Abstract
Background
Among hemodialysis patients, probing dry weight is an effective strategy for improving control of hypertension. Whether controlling hypertension improves or worsens symptoms among such patients remains unclear. The purpose of the study was to develop a tool to evaluate symptoms and examine the relationship of the change in these symptoms with blood pressure (BP) control.
Methods
Among patients participating in the Hemodialysis Patients Treated with Atenolol or Lisinopril (HDPAL) randomized controlled trial, a confirmatory factor analysis (CFA) was performed to establish the relationship between symptoms and organ systems. Next, the change in symptom scores pertaining to organ systems was analyzed using a mixed model. Finally, the independent effect of lowering home BP on change in symptoms was evaluated.
Results
Among 133 participants where symptoms were available at baseline, CFA revealed four level 1 domains: gastrointestinal symptoms, dialysis-related symptoms, cardiovascular symptoms and general symptoms. All except dialysis-related symptoms were ascribed to uremia (level 2 domain). Uremic symptoms improved over 6 months and then increased. Dialysis-related symptoms (fatigue, cramps and orthostatic dizziness) did not worsen despite lowering home BP. Probing dry weight was independently associated with an improvement in cardiovascular symptoms such as shortness of breath.
Conclusions
Reducing BP through the use of a strategy that includes volume control and medication improves symptoms seemingly unrelated to volume excess. In long-term hemodialysis patients, treating hypertension using home BP measurements may improve well-being.
Keywords: symptoms, blood pressure, cardiovascular, hemodialysis, hypertension
INTRODUCTION
The management of hypertension among hemodialysis patients remains mired in controversy [1]. Two independent meta-analyses of randomized trials among dialysis patients suggest that blood pressure (BP) control among hemodialysis patients may be associated with reduced cardiovascular event rates [2, 3]; however, large cohort studies suggest otherwise [4, 5]. The cohort studies show a strong and consistent association between both a lower baseline BP and time-dependent decline in BP and subsequent all-cause mortality [6].
Probing dry weight through incremental reductions in achieved postdialysis weight is an effective strategy in lowering ambulatory BP among hemodialysis patients [7]. However, critics point out that lowering dry weight can provoke intradialytic hypotension and unpleasant symptoms and may even be dangerous since it may provoke myocardial stunning due to subclinical myocardial ischemia [8, 9]. Likewise, lowering BP may cause orthostatic hypotension, fatigue and cramps and may make adherence to antihypertensive therapy problematic.
While we await randomized trials among hemodialysis patients of strategies to control BP with antihypertensive therapy and probing dry weight, we can ask the question how the patients feel and function while their dry weight is being probed and their BP lowered. In this study, I hypothesize that symptoms in hemodialysis patient will improve with lowering BP. BP, and not weight, was chosen as a predictor of improvement in symptoms because it is the final outcome of effective lowering of dry weight.
MATERIALS AND METHODS
The Hypertension in Dialysis Patients Treated with Atenolol or Lisinopril (HDPAL) was a randomized controlled trial that assigned hypertensive hemodialysis patients to either atenolol- or lisinopril-based antihypertensive therapy and followed them for 1 year for the primary endpoint of regression of left ventricular hypertrophy [10]. Each group had a target home BP of <140/90 mmHg, and the primary strategy used to lower BP was to probe dry weight. The clinical trial was registered at clinicaltrials.gov with registration number NCT00582114, and primary results of this trial have been published [10]. The study was approved by the Institutional Review Board of Indiana University and the Research and Development Committee of the Roudebush VA Medical Center, Indianapolis, and all subjects gave written informed consent.
To accurately capture the adverse effects of atenolol and lisinopril in the HDPAL trial, we used a structured questionnaire. This questionnaire was administered at baseline prior to any administration of the drug and subsequently at monthly intervals over the duration of the trial. The adverse effects attributable to either atenolol or lisinopril included symptoms such as nightmares; peripheral vasoconstriction, such as cold hands and feet; upper respiratory infection, which is reported more commonly among angiotensin-converting enzyme inhibitor users; dry cough and depression. Other symptoms were more general in nature (e.g. gastrointestinal manifestations of medication intake) or those related to excessive lowering of BP, such as orthostatic hypotension or fatigue.
Each of the 20 questions was preceded by the following stem: ‘Over the last week, how frequently have you found yourself bothered by the following symptoms?’ The symptoms were as follows: fatigue or tiredness, chest pain, abdominal pain, cold hands or feet, dizziness on standing, muscle cramps, diarrhea, nausea, vomiting, dry cough, upper respiratory infection or common cold, shortness of breath, headaches, persistent dizziness, numbness in hands or feet, decreased sex drive, decreased ability to have sex, drowsiness or sleepiness, depression or feeling sad and nightmares. The responses were constantly, frequently, sometimes, rarely or never. Never was coded as 0, rarely as 1, sometimes 2, frequently 3 and constantly as 4.
Based on this questionnaire, adverse effects that have been typically attributed to atenolol or lisinopril were not found to be different between drugs over time. For example, those randomized to the lisinopril group did not have any increase in dry cough or upper respiratory infections. In comparison, those assigned to atenolol did not have an increased frequency of nightmares, cold hands and feet, drowsiness or depression. Because the symptoms were collected in a structured way over the course of the trial in this analysis, I asked the question whether the symptoms deteriorated or improved. Also was there a relationship between a change in symptoms and a change in home BP. Home BP was recorded every month by the patients twice daily (on waking up and before going to bed) in triplicate on each occasion after the midweek dialysis for 4 days using a self-inflating automatic oscillometric device (HEM-705 CP; Omron Healthcare). The strategy of dry weight reduction and antihypertensive therapy escalation has been described elsewhere [10].
Statistical methods
Establishing symptom domains
Although symptoms can be analyzed individually, conceptually symptoms are often clustered to reflect disease in a particular organ or system. For example, nausea, vomiting, diarrhea and abdominal pain can be analyzed individually, but it may be better to treat them as a cluster to reflect gastrointestinal disease. One approach would be to simply average the scores of each one of the symptoms, but more robust statistical techniques are now available. One such technique is confirmatory factor analysis (CFA). This technique allows evaluation of whether an observed symptom (e.g. nausea) is truly reflective of the latent variable (e.g. gastrointestinal domain). Unlike exploratory factor analysis, CFA does not assume that error terms are independent and normally distributed; therefore, it can fit a model more adequately. By providing a better fit, it provides a better measure of the latent variable by isolating noise. The reliability of the overall model and model fit can be calculated. Finally, modification indices allow for refining the model fit.
The CFA model developed used 133 participants with complete data at baseline. I first performed an exploratory factor analysis and found three factors using maximal likelihood estimation (MPlus 7.3.1; Muthen and Muthen, Statmodel, Los Angeles, CA, USA). However, informed by clinical experience, I divided the symptoms into domains that would reasonably be considered reflective of the organ systems that they represent. Dry cough, upper respiratory infection and nightmares were not included in the analyses, as these questions were designed more to evaluate the side effect profile of the drugs.
The final CFA model shows loading coefficients for each symptom to calculate the symptom score for each domain both at baseline and at each subsequent month. Modification indices suggested including a covariance in the residual error term for nausea and vomiting.
Evolution of symptoms over time
A mixed model was used to assess the evolution of symptoms over time [11]. The outcome variable was the symptom score. The independent variables were the assigned drug with two levels of atenolol or lisinopril and the visit as a continuous variable as well as an indicator variable (in a separate model) with 12 levels starting from baseline. An interaction term (drug × visit) was included. The random component of the model included an intercept for subjects and coefficient for visit. A maximum likelihood estimation method was used for model fitting. The statistical significance of the visit and visit × drug interaction was tested next. Data presented are the marginal means of the model and the standard errors.
After plotting the symptom domains over time, it became evident that the change in symptom scores was time dependent. Accordingly, a quadratic term for visit month was introduced and visit month was modeled as a continuous variable. The remaining part of the model is similar to that noted above.
Testing effect of lowering BP on symptom scores
To test whether improvement in symptoms is mediated through lowering of dry weight, a mixed effects model was again used. Given that the change in symptoms was U-shaped and the maximal change in symptoms occurred over the first 7 months, I used in the model noted above the baseline home systolic BP for each individual and change from baseline within individual in the mixed model. In addition, I used the change from baseline in postdialysis weight as an independent variable. A sensitivity analysis where the data from all 12 months were used with a quadratic term for time and a further interaction term of change from baseline within individual for home BP with time was performed.
To test the notion whether change in postdialysis weight influences the dialysis symptom score, a mixed model was used. The outcome variable was the dialysis symptom score, and the following fixed effects were used: time, time-squared, weight at baseline, change in weight from baseline within individual and an interaction term of the change in weight from baseline × time. Random intercept for subject and random slopes for time and change from baseline in weight was used. An unstructured covariance was not required.
Confirmatory factor analyses were performed using MPlus 7.3.1. Remaining statistical analyses were performed using Stata 14.0 (StataCorp, College Station, TX, USA).
RESULTS
The overall results of the study have been previously published. The mean age ± SD of this sample was 51.5 ± 12.4 years, 69% men, 81% blacks and 50% were randomized to atenolol. The mean hemoglobin was 11.1 ± 1.3 g/dL, serum albumin concentration 3.6 ± 0.5 g/dL, serum phosphorus concentration 6.1 ± 1.9 mg/dL and urea reduction ratio 74.4 ± 7.5%. The distribution of access was as follows: 58% fistula, 14% graft and 28% catheter. Baseline home BP was 165 ± 20/91 ± 12 mmHg with a pulse rate of 78 ± 11 bpm. Baseline weight was 82.2 ± 19.1 kg.
Table 1 shows the distribution of symptoms in 133 participants. The top five symptoms were fatigue, drowsiness, numbness in extremities, decreased sex drive and muscle cramps. The least common symptoms were nightmares, common cold, persistent dizziness, abdominal pain and chest pain.
Table 1.
Distribution of symptoms at baseline
| Symptoms | Never | Rare | Sometimes | Frequently | Constant | Mean ± SD |
|---|---|---|---|---|---|---|
| Fatigue | 21 (16%) | 23 (17%) | 52 (39%) | 25 (19%) | 12 (9%) | 47 ± 29 |
| Chest pain | 68 (51%) | 27 (20%) | 25 (19%) | 12 (9%) | 1 (1%) | 22 ± 26.5 |
| Abdominal pain | 68 (51%) | 27 (20%) | 27 (20%) | 8 (6%) | 3 (2%) | 22 ± 26.8 |
| Cold hands/feet | 43 (32%) | 23 (17%) | 40 (30%) | 14 (11%) | 13 (10%) | 37 ± 32.7 |
| Orthostasis | 52 (39%) | 33 (25%) | 38 (29%) | 6 (5%) | 4 (3%) | 26.9 ± 26.6 |
| Muscle cramps | 30 (23%) | 33 (25%) | 49 (37%) | 17 (13%) | 4 (3%) | 37.2 ± 26.8 |
| Diarrhea | 50 (38%) | 28 (21%) | 34 (26%) | 17 (13%) | 4 (3%) | 30.6 ± 29.3 |
| Nausea | 45 (34%) | 29 (22%) | 35 (26%) | 18 (14%) | 6 (5%) | 33.3 ± 30.1 |
| Vomiting | 65 (49%) | 28 (21%) | 29 (22%) | 8 (6%) | 3 (2%) | 22.9 ± 26.8 |
| Dry cough | 57 (43%) | 26 (20%) | 35 (26%) | 12 (9%) | 2 (2%) | 26.5 ± 27.4 |
| Common cold | 79 (59%) | 28 (21%) | 19 (14%) | 5 (4%) | 1 (1%) | 16.1 ± 22.9 |
| Short of breath | 51 (38%) | 32 (24%) | 40 (30%) | 8 (6%) | 2 (2%) | 27.1 ± 25.8 |
| Headache | 49 (37%) | 31 (23%) | 32 (24%) | 17 (13%) | 4 (3%) | 30.5 ± 29.1 |
| Dizzy all the time | 80 (60%) | 20 (15%) | 23 (17%) | 7 (5%) | 3 (2%) | 18.6 ± 26.6 |
| Numbness in extremities | 43 (32%) | 19 (14%) | 38 (29%) | 20 (15%) | 13 (10%) | 38.9 ± 33.5 |
| Decreased sex drive | 50 (38%) | 18 (14%) | 24 (18%) | 16 (12%) | 19 (14%) | 37.4 ± 37.1 |
| Decreased ability to have sex | 59 (44%) | 12 (9%) | 24 (18%) | 15 (11%) | 17 (13%) | 34.1 ± 37.2 |
| Drowsiness | 31 (23%) | 20 (15%) | 46 (35%) | 27 (20%) | 9 (7%) | 43 ± 30.5 |
| Depression or feeling sad | 53 (40%) | 27 (20%) | 35 (26%) | 9 (7%) | 9 (7%) | 30.1 ± 30.7 |
| Nightmares | 87 (65%) | 23 (17%) | 13 (10%) | 7 (5%) | 3 (2%) | 15.4 ± 25.3 |
Symptoms were coded as follows: never 0, rare 25, sometimes 50, frequently 75, constant 100.
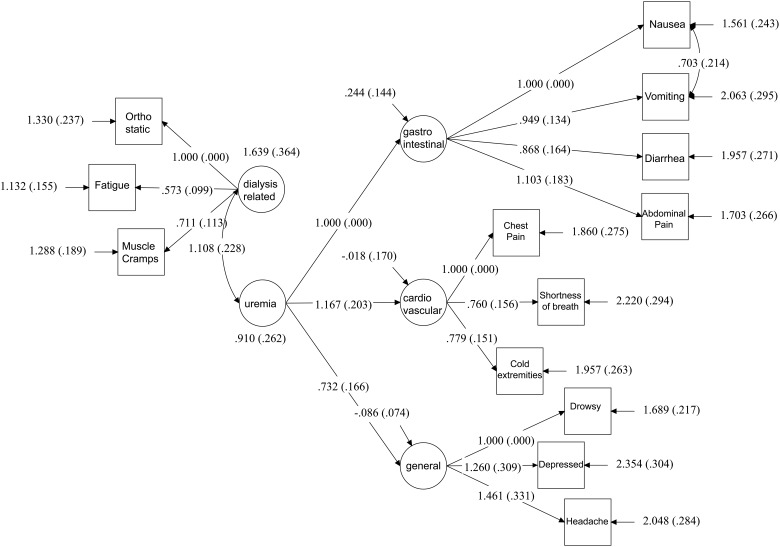
Figure 1 shows the CFA model of two domains of symptoms: dialysis-related symptoms and uremic symptoms. Uremic symptoms included gastrointestinal, cardiovascular and general symptoms. Thirteen symptoms encompassed these four domains/subdomains. Although 20 symptoms were evaluated, only 13 were retained in the final model based on factor loadings, conceptual construct and model fit. The model failed to converge with the sexual dysfunction domain, therefore it was removed. In the final model fit, each factor loading was >0.4, indicating statistical validity to include the symptom in the domain. The reliability of the overall model was high (ρ = 0.87).
FIGURE 1:
Hierarchical CFA of dialysis symptoms, final model. Four level 1 latent variables represented in ovals were gastrointestinal symptoms (gastrointestinal), cardiovascular symptoms (cardiovascular), dialysis symptoms (dialysis related) and general symptoms (general). One level 2 latent variable (uremia) is represented by three level 1 variables: gastrointestinal, cardiovascular and general. The arrows point to the observed symptoms contained in rectangles within each domain. Numbers next to the straight arrows are the factor loadings. Each loading was both significant and >0.4. The arrows pointing to the squares are the residual variances and standard error of the residual variance. The curved arrows show the covariances either among the error terms (vomiting and nausea) or covariances among the latent variables (dialysis-related and uremia). All covariances were statistically significant. Model fit indices show that the χ2 was not significant (P = 0.18), indicating that the structural equation model identified the covariance matrix adequately. The root mean square error of approximation (RMSEA) was 0.035 and the standardized root mean square residuals (SRMR) was 0.049. The comparative fit index (CFI) was 0.978 and the reliability coefficient of the model (ρ) was 0.86.
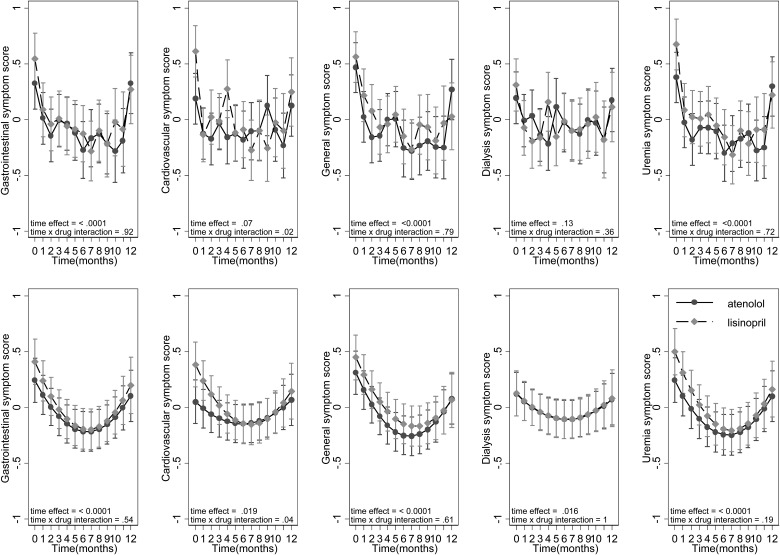
Figure 2 shows the evolution of symptoms over time among participants randomized to one of the two drugs. To facilitate comparison, all symptom scores were standardized to have a mean of 0 and SD of 1. The top five graphs treat time (visit month) as an indicator variable. The bottom five graphs treat time as a continuous variable with a quadratic term included for time. Except for cardiovascular symptoms that were greater in the lisinopril group, there were no differences in symptom scores in any domain between drugs at baseline. Over time, there was improvement in cardiovascular, gastrointestinal and general symptoms. There was no change in the dialysis symptom score if time was treated as an indicator variable. However, a significant U-shaped relationship emerged when time was treated as a continuous variable. There was no drug × time interaction for any of the symptoms except for cardiovascular symptoms, where a greater improvement was seen with lisinopril in the first 6 months.
FIGURE 2:
Symptom score over time in the randomized trial. Scores are standardized to have a mean of 0 and SD of 1 to facilitate comparison across groups. High scores represent more symptoms. The top five graphs show time as an indicator variable, whereas the bottom five treat time as a continuous variable with a quadratic term added for time. Marginal modeled means are shown with their standard errors. Significant improvement was seen with time in all but dialysis-related symptoms. Time effect reflects the P-value of time, while time × drug interaction is the P-value of the interaction term.
The trajectory of home BP and postdialysis weight and their change from baseline are shown in the Supplementary data, Appendix. Improvement in home BP was significant (P < 0.0001) and was independent of the drug assignment. Improvement in the postdialysis weight was significantly more in the lisinopril group (P < 0.0001).
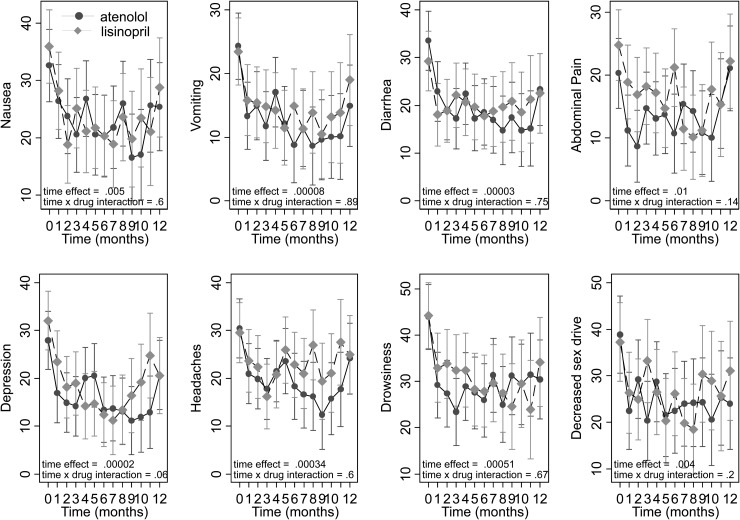
Figure 3 shows the eight symptoms that demonstrated improvement over time. Symptoms were scaled from 0 to 100 and a higher score represents more symptoms. Symptoms that improved included all gastrointestinal symptoms (nausea, vomiting, diarrhea and abdominal pain) and a few other symptoms (depression, headaches, drowsiness and sex drive). No time effect (indicating no significant change in symptom over time) or drug × time interaction effect (indicating no confounding effect of drug over time on symptoms) for any of the 12 remaining symptom questions was noted.
FIGURE 3:
Graph of eight individual symptoms that showed improvement over time. Treatment × time interaction was not significant, indicating no unique effect of the drug. All gastrointestinal symptoms (top four graphs) and general symptoms (next three graphs) showed improvement, as did sex drive. The last symptom was not included in the CFA model. Marginal modeled means are shown with their standard errors. All symptoms are scaled from 0 to 100, where 100 represents most symptoms.
Table 2 shows the mixed model derived symptom scores for each of the domains. Since the relationship of symptoms over time was U-shaped, to facilitate model interpretation the data were truncated at 7 months because linearity was evident until this point. However, a model that included all 12 months of data with quadratic terms for time and appropriate interaction terms for time-dependent BP change revealed similar results. In each instance of uremic symptoms, there was a significant direct relationship between the change from baseline in systolic BP and improvement in symptom scores. For example, a 10 mmHg reduction in home systolic BP reduced symptoms by 0.175 points for cardiovascular symptoms. In the case of dialysis-related symptoms, there was no significant relationship between lowering of home systolic BP and worsening of symptoms. At least the coefficient was not negative, which would have indicated a worsening of symptoms with lowering of home systolic BP. In the case of cardiovascular symptoms, there was a significant and direct relationship between lowering of postdialysis weight and improvement in symptoms.
Table 2.
Mixed model parameters for symptom scores
| Parameter | Symptom score |
||||
|---|---|---|---|---|---|
| Cardiovascular | Gastrointestinal | General | Uremia | Dialysis related | |
| Intercept | 4.22*** ± 0.30 | 6.23*** ± 0.50 | 6.736*** ± 0.46 | 16.1*** ± 1.04 | 5.03*** ± 0.27 |
| Month | −0.122** ± 0.042 | −0.301*** ± 0.06 | −0.335*** ± 0.065 | −0.726*** ± 0.136 | −0.063** ± 0.039 |
| BL home SBP (>140 mmHg) | 0.008 ± 0.009 | 0.018 ± 0.016 | 0.025 ± 0.014 | 0.047 ± 0.033 | 0.003 ± 0.008 |
| CFB home SBP (/10 mmHg) | 0.175*** ± 0.051 | 0.209** ± 0.081 | 0.250*** ± 0.075 | 0.611*** ± 0.155 | 0.047 ± 0.048 |
| CFB post wt (kg) | 0.088* ± 0.044 | 0.05 ± 0.066 | −0.004 ± 0.069 | 0.106 ± 0.137 | 0.045 ± 0.037 |
| SD intercept | 1.88 | 3.12 | 2.9 | 6.87 | 1.6 |
| SD month | 0.177 | 0.396 | 0.363 | 0.817 | 0.175 |
| SD CFB post wt | 0.153 | 0 | 0.254 | 0.344 | 0 |
| σ | 2.293 | 3.615 | 3.282 | 6.664 | 2.210 |
Given that the relationship of symptoms over time was U-shaped, to facilitate model interpretation the data shown are for the first 7 months when the relationship was linear.
BL, baseline; CFB, change from baseline; SD, standard deviation derived from the random component of the model; SBP, systolic blood pressure; post wt, postdialysis weight.
*P < 0.05, **P < 0.01, ***P < 0.001.
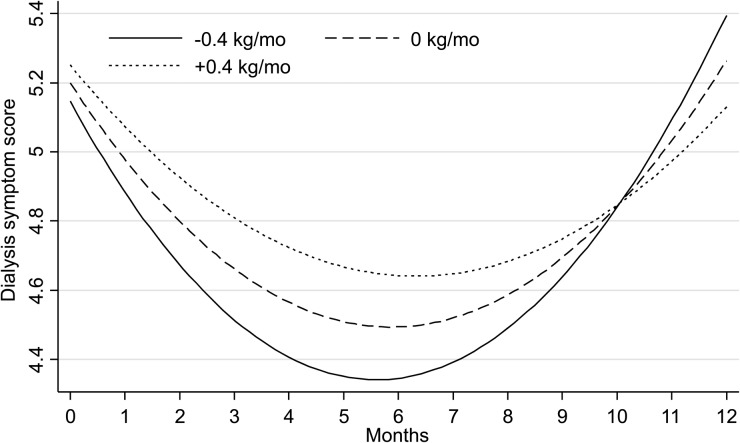
The independent effect of postdialysis weight on dialysis-related symptoms is shown in Figure 4. There was a significant effect on dialysis symptom score of time (−0.192 ± 0.06, P = 0.001) and time-squared (0.015 ± 0.005, P = 0.003), but in addition there was a significant effect of the change from baseline in postdialysis weight in dialysis symptom score (0.13/kg ± 0.05, P = 0.012) that was mitigated over time [−0.93 ± 0.006 (P = 0.028)].
FIGURE 4:
Relationship of the change in postdialysis weight with dialysis-related symptom score over time. Estimated dialysis-related symptom score is shown on the ordinate and time in months on the abscissa for an 80-kg participant. A U-shaped relationship emerged between dialysis symptoms and time even when no change in post-dialysis weight occurred. However, a greater reduction in weight over the first few months was associated with a greater improvement in symptoms. See the text for details.
DISCUSSION
At baseline, symptoms could be classified as dialysis-related symptoms and a hierarchical group of uremic symptoms which were reflected by three level 1 domains: gastrointestinal symptoms, cardiovascular symptoms and general symptoms. The gastrointestinal symptoms were elicited by questions relating to nausea, vomiting, diarrhea and abdominal pain; cardiovascular symptoms by chest pain, shortness of breath and cold extremities; general symptoms by evaluation of drowsiness, headaches and depression; the dialysis-related symptoms were elicited by three questions on fatigue, cramps and orthostatic dizziness. The remaining questions could not be mapped to any specific domains by CFA and therefore were excluded. The CFA model had a good fit as noted by the following observations: a non-significant χ2-test for model fit, P-value <0.05 for RMSEA, CFI > 0.95, SRMR < 0.05 and a reliability coefficient of 0.86. By usual metrics of CFA, these are considered adequate. Given that the symptom burden in the hemodialysis patient is high [12, 13], the study provides a framework by which symptoms could be evaluated and mitigated in the long term.
The most common symptom in this study was fatigue, the mean score of which was 47, and 67% of the participants had experienced fatigue at least some of the time. In fact, 28% felt fatigue frequently or constantly. This is in line with what has been reported by others [14–16]. Fatigue is directly related to survival [17]. Furthermore, a single item question, ‘how long does it take you to recover from a dialysis session?’ was related to fatigue (r = 0.38, P < 0.0001) [15] and is directly related to survival [18]. Even among patients with CKD not on dialysis, fatigue is the most common symptom reported [19]. However, no improvement in fatigue was noted in this study. Perhaps factors other than BP and volume, such as cytokine levels, are more important in causing fatigue [16, 20].
An improvement in most symptoms was seen in the study. Of the gastrointestinal symptoms, all four symptoms improved. Of the general symptoms, there was improvement in all three symptoms, notably drowsiness, depression and headaches. More importantly, there was no worsening seen in the dialysis symptoms such as fatigue, orthostatic dizziness and muscle cramps. In fact, a reduction in dry weight and lowering BP may increase symptoms of fatigue, orthostatic hypotension and muscle cramps. However, if anything, there was a slight improvement, not deterioration, of the symptoms. Notably, the improvement occurred at a time when dry weight was being probed. This suggests that although in the short term probing dry weight may lead to uncomfortable symptoms, in the long term, lowering BP measured by using home BP monitoring does not result in an increase in symptoms. Excess fluid that may accumulate in the liver tract may elicit gastrointestinal symptoms. Therefore, it is biologically plausible that a reduction in BP (which we achieved predominantly by probing dry weight) may improve such symptoms. Likewise depression, drowsiness, headaches and sexual dysfunction, though not being due to excess volume, may represent modifiable symptoms addressable by the simple expedient of probing dry weight that is manifested by a lower BP.
Further evidence for the improvement of cardiovascular symptoms due to probing dry weight comes from the observation that a direct relationship existed between change from baseline in postdialysis weight from 1 month to the next and improvement in symptoms above and beyond BP. This was not so for the gastrointestinal and general symptoms. In other words, in a patient with cardiovascular symptoms, both lowering dry weight and lowering BP can improve symptoms. We reported earlier that patients in the lisinopril group remained more hypertensive and required greater lowering of dry weight. It is possible that the early improvement in symptoms in the lisinopril group may have been due to lowering of dry weight.
It may be expected that probing dry weight may increase dialysis-related symptoms. Figure 4 shows that the dialysis symptom score improved early and worsened later when there was no change in weight from baseline (this is expected since the time and time-squared coefficients were significant). However, with lowering of postdialysis weights there was a greater improvement in dialysis-related symptoms early, but also greater worsening later. Contrary to expectations, dry-weight reduction early was associated with improvement in symptoms. One may argue that after 10 months postdialysis weight may be increased to mitigate symptoms. However, post-dialysis weight may measure different parameters before and after 6 months. Postdialysis weight changes in the first few months may reflect a change in volume status. Mitigation of gastrointestinal symptoms may cause weight gain. Thus, the change from baseline in weight after the first 6 months may reflect an accumulation of fat or lean body mass. If this is true, then the U-shaped relationship between symptoms and weight change can be explained based on changes in body composition.
Some limitations must be pointed out. First, the questionnaire to assess symptoms was administered to only some of the participants. This was because the idea of assessment of symptoms was developed when the study was under way. Second, not all participants completed the survey in each of the 12 months because the study was terminated early. Nonetheless, we had sufficient responses to model changes in symptom scores. We did not have a control group of placebo-treated patients. So it is difficult to be certain whether a cause-and-effect relationship exists. The improvement in symptoms waned in the last 4–5 months of the trial. A placebo control would have detected this effect more clearly. As a cautionary remark, it is unlikely that symptoms and BP will be correlated outside an interventional study. In other words, if we simply measured spontaneous changes in BP and symptoms they are unlikely to be related. However, in the context of an interventional study, where BP is controlled through a deliberate reduction in dry weight and medication use, improvement in symptoms is likely.
There are some strengths of this study. Incorporation of patient-reported symptoms is novel and something that is directly interpretable by the patients. Other novel features of this study are the application of CFA to the study of symptoms in a randomized trial and demonstration that lowering BP can effectively improve symptom scores.
This study demonstrates that it is possible to incorporate the study of symptoms among a group of sick patients that should take no more than 5 min to complete. Symptom evaluation such as this may be particularly relevant to the dialysis patient. Although the lifespan of dialysis patients may not necessarily be prolonged without transplantation, lowering BP (predominantly by probing dry weight) may keep them less symptomatic. It also provides those caring for these patients with evidence to share with their patients that lowering BP, in part through probing dry weight, may improve well-being in the long term.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by the National Institutes of Health NIDDK 2R01-DK062030-10.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Agarwal R. Hypertension and survival in chronic hemodialysis patients—past lessons and future opportunities. Kidney Int 2005; 67: 1–13 [DOI] [PubMed] [Google Scholar]
- 2.Heerspink HJ, Ninomiya T, Zoungas S et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet 2009; 373: 1009–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal R, Peixoto AJ, Santos SF et al. Out-of-office blood pressure monitoring in chronic kidney disease. Blood Press Monit 2009; 14: 2–11 [DOI] [PubMed] [Google Scholar]
- 4.Port FK, Hulbert-Shearon TE, Wolfe RA et al. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 1999; 33: 507–517 [DOI] [PubMed] [Google Scholar]
- 5.Zager PG, Nikolic J, Brown RH et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc . Kidney Int 1998; 54: 561–569 [published erratum Kidney Int 1998; 54: 1417] [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Lacson E Jr, Lowrie EG et al. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 2006; 48: 606–615 [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Alborzi P, Satyan S et al. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension 2009; 53: 500–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McIntyre CW. Recurrent circulatory stress: the dark side of dialysis. Semin Dial 2010; 23: 449–451 [DOI] [PubMed] [Google Scholar]
- 9.McIntyre CW. Effects of hemodialysis on cardiac function. Kidney Int 2009; 76: 371–375 [DOI] [PubMed] [Google Scholar]
- 10.Agarwal R, Sinha AD, Pappas MK et al. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant 2014; 29: 672–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden JE, Kelley K, Agarwal R. Analyzing change: a primer on multilevel models with applications to nephrology. Am J Nephrol 2008; 28: 792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol 2009; 4: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weisbord SD, Fried LF, Arnold RM et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage 2004; 27: 226–240 [DOI] [PubMed] [Google Scholar]
- 14.Weisbord SD, Fried LF, Arnold RM et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol 2005; 16: 2487–2494 [DOI] [PubMed] [Google Scholar]
- 15.Lindsay RM, Heidenheim PA, Nesrallah G et al. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol 2006; 1: 952–959 [DOI] [PubMed] [Google Scholar]
- 16.Jhamb M, Weisbord SD, Steel JL et al. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis 2008; 52: 353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jhamb M, Pike F, Ramer S et al. Impact of fatigue on outcomes in the hemodialysis (HEMO) study. Am J Nephrol 2011; 33: 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rayner HC, Zepel L, Fuller DS et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2014; 64: 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal R. Developing a self-administered CKD symptom assessment instrument. Nephrol Dial Transplant 2010; 25: 160–166 [DOI] [PubMed] [Google Scholar]
- 20.Jhamb M, Argyropoulos C, Steel JL et al. Correlates and outcomes of fatigue among incident dialysis patients. Clin J Am Soc Nephrol 2009; 4: 1779–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.