmoplastic types of melanomas.17 However, its utility to identify DM remains to be proven. Due to the clinical subtlety of DM, there is limited information about its dermoscopic characteristics. Debarbieux and colleagues examined the dermoscopic presentation of six patients with DM.18 Five had an associated non-desmoplastic melanoma subtype (2 SSM, 2 LMM, 1 acral lentiginous melanoma), but only three revealed one or more melanocytic structures (i.e. pigment network, aggregated globules and streaks). For hypopigmented or amelanotic lesions, the authors concluded that the presence of white scar-like structureless areas and abnormal vascular patterns, such as linear-irregular vessels (also known as serpentine vessels) and milky-red areas were the only potential visual clues.
We analyzed the clinical and dermoscopic characteristics of 37 cases of DM from eight high-risk clinics (submitted): 43% of the lesions revealed melanocytic structures, including atypical globules, atypical pigment network and features associated with LMM such as polygonal lines and asymmetric annular granular pattern. All of the lesions revealed at least one melanoma-specific structure, particularly atypical vascular structures and regression structures such as peppering.
Histopathologic Features
Under the light microscope, DM is characterized by the association of invasive melanoma with abundant collagenous matrix giving the tumor silhouette a pink scar-like appearance on routine hematoxylin and eosin-stained sections (Figure 3-4). The extent of desmoplasia in an invasive melanoma required for a diagnosis of DM varies considerably in the literature. The morphologic heterogeneity of DM prompted a new histologic classification by Busam et al.8 pDM is typically pauci-cellular, with individual tumor cells dispersed in and separated from each other by fibrous tissue (Figure 3). Desmoplasia should be prominent throughout the vast majority of the tumor. In contrast, mDM has higher cell density than pDM, which may be throughout the entire tumor or manifest as nodule(s) of solid spindle and/or epithelioid melanocytes in a background of otherwise classic pauci-cellular DM (Figure 4). pDM or mDM may or may not be associated with in situ melanoma in the epidermis and/or follicular epithelium.
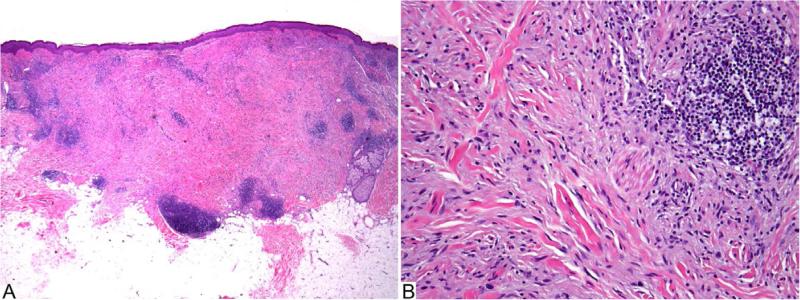
Figure 3.
Desmoplastic melanoma, pure type (H&E). A: Amelanotic spindle cell melanoma is associated with collagenous stroma and lymphocytic aggregates. B: Individual hyperchromatic fusiform melanocytes are dispersed in a fibrous matrix.
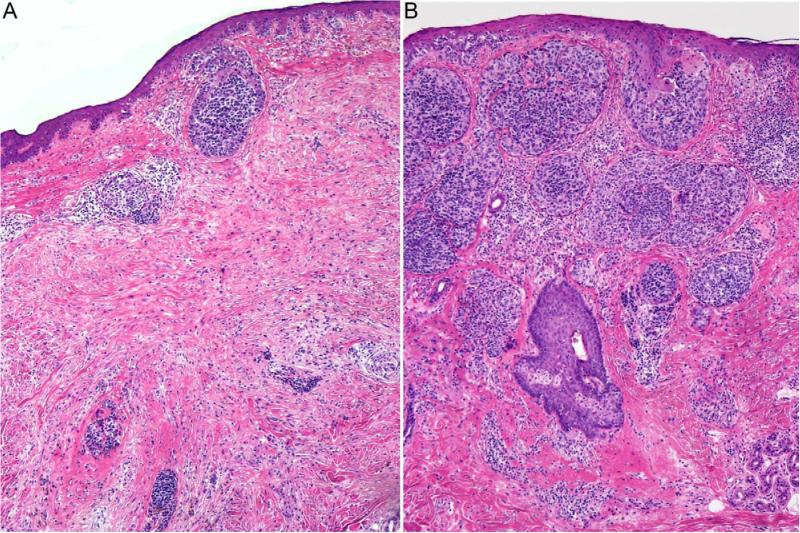
Figure 4.
Desmoplastic melanoma, mixed type (H&E). A: One part of the invasive melanoma displays a fibrosing spindle cell component. B: Another part of the melanoma is composed of densely cellular aggregates of epithelioid tumor cells.
Nerve involvement by DM is common and most often associated with deeply infiltrating tumors of the head and neck region. The extent of nerve involvement may vary from focal perineural invasion to extensive neurotropism. The latter term is used when melanoma cells are found around or within small nerve bundles peripheral and/or deep to the main tumor mass. In a two-dimensional view of a cross sectional profile, there may be skip areas of several millimeters separating peri- or intraneural tumor cells from the main tumor, which poses a challenge for margin assessment and control, and tumors may recur from such foci. In a large meta-analysis, the presence of neurotropism ranged between 16.7%-77.8% and amelanosis ranged from 46.2%-93%.19
In approximately one third of DM, there is no identifiable in situ melanoma and recognition of an amelanotic dermal spindle cell tumor as melanoma can be difficult. The cytologic features of DM can be deceptively bland and lead to confusion with benign entities.7, 20 Careful attention to the presence of focal hyperchromatic spindle cells and possible associated lymphocytic aggregates may provide clues for a possible diagnosis of DM and prompt the use of ancillary studies such as immunostaining.11
Immunohistochemical studies are often needed for the optimal assessment of tumor thickness of a DM and for its distinction from non-melanocytic simulants (Table 1).21, 22 The most valuable marker for the diagnosis of DM is S100 protein; antibodies nerve growth factor receptor and Sox 10 might also be helpful. Caution is warranted when using immunohistochemistry in the detection of a possible residual DM component within a scar: typically, scars contain only scattered isolated S-100-positive cells whereas DM is typically strongly and diffusely positive for S-100 protein.11 Furthermore, S-100 protein does not distinguish DM from tumors of Schwann cells (Schwannoma, neurofibroma and malignant peripheral nerve sheath tumor).
Table 1.
Distinction of desmoplastic melanoma from non-melanocytic mimickers by immunohistochemical stains
| Immunohistochemical stain | Desmoplastic melanoma | Spindle cell carcinoma | Sarcoma |
|---|---|---|---|
| S100 protein | Diffusely positive | Usually negativea | Usually negativea |
| Melanocyte Differentiation antigens (gp100, Melan-A/Mart-1, tyrosinase, MITF) | May be focally positive | Negative | Negative |
| Muscle markers (SMA, Desmin) | Usually negative; may be positiveb | Usually negative | May be positivec |
| Epithelial Markers (p63, CK5/6, MNF116, 34BE12) | Negative | Positive | Usually negative |
May be positive in some tumors (e.g., myoepithelial carcinoma or malignant nerve sheath tumor)
SMA is not uncommonly weakly positive in desmoplastic melanoma; strong labeling for desmin is rare
Strong staining for SMA and desmin, with no or only weak labeling for S100 protein suggest leiomyosarcoma
Abbreviations: SMA: smooth muscle antibody, MITF: Microphthalmia-associated transcription factor
Molecular Profile
In gene expression profiling, DM demonstrated a decrease in a number of genes involved in melanin synthesis, which is purported to be the reason that many of these lesions are amelanotic.23 There was increased expression of the protein clusterin, a ubiquitous glycoprotein involved in tissue remodeling and fibrogenic processes, leading to speculation of its involvement in the generation of the fibrous stroma associated with DM.23 Fluorescence in situ hybridization (FISH) has been shown to have limited diagnostic value in differentiating DM from benign melanocytic lesions based upon probing for specific chromosomal aberrations.24, 25 The vast majority of DM lacks a known mutation, though a rare mDM associated with a SSM may harbor a BRAFV600E mutation.26 Additionally, in the personal experience of one author (KJB), a rare acral DM has been found to harbor a c-kit mutation.
Rendering the final diagnosis
The fact that melanoma often does not enter the differential diagnosis, either clinically or histopathologically, is a common problem for lesions that prove to be DM. The low suspicion for melanoma often leads to partial biopsies which, by definition, takes only a sample of the lesion and may miss an area that is most diagnostic. The odds of melanoma misdiagnosis are higher for partial biopsies than excisional biopsies and may result in potential legal ramifications.27, 28 In lesions where any doubt persists on either side, a repeat biopsy is strongly recommended.
PROGNOSTIC FACTORS
The five-year overall survival (OS) for DM ranges between 67-89%.29 Advancing age, male gender and head and neck location are associated with an increased risk of DM-specific death.2 Controversy persists regarding the prognosis of DM compared with non-desmoplastic melanomas. Initial case series suggested that DM has a less favorable prognosis, based on its apparently aggressive clinical behavior.30, 31 Most of the lesions described were deeply invasive and advanced primary tumors, though no information was included about thickness rate of growth or histologic subtype.
Tumor thickness
Most DMs at the time of diagnosis tend to be thick, with the majority of tumors presenting with Clark levels IV and V, and the mean Breslow depth ranging between 2.0mm to 6.5mm.2, 19 We speculate the increased depth at presentation of DM to be due to the difficulties in the initial clinical recognition. When separating by subtype, pDM (3.6mm) and mDM (5.5mm) are both significantly thicker than non-desmoplastic melanomas (2.1mm; p<0.001).32
Recent studies adjusting for tumor thickness found a more favorable prognosis for DM compared to non-desmoplastic melanoma of similar thickness.33-36 Carlson et al. found higher 5-year survival rates for DM tumors greater than 4 mm in thickness compared to other types of cutaneous melanoma greater than 4 mm (72% versus 37%-48%).33 Similarly, Skelton et al. also found higher survival rates in DM greater than 4 mm thickness as compared to non-desmoplastic melanoma (61% versus 40%-41%).34 However, when DMs of all thicknesses were included in a large case series from the SMU, there was no significant difference in survival rates between patients with DM and non-desmoplastic melanoma.1 These differing results were likely due to the inclusion of many thin DM tumors. To reduce the thickness-related biases, the first case-control study matching for tumor thickness was performed with 89 DM cases and 178 non-desmoplastic melanomas in the Massachusetts General Hospital melanoma database.37 When compared to thickness-matched controls, DM patients had similar survival rates to controls (5- year OS 72.6% versus 76.9%, respectively).
The conflicting reports about prognosis may be due to the lack of precise and uniformly applied pathologic criteria for classification and the inclusion of a heterogenous mix of DM cases. Prior to the separation of pDM and mDM, various studies included tumors with variable degrees of desmoplasia and neurotropism, leading to the inclusion of spindle cell neurotropic tumors with only minor desmoplasia as DM.1, 33, 34, 38 Therefore, it is recommended that these pathologic characteristics be reported as distinctive findings.
Neurotropism
Reed and Leonard were the first to report on 22 cases of neurotropic DM (NDM) with clinical characteristics of local infiltration, multiple recurrences and metastases.38 NDM tumors reveal deeper Clark levels (IV and V) and greater mitotic activity compared to DM without neurotropism.1 Because the majority of NDM occurs on the head and neck, NDM can give rise to facial nerve neuropathies due to direct tumor extension.36, 39 Regarding the impact of neurotropism on survival, one small case series found that NDM was associated with a 30% decrease in 8-year survival compared to DM without neurotropism.36 However, in other series, the presence of neurotropism was not found to be associated with a worse survival, but was associated with higher local recurrence rates (20% versus 6.8% in non-neurotropic DM).1, 37
Pure and Mixed subtype
To distinguish the clinically relevant outcomes between histologic subtypes of DM, Busam et al.8 classified 92 DM cases into 55 pure and 37 mixed subtype. This demonstrated significant prognostic differences in disease-free survival. Patients with mDM had a 3.5 fold greater risk for death or metastases than those with pDM. Subsequent retrospective studies found significant differences in the clinical behavior between pDM and mDM (Table 2). Hawkins et al.32 confirmed the worse prognosis of mDM compared to pDM (five-year melanoma-specific mortality, 31% vs. 11%; P<0.01). One contributing factor is the higher frequency of regional lymph node involvement in patients with mDM (10%) versus pDM (1%), and higher rates of locoregional recurrence and a shorter time to recurrence for mDM.40 Furthermore, it has been observed that mDM has a higher mitotic index and Ki-67 proliferative index.26, 41 In contrast, pDM was found to have similar mortality rates to other types of melanoma despite a 3-fold difference in median tumor thickness, perhaps lending credence to claims that thickness for thickness, pDM has a more favorable prognosis. These findings may begin to explain the phenotypic differences between the two DM groups. Larger studies need to be performed in order to better correlate DM subtype and clinical outcomes.
Table 2.
Key features of Pure and Mixed Desmoplastic Melanoma
| Features | Pure DM | Mixed DM |
|---|---|---|
| Clinical | Frequently, a dermal nodule or plaque Less often contains clinical pigmentation |
More often associated with LM or SSM More often contains clinical pigmentation |
| Histologic | Pauci-cellular Prominent desmoplasia throughout entire tumor |
Higher cellular density Higher mitotic index, increased CD117 staining and higher Ki-67 proliferative index (Miller) |
| Locoregional Recurrence 32,40 | 4.1%-26% | 12.4%-39% |
| Regional lymph node involvement 32, 40, 41, 51 | 0-2.2% | 8.5%-22% |
| Distant Metastasis 40 | 11.3% | 12.4% |
MANAGEMENT
Wide Local Excision (WLE)
The first line of treatment for any primary cutaneous melanoma is surgical management. The present recommendations are margins from 1 to 2 cm for lesions 1.01 to 2.0 mm in thickness and 2 cm margins for melanomas greater than 2.0mm.42 A large case series study of 1,735 cases from the SEER registry demonstrates that for DM the extent of surgical resection, independent of tumor thickness, is a significant predictor for survival, with the minimal acceptable margins of WLE to be 1 cm.29 Patients undergoing WLE (greater than 1cm) had better OS than excision less than 1 cm (67% versus 60%; p=0.029).
Another reason for maintaining wide margins is because of its propensity for local recurrence, ranging between 6.7% and 56.0%.19 “Local recurrence” of DM is likely due to persistent tumors resulting from missing a focus of positivity at a margin or from perineural skips areas. Not surprisingly, patients with DM with positive margins have higher local recurrence rates than those in patients with negative margins.1, 39, 43, 44 One Australian series reported significantly increased local recurrence rate in lesions excised with margins <1 cm than margins >2 cm (p=−.001).1 Maurichi et al. examined the differences in surgical margins between thin (<2mm) and thick (>2mm) pDM and mDM.45 In patients with thin pDM, those with 1 cm margins had a higher rate of recurrence as well as worse 5-year OS compared to those treated with 2 cm margins (60% versus 85.2%, P=0.014). Thick pDM treated with 2 cm margins had similar survival to thin pDM treated with the same (86.6% versus 85.2%). In contrast, the mortality risk in mDM increased with stage but was independent of width of excision margins, behaving more like nondesmoplastic melanomas.
The difficulty in achieving wide and deep excisions may be due to increased tumor depth and anatomic site.48 Since DM frequently affects the head and neck region, cosmetic and anatomic restraints may prevent excision with adequate margins. In addition, higher recurrence rates have been attributed to nerve involvement in DM because surgical margins may fail to detect focal persistent tumor deposits around nerve trunks. The predilection for local recurrence and neurotropism support the current guidelines that wider margins of at least 2 cm are preferred for DM.
Sentinel Lymph Node Biopsy (SLNB)
Sentinel lymph node (LN) status is the most important survival predictor for patients with primary localized cutaneous melanoma.46 Regional LN involvement at the time of DM diagnosis occurs less frequently than in other cutaneous melanomas, with a reported range of 0 to 18.8%. 19, 47-49 However, LN status on survival in DM remains conflicting. From the most recent SEER data, nodal status had no impact on 5-year OS (65% for node negative, 64% node positive, p=0.86).29 A recent study from DM cases of the head and neck demonstrated that SLN-positive and SLN-negative patients have no significant differences in survival.50 In contrast, a study using the same population-registry database found that risk of death was three fold higher in those with positive LN involvement compared to no LN involvement (95% CI: 1.94-4.65).2 The inconsistencies of these studies are likely due to the low incidence of lymph node disease in this rare variant of melanoma.
Given the low risk of LN involvement and questionable survival benefits of SLNB, some are questioning its clinical utility and recommending against its routine use in patients with DM.29, 41, 48, 51, 52 Classifying DM into histological subtype could better inform this management decision. George et al. found only 2 out of 155 (1.4%) of pDM patients versus 14 out of 81 (18.5%) with mDM had positive regional LN at time of initial surgical staging.41 These results suggest that the incidence of LN involvement of mDM is comparable to that of non-desmoplastic melanomas. In a recent series from Memorial Sloan-Kettering Cancer Center of 47 patients with DM of the head and neck, no patients were found to have a positive SLN out of the 23 who underwent SLN mapping (15 pDM and 8 mDM).52 These studies bring into question the necessity of SLNB for patients with the pDM subtype.
However, rather than eliminating SLNB altogether, some have suggested selective SLNB for patients with additional high-risk factors. Though the prognostic significance of these characteristics is not fully understood, there is increasing evidence that patients with DM with neurotropism, high mitotic rate and ulceration may be considered for SLNB.37, 53 The National Comprehensive Cancer Network guidelines advise: “an experienced dermatopathologist examine the entire lesion before making the decision to perform a SLNB.”54 Though the two histopathologic DM subtypes may have distinct tendencies for LN involvement, this classification must be more clearly defined and correlated with LN status and survival outcomes to determine the best recommendations for or against SLNB.
Radiation
Because of the relatively high DM recurrence rates at the site of local excision, several groups have advocated adjuvant local radiation therapy. Vontgama et al. conducted a retrospective cohort study of 15 patients with locally recurrent DM who received adjuvant radiation therapy (most received 50Gy in 25-28 fractions).55 None of these patients experienced a local recurrence. Compared to the 29 patients that underwent surgery alone, four patients in that group experienced a local recurrence, suggesting an association with adjuvant radiation therapy and improved local control. The SMU recently reported the outcome of patients with NDM thought to be at high risk for local recurrence who were treated with adjuvant radiation therapy (most received 48-50 Gy in 20-25 fractions).44 Among those treated, significantly more were men, had head and neck primary tumors that were thick (>4 mm thick or Clark level V involvement), ulcerated, and underwent excision with positive or narrow margins. Similar rates of local failure were reported compared to the group of patients who underwent surgery alone (6% and 7%, respectively). Because the results of treatment in in pDM versus mDM were not reported separately, no conclusions about the effectiveness of radiation therapy in pDM or mDM can be made.
Based on the aforementioned literature adjuvant radiotherapy may be valuable for patients with locally recurrent DM, residual gross tumor, or narrow/positive excision margins (especially in cosmetically or functionally sensitive areas on the head and neck, where wide excision and local failure can be morbid). The presence of neurotropism portends a poor prognosis in many cutaneous malignancies and consideration of adjuvant therapy might also be worthwhile in this situation.56, 57 An ongoing randomized controlled trial of adjuvant radiation therapy versus observation in patients with neurotropic melanoma of the head and neck (NCT00975520) will help clarify the role of radiation therapy in this patient population.
Systemic therapies
Systemic metastasis occurs in 7% to 44% of DM cases with the most common involved areas being the lung, liver and bone.19 The histopathological type of DM also determines the risk for distant metastasis. For instance, mDM has more LN and distant metastases than pDM.45 If metastases are present, the time it takes to develop is shorter in mDM.
Whether systemic treatments for non-desmoplastic melanoma, such as ipilimumab or vemurafenib, can be successfully applied to DM remains to be determined. The observation that genetic mutations such as BRAFV600E and c-KIT mutation are frequently absent in DM should be taken into consideration when discussing these targeted therapies for melanoma.26, 58
CONCLUSIONS AND RECOMMENDATIONS
DM can be a diagnostic challenge for clinicians and pathologists alike, because of its non-specific and often banal appearance. Multiple pitfalls exist in achieving the correct diagnosis including the initial clinical diagnosis as a benign entity, partial biopsies that are insufficient to render a pathologic diagnosis, and interpretation error under the microscope. We offer some final recommendations which can aid in the diagnostic and management decisions
Any clinically suspect LMM should be palpated due to the association of DM with LMM. If a dermal firm nodule is noted, one needs to consider the possibility of a DM component.
An excisional biopsy, whenever feasible, is the preferred biopsy method for suspect lesions.
The pathologic distinction between pure and mixed DM should be documented since this distinction is likely to help inform diagnostic and management decisions.
WLE with clear margins (when feasible, a 2 cm margin) is important for all types of DM in order to reduce local recurrence.
SLNB may be unnecessary for patients with pure DM, however, considered in patients with mDM, especially in those manifesting high-risk DM tumor characteristics such as deep infiltration, neurotropism, ulceration and/or higher mitotic rate.
Following surgery, adjuvant radiotherapy appears beneficial in patients with locally recurrent DM, residual gross tumors, DM with perineural involvement, or DM excised with narrow margins.
Further studies should help improve our understanding of the molecular underpinnings of DM's growth and progression, and result in improved risk stratification of patients and help researchers formulate better directed therapies towards controlling this malignancy.
CAPSULE SUMMARY.
The diagnosis and management of desmoplastic melanoma is challenging because it is an infrequently encountered subtype of melanoma and, it often has a banal clinical morphology and its biology is not fully understood.
Distinction between pure and mixed desmoplastic melanoma has been shown to have clinical, therapeutic and prognostic significance.
Knowledge of the distinct histologic subtypes of desmoplastic melanoma, their risk factors, as well as their clinical and dermoscopic characteristics, can aid in their detection and management.
Acknowledgments
Funding sources: none
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Quinn MJ, Crotty KA, Thompson JF, Coates AS, O'Brien CJ, McCarthy WH. Desmoplastic and desmoplastic neurotropic melanoma: experience with 280 patients. Cancer. 1998;83:1128–35. [PubMed] [Google Scholar]
- 2.Feng Z, Wu X, Chen V, Velie E, Zhang Z. Incidence and survival of desmoplastic melanoma in the United States, 1992-2007. J Cutan Pathol. 2011;38:616–24. doi: 10.1111/j.1600-0560.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- 3.Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of spindle cell melanoma). Cancer. 1971;28:914–36. doi: 10.1002/1097-0142(1971)28:4<914::aid-cncr2820280415>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Labrecque PG, Hu CH, Winkelmann RK. On the nature of desmoplastic melanoma. Cancer. 1976;38:1205–13. doi: 10.1002/1097-0142(197609)38:3<1205::aid-cncr2820380322>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Bernaba BN, Vogiatzis PI, Binder SW, Cassarino DS. Potentially useful markers for desmoplastic melanoma: an analysis of KBA.62, p-AKT, and ezrin. Am J Dermatopathol. 2011;33:333–7. doi: 10.1097/DAD.0b013e3181e5a2b4. quiz 8-40. [DOI] [PubMed] [Google Scholar]
- 6.Daimaru Y, Hashimoto H, Enjoji M. Malignant peripheral nerve-sheath tumors (malignant schwannomas). An immunohistochemical study of 29 cases. Am J Surg Pathol. 1985;9:434–44. doi: 10.1097/00000478-198506000-00005. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445–51. doi: 10.1080/00313020412331285336. [DOI] [PubMed] [Google Scholar]
- 8.Busam KJ, Mujumdar U, Hummer AJ, Nobrega J, Hawkins WG, Coit DG, et al. Cutaneous desmoplastic melanoma: reappraisal of morphologic heterogeneity and prognostic factors. Am J Surg Pathol. 2004;28:1518–25. doi: 10.1097/01.pas.0000141391.91677.a4. [DOI] [PubMed] [Google Scholar]
- 9.Howlader NNA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2008. 2010 Available at: http://seer.cancer.gov/csr/1975_2008/
- 10.Melanoma Incidence and Mortality. Surveillance Epidemiology and End Results. 2011 [Google Scholar]
- 11.Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321–30. doi: 10.1016/j.cll.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Prasad ML, Patel SG, Busam KJ. Primary mucosal desmoplastic melanoma of the head and neck. Head Neck. 2004;26:373–7. doi: 10.1002/hed.10384. [DOI] [PubMed] [Google Scholar]
- 13.Chung KC, Calkins ER, Crawford R, Rees RS. Desmoplastic melanoma of the hand: case reports and review of the literature. J Hand Surg Am. 1995;20:873–6. doi: 10.1016/S0363-5023(05)80447-2. [DOI] [PubMed] [Google Scholar]
- 14.Kilpatrick SE, White WL, Browne JD. Desmoplastic malignant melanoma of the oral mucosa. An underrecognized diagnostic pitfall. Cancer. 1996;78:383–9. doi: 10.1002/(SICI)1097-0142(19960801)78:3<383::AID-CNCR1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. A study of 45 cases. Am J Surg Pathol. 1989;13:358–73. doi: 10.1097/00000478-198905000-00003. [DOI] [PubMed] [Google Scholar]
- 16.de Almeida LS, Requena L, Rutten A, Kutzner H, Garbe C, Pestana D, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207–15. doi: 10.1097/DAD.0b013e3181716e6b. [DOI] [PubMed] [Google Scholar]
- 17.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–65. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 18.Debarbieux S, Ronger-Salve S, Dalle S, Balme B, Thomas L. Dermoscopy of desmoplastic melanoma: report of six cases. Br J Dermatol. 2008;159:360–3. doi: 10.1111/j.1365-2133.2008.08687.x. [DOI] [PubMed] [Google Scholar]
- 19.Lens MB, Newton-Bishop JA, Boon AP. Desmoplastic malignant melanoma: a systematic review. Br J Dermatol. 2005;152:673–8. doi: 10.1111/j.1365-2133.2005.06462.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaneishi NK, Cockerell CJ. Histologic differentiation of desmoplastic melanoma from cicatrices. Am J Dermatopathol. 1998;20:128–34. doi: 10.1097/00000372-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Busam KJ, Iversen K, Coplan KC, Jungbluth AA. Analysis of microphthalmia transcription factor expression in normal tissues and tumors, and comparison of its expression with S-100 protein, gp100, and tyrosinase in desmoplastic malignant melanoma. Am J Surg Pathol. 2001;25:197–204. doi: 10.1097/00000478-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Longacre TA, Egbert BM, Rouse RV. Desmoplastic and spindle-cell malignant melanoma. An immunohistochemical study. Am J Surg Pathol. 1996;20:1489–500. doi: 10.1097/00000478-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Busam KJ, Zhao H, Coit DG, Kucukgol D, Jungbluth AA, Nobrega J, et al. Distinction of Desmoplastic Melanoma from Non-Desmoplastic Melanoma by Gene Expression Profiling. J Investig Dermatol. 2005;124:412–9. doi: 10.1111/j.0022-202X.2004.23600.x. [DOI] [PubMed] [Google Scholar]
- 24.Clemente C, Bettio D, Venci A, Scopsi L, Rao S, Ferrari A, et al. A fluorescence in situ hybridization (FISH) procedure to assist in differentiating benign from malignant melanocytic lesions. Pathologica. 2009;101:169–74. [PubMed] [Google Scholar]
- 25.Gerami P, Beilfuss B, Haghighat Z, Fang Y, Jhanwar S, Busam KJ. Fluorescence in situ hybridization as an ancillary method for the distinction of desmoplastic melanomas from sclerosing melanocytic nevi. J Cutan Pathol. 2011;38:329–34. doi: 10.1111/j.1600-0560.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- 26.Miller DD, Emley A, Yang S, Richards JE, Lee JE, Deng A, et al. Mixed versus pure variants of desmoplastic melanoma: a genetic and immunohistochemical appraisal. Mod Pathol. 2011 doi: 10.1038/modpathol.2011.196. [DOI] [PubMed] [Google Scholar]
- 27.Ng JC, Swain S, Dowling JP, Wolfe R, Simpson P, Kelly JW. The Impact of Partial Biopsy on Histopathologic Diagnosis of Cutaneous Melanoma: Experience of an Australian Tertiary Referral Service. Arch Dermatol. 2010;146:234–9. doi: 10.1001/archdermatol.2010.14. [DOI] [PubMed] [Google Scholar]
- 28.Troxel DB. Pitfalls in the diagnosis of malignant melanoma: findings of a risk management panel study. Am J Surg Pathol. 2003;27:1278–83. doi: 10.1097/00000478-200309000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Wasif N, Gray RJ, Pockaj BA. Desmoplastic melanoma - the step-child in the melanoma family? J Surg Oncol. 2011;103:158–62. doi: 10.1002/jso.21778. [DOI] [PubMed] [Google Scholar]
- 30.Reiman HM, Goellner JR, Woods JE, Mixter RC. Desmoplastic melanoma of the head and neck. Cancer. 1987;60:2269–74. doi: 10.1002/1097-0142(19871101)60:9<2269::aid-cncr2820600928>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Egbert B, Kempson R, Sagebiel R. Desmoplastic malignant melanoma. A clinicohistopathologic study of 25 cases. Cancer. 1988;62:2033–41. doi: 10.1002/1097-0142(19881101)62:9<2033::aid-cncr2820620927>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 32.Hawkins WG, Busam KJ, Ben-Porat L, Panageas KS, Coit DG, Gyorki DE, et al. Desmoplastic melanoma: a pathologically and clinically distinct form of cutaneous melanoma. Ann Surg Oncol. 2005;12:207–13. doi: 10.1245/ASO.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 33.Carlson JA, Dickersin GR, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. A clinicopathologic analysis of 28 cases. Cancer. 1995;75:478–94. doi: 10.1002/1097-0142(19950115)75:2<478::aid-cncr2820750211>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.Skelton HG, Smith KJ, Laskin WB, McCarthy WF, Gagnier JM, Graham JH, et al. Desmoplastic malignant melanoma. J Am Acad Dermatol. 1995;32:717–25. doi: 10.1016/0190-9622(95)91448-x. [DOI] [PubMed] [Google Scholar]
- 35.Walsh NM, Roberts JT, Orr W, Simon GT. Desmoplastic malignant melanoma. A clinicopathologic study of 14 cases. Arch Pathol Lab Med. 1988;112:922–7. [PubMed] [Google Scholar]
- 36.Baer SC, Schultz D, Synnestvedt M, Elder DE. Desmoplasia and neurotropism. Prognostic variables in patients with stage I melanoma. Cancer. 1995;76:2242–7. doi: 10.1002/1097-0142(19951201)76:11<2242::aid-cncr2820761110>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Livestro DP, Muzikansky A, Kaine EM, Flotte TJ, Sober AJ, Mihm MC, Jr., et al. Biology of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739–46. doi: 10.1200/JCO.2005.04.515. [DOI] [PubMed] [Google Scholar]
- 38.Reed RJ, Leonard DD. Neurotropic melanoma. A variant of desmoplastic melanoma. Am J Surg Pathol. 1979;3:301–11. doi: 10.1097/00000478-197908000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Smithers BM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186–90. doi: 10.1007/BF02071519. [DOI] [PubMed] [Google Scholar]
- 40.Murali R, Shaw HM, Lai K, McCarthy SW, Quinn MJ, Stretch JR, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010;116:4130–8. doi: 10.1002/cncr.25148. [DOI] [PubMed] [Google Scholar]
- 41.George E, McClain SE, Slingluff CL, Polissar NL, Patterson JW. Subclassification of desmoplastic melanoma: pure and mixed variants have significantly different capacities for lymph node metastasis. J Cutan Pathol. 2009;36:425–32. doi: 10.1111/j.1600-0560.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 42.Bichakjian CK, Halpern AC, Johnson TM, Foote Hood A, Grichnik JM, Swetter SM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65:1032–47. doi: 10.1016/j.jaad.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 43.Jaroszewski DE, Pockaj BA, DiCaudo DJ, Bite U. The clinical behavior of desmoplastic melanoma. Am J Surg. 2001;182:590–5. doi: 10.1016/s0002-9610(01)00819-4. [DOI] [PubMed] [Google Scholar]
- 44.Chen JY, Hruby G, Scolyer RA, Murali R, Hong A, Fitzgerald P, et al. Desmoplastic neurotropic melanoma: a clinicopathologic analysis of 128 cases. Cancer. 2008;113:2770–8. doi: 10.1002/cncr.23895. [DOI] [PubMed] [Google Scholar]
- 45.Maurichi A, Miceli R, Camerini T, Contiero P, Patuzzo R, Tragni G, et al. Pure desmoplastic melanoma: a melanoma with distinctive clinical behavior. Ann Surg. 2010;252:1052–7. doi: 10.1097/SLA.0b013e3181efc23c. [DOI] [PubMed] [Google Scholar]
- 46.Balch CM, Morton DL, Gershenwald JE, McMasters KM, Nieweg OE, Powell B, et al. Sentinel node biopsy and standard of care for melanoma. J Am Acad Dermatol. 2009;60:872–5. doi: 10.1016/j.jaad.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 47.Sassen S, Shaw HM, Colman MH, Scolyer RA, Thompson JF. The complex relationships between sentinel node positivity, patient age, and primary tumor desmoplasia: analysis of 2303 melanoma patients treated at a single center. Ann Surg Oncol. 2008;15:630–7. doi: 10.1245/s10434-007-9684-1. [DOI] [PubMed] [Google Scholar]
- 48.Gyorki DE, Busam K, Panageas K, Brady MS, Coit DG. Sentinel lymph node biopsy for patients with cutaneous desmoplastic melanoma. Ann Surg Oncol. 2003;10:403–7. doi: 10.1245/aso.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 49.Cummins DL, Esche C, Barrett TL, Balch CM, Mofid M. Lymph node biopsy results for desmoplastic malignant melanoma. Cutis. 2007;79:390–4. [PubMed] [Google Scholar]
- 50.Smith VA, Lentsch EJ. Sentinel node biopsy in head and neck desmoplastic melanoma: An analysis of 244 cases. Laryngoscope. 2011 doi: 10.1002/lary.22445. [DOI] [PubMed] [Google Scholar]
- 51.Pawlik TM, Ross MI, Prieto VG, Ballo MT, Johnson MM, Mansfield PF, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900–6. doi: 10.1002/cncr.21635. [DOI] [PubMed] [Google Scholar]
- 52.Mohebati A, Ganly I, Busam KJ, Coit D, Kraus DH, Shah JP, et al. The Role of Sentinel Lymph Node Biopsy in the Management of Head and Neck Desmoplastic Melanoma. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2468-2. [DOI] [PubMed] [Google Scholar]
- 53.Arora A, Lowe L, Su L, Rees R, Bradford C, Cimmino VC, et al. Wide excision without radiation for desmoplastic melanoma. Cancer. 2005;104:1462–7. doi: 10.1002/cncr.21311. [DOI] [PubMed] [Google Scholar]
- 54.The NCCN Clinical Practice Guidelines in Oncology: Melanoma. Version 3.2012. National Comprehensive Cancer Network, Inc.; [March, 2012]. Available at: http://www.nccn.org. [Google Scholar]
- 55.Vongtama R, Safa A, Gallardo D, Calcaterra T, Juillard G. Efficacy of radiation therapy in the local control of desmoplastic malignant melanoma. Head Neck. 2003;25:423–8. doi: 10.1002/hed.10263. [DOI] [PubMed] [Google Scholar]
- 56.Mendenhall WM, Amdur RJ, Hinerman RW, Werning JW, Malyapa RS, Villaret DB, et al. Skin cancer of the head and neck with perineural invasion. Am J Clin Oncol. 2007;30:93–6. doi: 10.1097/01.coc.0000251224.16075.60. [DOI] [PubMed] [Google Scholar]
- 57.Newlin HE, Morris CG, Amdur RJ, Mendenhall WM. Neurotropic melanoma of the head and neck with clinical perineural invasion. Am J Clin Oncol. 2005;28:399–402. doi: 10.1097/01.coc.0000144853.76112.3d. [DOI] [PubMed] [Google Scholar]
- 58.Davison JM, Rosenbaum E, Barrett TL, Goldenberg D, Hoque MO, Sidransky D, et al. Absence of V599E BRAF mutations in desmoplastic melanomas. Cancer. 2005;103:788–92. doi: 10.1002/cncr.20861. [DOI] [PubMed] [Google Scholar]