Abstract
Gonadotropin releasing hormone (GnRH) neurons originate the nasal placode and migrate into the brain during prenatal development. Once within the brain, these cells become integral components of the hypothalamic-pituitary-gonadal axis, essential for reproductive function. Disruption of this system causes hypogonadotropic hypogonadism (HH). HH associated with anosmia is clinically defined as Kallman syndrome (KS). Recent work examining the developing nasal region has shed new light on cellular composition, cell interactions and molecular cues responsible for the development of this system in different species. This review discusses some developmental aspects, animal models and current advancements in our understanding of pathologies affecting GnRH. In addition we discuss how development of neural crest derivatives such as the glia of the olfactory system and craniofacial structures control GnRH development and reproductive function.
The GnRH neurons – Regulators of fertility
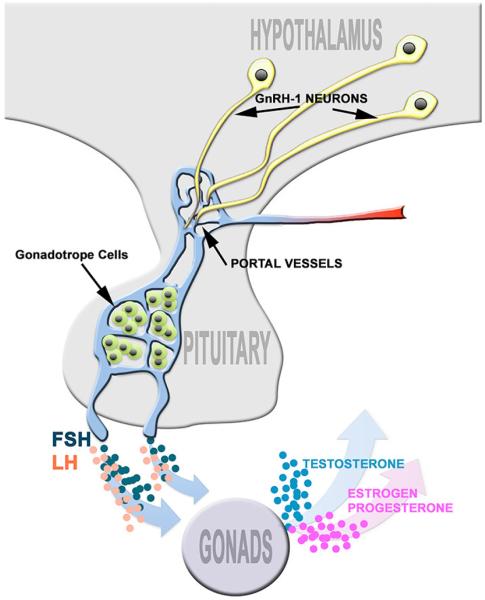
The hypothalamic-pituitary-gonadal (HPG) axis is considered to be an evolutionary innovation specific to vertebrates [1; 2]. The neuroendocrine gonadotropin releasing hormone (GnRH) neurons are integral components of this axis, regulating sexual development and reproductive function (see Figure 1). In mammals, the primary terminal field of the neurosecretory GnRH neurons is the median eminence. Here, the cleaved and amidated GnRH decapeptide is released into portal vessels where it is transported to the anterior pituitary gland. GnRH activates receptors on pituitary gonadotropes, triggering synthesis and release of the gonadotropins luteinizing hormone (LH) and follicle stimulating (FSH). LH and FSH are necessary for gonadal function. In the ovary, LH acts upon theca cells that produce the androgen substrate required for ovarian estrogen biosynthesis. In the testis, LH induces Leydig cells to produce testosterone. In both males and females, FSH stimulates maturation of germ cells. Thus, if GnRH release is compromised it translates into impaired reproductive maturation and function - e.g. hypogonadotropic hypogonadism (HH). HH can result from defects in GnRH neuronal development [3; 4; 5], GnRH synthesis [6], release [7; 8; 9; 10] or ligand/receptor (GnRH/GnRHR) pairing [4; 11; 12; 13].
Fig. 1. Hypothalamic-pituitary-gonadal (HPG) axis.
GnRH-1 cells extend their axons to the median eminence where they release GnRH-1 into the portal capillary system. Gonadotrope cells of the anterior pituitary, synthesize and release gonadotropins, LH and FSH. Pituitary release of FSH and LH, control sex steroid synthesis in the gonads.
Recent work has given us a better understanding of the cellular composition of the developing olfactory area, development of the GnRH cells and perturbations leading to HH. However, we still do not fully understand the cell types involved in GnRH neuronal development. This review discusses some of the similarities and differences of the GnRH system among animal models. In addition, we highlight some recent animal studies on GnRH neuronal embryonic lineage, craniofacial development and olfactory ensheathing cells that may explain the diversity of phenotypes observed in HH patients that resulted from a developmental perturbation.
GnRH Peptides
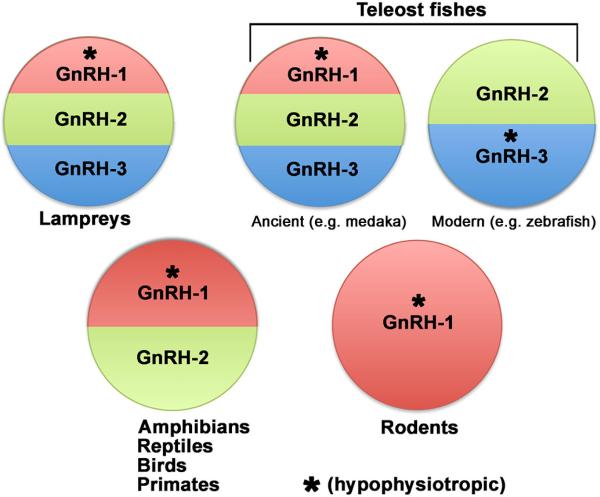
During early evolution, three paralogous GnRH genes (gnrh1, gnrh2 and gnrh3) arose from two rounds of genome duplication [14]. Various isoforms of GnRH decapeptides have been found in all vertebrates: ranging from agnathans to mammals [15; 16; 17]. Characterization of non-mammalian vertebrates species including fishes, amphibians and reptiles identified up to 15 different GnRH variants [18; 19] that were classified into three main phylogenetic groups, [17; 20; 21; 22; 23; 24; 25], prior to gene identification, and historically named after the first class or species in which they were characterized [24; 26; 27; 28; 29; 30]. However, identification of the gnrh genes revealed a genetic twist - the gnrh genes expressed in vertebrates varies within teleosts as well as within mammals (Figure 2). All 3 GnRH genes are found in ancient teleosts including medaka [31; 32; 33]. However, genetic studies have shown that though multiple GnRH paralogs originated during evolution, the GnRH-3 family was lost in the tetrapod lineage [34]. In most teleosts, GnRH-3 is expressed by neurons of the terminal nerve/olfactory region and believed to function as a neuromodulator and to be indirectly linked to the reproductive neuroendocrine axis [31]. Observations made in sea bass indicate that GnRH-3 neuronal projections can innervate the retina and modulate retinal function [35]. Gnrh2 (Human chromosome 20) is the most ancient form of GnRH. GnRH-2 peptide is often referred to as mesencephalic GnRH based on the anatomical location of the cells expressing the peptide. Studies in fish suggest that GnRH-2 might be a neuromodulator in the auditory system [31; 36; 37] or a melatonin-releasing factor in the pineal gland, participating in sleep/wake cycles [38]. However, during evolution, the preproGnRH-2 gene as well as the GnRH-2 receptor has been deleted or inactivated from the genome of many mammals [39]. Thus, a physiological role for GnRH-2 in mammals remains controversial. Gnrh1 is present in most vertebrates (Human chromosome 8) but notably absent in modern teleosts including zebrafish [40; 41] [42; 43]. In modern teleosts, GnRH-3 adopted the role of GnRH-1 in reproduction [44]. GnRH-1 is the only form of the GnRH gene that exists in the rodent genome [45] (Summarized in Fig. 2). In mammals, GnRH-1 cells are located in the forebrain, distributed bilaterally on either side of midline. The actual location of GnRH-1 cells can vary rostrally to caudally depending on the species [46]. The pivotal function of the GnRH-1 peptide in controlling the HPG axis was proven in mice carrying a loss of function mutation in GnRH-1 gene, hpg mice [47]. These mice exhibit deficient pituitary gonadotropin secretion and failure of gonadal postnatal development (either testes or ovary). In addition, GnRH-1 function in controlling the HPG axis was elegantly demonstrated by one of the very first examples of gene therapy/rescue experiment in animals. In 1986, A. J. Mason and coworkers showed that transgenic expression of GnRH-1 was sufficient to re-established reproductive competence in both hpg male and female mice [47; 48]. Proof of the same role for the GnRH-1 system in humans came from the identification of homozygous loss of function mutations in the GnRH1 gene in a family carrying normosmic HH [49].
Fig.2.
Schematic illustrating the different GnRH paralogs expressed by vertebrate species. * indicates hypophysiotropic form.
Embryonic development of GnRH neurons
Deficits in the sense of smell and hypogonadism were first reported in 1856 by A. Maestre de San Juan and later, in 1944, by F. J. Kallmann [50]. Kallmann noticed a co-segregation of anosmia and hypogonadism in individuals from three families and suggested a hereditary nature of this syndrome, now commonly know as Kallmann syndrome (KS). The anatomical link between anosmia and hypogonadism would remain unknown for four decades.
Although genetic changes have added unexpected twists to understanding the hypophysiotropic GnRH system (for other reviews see [5; 14; 51; 52]) a striking similarity has been conserved throughout evolution in the development of the GnRH neurons responsible for controlling the HPG axis. In the late 1980s, developmental studies in mice, by two independent groups [53; 54; 55], revealed that GnRH-1 neurons migrate from the nose to the brain in association with developing sensory axons of the olfactory/vomeronasal/terminal nerve system (see Figure 3). These studies found the crucial developmental link between the two main components affected in syndromic forms of HH (e.g. KS) [3]. Further characterization in mice indicated that the GnRH-1 cells migrated along a pathway [56] that is a subset of putative vomeronasal/terminal nerve fibers [56; 57]. GnRH-1 neurons have been subsequently described to originate in the developing nose and migrate along subsets of fibers to the brain in birds, amphibians, reptiles, medaka and mammals.
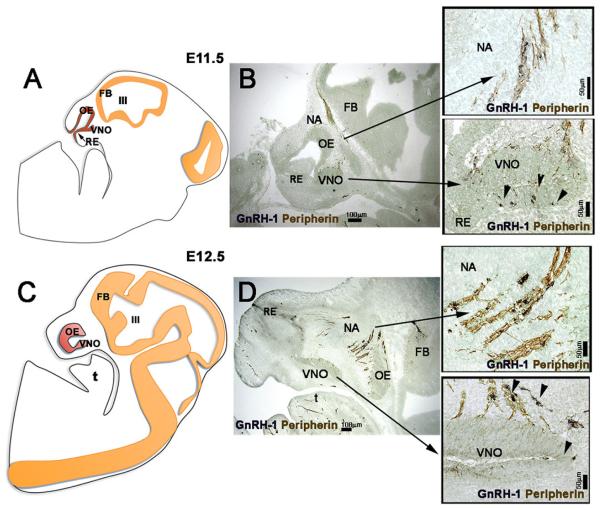
Fig. 3. GnRH neuronal migration in the developing nasal region in mouse.
Left panels: schematics of E11.5 and E12.5 mouse sections showing developmental changes in the nasal placode (red). Nose is toward the left. Right panels – corresponding stage immunocytochemically stained for GnRH (blue, arrowheads) and peripherin (sensory axon marker, brown). GnRH neurons are apposed to axons as they migrate from the VNO to the brain. OE=olfactory epithelium, RE = respiratory epithelium, VNO= vomeronasal organ, III= 3rd ventricle, FB = forebrain. NA=nasal area.
The Olfactory placode
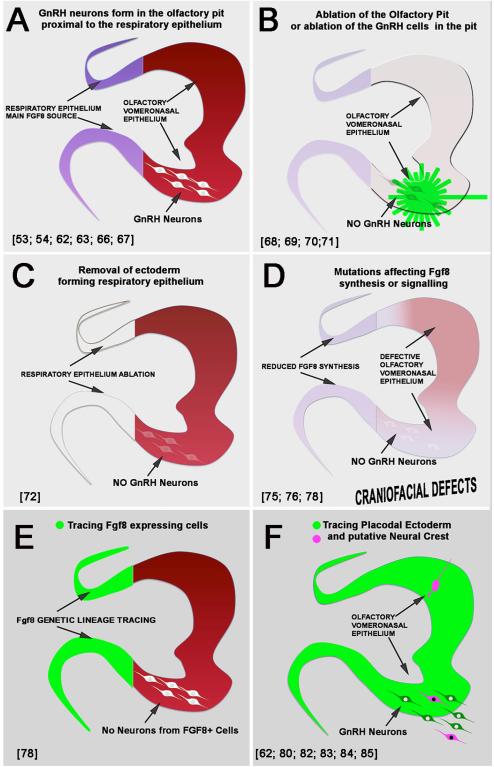
Although GnRH-1 gene expression has been reported at morula and blastocyst stages , and transient expression is observed in both neuronal and non-neuronal cell types [5], birth dating studies in mice have shown that GnRH expressing neurons responsible for the HPG axis are born and fate specified around mid-gestation [53; 54; 63; 66; 67]. GnRH neurons form in a niche at the border between respiratory epithelium and vomeronasal/olfactory epithelium (Fig. 3 and 4A). Removal of the olfactory placode in amphibian and birds [68; 69], mouse models with lack olfactory placodal development [70] and laser ablation of GnRH cells from the nasal area in fish [71], support the notion that GnRH neurons with hypophysiotropic function arise in the olfactory pits, a structure that forms as the olfactory placode invaginates (summarized in Fig. 4B).
Fig.4. Cartoons summarizing key developmental studies (see references in figure) indicating the origin of GnRH neurons from the olfactory pit (A, B) and embryonic (C,D) and genetic (E, F) lineage of the progenitors.
Though studies have been performed on different animal models the shape of the olfactory pit was adapted to the one of the mouse.
Tissue ablation of portions of ectoderm of the developing nose in chick and analysis of mice lacking AP-2α, which delineates presumptive respiratory epithelia from olfactory epithelia [73], suggested that GnRH-1 neurons might originate from an area of the olfactory pit that included the ectoderm responsible for the formation of the respiratory epithelium [72] (Figure 4C). Although ablation experiments (Fig. 4B,C) confirmed that the olfactory pit and putative respiratory epithelium were important for the differentiation of GnRH neurons, experiments of this kind could never fully rule out that: a) the analyzed GnRH cells originated somewhere else and then migrated and matured in the pit [74] or b) the ablated tissue (e.g. respiratory epithelium) was the source of necessary trophic factors needed for GnRH neuron differentiation or survival, rather than the site of origin of GnRH neurons themselves [72; 75; 76; 77] (Figure 4D). In fact, recent genetic tracing of Fgf8 producing cells of the respiratory epithelium indicated that these cells lacked neurogenic ability [78] (Figure 4E). These data are consistent with GnRH cells associated with the respiratory epithelium originating elsewhere, but does not address whether trophic factors within the respiratory epithelium are important for GnRH neuron differentiation or survival (see below).
Classically, the olfactory placode was believed to give rise to nonsensory respiratory, sensory olfactory epithelium, GnRH neurons and olfactory ensheathing cells and, unlike other sensory placodes, not to have a neural crest contribution [51; 79]. Recent cell and lineage tracings in chick and mouse confirmed that GnRH-1 neurons originate from cells that belong to the olfactory placode (Fig. 4F) [62; 80] and highlighted that the embryonic origin and/or molecular profile of the GnRH-1 neuronal progenitors within the placode is not homogeneous as previously believed [80]. In fact Forni and coworkers [80; 81] proposed that progenitor cells of putative neural crest origin and placodal origin contribute to the GnRH-1 neuron cell lineage. In addition, this work also proposed that that neural crest cells contributed to different cell populations in the olfactory epithelium (Figure 4F). Though multiple reports now support a neural crest contribution to the cells of the olfactory pit [82; 83; 84; 85] the embryonic heterogeneity of the GnRH-1 in mammals is still debated [62; 81; 86; 87; 88], perhaps exacerbated by controversies in teleosts.
Studies using a genetically modified Medaka fish model [64] showed that GnRH-3 and GnRH-1 neurons share the same olfactory embryonic origin and migratory path from nose to the brain, but did not address their lineage. Two recent studies using zebrafish expressing EGFP under the control of the GnRH-3 promoter obtained different results with respect to origin of GnRH-3 neurons. The first study showed that laser ablation of the soma of GnRH-3 neurons in the nasal area during early development resulted in a complete lack of olfactory, terminal nerve, preoptic area, and hypothalamic GnRH-3 neurons [40; 71]. At later developmental stages, these fish had arrested oocyte development and reduced average oocyte diameter [71], consistent with the lack of GnRH-3 neurons and loss of hypophysiotropic function. This study suggests that all GnRH-3 neurons originated from the olfactory region and migrate to the brain. However, a second study using a different transgenic zebrafish line concluded that the GnRH-3 system is actually formed by distinct GnRH-3 expressing neuronal populations that independently form in the olfactory region, hypothalamus, preoptic area, and trigeminal ganglion [77]. However, earlier studies in zebrafish [74; 77], based on lineage cell fate tracing, suggested that GnRH-3 neurons originated from developing neural crest [74; 89], similar to the GnRH-1 subpopulation recently detected in mouse [80].
Cranial morphogenesis and GnRH neurogenesis
The frontonasal mesenchyme adjacent to the developing olfactory placode is mainly composed of cells of neural crest origin that differentiate into cells of bone, cartilage and connective tissue of the nose [90]. The olfactory pit is the source of most of the migratory neurons, olfactory neurons and the respiratory epithelium of the nasal cavity. Molecular cross talk between the nasal mesenchyme and the olfactory pit are necessary for invagination of the olfactory pit, patterning of neurogenic areas and definition of precise milieus responsible for development of placodal derivatives, including GnRH-1 cells [91; 92; 93; 94]. Correct development of nasal cartilage, olfactory cavities and nasal connective tissue depends on molecular cues secreted by the invaginating placode [75; 95; 96; 97; 98]. Tipping the balance of cross talk between the nasal mesenchyme and the olfactory pit can have dramatic consequences, ultimately resulting in reproductive dysfunction as described below.
Trophic factors and craniofacial development
In the developing olfactory pit, the non-neurogenic respiratory epithelium is the main source of FGF8 (Figure 5A-C), a molecule that plays a key role in craniofacial development [78; 99]. FGF8 levels are crucial for midline facial integration and correct formation of nasal regions [75; 99; 100]. Mechanical excision of the respiratory epithelium and therefore the Fgf8 source, like loss of function of the fgf8 gene, disrupts early stages in the formation of GnRH-1 neurons [76] (Fig. 4C, D). In humans and rodents, even partial loss of function in FGF8 signaling results in craniofacial defects, aberrant olfactory development and lack of GnRH-1 neurogenesis [75; 76; 101; 102; 103]. In humans [101], nonsense mutations in the FGF8 gene have been linked with different penetrance and degrees of 1) cranial facial defects, including cleft lip and palate, osteoporosis, hearing loss, hypertelorism (abnormal distance between eye orbits), flat cleft palate and nasal bridge, and 2) gonadotropin-releasing deficiency and delayed puberty.
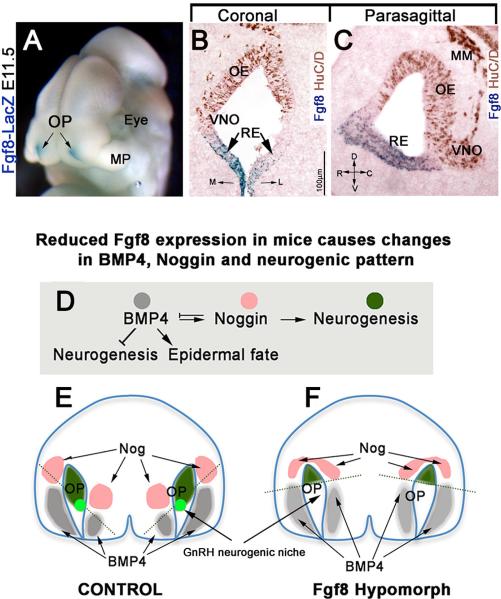
Fig.5. Fgf8 affects craniofacial growth and craniofacial signals.
A) (E11.5) Whole head of Fgf8Lacz knock-in mouse showing Fgf8 (blue) is expressed in the rostral portion of olfactory pits (OP); MP-maxillary process. M=Medial; L= Lateral. B,C Coronal and parasagittal sections of E11.5 mouse nasal sections reveal that Fgf8 expression is limited to the non neurogenic portions of the olfactory pit, putative respiratory epithelium (RE), negative for the neuronal marker HuC/D (brown). R=rostral; D=dorsal; C=caudal; V=ventral. D-F) BMP4, NOG and neurogenesis. BMP4 (gray) represses neuronal cell fate acquisition leading surface ectodermal cells to acquire epidermal fate. BMP4 directly induces Nog expression (pink). Nog, by blocking BMP4, defines neurogenic permissive areas (green). Schematic, frontal view, mouse nasal area at E10.5 (based on [78; 99]) in control (E) and Fgf8 hypomorph (F). Mesenchymal BMP4 (gray) induces Nog expression (pink) in the medial and lateral mesenchyme. These sources of Nog define neurogenic areas (green) of the olfactory pit (OP). The GnRH neurons niche (light green) is defined by ventral mesenchymal sources of Nog. In response to reduced Fgf8 gene expression (F), facial BMP4 expression expanded, causing changes in Nog gene expression pattern and the neurogenic permissive areas of the olfactory pit (compare green areas and dotted line in E and F). The GnRH neurogenic niche is lost in FgF8 mutants as Nog moves from the ventral position of the pit.
Recent work has shown how loss of FGF8 in the nasal region tips the balance between signals that subsequently alter the olfactory pit, GnRH-1 neurogenesis, and thus reproductive function [78] (Figure 5D-F). Areas that will give rise to neurons (neurogenic niches) in the olfactory placode are defined by pro-neurogenic signals such as Fgf8 and TGF-β factor antagonists (e.g. Noggin), in conjunction with neurogenic repressors signals such as Bone Morphogenic Protein-4 (BMP4) [104; 105]. Neurogenic repressors induce ectodermal cells to acquire epidermal cell fates [106]. Analysis of mutant mice with impaired Fgf8 expression revealed an important relationship between dysmorphic growth of the nasal mesenchyme and neurogenesis in the olfactory pit (Fig. 5E, F). Neurogenic defects in the developing olfactory pit were a reflection of aberrant growth of the nasal mesenchyme and associated changes in morphogenic signals. Two independent studies showed that reduced Fgf8 expression translated into expansion of BMP4 expression areas in nasal mesenchyme [78; 99]. BMP signaling can trigger the expression of BMP antagonists, such as Nog, Chordin and Follisatin [107]. BMP antagonists play a key role in controlling olfactory neurogenesis [105; 108]. In line with this BMP4, was found to be a direct inducer of Nog gene expression (pro-neurogenic) in nasal mesenchyme [78]. Thus, BMP4 expression, by inducing Nog, actually defined the neurogenic permissive milieu in the olfactory placode. In mice carrying reduced fgf8 expression, expansion of BMP4 altered noggin expression away from the region where GnRH-1 cells normally arise (respiratory/olfactory epithelium border). Though through which mechanism Fgf8 alters BMP expression is still unclear. Olfactory sensory cells still developed but GnRH-1 cells were absent. These experiments indicate that perturbing one signal during development cascaded forward to lead to a loss of a specific cell type, GnRH-1 neurons. Thus, correct formation of craniofacial structures (cartilage, mesenchyme and bones) is necessary for defining the neurogenic niches in the developing olfactory pit [55; 99; 104; 105]. These results imply that a broad analysis of mutations affecting craniofacial morphogenesis could reveal new important aspects at the base of olfactory sensory loss and HH.
The migratory track
The exact nature of the scaffold upon which GnRH neurons migrate on from the nose to the brain is still poorly defined. Olfactory sensory axons, vomeronasal sensory axons, terminal nerve axons, neuronal cells, glial cells, transient axons, and blood vessels have all been identified to bundle together [5]. The terminal nerve is defined as a cranial nerve that serves as part of the accessory olfactory system with a role in modulating the activity of the olfactory epithelium [109]. At early developmental stages, specific markers (with consistent expression throughout species) have not yet been found for most of the axonal groups crossing from the olfactory placode to the brain. Moreover, a number of different cell types have been described migrating at the same time and along a similar route to the one of GnRH-1 neurons ([51]). The various markers expressed by these migrating cells include olfactory marker protein (OMP) [110], gamma-aminobutyric acid (GABA) [111], thyrosine hydroxylase (TH), acetylcholinesterase NOS, neuropeptide Y (NPY) [109; 114], cardioexcitatory tetrapeptide (FMRF-amide), galanin neuropilin-1 and neuropilin-2 [117; 118; 119]. The fate of these migratory cells still needs to be clarified, as well as their physiological function, if any. A number of molecules such as stromal cell-derived factor 1 (SDF1)/chemokine receptor (CXCR-4), GABA, Hepatocyte growth factor (HGF)/Met receptor, PlexinB1, Semaphorins (3A, F, 4D, 7A), Nasal embryonic LHRH factor (NELF) and CCK, have been shown to influence the migration of GnRH-1 neurons [51; 117; 118; 119; 120; 121]. Several recent reviews of molecules involved in the migration of GnRH cells are available [51; 122; 123; 124]. However the cellular/molecular mechanism through which specific genetic mutations influence GnRH neuronal migration is often hard to unravel, due to the complex anatomy of the nasal region and broad expression of the same molecules among olfactory axons, migratory cells, mesenchyme and endothelial cells of blood vessels.
Kallmann syndrome
Many developmental aspects of the GnRH system that control the reproductive axis still need to be addressed e.g. embryonic lineage of the initial progenitor cells as well as whether different GnRH lineage derived-subpopulations play distinct physiological roles. However, it is broadly accepted in mammals, at least after specification, that 1) GnRH-1 neurons migrate from the nasal area to the central nervous system, 2) GnRH-1 neuronal migration is axophilic in that they use axons of the terminal nerve/olfactory pathways to reach their final position [120; 125], and 3) GnRH-1 neurons migrate with other migratory neurons [126] and olfactory ensheathing cells [127; 128]. KS patients have mutations that alter 1 or more of these events.
It is known that KS is a developmental pathology with complex and heterogeneous genetic etiology [129; 130]. Observations, using different mouse models, indicate that syndromic anosmia and defective GnRH neuronal development may result from very different developmental events. Mouse models (summarized in Fig. 6), suggest that KS phenotypes can occur from gene mutations that broadly affect placodal development and therefore correct neurogenesis in the nasal area and craniofacial development. These kinds of mutations will impair the onset of GnRH neurons as well the onset of olfactory vomeronasal/terminal nerve cells [75; 76; 131; 132]. Genes in this category include Fgf8 (see section above) [76; 101; 102; 103; 133], fibroblast growth factor receptor 1 (FGFR1) [134; 135; 136], ‘Fgf8 synexpression genes’ [137], Pax6 [138; 139], and chromodomain helicase DNA-binding protein 7 gene (CHD7) [131; 132; 140; 141].
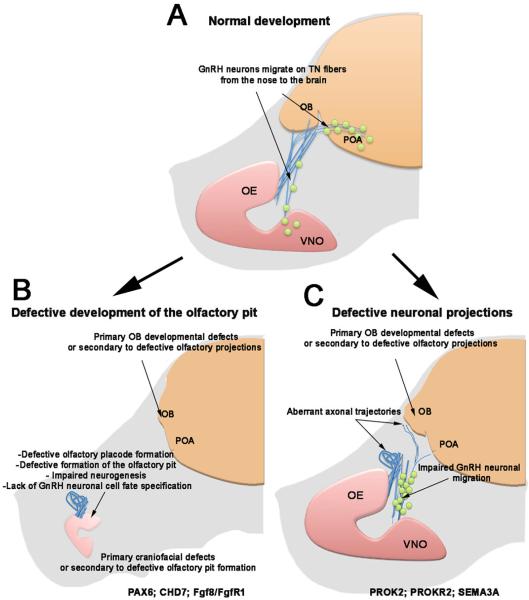
Fig. 6. What happens in KS?
Schematic of mouse embryo: (A) normal projections of olfactory, vomeronasal/terminal fibers (light blue) from the olfactory epithelium (OE) and VNO to the olfactory bulb (OB); GnRH neurons (green) migrating along the putative terminal nerve (TN) to the preoptic area of the hypothalamus (POA). B,C Two different KS developmental scenarios. B) Mouse models with defective olfactory pit formation or olfactory, vomeronasal and/or GnRH-1 neuronal onset. C) Mouse models carrying mutations that affect olfactory/terminal nerve growth or path finding. Defects in OB formation can be secondary to defective neuronal projection to the brain or directly affect the ability of the olfactory fibers to connect to the brain. These cartoons summarize mouse models that recapitulate KS phenotypes linked to mutations on the listed genes.
KS has also been linked to genetic mutations affecting cell adhesion molecules, molecules involved in neuronal path finding, and cell migration in nasal areas (mouse models in Fig. 6C). These genes include anosmin-1 (KAL-1) [129; 142], nasal embryonic LHRH factor NELF [142; 143; 144], SEMA7A [146; 147; 148], chromodomain helicase DNA-binding protein 7 gene (CHD7) [131; 132; 140], SEMA3A [149] and SOX10 [150]. Kal-1 was the first gene identified to co-segregate with the KS phenotype but its mechanism of action in mammals is still unclear and difficult to investigate, as Kal-1 is absent from the mouse genome [151]. Kal-1 gene encodes for a secreted heparin-binding protein (anosmin-1) that interacts with multiple heparan sulfate proteoglycans and has a role in neuroblast migration [152; 153]. Inactivation of Kal-1 in zebrafish and medaka was observed to affect fasciculation and targeting of olfactory sensory neurons, and to disrupt forebrain GnRH neuronal migration [42; 64; 89]. Notably recent data in chicken showed a key role for Anosmin in modulating FGF8 and BMP5 gene expression, cranial neural crest formation, and craniofacial development.
As suggested by mouse models in which prokineticin 2 ligand and receptor (PROK2/PROKR2) [155; 156; 157; 158; 159; 160], SEMA3A [149] or SOX10 genes have been knocked out or mutated [150], other forms of KS may be due to failure of the olfactory/terminal nerve fibers to establish proper contact with the forebrain [117; 147; 158; 161]. Lack of these fibers connecting with the brain can result from cell autonomous defects in olfactory neurons or defective formation of olfactory bulb (OB). Impaired OB development is a common phenotype in patients with KS. For a long time, OB induction was thought to depend on connections of olfactory fibers to the brain and not that OB induction influenced olfactory fiber connections [162; 163; 164; 165]. However, it is now known that in mouse lines where the olfactory epithelium fails to form or cannot innervate the brain (e.g. Pax-6 SeyNeu/SeyNeu and Dlx5 KO), OB-like structures still develop [166; 167] while in PROKR2/PROK2 mouse mutants the OBs do not form correctly and the olfactory fibers fail to connect to the brain [158; 168]. These mouse models suggest that genetic mutations affecting central components of the olfactory system could be the cause for both anosmia and HH in specific forms of KS [158; 168] such as mutations in PROKR2, a gene that is involved in both monogenic recessive and digenic/oligogenic KS transmission modes [157].
Olfactory ensheathing cells are they new players in KS defects?
OECs are the glial population of the olfactory system. Recently, two groups [169] [80; 83] showed that OECs, like Schwann cells of the peripheral nervous system, are neural crest derivatives (Fig. 7A, B). Notably, OECs being neural crest derivatives challenged the dogma that the olfactory system was composed of only placodal derivatives and has offered new insight into interpreting human pathologies such as KS that often include multiple neural crest defects.
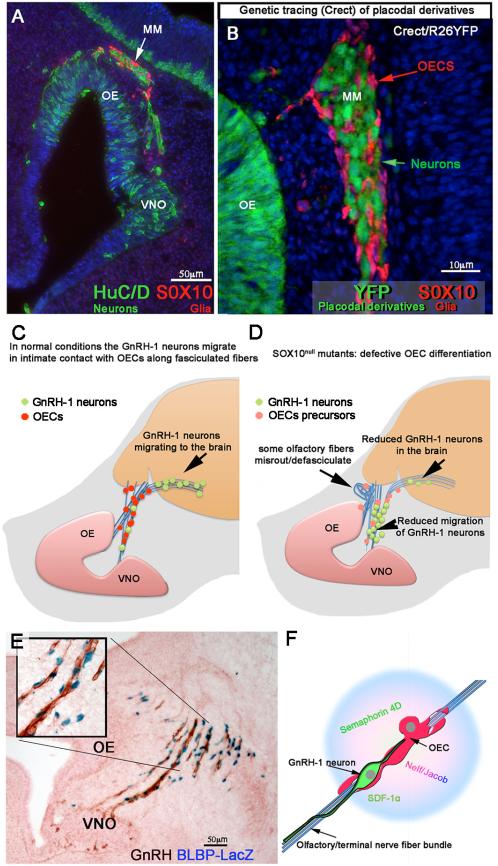
Fig. 7. The olfactory system is composed of placodal and neural crest cells. The OECs are important players in GnRH migration.
A) Section of mouse embryo E11.5 immunostained against HuCD (green, neurons) and Sox10 (Red, olfactory ensheathing cells (OECS)). Over development, the migratory mass (MM) composed of neurons and glia moves away from the olfactory (OE)/vomeronasal epithelia (VNO) toward the brain. B) Crect/RosaYFP genetic lineage tracing reveals placodal derivatives as positive for YFP expression (green). The OECs of the migratory mass are not derived from placodal progenitors. C and D) Diagrams (based on [150; 175]) representing normal GnRH neurons migrating along the olfactory/terminal nerve fibers from the nose to the brain in control animals; (C) In Sox10null mice, where the OECs do not complete maturation (pink dots), fewer GnRH neurons migrate into the brain and olfactory sensory axons misroute/defasisculate (D). Olfactory, vomeronasal and terminal fibers (blue), GnRH neurons green, OECs red in control, pink OECs precursors in Sox10null mutant. E) BLBP is expressed by the OECS; X-Gal staining (blue) on a section from BLBPCreIRESLacZ, [207], embryo, E12.5 immunostained for GnRH-1 (brown). GnRH-1 neurons migrate (brown) in close contact to maturing OECs (blue nuclei) (see insert). F) Model based on [127] representing OECS releasing molecules important for GnRH neuronal migration/motility on olfactory/terminal fibers.
The transcription factor SOX10, a member of the SRY-related HMG-box family, has a key role in defining the identity of several neural crest-derived cell populations, including Schwann cells and OECs [170; 171; 172; 173]. Recent studies showed that SOX10 is required for terminal glial differentiation [170; 172; 174] and that loss of function of the Sox10 gene leads to complete absence of mature Schwann cells and impaired OEC differentiation [171; 175]. Animal models [176; 177; 178; 179] have linked SOX10 gene mutations to impaired maturation of OECs, tangle formation and misrouting of some olfactory axons, and defective GnRH-1 neuron migration to the brain (Figure 7C,D). Genetic studies in humans have linked SOX10 gene mutations to demyelinating neuropathies and Waardenburg-Hirschsprung syndrome [176; 177; 178; 179]. This syndrome is a rare genetic disorder characterized by a spectrum of developmental defects that include pigmentation abnormalities in hair, eyes and skin, craniofacial defects, deafness, various neurologic manifestations and olfactory bulb agenesis. Examination of KS patients with deafness revealed that ~1/3 of these patients carry a SOX10 loss-of-function mutation, indicating a substantial involvement of this gene in some forms of KS [150].
Although OECs share a high level of genetic and molecular similarities with Schwann cells [127; 181; 182; 183; 184; 185; 186; 187; 188; 189; 190], OECs do not myelinate olfactory axons but surround (ensheath) them as they exit the olfactory epithelium and and project to the olfactory bulb [162; 163; 190; 191]. OECs are found in close apposition to developing olfactory axons from the earliest stages of olfactory development, E10.5-E11.5 in mouse [81; 192]. At this stage, pioneer olfactory axons, terminal nerve fibers and migratory neurons (together forming the migratory mass) start to emerge from the olfactory epithelium [192; 193], also reviewed in [81]. GnRH-1 neurons, as part of the migratory mass, travel together with other neuronal cells from the olfactory pit apposed to growing fibers and OECs [127; 192] (Fig. 7E). A potential guidance role for maturing OECs or OECs precursors in the migratory mass was suggested almost two decades ago [194]. OECs play an active role in growth, fasciculation, axon motility and patterning of primary olfactory neurons [191; 195; 196; 197; 198; 199; 200]. Since OECs extend processes early in development, it is possible that olfactory axon growth and pathfinding, is influenced by OECs. Notably some of the molecules known to be crucial in controlling GnRH-1 neuronal migration, such as Semaphorin 4D, NELF/Jacob, and SDF-1α [43; 142; 143; 144; 145; 201] are expressed by OECs [127; 202; 203] (Fig. 7F). Thus, OECs might be important sources of factors needed for appropriate migratory mass movement [127; 204]. However, it is worth noting that even in Sox 10 gene null mouse embryos, ~20-30% of the total GnRH-1 neuronal population migrated into the brain, and OECs precursors (even if in an immature state) were identified along the olfactory axon/GnRH-1 neuron migratory route to the brain. Thus, as one would predict for such an important physiological system, multiple and probably redundant mechanisms are in place to ensure enough GnRH-1 neurons [205] enter the brain for reproductive competency.
From the examples above, it is clear that the neural crest derived OECs may be important players in both normal and abnormal olfactory development and GnRH-1 neuronal migration. As such, the role of these cells in the etiology of KS defects needs to be further investigated. In fact, HH can be syndromic with a number of neurological symptoms such as bimanual synkinesia (mirror movements of the hands), abnormal visual spatial attention, ocular motor abnormalities, sensory neural hearing loss and various degrees of cerebellar ataxia. These “satellite” defects could be, as Sox10 gene mutations suggest [150; 171; 177], manifestations of broad gliopathies affecting both central and peripheral system, and therefore a more global cell type to consider in HH.
Conclusions
In the last thirty years we have reached a better understanding of the cellular composition of the developing olfactory area, development of the GnRH-1 cells and perturbations leading to HH/KS. However, we still do not fully understand the cell types involved in GnRH-1 neuronal development. Analysis of different animal models have revealed that GnRH-1 and its paralogs are broadly and differentially expressed in the animal kingdom. Evidence indicates that GnRH peptide isoforms that control gonadotropin function are associated with the olfactory/terminal nerve system. A mix embryonic lineage or genetic heterogeneity has been shown for GnRH-1 neurons and for the cells of the olfactory/vomeronasal systems [80; 82; 83; 84]. The physiological functional significance of this, if any, is currently unknown. New evidence suggests that the OECs and certainly the nasal mesenchyme are important for normal GnRH neuronal development. These newly discovered developmental relationships highlight unexplored genetic mutations that may underlie the etiology and associated symptoms of HH and as such, may allow better clinical diagnosis and potential classification of the related syndromes. As we identify new molecules that are important for craniofacial development and olfactory/GnRH neurogenesis, such as Fgf8, we need to understand if KS and normosmic forms of HH reflect a spectrum of neurocristopathies as suggested by “satellite” defects such as lack of teeth, plate cleft, craniofacial defects, pigmentation defects and other neurological defects. Clearly, early changes in neural crest development and craniofacial development can lead to acute disruption in factors balancing GnRH neurogenesis and, as such, be the direct cause of some forms of HH.
Highlights.
A general overview of the Hypothalamic–Pituitary–Gonadal (HPG) axis is provided
What are the different forms of GnRH peptides in vertebrates?
Developmental defects linking anosmia and hypogonadotropic hypogonadism in mouse models and humans
Neural crest derivatives such as craniofacial tissues and olfactory ensheathing cells play key roles in controlling neurogenesis and migration of the GnRH neurons.
Acknowledgments
Funding –This work was supported by the Intramural Research Program of the NIH, NINDS. Paolo E. Forni's research is supported by Departmental Startup Grant SUNY, Albany.
ABBREVIATIONS USED
- GnRH-1
Gonadotropin Releasing Hormone
- HPG
Hypothalamic–Pituitary–Gonadal
- LH
luteinizing hormone
- FSH
and follicle stimulating hormone
- GnRHR
Gonadotropin Releasing Hormone Receptor
- HH
hypogonadotropic hypogonadism
- TN
terminal nerve
- OMP
olfactory marker protein
- KS
Kallmann syndrome
- OB
olfactory Bulb
- OECs
Olfactory ensheathing cells
- OP
- E
embryonic day
- FGF8
fibroblast growth factor 8
- BMP
bone morphogenic protein
- BLBP
Brain lipid-binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sower SA, Freamat M, Kavanaugh SI. The origins of the vertebrate hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-thyroid (HPT) endocrine systems: new insights from lampreys. General and comparative endocrinology. 2009;161:20–9. doi: 10.1016/j.ygcen.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 2.Campbell RK, Satoh N, Degnan BM. Piecing together evolution of the vertebrate endocrine system. Trends Genet. 2004;20:359–66. doi: 10.1016/j.tig.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain research. Molecular brain research. 1989;6:311–26. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- 4.Quinton R, Hasan W, Grant W, Thrasivoulou C, Quiney RE, Besser GM, Bouloux PM. Gonadotropin-releasing hormone immunoreactivity in the nasal epithelia of adults with Kallmann's syndrome and isolated hypogonadotropic hypogonadism and in the early midtrimester human fetus. The Journal of clinical endocrinology and metabolism. 1997;82:309–14. doi: 10.1210/jcem.82.1.3673. [DOI] [PubMed] [Google Scholar]
- 5.Wray S. Development of gonadotropin-releasing hormone-1 neurons. Front Neuroendocrinol. 2002;23:292–316. doi: 10.1016/s0091-3022(02)00001-8. [DOI] [PubMed] [Google Scholar]
- 6.Cattanach BM, Iddon CA, Charlton HM, Chiappa SA, Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–40. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- 7.Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schutz G, Herbison AE. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. doi: 10.1038/ncomms3492. [DOI] [PubMed] [Google Scholar]
- 8.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr., Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 9.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacobini P, Parkash J, Campagne C, Messina A, Casoni F, Vanacker C, Langlet F, Hobo B, Cagnoni G, Gallet S, Hanchate NK, Mazur D, Taniguchi M, Mazzone M, Verhaagen J, Ciofi P, Bouret SG, Tamagnone L, Prevot V. Brain endothelial cells control fertility through ovariansteroid-dependent release of semaphorin 3A. PLoS biology. 2014;12:e1001808. doi: 10.1371/journal.pbio.1001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tello JA, Newton CL, Bouligand J, Guiochon-Mantel A, Millar RP, Young J. Congenital hypogonadotropic hypogonadism due to GnRH receptor mutations in three brothers reveal sites affecting conformation and coupling. PLoS One. 2012;7:e38456. doi: 10.1371/journal.pone.0038456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chevrier L, Guimiot F, de Roux N. GnRH receptor mutations in isolated gonadotropic deficiency. Molecular and cellular endocrinology. 2011;346:21–8. doi: 10.1016/j.mce.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Pralong FP, Gomez F, Castillo E, Cotecchia S, Abuin L, Aubert ML, Portmann L, Gaillard RC. Complete hypogonadotropic hypogonadism associated with a novel inactivating mutation of the gonadotropin-releasing hormone receptor. The Journal of clinical endocrinology and metabolism. 1999;84:3811–6. doi: 10.1210/jcem.84.10.6042. [DOI] [PubMed] [Google Scholar]
- 14.Okubo K, Nagahama Y. Structural and functional evolution of gonadotropin-releasing hormone in vertebrates. Acta Physiol (Oxf) 2008;193:3–15. doi: 10.1111/j.1748-1716.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 15.Kelsall R, Coe IR, Sherwood NM. Phylogeny and ontogeny of gonadotropin-releasing hormone: comparison of guinea pig, rat, and a protochordate. General and comparative endocrinology. 1990;78:479–94. doi: 10.1016/0016-6480(90)90037-m. [DOI] [PubMed] [Google Scholar]
- 16.Montaner AD, Somoza GM, King JA, Bianchini JJ, Bolis CG, Affanni JM. Chromatographic and immunological identification of GnRH (gonadotropin-releasing hormone) variants. Occurrence of mammalian and a salmon-like GnRH in the forebrain of an eutherian mammal: Hydrochaeris hydrochaeris (Mammalia, Rodentia). Regul Pept. 1998;73:197–204. doi: 10.1016/s0167-0115(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 17.Uchida K, Moriyama S, Chiba H, Shimotani T, Honda K, Miki M, Takahashi A, Sower SA, Nozaki M. Evolutionary origin of a functional gonadotropin in the pituitary of the most primitive vertebrate, hagfish. Proc Natl Acad Sci U S A. 2010;107:15832–7. doi: 10.1073/pnas.1002208107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavanaugh SI, Nozaki M, Sower SA. Origins of gonadotropin-releasing hormone (GnRH) in vertebrates: identification of a novel GnRH in a basal vertebrate, the sea lamprey. Endocrinology. 2008;149:3860–9. doi: 10.1210/en.2008-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lethimonier C, Madigou T, Munoz-Cueto JA, Lareyre JJ, Kah O. Evolutionary aspects of GnRHs, GnRH neuronal systems and GnRH receptors in teleost fish. General and comparative endocrinology. 2004;135:1–16. doi: 10.1016/j.ygcen.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Powell RC, Ciarcia G, Lance V, Millar RP, King JA. Identification of diverse molecular forms of GnRH in reptile brain. Peptides. 1986;7:1101–8. doi: 10.1016/0196-9781(86)90140-3. [DOI] [PubMed] [Google Scholar]
- 21.Calvin JL, Slater CH, Bolduc TG, Laudano AP, Sower SA. Multiple molecular forms of gonadotropin-releasing hormone in the brain of an elasmobranch: evidence for IR-lamprey GnRH. Peptides. 1993;14:725–9. doi: 10.1016/0196-9781(93)90104-o. [DOI] [PubMed] [Google Scholar]
- 22.Meglio M, Masucci M, D'Aniello B, Lela L, Rastogi RK. Immunohistochemical localization of multiple forms of gonadotropin-releasing hormone in the brain of the adult frog. Journal of neuroendocrinology. 1991;3:363–8. doi: 10.1111/j.1365-2826.1991.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 23.Sherwood NM, Zoeller RT, Moore FL. Multiple forms of gonadotropin-releasing hormone in amphibian brains. General and comparative endocrinology. 1986;61:313–22. doi: 10.1016/0016-6480(86)90208-x. [DOI] [PubMed] [Google Scholar]
- 24.King JA, Millar RP. Multiple molecular forms of gonadotropin-releasing hormone in teleost fish brain. Peptides. 1985;6:689–94. doi: 10.1016/0196-9781(85)90173-1. [DOI] [PubMed] [Google Scholar]
- 25.Hayes WP, Wray S, Battey JF. The frog gonadotropin-releasing hormone-I (GnRH-I) gene has a mammalian-like expression pattern and conserved domains in GnRH-associated peptide, but brain onset is delayed until metamorphosis. Endocrinology. 1994;134:1835–45. doi: 10.1210/endo.134.4.8137750. [DOI] [PubMed] [Google Scholar]
- 26.Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, Nair RM, Debeljuk L, White WF. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971;173:1036–8. doi: 10.1126/science.173.4001.1036. [DOI] [PubMed] [Google Scholar]
- 27.Gordon JH, Reichlin S. Changes in pituitary responsiveness to luteinizing hormone-releasing factor during the rat estrous cycle. Endocrinology. 1974;94:974–8. doi: 10.1210/endo-94-4-974. [DOI] [PubMed] [Google Scholar]
- 28.King JA, Millar RP. Structure of chicken hypothalamic luteinizing hormone-releasing hormone. II. Isolation and characterization. The Journal of biological chemistry. 1982;257:10729–32. [PubMed] [Google Scholar]
- 29.Miyamoto K, Hasegawa Y, Minegishi T, Nomura M, Takahashi Y, Igarashi M, Kangawa K, Matsuo H. Isolation and characterization of chicken hypothalamic luteinizing hormone-releasing hormone. Biochem Biophys Res Commun. 1982;107:820–7. doi: 10.1016/0006-291x(82)90596-4. [DOI] [PubMed] [Google Scholar]
- 30.Sherwood N, Eiden L, Brownstein M, Spiess J, Rivier J, Vale W. Characterization of a teleost gonadotropin-releasing hormone. Proc Natl Acad Sci U S A. 1983;80:2794–8. doi: 10.1073/pnas.80.9.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oka Y. Three types of gonadotrophin-releasing hormone neurones and steroid-sensitive sexually dimorphic kisspeptin neurones in teleosts. Journal of neuroendocrinology. 2009;21:334–8. doi: 10.1111/j.1365-2826.2009.01850.x. [DOI] [PubMed] [Google Scholar]
- 32.Kawabata Y, Hiraki T, Takeuchi A, Okubo K. Sex differences in the expression of vasotocin/isotocin, gonadotropin-releasing hormone, and tyrosine and tryptophan hydroxylase family genes in the medaka brain. Neuroscience. 2012;218:65–77. doi: 10.1016/j.neuroscience.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Karigo T, Aikawa M, Kondo C, Abe H, Kanda S, Oka Y. Whole brain-pituitary in vitro preparation of the transgenic medaka (Oryzias latipes) as a tool for analyzing the differential regulatory mechanisms of LH and FSH release. Endocrinology. 2014;155:536–47. doi: 10.1210/en.2013-1642. [DOI] [PubMed] [Google Scholar]
- 34.Tostivint H. Evolution of the gonadotropin-releasing hormone (GnRH) gene family in relation to vertebrate tetraploidizations. General and comparative endocrinology. 2011;170:575–81. doi: 10.1016/j.ygcen.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 35.Servili A, Herrera-Perez P, Kah O, Munoz-Cueto JA. The retina is a target for GnRH-3 system in the European sea bass, Dicentrarchus labrax. General and comparative endocrinology. 2012;175:398–406. doi: 10.1016/j.ygcen.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Kanda S, Nishikawa K, Karigo T, Okubo K, Isomae S, Abe H, Kobayashi D, Oka Y. Regular pacemaker activity characterizes gonadotropin-releasing hormone 2 neurons recorded from green fluorescent protein-transgenic medaka. Endocrinology. 2010;151:695–701. doi: 10.1210/en.2009-0842. [DOI] [PubMed] [Google Scholar]
- 37.Maruska KP, Tricas TC. Gonadotropin-releasing hormone (GnRH) modulates auditory processing in the fish brain. Horm Behav. 2011;59:451–64. doi: 10.1016/j.yhbeh.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Servili A, Lethimonier C, Lareyre JJ, Lopez-Olmeda JF, Sanchez-Vazquez FJ, Kah O, Munoz-Cueto JA. The Highly conserved gonadotropin-releasing hormone-2 form acts as a melatonin-releasing factor in the pineal of a teleost fish, the european sea bass Dicentrarchus labrax. Endocrinology. 2010;151:2265–75. doi: 10.1210/en.2009-1207. [DOI] [PubMed] [Google Scholar]
- 39.Stewart AJ, Katz AA, Millar RP, Morgan K. Retention and silencing of prepro-GnRH-II and type II GnRH receptor genes in mammals. Neuroendocrinology. 2009;90:416–32. doi: 10.1159/000233303. [DOI] [PubMed] [Google Scholar]
- 40.Abraham E, Palevitch O, Gothilf Y, Zohar Y. The zebrafish as a model system for forebrain GnRH neuronal development. General and comparative endocrinology. 2009;164:151–60. doi: 10.1016/j.ygcen.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Zohar Y, Munoz-Cueto JA, Elizur A, Kah O. Neuroendocrinology of reproduction in teleost fish. Gen Comp Endocrinol. 2010;165:438–55. doi: 10.1016/j.ygcen.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Yanicostas C, Herbomel E, Dipietromaria A, Soussi-Yanicostas N. Anosmin-1a is required for fasciculation and terminal targeting of olfactory sensory neuron axons in the zebrafish olfactory system. Molecular and cellular endocrinology. 2009;312:53–60. doi: 10.1016/j.mce.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Palevitch O, Abraham E, Borodovsky N, Levkowitz G, Zohar Y, Gothilf Y. Cxcl12a-Cxcr4b signaling is important for proper development of the forebrain GnRH system in zebrafish. General and comparative endocrinology. 2010;165:262–8. doi: 10.1016/j.ygcen.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Palevitch O, Kight K, Abraham E, Wray S, Zohar Y, Gothilf Y. Ontogeny of the GnRH systems in zebrafish brain: in situ hybridization and promoter-reporter expression analyses in intact animals. Cell and tissue research. 2007;327:313–22. doi: 10.1007/s00441-006-0279-0. [DOI] [PubMed] [Google Scholar]
- 45.Fernald RD, White RB. Gonadotropin-releasing hormone genes: phylogeny, structure, and functions. Front Neuroendocrinol. 1999;20:224–40. doi: 10.1006/frne.1999.0181. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman GE, Lee W-S, Wray S. Gonadotropin Releasing Hormone (GnRH). In: C.B.N., editor. Neuroendocrinology. CRC Press, Inc.; Boca Raton, Florida: 1992. [Google Scholar]
- 47.Mason AJ, Hayflick JS, Zoeller RT, Young WS, 3rd, Phillips HS, Nikolics K, Seeburg PH. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986;234:1366–71. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- 48.Mason AJ, Pitts SL, Nikolics K, Szonyi E, Wilcox JN, Seeburg PH, Stewart TA. The hypogonadal mouse: reproductive functions restored by gene therapy. Science. 1986;234:1372–8. doi: 10.1126/science.3097822. [DOI] [PubMed] [Google Scholar]
- 49.Bouligand J, Ghervan C, Tello JA, Brailly-Tabard S, Salenave S, Chanson P, Lombes M, Millar RP, Guiochon-Mantel A, Young J. Isolated familial hypogonadotropic hypogonadism and a GNRH1 mutation. N Engl J Med. 2009;360:2742–8. doi: 10.1056/NEJMoa0900136. [DOI] [PubMed] [Google Scholar]
- 50.Kallmann FJ BS. The genetic aspects of primary eunuchoidism. J Ment Defic. 1944;48:203–236. [Google Scholar]
- 51.Wray S. From nose to brain: development of gonadotrophin-releasing hormone-1 neurones. Journal of neuroendocrinology. 2010;22:743–53. doi: 10.1111/j.1365-2826.2010.02034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Karigo T, Oka Y. Neurobiological Study of Fish Brains Gives Insights into the Nature of Gonadotropin-Releasing Hormone 1-3 Neurons. Frontiers in endocrinology. 2013;4:177. doi: 10.3389/fendo.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–4. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 54.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86:8132–6. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wray S, Nieburgs A, Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain research. Developmental brain research. 1989;46:309–18. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]
- 56.Wray S, Key S, Qualls R, Fueshko SM. A subset of peripherin positive olfactory axons delineates the luteinizing hormone releasing hormone neuronal migratory pathway in developing mouse. Dev Biol. 1994;166:349–54. doi: 10.1006/dbio.1994.1320. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida K, Tobet SA, Crandall JE, Jimenez TP, Schwarting GA. The migration of luteinizing hormone-releasing hormone neurons in the developing rat is associated with a transient, caudal projection of the vomeronasal nerve. J Neurosci. 1995;15:7769–77. doi: 10.1523/JNEUROSCI.15-12-07769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakami S, Seki T, Wakabayashi K, Arai Y. The ontogeny of luteinizing hormone-releasing hormone (LHRH) producing neurons in the chick embryo: possible evidence for migrating LHRH neurons from the olfactory epithelium expressing a highly polysialylated neural cell adhesion molecule. Neurosci Res. 1991;12:421–31. doi: 10.1016/0168-0102(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 59.Rastogi RK, Meyer DL, Pinelli C, Fiorentino M, D'Aniello B. Comparative analysis of GnRH neuronal systems in the amphibian brain. General and comparative endocrinology. 1998;112:330–45. doi: 10.1006/gcen.1998.7144. [DOI] [PubMed] [Google Scholar]
- 60.Iela L, Powell JF, Sherwood NM, D'Aniello B, Rastogi RK, Bagnara JT. Reproduction in the Mexican leaf frog, Pachymedusa dacnicolor. VI. Presence and distribution of multiple GnRH forms in the brain. General and comparative endocrinology. 1996;103:235–43. doi: 10.1006/gcen.1996.0117. [DOI] [PubMed] [Google Scholar]
- 61.D'Aniello B, Pinelli C, King JA, Rastogi RK. Neuroanatomical organization of GnRH neuronal systems in the lizard (Podarcis s. sicula) brain during development. Brain Res. 1994;657:221–6. doi: 10.1016/0006-8993(94)90971-7. [DOI] [PubMed] [Google Scholar]
- 62.Sabado V, Barraud P, Baker CV, Streit A. Specification of GnRH-1 neurons by antagonistic FGF and retinoic acid signaling. Developmental biology. 2011 doi: 10.1016/j.ydbio.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skynner MJ, Slater R, Sim JA, Allen ND, Herbison AE. Promoter transgenics reveal multiple gonadotropin-releasing hormone-I-expressing cell populations of different embryological origin in mouse brain. J Neurosci. 1999;19:5955–66. doi: 10.1523/JNEUROSCI.19-14-05955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okubo K, Sakai F, Lau EL, Yoshizaki G, Takeuchi Y, Naruse K, Aida K, Nagahama Y. Forebrain gonadotropin-releasing hormone neuronal development: insights from transgenic medaka and the relevance to X-linked Kallmann syndrome. Endocrinology. 2006;147:1076–84. doi: 10.1210/en.2005-0468. [DOI] [PubMed] [Google Scholar]
- 65.Casan EM, Raga F, Polan ML. GnRH mRNA and protein expression in human preimplantation embryos. Molecular human reproduction. 1999;5:234–9. doi: 10.1093/molehr/5.3.234. [DOI] [PubMed] [Google Scholar]
- 66.Jasoni CL, Porteous RW, Herbison AE. Anatomical location of mature GnRH neurons corresponds with their birthdate in the developing mouse. Dev Dyn. 2009;238:524–31. doi: 10.1002/dvdy.21869. [DOI] [PubMed] [Google Scholar]
- 67.Forni PE, Fornaro M, Guenette S, Wray S. A role for FE65 in controlling GnRH-1 neurogenesis. J Neurosci. 2011;31:480–91. doi: 10.1523/JNEUROSCI.4698-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akutsu S, Takada M, Ohki-Hamazaki H, Murakami S, Arai Y. Origin of luteinizing hormone-releasing hormone (LHRH) neurons in the chick embryo: effect of the olfactory placode ablation. Neuroscience letters. 1992;142:241–4. doi: 10.1016/0304-3940(92)90382-h. [DOI] [PubMed] [Google Scholar]
- 69.Murakami S, Kikuyama S, Arai Y. The origin of the luteinizing hormone-releasing hormone (LHRH) neurons in newts (Cynops pyrrhogaster): the effect of olfactory placode ablation. Cell and tissue research. 1992;269:21–7. doi: 10.1007/BF00384722. [DOI] [PubMed] [Google Scholar]
- 70.Dellovade TL, Pfaff DW, Schwanzel-Fukuda M. The gonadotropin-releasing hormone system does not develop in Small-Eye (Sey) mouse phenotype. Brain research. Developmental brain research. 1998;107:233–40. doi: 10.1016/s0165-3806(98)00007-8. [DOI] [PubMed] [Google Scholar]
- 71.Abraham E, Palevitch O, Gothilf Y, Zohar Y. Targeted gonadotropin-releasing hormone-3 neuron ablation in zebrafish: effects on neurogenesis, neuronal migration, and reproduction. Endocrinology. 2010;151:332–40. doi: 10.1210/en.2009-0548. [DOI] [PubMed] [Google Scholar]
- 72.el Amraoui A, Dubois PM. Experimental evidence for an early commitment of gonadotropin-releasing hormone neurons, with special regard to their origin from the ectoderm of nasal cavity presumptive territory. Neuroendocrinology. 1993;57:991–1002. doi: 10.1159/000126490. [DOI] [PubMed] [Google Scholar]
- 73.Kramer PR, Guerrero G, Krishnamurthy R, Mitchell PJ, Wray S. Ectopic expression of luteinizing hormone-releasing hormone and peripherin in the respiratory epithelium of mice lacking transcription factor AP-2alpha. Mech Dev. 2000;94:79–94. doi: 10.1016/s0925-4773(00)00316-6. [DOI] [PubMed] [Google Scholar]
- 74.Whitlock KE. Origin and development of GnRH neurons. Trends Endocrinol Metab. 2005;16:145–51. doi: 10.1016/j.tem.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–23. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- 76.Chung WC, Moyle SS, Tsai PS. Fibroblast growth factor 8 signaling through fibroblast growth factor receptor 1 is required for the emergence of gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:4997–5003. doi: 10.1210/en.2007-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y, Lin MC, Farajzadeh M, Wayne NL. Early development of the gonadotropin-releasing hormone neuronal network in transgenic zebrafish. Front Endocrinol (Lausanne) 2013;4:107. doi: 10.3389/fendo.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Forni PE, Bharti K, Flannery EM, Shimogori T, Wray S. The indirect role of fibroblast growth factor-8 in defining neurogenic niches of the olfactory/GnRH systems. J Neurosci. 2013;33:19620–34. doi: 10.1523/JNEUROSCI.3238-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Couly G, Grapin-Botton A, Coltey P, Ruhin B, Le Douarin NM. Determination of the identity of the derivatives of the cephalic neural crest: incompatibility between Hox gene expression and lower jaw development. Development. 1998;125:3445–59. doi: 10.1242/dev.125.17.3445. [DOI] [PubMed] [Google Scholar]
- 80.Forni PE, Taylor-Burds C, Melvin VS, Williams T, Wray S. Neural crest and ectodermal cells intermix in the nasal placode to give rise to GnRH-1 neurons, sensory neurons, and olfactory ensheathing cells. J Neurosci. 2011;31:6915–27. doi: 10.1523/JNEUROSCI.6087-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Forni PE, Wray S. Neural crest and olfactory system: new prospective. Molecular neurobiology. 2012;46:349–60. doi: 10.1007/s12035-012-8286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Saxena A, Peng BN, Bronner ME. Sox10-dependent neural crest origin of olfactory microvillous neurons in zebrafish. eLife. 2013;2:e00336. doi: 10.7554/eLife.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katoh H, Shibata S, Fukuda K, Sato M, Satoh E, Nagoshi N, Minematsu T, Matsuzaki Y, Akazawa C, Toyama Y, Nakamura M, Okano H. The dual origin of the peripheral olfactory system: placode and neural crest. Mol Brain. 2011;4:34. doi: 10.1186/1756-6606-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki J, Yoshizaki K, Kobayashi T, Osumi N. Neural crest-derived horizontal basal cells as tissue stem cells in the adult olfactory epithelium. Neurosci Res. 2013;75:112–20. doi: 10.1016/j.neures.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 85.Harden MV, Pereiro L, Ramialison M, Wittbrodt J, Prasad MK, McCallion AS, Whitlock KE. Close association of olfactory placode precursors and cranial neural crest cells does not predestine cell mixing. Dev Dyn. 2012;241:1143–54. doi: 10.1002/dvdy.23797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steventon B, Mayor R, Streit A. Neural crest and placode interaction during the development of the cranial sensory system. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patthey C, Schlosser G, Shimeld SM. The evolutionary history of vertebrate cranial placodes -I: Cell type evolution. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 88.Stevenson EL, Corella KM, Chung WC. Ontogenesis of gonadotropin-releasing hormone neurons: a model for hypothalamic neuroendocrine cell development. Front Endocrinol (Lausanne) 2013;4:89. doi: 10.3389/fendo.2013.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whitlock KE, Smith KM, Kim H, Harden MV. A role for foxd3 and sox10 in the differentiation of gonadotropin-releasing hormone (GnRH) cells in the zebrafish Danio rerio. Development. 2005;132:5491–502. doi: 10.1242/dev.02158. [DOI] [PubMed] [Google Scholar]
- 90.Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–29. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- 91.Rawson NE, Lischka FW, Yee KK, Peters AZ, Tucker ES, Meechan DW, Zirlinger M, Maynard TM, Burd GB, Dulac C, Pevny L, LaMantia AS. Specific mesenchymal/epithelial induction of olfactory receptor, vomeronasal, and gonadotropin-releasing hormone (GnRH) neurons. Dev Dyn. 2010;239:1723–38. doi: 10.1002/dvdy.22315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rawson NE, LaMantia AS. Once and again: retinoic acid signaling in the developing and regenerating olfactory pathway. J Neurobiol. 2006;66:653–76. doi: 10.1002/neu.20236. [DOI] [PubMed] [Google Scholar]
- 93.LaMantia AS, Bhasin N, Rhodes K, Heemskerk J. Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron. 2000;28:411–25. doi: 10.1016/s0896-6273(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 94.Metzis V, Courtney AD, Kerr MC, Ferguson C, Rondon Galeano MC, Parton RG, Wainwright BJ, Wicking C. Patched1 is required in neural crest cells for the prevention of orofacial clefts. Human molecular genetics. 2013 doi: 10.1093/hmg/ddt353. [DOI] [PubMed] [Google Scholar]
- 95.Duggan CD, DeMaria S, Baudhuin A, Stafford D, Ngai J. Foxg1 is required for development of the vertebrate olfactory system. J Neurosci. 2008;28:5229–39. doi: 10.1523/JNEUROSCI.1134-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, Bober E, Barbieri O, Simeone A, Levi G. Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development. 1999;126:3795–809. doi: 10.1242/dev.126.17.3795. [DOI] [PubMed] [Google Scholar]
- 97.Collinson JM, Quinn JC, Hill RE, West JD. The roles of Pax6 in the cornea, retina, and olfactory epithelium of the developing mouse embryo. Dev Biol. 2003;255:303–12. doi: 10.1016/s0012-1606(02)00095-7. [DOI] [PubMed] [Google Scholar]
- 98.Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev Biol. 2009;326:75–85. doi: 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Griffin JN, Compagnucci C, Hu D, Fish J, Klein O, Marcucio R, Depew MJ. Fgf8 dosage determines midfacial integration and polarity within the nasal and optic capsules. Dev Biol. 2013;374:185–97. doi: 10.1016/j.ydbio.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Creuzet S, Schuler B, Couly G, Le Douarin NM. Reciprocal relationships between Fgf8 and neural crest cells in facial and forebrain development. Proc Natl Acad Sci U S A. 2004;101:4843–7. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trarbach EB, Abreu AP, Silveira LF, Garmes HM, Baptista MT, Teles MG, Costa EM, Mohammadi M, Pitteloud N, Mendonca BB, Latronico AC. Nonsense mutations in FGF8 gene causing different degrees of human gonadotropin-releasing deficiency. J Clin Endocrinol Metab. 2010;95:3491–6. doi: 10.1210/jc.2010-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–31. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dode C, Hardelin JP. Kallmann syndrome: fibroblast growth factor signaling insufficiency? J Mol Med. 2004;82:725–34. doi: 10.1007/s00109-004-0571-y. [DOI] [PubMed] [Google Scholar]
- 104.Maier E, Nord H, von Hofsten J, Gunhaga L. A balance of BMP and notch activity regulates neurogenesis and olfactory nerve formation. PLoS One. 2011;6:e17379. doi: 10.1371/journal.pone.0017379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maier E, von Hofsten J, Nord H, Fernandes M, Paek H, Hebert JM, Gunhaga L. Opposing Fgf and Bmp activities regulate the specification of olfactory sensory and respiratory epithelial cell fates. Development. 2010;137:1601–11. doi: 10.1242/dev.051219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–3. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 107.Stottmann RW, Anderson RM, Klingensmith J. The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Developmental biology. 2001;240:457–73. doi: 10.1006/dbio.2001.0479. [DOI] [PubMed] [Google Scholar]
- 108.Kawauchi S, Kim J, Santos R, Wu HH, Lander AD, Calof AL. Foxg1 promotes olfactory neurogenesis by antagonizing Gdf11. Development. 2009;136:1453–64. doi: 10.1242/dev.034967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mousley A, Polese G, Marks NJ, Eisthen HL. Terminal nerve-derived neuropeptide y modulates physiological responses in the olfactory epithelium of hungry axolotls (Ambystoma mexicanum). J Neurosci. 2006;26:7707–17. doi: 10.1523/JNEUROSCI.1977-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Valverde F, Heredia M, Santacana M. Characterization of neuronal cell varieties migrating from the olfactory epithelium during prenatal development in the rat. Immunocytochemical study using antibodies against olfactory marker protein (OMP) and luteinizing hormone-releasing hormone (LH-RH). Brain research. Developmental brain research. 1993;71:209–20. doi: 10.1016/0165-3806(93)90173-8. [DOI] [PubMed] [Google Scholar]
- 111.Tobet SA, Chickering TW, King JC, Stopa EG, Kim K, Kuo-Leblank V, Schwarting GA. Expression of gamma-aminobutyric acid and gonadotropin-releasing hormone during neuronal migration through the olfactory system. Endocrinology. 1996;137:5415–20. doi: 10.1210/endo.137.12.8940365. [DOI] [PubMed] [Google Scholar]
- 112.Verney C, el Amraoui A, Zecevic N. Comigration of tyrosine hydroxylase- and gonadotropin-releasing hormone-immunoreactive neurons in the nasal area of human embryos. Brain research. Developmental brain research. 1996;97:251–9. doi: 10.1016/s0165-3806(96)00147-2. [DOI] [PubMed] [Google Scholar]
- 113.Wirsig CR, Leonard CM. Acetylcholinesterase and luteinizing hormone-releasing hormone distinguish separate populations of terminal nerve neurons. Neuroscience. 1986;19:719–40. doi: 10.1016/0306-4522(86)90295-2. [DOI] [PubMed] [Google Scholar]
- 114.Vecino E, Ekstrom P. Colocalization of neuropeptide Y (NPY)-like and FMRFamide-like immunoreactivities in the brain of the Atlantic salmon (Salmo salar). Cell and tissue research. 1992;270:435–42. doi: 10.1007/BF00645044. [DOI] [PubMed] [Google Scholar]
- 115.Wright DE, Demski LS. Organization of GnRH and FMRF-amide systems in two primitive bony fishes (order polypteriformes). Brain, behavior and evolution. 1996;47:267–78. doi: 10.1159/000113246. [DOI] [PubMed] [Google Scholar]
- 116.Key S, Wray S. Two olfactory placode derived galanin subpopulations: luteinizing hormone-releasing hormone neurones and vomeronasal cells. Journal of neuroendocrinology. 2000;12:535–45. doi: 10.1046/j.1365-2826.2000.00486.x. [DOI] [PubMed] [Google Scholar]
- 117.Cariboni A, Davidson K, Rakic S, Maggi R, Parnavelas JG, Ruhrberg C. Defective gonadotropin-releasing hormone neuron migration in mice lacking SEMA3A signalling through NRP1 and NRP2: implications for the aetiology of hypogonadotropic hypogonadism. Human molecular genetics. 2011;20:336–44. doi: 10.1093/hmg/ddq468. [DOI] [PubMed] [Google Scholar]
- 118.Giacobini P, Prevot V. Semaphorins in the development, homeostasis and disease of hormone systems. Seminars in cell & developmental biology. 2013;24:190–8. doi: 10.1016/j.semcdb.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 119.Messina A, Giacobini P. Semaphorin Signaling in the Development and Function of the Gonadotropin Hormone-Releasing Hormone System. Front Endocrinol (Lausanne) 2013;4:133. doi: 10.3389/fendo.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Casoni F, Hutchins BI, Donohue D, Fornaro M, Condie BG, Wray S. SDF and GABA interact to regulate axophilic migration of GnRH neurons. J Cell Sci. 2012;125:5015–25. doi: 10.1242/jcs.101675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Metz H, Wray S. Use of mutant mouse lines to investigate origin of gonadotropin-releasing hormone-1 neurons: lineage independent of the adenohypophysis. Endocrinology. 2010;151:766–73. doi: 10.1210/en.2009-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wierman ME, Kiseljak-Vassiliades K, Tobet S. Gonadotropin-releasing hormone (GnRH) neuron migration: initiation, maintenance and cessation as critical steps to ensure normal reproductive function. Frontiers in neuroendocrinology. 2011;32:43–52. doi: 10.1016/j.yfrne.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Messina A, Giacobini P. Semaphorin Signaling in the Development and Function of the Gonadotropin Hormone-Releasing Hormone System. Frontiers in endocrinology. 2013;4:133. doi: 10.3389/fendo.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martin C, Balasubramanian R, Dwyer AA, Au MG, Sidis Y, Kaiser UB, Seminara SB, Pitteloud N, Zhou QY, Crowley WF., Jr. The role of the prokineticin 2 pathway in human reproduction: evidence from the study of human and murine gene mutations. Endocrine reviews. 2011;32:225–46. doi: 10.1210/er.2010-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hutchins BI, Klenke U, Wray S. Calcium release-dependent actin flow in the leading process mediates axophilic migration. J Neurosci. 2013;33:11361–71. doi: 10.1523/JNEUROSCI.3758-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fornaro M, Geuna S, Fasolo A, Giacobini-Robecchi MG. HuC/D confocal imaging points to olfactory migratory cells as the first cell population that expresses a post-mitotic neuronal phenotype in the chick embryo. Neuroscience. 2003;122:123–8. doi: 10.1016/j.neuroscience.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 127.Geller S, Kolasa E, Tillet Y, Duittoz A, Vaudin P. Olfactory ensheathing cells form the microenvironment of migrating GnRH-1 neurons during mouse development. Glia. 2013;61:550–66. doi: 10.1002/glia.22455. [DOI] [PubMed] [Google Scholar]
- 128.Raucci F, Tiong JD, Wray S. P75 nerve growth factor receptors modulate development of GnRH neurons and olfactory ensheating cells. Frontiers in neuroscience. 2013;7:262. doi: 10.3389/fnins.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hardelin JP, Dode C. The complex genetics of Kallmann syndrome: KAL1, FGFR1, FGF8, PROKR2, PROK2, et al. Sex Dev. 2008;2:181–93. doi: 10.1159/000152034. [DOI] [PubMed] [Google Scholar]
- 130.Hermanussen M, Sippell WG. Heterogeneity of Kallmann's syndrome. Clin Genet. 1985;28:106–11. doi: 10.1111/j.1399-0004.1985.tb00368.x. [DOI] [PubMed] [Google Scholar]
- 131.Layman WS, Hurd EA, Martin DM. Reproductive dysfunction and decreased GnRH neurogenesis in a mouse model of CHARGE syndrome. Human molecular genetics. 2011;20:3138–50. doi: 10.1093/hmg/ddr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ogata T, Fujiwara I, Ogawa E, Sato N, Udaka T, Kosaki K. Kallmann syndrome phenotype in a female patient with CHARGE syndrome and CHD7 mutation. Endocr J. 2006;53:741–3. doi: 10.1507/endocrj.k06-099. [DOI] [PubMed] [Google Scholar]
- 133.Chung WC, Tsai PS. Role of fibroblast growth factor signaling in gonadotropin-releasing hormone neuronal system development. Front Horm Res. 2010;39:37–50. doi: 10.1159/000312692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bailleul-Forestier I, Gros C, Zenaty D, Bennaceur S, Leger J, de Roux N. Dental agenesis in Kallmann syndrome individuals with FGFR1 mutations. International journal of paediatric dentistry / the British Paedodontic Society [and] the International Association of Dentistry for Children. 2010;20:305–12. doi: 10.1111/j.1365-263X.2010.01056.x. [DOI] [PubMed] [Google Scholar]
- 135.Dode C, Fouveaut C, Mortier G, Janssens S, Bertherat J, Mahoudeau J, Kottler ML, Chabrolle C, Gancel A, Francois I, Devriendt K, Wolczynski S, Pugeat M, Pineiro-Garcia A, Murat A, Bouchard P, Young J, Delpech M, Hardelin JP. Novel FGFR1 sequence variants in Kallmann syndrome, and genetic evidence that the FGFR1c isoform is required in olfactory bulb and palate morphogenesis. Human mutation. 2007;28:97–8. doi: 10.1002/humu.9470. [DOI] [PubMed] [Google Scholar]
- 136.Hu Y, Poopalasundaram S, Graham A, Bouloux PM. GnRH neuronal migration and olfactory bulb neurite outgrowth are dependent on FGF receptor 1 signaling, specifically via the PI3K p110alpha isoform in chick embryo. Endocrinology. 2013;154:388–99. doi: 10.1210/en.2012-1555. [DOI] [PubMed] [Google Scholar]
- 137.Miraoui H, Dwyer AA, Sykiotis GP, Plummer L, Chung W, Feng B, Beenken A, Clarke J, Pers TH, Dworzynski P, Keefe K, Niedziela M, Raivio T, Crowley WF, Jr., Seminara SB, Quinton R, Hughes VA, Kumanov P, Young J, Yialamas MA, Hall JE, Van Vliet G, Chanoine JP, Rubenstein J, Mohammadi M, Tsai PS, Sidis Y, Lage K, Pitteloud N. Mutations in FGF17, IL17RD, DUSP6, SPRY4, and FLRT3 are identified in individuals with congenital hypogonadotropic hypogonadism. American journal of human genetics. 2013;92:725–43. doi: 10.1016/j.ajhg.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nomura T, Haba H, Osumi N. Role of a transcription factor Pax6 in the developing vertebrate olfactory system. Development, growth & differentiation. 2007;49:683–90. doi: 10.1111/j.1440-169X.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 139.Compagnucci C, Fish JL, Schwark M, Tarabykin V, Depew MJ. Pax6 regulates craniofacial form through its control of an essential cephalic ectodermal patterning center. Genesis. 2011;49:307–25. doi: 10.1002/dvg.20724. [DOI] [PubMed] [Google Scholar]
- 140.Jongmans MC, van Ravenswaaij-Arts CM, Pitteloud N, Ogata T, Sato N, Claahsen-van der Grinten HL, van der Donk K, Seminara S, Bergman JE, Brunner HG, Crowley WF, Jr., Hoefsloot LH. CHD7 mutations in patients initially diagnosed with Kallmann syndrome--the clinical overlap with CHARGE syndrome. Clin Genet. 2009;75:65–71. doi: 10.1111/j.1399-0004.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schulz Y, Wehner P, Opitz L, Salinas-Riester G, Bongers EM, van Ravenswaaij-Arts CM, Wincent J, Schoumans J, Kohlhase J, Borchers A, Pauli S. CHD7, the gene mutated in CHARGE syndrome, regulates genes involved in neural crest cell guidance. Human genetics. 2014 doi: 10.1007/s00439-014-1444-2. [DOI] [PubMed] [Google Scholar]
- 142.Trarbach EB, Baptista MT, Garmes HM, Hackel C. Molecular analysis of KAL-1, GnRH-R, NELF and EBF2 genes in a series of Kallmann syndrome and normosmic hypogonadotropic hypogonadism patients. The Journal of endocrinology. 2005;187:361–8. doi: 10.1677/joe.1.06103. [DOI] [PubMed] [Google Scholar]
- 143.Kramer PR, Wray S. Nasal embryonic LHRH factor (NELF) expression within the CNS and PNS of the rodent. Brain research. Gene expression patterns. 2001;1:23–6. doi: 10.1016/s1567-133x(01)00004-7. [DOI] [PubMed] [Google Scholar]
- 144.Xu N, Bhagavath B, Kim HG, Halvorson L, Podolsky RS, Chorich LP, Prasad P, Xiong WC, Cameron RS, Layman LC. NELF is a nuclear protein involved in hypothalamic GnRH neuronal migration. Molecular and cellular endocrinology. 2010;319:47–55. doi: 10.1016/j.mce.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Giacobini P, Messina A, Morello F, Ferraris N, Corso S, Penachioni J, Giordano S, Tamagnone L, Fasolo A. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. The Journal of cell biology. 2008;183:555–66. doi: 10.1083/jcb.200806160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Messina A, Ferraris N, Wray S, Cagnoni G, Donohue DE, Casoni F, Kramer PR, Derijck AA, Adolfs Y, Fasolo A, Pasterkamp RJ, Giacobini P. Dysregulation of Semaphorin7A/beta1-integrin signaling leads to defective GnRH-1 cell migration, abnormal gonadal development and altered fertility. Human molecular genetics. 2011;20:4759–74. doi: 10.1093/hmg/ddr403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Parkash J, Cimino I, Ferraris N, Casoni F, Wray S, Cappy H, Prevot V, Giacobini P. Suppression of beta1-integrin in gonadotropin-releasing hormone cells disrupts migration and axonal extension resulting in severe reproductive alterations. J Neurosci. 2012;32:16992–7002. doi: 10.1523/JNEUROSCI.3057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kansakoski J, Fagerholm R, Laitinen EM, Vaaralahti K, Hackman P, Pitteloud N, Raivio T, Tommiska J. Mutation screening of SEMA3A and SEMA7A in patients with congenital hypogonadotropic hypogonadism. Pediatric research. 2014;75:641–4. doi: 10.1038/pr.2014.23. [DOI] [PubMed] [Google Scholar]
- 149.Hanchate NK, Giacobini P, Lhuillier P, Parkash J, Espy C, Fouveaut C, Leroy C, Baron S, Campagne C, Vanacker C, Collier F, Cruaud C, Meyer V, Garcia-Pinero A, Dewailly D, Cortet-Rudelli C, Gersak K, Metz C, Chabrier G, Pugeat M, Young J, Hardelin JP, Prevot V, Dode C. SEMA3A, a gene involved in axonal pathfinding, is mutated in patients with Kallmann syndrome. PLoS genetics. 2012;8:e1002896. doi: 10.1371/journal.pgen.1002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pingault V, Bodereau V, Baral V, Marcos S, Watanabe Y, Chaoui A, Fouveaut C, Leroy C, Verier-Mine O, Francannet C, Dupin-Deguine D, Archambeaud F, Kurtz FJ, Young J, Bertherat J, Marlin S, Goossens M, Hardelin JP, Dode C, Bondurand N. Loss-of-function mutations in SOX10 cause Kallmann syndrome with deafness. American journal of human genetics. 2013;92:707–24. doi: 10.1016/j.ajhg.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Choy C, Kim SH. Biological actions and interactions of anosmin-1. Front Horm Res. 2010;39:78–93. doi: 10.1159/000312695. [DOI] [PubMed] [Google Scholar]
- 152.Maggi R, Cariboni A, Zaninetti R, Samara A, Stossi F, Pimpinelli F, Giacobini P, Consalez GG, Rugarli E, Piva F. Factors involved in the migration of neuroendocrine hypothalamic neurons. Archives italiennes de biologie. 2005;143:171–8. [PubMed] [Google Scholar]
- 153.Hudson ML, Kinnunen T, Cinar HN, Chisholm AD. C. elegans Kallmann syndrome protein KAL-1 interacts with syndecan and glypican to regulate neuronal cell migrations. Dev Biol. 2006;294:352–65. doi: 10.1016/j.ydbio.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 154.Endo Y, Ishiwata-Endo H, Yamada KM. Extracellular matrix protein anosmin promotes neural crest formation and regulates FGF, BMP, and WNT activities. Developmental cell. 2012;23:305–16. doi: 10.1016/j.devcel.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Abreu AP, Trarbach EB, de Castro M, Frade Costa EM, Versiani B, Matias Baptista MT, Garmes HM, Mendonca BB, Latronico AC. Loss-of-function mutations in the genes encoding prokineticin-2 or prokineticin receptor-2 cause autosomal recessive Kallmann syndrome. J Clin Endocrinol Metab. 2008;93:4113–8. doi: 10.1210/jc.2008-0958. [DOI] [PubMed] [Google Scholar]
- 156.Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc JE, Wolczynski S, Delpech M, Petit C, Young J, Hardelin JP. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS genetics. 2006;2:e175. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dode C, Rondard P. PROK2/PROKR2 Signaling and Kallmann Syndrome. Front Endocrinol (Lausanne) 2013;4:19. doi: 10.3389/fendo.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103:4140–5. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ragancokova D, Rocca E, Oonk AM, Schulz H, Rohde E, Bednarsch J, Feenstra I, Pennings RJ, Wende H, Garratt AN. TSHZ1-dependent gene regulation is essential for olfactory bulb development and olfaction. J Clin Invest. 2014;124:1214–27. doi: 10.1172/JCI72466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley WF., Jr. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2007;104:17447–52. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Berghard A, Hagglund AC, Bohm S, Carlsson L. Lhx2-dependent specification of olfactory sensory neurons is required for successful integration of olfactory, vomeronasal, and GnRH neurons. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:3464–72. doi: 10.1096/fj.12-206193. [DOI] [PubMed] [Google Scholar]
- 162.Graziadei PP, Monti-Graziadei AG. The influence of the olfactory placode on the development of the telencephalon in Xenopus laevis. Neuroscience. 1992;46:617–29. doi: 10.1016/0306-4522(92)90149-v. [DOI] [PubMed] [Google Scholar]
- 163.Stout RP, Graziadei PP. Influence of the olfactory placode on the development of the brain in Xenopus laevis (Daudin). I. Axonal growth and connections of the transplanted olfactory placode. Neuroscience. 1980;5:2175–86. doi: 10.1016/0306-4522(80)90134-7. [DOI] [PubMed] [Google Scholar]
- 164.Byrd CA, Burd GD. Morphological and quantitative evaluation of olfactory bulb development in Xenopus after olfactory placode transplantation. The Journal of comparative neurology. 1993;331:551–63. doi: 10.1002/cne.903310410. [DOI] [PubMed] [Google Scholar]
- 165.De Carlos JA, Lopez-Mascaraque L, Valverde F. The telencephalic vesicles are innervated by olfactory placode-derived cells: a possible mechanism to induce neocortical development. Neuroscience. 1995;68:1167–78. doi: 10.1016/0306-4522(95)00199-s. [DOI] [PubMed] [Google Scholar]
- 166.Lopez-Mascaraque L, de Castro F. The olfactory bulb as an independent developmental domain. Cell death and differentiation. 2002;9:1279–86. doi: 10.1038/sj.cdd.4401076. [DOI] [PubMed] [Google Scholar]
- 167.Levi G, Puche AC, Mantero S, Barbieri O, Trombino S, Paleari L, Egeo A, Merlo GR. The Dlx5 homeodomain gene is essential for olfactory development and connectivity in the mouse. Molecular and cellular neurosciences. 2003;22:530–43. doi: 10.1016/s1044-7431(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 168.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–7. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 169.Barraud P, Seferiadis AA, Tyson LD, Zwart MF, Szabo-Rogers HL, Ruhrberg C, Liu KJ, Baker CV. Neural crest origin of olfactory ensheathing glia. Proc Natl Acad Sci U S A. 2010;107:21040–5. doi: 10.1073/pnas.1012248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bosl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. The Journal of cell biology. 2010;189:701–12. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–50. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pozniak CD, Langseth AJ, Dijkgraaf GJ, Choe Y, Werb Z, Pleasure SJ. Sox10 directs neural stem cells toward the oligodendrocyte lineage by decreasing Suppressor of Fused expression. Proc Natl Acad Sci U S A. 2010;107:21795–800. doi: 10.1073/pnas.1016485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–70. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Barraud P, St John JA, Stolt CC, Wegner M, Baker CV. Olfactory ensheathing glia are required for embryonic olfactory axon targeting and the migration of gonadotropin-releasing hormone neurons. Biology open. 2013;2:750–9. doi: 10.1242/bio.20135249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Inoue K, Tanabe Y, Lupski JR. Myelin deficiencies in both the central and the peripheral nervous systems associated with a SOX10 mutation. Annals of neurology. 1999;46:313–8. doi: 10.1002/1531-8249(199909)46:3<313::aid-ana6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 177.Pingault V, Bondurand N, Kuhlbrodt K, Goerich DE, Prehu MO, Puliti A, Herbarth B, Hermans-Borgmeyer I, Legius E, Matthijs G, Amiel J, Lyonnet S, Ceccherini I, Romeo G, Smith JC, Read AP, Wegner M, Goossens M. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet. 1998;18:171–3. doi: 10.1038/ng0298-171. [DOI] [PubMed] [Google Scholar]
- 178.Kuhlbrodt K, Schmidt C, Sock E, Pingault V, Bondurand N, Goossens M, Wegner M. Functional analysis of Sox10 mutations found in human Waardenburg-Hirschsprung patients. The Journal of biological chemistry. 1998;273:23033–8. doi: 10.1074/jbc.273.36.23033. [DOI] [PubMed] [Google Scholar]
- 179.Herbarth B, Pingault V, Bondurand N, Kuhlbrodt K, Hermans-Borgmeyer I, Puliti A, Lemort N, Goossens M, Wegner M. Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc Natl Acad Sci U S A. 1998;95:5161–5. doi: 10.1073/pnas.95.9.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ulrich R, Imbschweiler I, Kalkuhl A, Lehmbecker A, Ziege S, Kegler K, Becker K, Deschl U, Wewetzer K, Baumgartner W. Transcriptional profiling predicts overwhelming homology of schwann cells, olfactory ensheathing cells, and schwann cell-like glia. Glia. 2014 doi: 10.1002/glia.22700. [DOI] [PubMed] [Google Scholar]
- 181.Williams SK, Franklin RJ, Barnett SC. Response of olfactory ensheathing cells to the degeneration and regeneration of the peripheral olfactory system and the involvement of the neuregulins. The Journal of comparative neurology. 2004;470:50–62. doi: 10.1002/cne.11045. [DOI] [PubMed] [Google Scholar]
- 182.Vincent AJ, Taylor JM, Choi-Lundberg DL, West AK, Chuah MI. Genetic expression profile of olfactory ensheathing cells is distinct from that of Schwann cells and astrocytes. Glia. 2005;51:132–47. doi: 10.1002/glia.20195. [DOI] [PubMed] [Google Scholar]
- 183.Roet KC, Bossers K, Franssen EH, Ruitenberg MJ, Verhaagen J. A meta-analysis of microarray-based gene expression studies of olfactory bulb-derived olfactory ensheathing cells. Experimental neurology. 229:10–45. doi: 10.1016/j.expneurol.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 184.Roet KC, Bossers K, Franssen EH, Ruitenberg MJ, Verhaagen J. A meta-analysis of microarray-based gene expression studies of olfactory bulb-derived olfactory ensheathing cells. Experimental neurology. 2011;229:10–45. doi: 10.1016/j.expneurol.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 185.Barnett SC. Olfactory ensheathing cells: unique glial cell types? J Neurotrauma. 2004;21:375–82. doi: 10.1089/089771504323004520. [DOI] [PubMed] [Google Scholar]
- 186.Chuah MI, West AK. Cellular and molecular biology of ensheathing cells. Microscopy research and technique. 2002;58:216–27. doi: 10.1002/jemt.10151. [DOI] [PubMed] [Google Scholar]
- 187.Wewetzer K, Brandes G. Axonal signalling and the making of olfactory ensheathing cells: a hypothesis. Neuron Glia Biol. 2006;2:217–24. doi: 10.1017/S1740925X06000305. [DOI] [PubMed] [Google Scholar]
- 188.Lakatos A, Franklin RJ, Barnett SC. Olfactory ensheathing cells and Schwann cells differ in their in vitro interactions with astrocytes. Glia. 2000;32:214–25. doi: 10.1002/1098-1136(200012)32:3<214::aid-glia20>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 189.Doucette JR. The glial cells in the nerve fiber layer of the rat olfactory bulb. Anat Rec. 1984;210:385–91. doi: 10.1002/ar.1092100214. [DOI] [PubMed] [Google Scholar]
- 190.Ramon-Cueto A, Avila J. Olfactory ensheathing glia: properties and function. Brain research bulletin. 1998;46:175–87. doi: 10.1016/s0361-9230(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 191.Steven C, Lehnen N, Kight K, Ijiri S, Klenke U, Harris WA, Zohar Y. Molecular characterization of the GnRH system in zebrafish (Danio rerio): cloning of chicken GnRH-II, adult brain expression patterns and pituitary content of salmon GnRH and chicken GnRH-II. General and comparative endocrinology. 2003;133:27–37. doi: 10.1016/s0016-6480(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 192.Miller AM, Treloar HB, Greer CA. Composition of the migratory mass during development of the olfactory nerve. The Journal of comparative neurology. 2010;518:4825–41. doi: 10.1002/cne.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Valverde F, Santacana M, Heredia M. Formation of an olfactory glomerulus: morphological aspects of development and organization. Neuroscience. 1992;49:255–75. doi: 10.1016/0306-4522(92)90094-i. [DOI] [PubMed] [Google Scholar]
- 194.Cummings DM, Brunjes PC. Migrating luteinizing hormone-releasing hormone (LHRH) neurons and processes are associated with a substrate that expresses S100. Brain research. Developmental brain research. 1995;88:148–57. doi: 10.1016/0165-3806(95)00091-q. [DOI] [PubMed] [Google Scholar]
- 195.Ekberg JA, Amaya D, Mackay-Sim A, St John JA. The migration of olfactory ensheathing cells during development and regeneration. Neuro-Signals. 2012;20:147–58. doi: 10.1159/000330895. [DOI] [PubMed] [Google Scholar]
- 196.Graziadei PP, Karlan MS, Graziadei GA, Bernstein JJ. Neurogenesis of sensory neurons in the primate olfactory system after section of the fila olfactoria. Brain Res. 1980;186:289–300. doi: 10.1016/0006-8993(80)90976-2. [DOI] [PubMed] [Google Scholar]
- 197.Doucette R. Development of the nerve fiber layer in the olfactory bulb of mouse embryos. The Journal of comparative neurology. 1989;285:514–27. doi: 10.1002/cne.902850407. [DOI] [PubMed] [Google Scholar]
- 198.Windus LC, Chehrehasa F, Lineburg KE, Claxton C, Mackay-Sim A, Key B, St John JA. Stimulation of olfactory ensheathing cell motility enhances olfactory axon growth. Cellular and molecular life sciences : CMLS. 2011;68:3233–47. doi: 10.1007/s00018-011-0630-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kafitz KW, Greer CA. The influence of ensheathing cells on olfactory receptor cell neurite outgrowth in vitro. Annals of the New York Academy of Sciences. 1998;855:266–9. doi: 10.1111/j.1749-6632.1998.tb10580.x. [DOI] [PubMed] [Google Scholar]
- 200.Kafitz KW, Greer CA. Olfactory ensheathing cells promote neurite extension from embryonic olfactory receptor cells in vitro. Glia. 1999;25:99–110. [PubMed] [Google Scholar]
- 201.Toba Y, Tiong JD, Ma Q, Wray S. CXCR4/SDF-1 system modulates development of GnRH-1 neurons and the olfactory system. Developmental neurobiology. 2008;68:487–503. doi: 10.1002/dneu.20594. [DOI] [PubMed] [Google Scholar]
- 202.Shyu WC, Liu DD, Lin SZ, Li WW, Su CY, Chang YC, Wang HJ, Wang HW, Tsai CH, Li H. Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke. J Clin Invest. 2008;118:2482–95. doi: 10.1172/JCI34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tham TN, Lazarini F, Franceschini IA, Lachapelle F, Amara A, Dubois-Dalcq M. Developmental pattern of expression of the alpha chemokine stromal cell-derived factor 1 in the rat central nervous system. The European journal of neuroscience. 2001;13:845–56. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- 204.Haas P, Gilmour D. Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Developmental cell. 2006;10:673–80. doi: 10.1016/j.devcel.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 205.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149:597–604. doi: 10.1210/en.2007-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Garcia-Gonzalez D, Murcia-Belmonte V, Clemente D, Castro FD. Olfactory System and Demyelination. Anat Rec (Hoboken) 2013 doi: 10.1002/ar.22736. [DOI] [PubMed] [Google Scholar]
- 207.Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell stem cell. 2007;1:443–57. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]