Abstract
BACKGROUND
Maternal folate intake and related biomarkers have been inconsistently associated with a risk of oral clefts.
METHODS
Maternal concentrations of plasma folate (PF) and erythrocyte folate (EF), plasma pyridoxal-5′-phosphate (PLP; active vitamin B6) and total plasma homocysteine (tHcy) were measured in a Utah study with 347 cases and 469 controls.
RESULTS
Risk of all clefts combined, including cleft lip with or without cleft palate (CL/P) and cleft palate only (CP), was 65% lower in the highest versus lowest PF quartile (odds ratio [OR], 0.35; 95% confidence interval [CI], 0.23–0.53; p-trend < 0.001). Results remained significant in the subgroups with isolated CL/P and CP (p-trend < 0.001 in each). EF results were similar. In the highest versus lowest PLP quartile, risk of CP with other malformations was lower (OR, 0.25; 95% CI, 0.07–0.95); however, no other associations were significant for PLP or tHcy. Differences in mean bio-marker levels between cases and controls widened with an increasing interval between delivery and maternal blood collection. Decreased cleft risk with increasing quartiles of PF, EF, and PLP and decreasing tHcy was more apparent in mothers with a longer versus shorter interval between the index child delivery and blood collection.
CONCLUSION
Low maternal blood folate concentration was associated with an increased risk of clefts, and the differences in mean case and control PF, EF, PLP, and tHcy concentrations widened over time. Additional mechanistic studies are warranted to elucidate whether an acquired or inherited disorder of folate metabolism plays a role in the etiology of clefts.
Keywords: oral cleft, folate, vitamin B6, homocysteine, case-control study
INTRODUCTION
Oral clefts are among the most common birth defects; they comprise a broad spectrum of anomalies including cleft lip (CL), cleft palate (CP), or both, in either isolation or association with other malformations. Common groupings of clefts for surveillance and etiologic studies include CL, with or without cleft palate (CL/P) and CP. Considerable variation exists in the occurrence of clefts by social class, by geographic area, and between resident and migrant groups of the same ethnicity, indicating an environmental component to the causes (Mossey et al., 2009). Among the U.S. states with statewide birth defects registries with active surveillance, Utah has the highest birth prevalence of clefts and the rates are generally higher in western states compared to eastern states (Gebreab et al., 2008).
Maternal deficiency of a wide variety of nutrients has long been related to the risk for clefts in experimental animals (Munger, 2002). In meta-analyses of human observational studies, maternal multivitamin use was associated with a reduced risk of clefts (Badovinac et al., 2007; Johnson and Little, 2008), although an analysis of data from the U.S. National Birth Defects Prevention Study (a case-control study) indicated no association (Shaw et al., 2006).
The well-established relationship between periconceptional folic acid supplementation and reduced risk of neural tube defects (NTDs; MRC Vitamin Study Research Group, 1991) suggests that a similar relationship may exist for clefts, because certain structures of the craniofacial region are derived from cephalic neural crest cells. Therefore, it is plausible that these birth defects share common developmental origins and risk factors for abnormal development (Been and Lieuw Kie Song, 1978; Lammer et al., 1985). Epidemiologic data, however, reveal important differences between NTDs and clefts in their patterns of geographic occurrence and in changes in birth prevalence over time; therefore, the causes of clefts may be different from NTDs. In China, NTD rates are higher in the north than the south, whereas clefts do not follow this pattern. (Berry et al., 1999; Dai et al., 2004) In most countries where sharp declines in the NTD rates were observed over the past decades, cleft rates have been relatively stable (International Clearinghouse for Birth Defects Surveillance and Research, 2008). Studies of dietary folate intake (Wilcox et al., 2007; Johnson and Little, 2008; Little et al., 2008a;) and of folate-related genes (Mossey et al., 2009) have resulted in inconsistent associations with the risk of clefts. If folate nutritional status is related to the etiology of clefts, this association and its modifying factors may differ from that of NTDs.
Folate plays important roles in one-carbon transfer reactions, including amino acid metabolism, formate oxidation, and purine and thymidylate biosynthesis, which are building blocks of DNA and RNA. Therefore, adequate status of folate is important for growth and development early in life (Tamura and Picciano, 2006). Folate-related biomarkers involved in one-carbon metabolism may be useful in studies of the association between maternal folate nutrition and clefts. However, findings of studies associating these biomarkers with the risk of clefts have been inconsistent. In the Netherlands, case mothers of children with clefts had higher serum and erythrocyte folate concentrations, but lower plasma pyridoxal-5′-phosphate (PLP) and higher total homocysteine (tHcy) compared with control mothers of children without malformations (Wong et al., 1999). In a subsequent Netherlands study, case mothers with low vitamin B12 and B6 levels had an increased risk of clefts in their children, whereas folate concentrations were not significantly different between case and control mothers (van Rooij et al., 2003). In a U.K.-based case-control study, higher serum and erythrocyte folate concentrations were associated with decreased risk of CLP but an increased risk of CP (Little et al., 2008b). In the Philippines, low plasma PLP concentrations were common and associated with increased cleft risk, although plasma and erythrocyte folate concentrations were inconsistently associated with cleft risk. We suggest that these associations are modified by inadequate vitamin B6 status in the population (Munger et al., 2004; Tamura et al., 2007).
Our objective was to determine whether biomarkers related to one-carbon metabolism, including concentrations of plasma and erythrocyte folate, plasma PLP, and plasma tHcy in mothers, were associated with risk of clefts in their children.
MATERIALS AND METHODS
Subjects and Samples
A state-wide case-control study of clefts was conducted in Utah during 2000 to 2005 in collaboration with the Utah Birth Defects Network (UBDN), a state-wide birth defects registry operated by the Utah Department of Health (UDOH). All study procedures were reviewed and approved by the institutional review boards of Utah State University (USU), the University of Utah, the UDOH, and the University of Alabama at Birmingham. Eligible case mothers included Utah residents with a live-born or stillborn child with a cleft between January 1, 1995, and June 30, 2004. Clefts and associated birth defects were classified after a review of all available medical records by the UBDN, including a medical geneticist (J.C.C.). Cleft cases with known genetic syndromes or chromosomal abnormalities were excluded from the present analyses. Control mothers were randomly selected using Utah birth certificate files that were frequency matched to cases by month and year of delivery and sex of the child; they were recruited at a rate projected to result in a 1:1 ratio of completed interviews with cases. The UDOH authorized the limited use of UBDN data to recruit case mothers of children with clefts and the use of Utah birth certificate files to recruit control mothers of children without birth defects. The UBDN staff members attempted to contact potential case and control mothers by mail to obtain consent for release of their names to USU investigators. Address updates were sought using available internet services. If no mailing address was available, attempts were made to locate the mothers in person by field tracing that included visits to the last known home address and inquiries with neighbors.
Interviews with mothers were conducted primarily by telephone; however, personal interviews were completed if no telephone was available. The interview included questions on demographic characteristics of the biologic parents, a reproductive health and pregnancy history, supplement use, medications, medical conditions, and smoking and alcohol use. Each mother received an individualized, color-coded pregnancy calendar that was generated based on the date of delivery of her index child and the self-reported gestational length. This visual aid was intended to assist mothers in recalling activities and timing of events during various periods referred to. Color-coding of the calendars indicated the reference periods including the 3-month period before the estimated date of conception and three trimesters. Interview materials were translated into Spanish, and mothers speaking Spanish only were contacted by a bilingual interviewer.
Maternal blood samples were obtained in evacuated tubes containing EDTA (BD Vacutainer; Preanalytical Solutions, Franklin Lake, NJ) from mothers ≥12 months after the end of their last pregnancy to avoid artifacts of the effects of pregnancy and lactation (Murphy et al., 2002). Blood samples were kept on ice and processed within 2 hours of collection. Aliquots of whole blood for folate assays were mixed with a 1% ascorbic acid solution. Samples were shipped on dry ice to Birmingham, Alabama, and were kept at −80°C until analysis.
Laboratory Analyses
Plasma and erythrocyte folate concentrations were determined by microbiologic assay using Lactobacillus rhamnosus as described previously (Tamura, 1990). Erythrocyte folate was assayed after whole blood lysates were incubated for 60 minutes at 37°C (pH 4.2) to hydrolyze polyglutamyl folates using endogenous plasma folate conjugase. The calculation of erythrocyte folate was done by the following formula: erythrocyte folate concentration = [Whole blood folate concentration − Plasma folate concentration × (1 − Hematocrit)] ÷ Hematocrit. Plasma tHcy assay was performed by a high-pressure liquid chromatography-fluorescent method (Tamura et al., 1996). Plasma PLP concentrations were measured by the tyrosine-apodecarboxylase method using [3H]-tyrosine as a substrate (Alpco Diagnostics, Windham, NH).
Data Management and Statistical Methods
An integrated data management system allowed tracking of recruitment progress, data, and blood collection across study sites. Quality-assurance procedures consisted of two independent reviews of interview forms and coding. Data entry forms were created in SPSS Data Builder (SPSS, Inc., Chicago, IL), and two independent data entries were made with the second data entry technician responsible for reconciliation. SPSS and SAS (SAS Institute, Inc., Cary, NC) data files were created for data analyses.
The means of continuous variables of case and control mothers were compared and evaluated with the t test after the values were log-transformed, if they were not normally distributed. The chi-square test was used to test for differences in the distribution of categorical variables between case and control mothers. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the relative risk of clefts across quartiles of plasma and erythrocyte folate, plasma PLP, and plasma tHcy. Quartiles of each variable were defined using data from case and control mothers combined, and the reference quartile was the lowest. Logistic-regression analyses were used to estimate ORs while adjusting for differences in covariates, including maternal age, education level of mother, alcohol use, smoking, multivitamin use, and the time interval between delivery of the index child and collection of the maternal blood sample. Tests for linear trends of ORs across quartiles of variables were performed using the median level within each quartile. Differences in biomarker means between groups defined by the interval between delivery of the index child and collection of the maternal blood sample (12–36, >36–60, ≥60 months) were evaluated with one-way ANOVA. Analyses of cleft risk by biomarker quartiles were also stratified by these intervals.
RESULTS
We identified 893 eligible case mothers who delivered a child with a cleft. Among these case mothers, 14.9% refused to allow the UBDN to release their name to USU for recruitment, and 11.2% could not be located; one case mother was deceased, two spoke neither English nor Spanish, and four had other reasons for not releasing their names. Among the control mothers, the rate of initial refusal for recruitment was higher (28.3%), as was the percentage that could not be located (14.6%); two control mothers were deceased; four spoke neither English nor Spanish; nine were excluded because they had delivered a child with a cleft; and 17 were excluded for other reasons. The names of 653 case mothers (73.1%) and 782 control mothers (54.8%) were released by the UBDN for recruitment. Recruitment of mothers involved direct mail and telephone contact with case and control mothers, with the result being that among the name-released mothers 86.8% of the cases and 84.8% of the controls completed interviews with USU staff members. Of the case mothers, 8.1% refused to be interviewed, and 5.1% could not be interviewed for other reasons. Of the control mothers, 12.7% refused to be interviewed, and 2.5 could not be interviewed for other reasons. The overall results of the two stages of recruitment were that interviews were completed with 565 (63.4%) of the identified case mothers and 660 (46.4%) selected control-mothers. Blood samples for laboratory analyses were collected from 404 (71.5%) of the interviewed case mothers and 469 (71.1%) of the interviewed control mothers. Cases with known genetic syndromes or chromosomal abnormalities (n = 57) were excluded from the data analyses.
Cleft subgroups in the analyses included isolated CL/P (CL/P-I) and isolated CP (CP-I) and CL/P and CP with multiple birth defects (CL/P-M and CP-M). The characteristics of mothers in each of the four case subgroups and control mothers appear in Table 1. The sex distribution of control and all case children combined was similar because of the frequency matching by sex. Among the cases, the sex distribution by cleft subtype was similar to findings elsewhere (Mossey et al., 2009), with a male predominance for CL/P-I and CL/P-M cases, an equal distribution for CP-I, and a female predominance for CP-M cases. The ethnicity of mothers reflected the Utah population, with 91% of case mothers and 89% of control mothers self-identifying as Caucasian. The mean maternal and paternal ages at the delivery of the index child were similar between the controls and case subgroups as were the means of maternal weight, height, and body mass index. Multivitamin use was common (>65% by the second month of pregnancy), but not significantly different between case and control mothers. Parity at the time of the index delivery and at the time of blood collection was not different between case and control mothers. The interval between the index delivery and maternal blood collection was longer for controls than cases: 4.3 years for controls (SD = 2.3); 3.8 (SD = 2.2) for CL/P-I; 3.4 (SD = 2.2) for CP-I; 3.5 (SD = 2.1) for CL/P-M; 3.6 (SD = 2.4) for CP-M.
Table 1.
Characteristics of Utah Mothers of Children with Oral Clefts and Controls
| Characteristics | Controls (n = 469)a | Oral cleft case mothers
|
|||
|---|---|---|---|---|---|
| Isolated clefts
|
Clefts with multiple congenital anomalies
|
||||
| CL/P (n = 212)a | CP (n = 87)a | CL/P (n = 25)a | CP (n = 23)a | ||
| Maternal age in years (SD) at birth of index child | 27.2 (5.5) | 27.4 (5.5) | 27.8 (5.6) | 26.3 (5.6) | 28.2 (5.8) |
| Paternal age in years (SD) at birth of index child | 29.4 (6.0) | 29.8 (6.2) | 30.6 (6.2) | 30.0 (7.4) | 30.6 (6.8) |
| Male:female (%) | 59:41b | 64:36 | 51:49 | 80:20 | 39:61 |
| Ethnicity (%) | |||||
| Caucasian | 89.3 | 89.2 | 94.3 | 96.0 | 95.7 |
| Hispanic/Latino | 5.8 | 5.2 | 2.3 | 0 | 0 |
| Other | 5.1 | 5.1 | 4.0 | 4.7 | 4.3 |
| Maternal weight at conception in kg (SD) | 67.0 (15.7) | 67.2 (15.8) | 66.5 (12.8) | 70.7 (20.6) | 65.0 (11.9) |
| Maternal height in cm (SD) | 165.4 (6.9) | 165.8 (7.3) | 165.2 (6.3) | 167.7 (6.6) | 167.0 (6.6) |
| Maternal body mass index in kg/m2 (SD) | 24.5 (5.6) | 24.3 (4.9) | 24.4 (4.6) | 24.9 (6.3) | 23.4 (4.6) |
| Infant birthweight in kg (SD) | 3.4 (0.6) | 3.3 (0.6) | 3.3 (0.4) | 2.8c (0.8) | 2.6c (0.7) |
| Infant birth length in cm (SD) | 50.5 (3.4) | 50.3 (3.1) | 50.6 (3.0) | 47.1c (4.7) | 47.7c (3.8) |
| Maternal education (%) | |||||
| High school or less | 29.0 | 36.3 | 34.5 | 40.0 | 34.8 |
| Post-high school training | 71.0 | 63.7 | 65.5 | 60.0 | 65.2 |
| Household income per year (%) | |||||
| <$20,000 | 18.2 | 21.9 | 15.3 | 20.0 | 31.8 |
| $20,000 to $40,000 | 42.4 | 39.8 | 42.4 | 60.0 | 31.8 |
| >$40,000 | 39.5 | 38.3 | 42.3 | 20.0 | 36.3 |
| Persons in household (SD) | 3.6 (1.6) | 3.6 (1.7) | 3.3 (1.5) | 2.7d (0.9) | 3.7 (2.4) |
| Multivitamin use (%) | |||||
| 3 months before conception | 27.9 | 31.0 | 28.0 | 18.2 | |
| 2 months before conception | 31.6 | 31.7 | 35.6 | 28.0 | 18.2 |
| 1 month before conception | 36.6 | 34.1 | 37.9 | 32.0 | 22.7 |
| First month of pregnancy | 44.4 | 43.3 | 46.0 | 24.0 | 31.8 |
| Second month of pregnancy | 66.9 | 64.9 | 71.3 | 76.0 | 68.2 |
| Third month of pregnancy | 74.7 | 70.7 | 74.7 | 80.0 | 68.2 |
| Smoked in periconceptional periodf(%) | 10.9 | 15.1e | 14.9 | 24.0 | 17.4 |
| Alcohol use in periconceptional periodf (%) | 7.0 | 7.1 | 8.0 | 8.0 | 8.7 |
| Index birth-blood sample interval (years) | 4.3 (2.3) | 3.8d (2.2) | 3.4c (2.2) | 3.5 (2.1) | 3.6 (2.4) |
| Plasma folate (nmol/L) | 64.6 (33.4) | 51.9c (26.3) | 53.4c (28.5) | 57.3 (32.6) | 57.2 (21.3) |
| No. of assaysg | n = 468 | n = 212 | n = 87 | n = 25 | n = 23 |
| Erythrocyte folate (nmol/L) | 2119 (826) | 1991e (826) | 1918e (803) | 1884 (716) | 1727e (436) |
| No. of of assaysg | n = 465 | n = 211 | n = 87 | n = 25 | n = 22 |
| Plasma PLP (nmol/L) | 62.7 (54.4) | 61.9 (56.7) | 58.6 (61.5) | 39.5c (37.7) | 74.0 (69.1) |
| No. of assaysg | n = 468 | n = 212 | n = 87 | n = 25 | n = 23 |
| Plasma tHCY (μmol/L) | 4.147 (1.32) | 4.34 (1.39) | 4.17 (1.34) | 4.21 (1.28) | 3.98 (1.36) |
| No. of assaysg | n = 426 | n = 199 | n = 83 | n = 24 | n = 21 |
Number of mothers with complete interview data and blood samples.
Sex of control children frequency-matched to sex distribution of all case children.
p < 0.001.
p < 0.01.
p < 0.05.
Periconception period defined as 3 months before conception and first trimester of pregnancy.
The number of assays completed varies slightly because of limited availability of some sample aliquots.
Mean plasma and erythrocyte folate and plasma PLP concentrations were lower in the case mothers than in control mothers, whereas mean plasma tHcy was similar between the two groups. The risk of having a child with any cleft (all subgroups combined) declined in a dose-response manner with increasing quartile of plasma folate (quartile 1 is reference, quartile 2 [OR, 0.83; 95% CI, 0.56–1.25], quartile 3 [OR, 0.59; CI, 0.39–0.89], quartile 4 [OR, 0.35; CI, 0.23–0.53]; p-trend < 0.001). This pattern was observed for the subgroups of CL/P-I and CP-I, and it was similar for CL/P-M and CP-M, although the results in these later two groups were not significant, perhaps owing to small sample sizes. The results for erythrocyte folate were similar but less striking. The risk of having a child with any cleft declined with increasing quartile of erythrocyte folate (quartile 1 is reference, quartile 2 [OR, 0.82; CI, 0.55–1.23], quartile 3 [OR, 0.56; CI, 0.38–0.85], quartile 4 [OR, 0.64; CI, 0.42–0.97]; p-trend = 0.01).
In the highest versus lowest PLP quartile, risk of CP-M was 75% less (OR, 0.25; 95% CI, 0.07–0.95), whereas there was not a dose-response gradient across quartiles (p-trend = 0.92) as in the plasma folate findings. There was a tendency of a reduction in risk of CL/P-I, CP-I, and CL/P-M in the highest quartile compared with the lowest quartile of PLP; however, these results did not achieve significance (Table 2). The means of plasma tHcy of all groups were low as a result of the era of folic acid fortification (Pfeiffer et al., 2007). Plasma tHcy was not significantly associated with cleft risk when either all clefts combined or individual cleft subgroups were evaluated (Table 2).
Table 2.
Risk of Oral Clefts by Type of Cleft and Quartiles of Maternal Biomarkers of Folate-Dependent One-Carbon Metabolism
| No. of mothersa
|
Adjustedb odds ratios and 95% confidence intervals
|
Combined
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Isolated
|
Multiple congenital anomalies
|
Isolated
|
Multiple congenital anomalies
|
||||||
| CL/P | CP | CL/P | CP | CL/P | CP | CL/P | CP | CL/P = CP | ||
| Quartile of plasma folate (nmol/L) | ||||||||||
| Quartile 1 (≤36.7) | 99 | 67 | 30 | 8 | 4 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 (36.7–53.6) | 102 | 61 | 21 | 7 | 10 | 0.86 (0.54–1.36) | 0.67 (0.35–1.27) | 0.74 (0.24–2.24) | 1.91 (0.54–6.76) | 0.83 (0.56–1.25) |
| Quartile 3 (53.7–74.0) | 118 | 50 | 19 | 3 | 4 | 0.66 (0.42–1.05) | 0.54 (0.28–1.03) | 0.34 (0.09–1.33) | 0.84 (0.20–3.54) | 0.59 (0.39–0.89) |
| Quartile 4 (≥74.1) | 149 | 34 | 17 | 7 | 5 | 0.34 (0.21–0.56) | 0.35 (0.18–0.68) | 0.63 (0.21–1.85) | 0.45 (0.09–2.14) | 0.35 (0.23–0.53) |
| p-trend | p < 0.001 | p = 0.002 | p = 0.278 | p = 0.143 | p < 0.001 | |||||
| Quartile of erythrocyte folate (nmol/L) | ||||||||||
| Quartile 1 (≤1488) | 98 | 56 | 28 | 8 | 7 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 (1489–1878) | 112 | 53 | 25 | 7 | 9 | 0.83 (0.52–1.33) | 0.78 (0.42–1.46) | 0.92 (0.30–2.79) | 1.06 (0.33–3.36) | 0.82 (0.55–1.23) |
| Quartile 3 (1879–2486) | 132 | 50 | 17 | 4 | 5 | 0.65 (0.41–1.04) | 0.46 (0.23–0.90) | 0.48 (0.13–1.72) | 0.62 (0.18–2.13) | 0.56 (0.38–0.85) |
| Quartile 4 (≥2487) | 123 | 52 | 17 | 6 | 1 | 0.78 (0.48–1.25) | 0.49 (0.24–0.97) | 0.81 (0.25–2.63) | 0.12 (0.01–1.07) | 0.64 (0.42–0.97) |
| p-trend | p = 0.20 | p = 0.01 | p = 0.51 | p = 0.03 | p = 0.01 | |||||
| Quartile of Plasma pyridoxal-5′-phosphate (nmol/L) | ||||||||||
| Quartile 1 (≤26.0) | 112 | 60 | 21 | 12 | 5 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 (26.1–45.0) | 116 | 48 | 25 | 4 | 2 | 0.79 (0.50–1.27) | 1.23 (0.64–2.35) | 1.27 (0.44–3.63) | 0.39 (0.19–1.29) | 0.41 (0.76–2.17) |
| Quartile 3 (45.1–78.0) | 114 | 48 | 27 | 6 | 10 | 0.77 (0.48–1.24) | 1.26 (0.66–2.43) | 0.74 (0.22–2.47) | 0.57 (0.19–1.65) | 1.29 (0.38–4.35) |
| Quartile 4 (≥78.1) | 126 | 56 | 14 | 3 | 6 | 0.83 (0.52–1.31) | 0.57 (0.27–1.19) | 0.81 (0.26–2.53) | 0.25 (0.07–0.95) | 0.80 (0.22–2.94) |
| p-trend | p = 0.43 | p = 0.19 | p = 0.049 | p = 0.92 | p = 0.12 | |||||
| Quartile of plasma homocysteine (μmol/L) | ||||||||||
| Quartile 1 (≤3.2) | 114 | 49 | 22 | 5 | 9 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Quartile 2 (3.21–4.0) | 107 | 42 | 20 | 10 | 4 | 0.95 (0.58–1.56) | 1.05 (0.54–2.06) | 2.11 (0.67–6.68) | 0.58 (0.16–2.03) | 1.01 (0.67–1.54) |
| Quartile 3 (4.1–5.0) | 114 | 53 | 20 | 3 | 5 | 1.19 (0.74–1.93) | 1.03 (0.53–2.03) | 0.73 (0.17–3.22) | 0.43 (0.11–1.73) | 1.04 (0.69–1.57) |
| Quartile 4 (≥5.1) | 91 | 55 | 21 | 6 | 3 | 1.43 (0.88–2.33) | 1.30 (0.66–2.55) | 1.56 (0.45–5.38) | 0.44 (0.11–1.75) | 1.33 (0.87–2.03) |
| p-trend | p = 0.10 | p = 0.49 | p = 0.18 | p = 0.88 | p = 0.21 | |||||
All cases with known genetic syndromes or chromosomal abnormalities were excluded from the analyses.
The number of assays completed varies slightly because of limited availability of some sample aliquots.
Adjusted for maternal age, education, alcohol use, smoking, multivitamin use, and interval between delivery of the index child and maternal blood collection in logistic regression models.
CL/P, cleft lip with or without cleft palate; CP, cleft palate; I, isolated oral cleft (no other birth defects); M, with multiple birth defects.
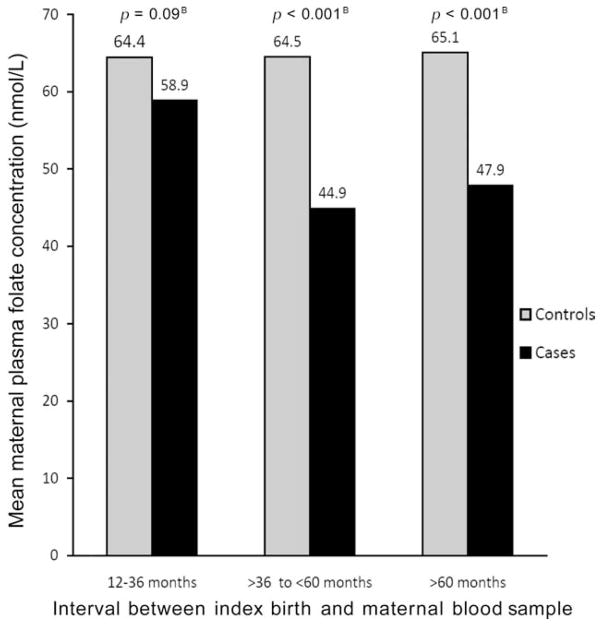
When mean plasma folate concentrations of case and control mothers were compared by intervals between index delivery and maternal blood collection (12–36, 36–60, and ≥60 months), an unexpected pattern emerged: a widening difference between the cases and controls with increasing time since the index delivery. This analysis combined all cases of clefts, because the analyses across additional strata exhausted the sample size of individual cleft subgroups. The means of plasma folate for controls versus all cleft cases were 64 versus 59 nmol/L for the 12- to 36-month interval (p = 0.09), 65 versus 45 for the >36- to 60-month interval (p < 0.001), and 65 versus 48 for the interval ≥60 months (p < 0.001; Fig. 1). As shown in Table 3, ANOVA models contrasting the plasma folate means of case and control mothers separately across the intervals indicated a significant decline of means of the case mothers after the 12- to 36-month interval (p < 0.001), whereas the means of control mothers were not significantly different across the intervals (p = 0.98). Similar results were observed for erythrocyte folate with a significant decline for case mothers (p = 0.01) and no significant differences for control-mothers over the interval (p = 0.13). PLP means declined over the periods for case mothers, although the differences were not significant. Means of tHcy demonstrated an inverse pattern with a significant stepwise increase with increasing time for case mothers (p = 0.01) and a nonsignificant increase in means for control mothers (p = 0.21).
Figure 1.
Mean maternal plasma folate concentrations of Utah oral cleft case (n=347) and control (n=468) mothers by interval between index birth and collection of maternal blood sample
Table 3.
Mean Levels of Maternal Folate-Dependent Biomarkers of One-Carbon Metabolism by Interval between Birth of Index Child and Collection of Maternal Blood Sample for Mothers of Children with Oral Cleftsa and Mothers of Controls
| Maternal biomarker | Maternal group and sample size | Interval between birth of index child and collection of maternal blood sample
|
|||
|---|---|---|---|---|---|
| 12–36 months | >36 to ≤60 months | >60 months | p-valueb | ||
| Plasma folate (nmol/L) | Oral cleft cases (n = 347) | 58.9 (29.4) | 44.9 (22.2) | 47.9 (23.4) | <0.001 |
| Controls (n = 468) | 64.4 (33.2) | 64.5 (33.7) | 65.1 (33.6) | <0.98 | |
| Erythrocyte folate (nmol/L) | Oral cleft cases (n = 345) | 2064 (840) | 1743 (621) | 1905 (803) | <0.01 |
| Controls (n = 465) | 2106 (797) | 2013 (778) | 2210 (881) | <0.13 | |
| Plasma pyridoxal-5′-phosphate (nmol/L) | Oral cleft cases (n = 347) | 65.9 (59.7) | 55.9 (57.9) | 53.6 (55.5) | <0.19 |
| Controls (n = 468) | 60.7 (44.0) | 67.0 (65.0) | 61.0 (55.0) | <0.56 | |
| Plasma total homocysteine (μmol/L) | Oral cleft cases (n = 327) | 4.05 (1.31) | 4.37 (1.37) | 4.56 (1.42) | <0.01 |
| Controls (n = 426) | 4.00 (1.40) | 4.17 (1.29) | 4.26 (1.24) | <0.21 | |
Cases include all oral clefts with the exclusion of known genetic syndromes and chromosomal abnormalities.
p values for ANOVA test of differences between means of biomarkers between intervals within cases or controls.
The modifying effect of the interval between index delivery and blood collection on the risk of clefts in association with laboratory indices was further evaluated in logistic-regression analyses (Table 4). In general, stronger associations emerged between increasing plasma folate quartiles and reduced cleft risk, with longer time elapsed between the index delivery and blood collection. The association was not significant for the 12- to 36-month interval, although a significant dose-response manner of protection was seen in the >36- to 60-month interval (p-trend < 0.001) and the >60-month interval (p-trend < 0.001). A similar pattern was observed for erythrocyte folate with no significant association in the 12- to 36-month interval (p-trend = 0.97) and a significant protective association in the 36- to 60-month interval (p-trend = 0.01) and the >60-month interval (p-trend = 0.004). The PLP data, stratified by the same intervals, were suggestive of a protective association in the longer interval, although this was not significant. An association between higher levels of tHcy and higher cleft risk was apparent in the >60-month interval (quartile 4 vs quartile 1: OR, 2.23; 95% CI, 1.01–4.91; p-trend 0.056), and this association was not apparent in the two earlier intervals.
Table 4.
Risk of Oral Cleftsa by Quartiles of Maternal Biomarkers of Folate-Dependent One-Carbon Metabolism and Interval between Birth of Index Child and Maternal Blood Specimen Collection
| Maternal biomarker (sample size) | Quartile | Adjustedb odds ratio and 95% confidence intervals by interval between birth of index child and collection of maternal blood specimen
|
||
|---|---|---|---|---|
| 12–36 months | >36–≤60 months | >60 months | ||
| Plasma folate (nmol/L; 347 cases, 468 controls) | Quartile 1 (≤36.7) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Quartile 2 (36.7–53.6) | 0.98 (0.52–1.83) | 0.63 (0.27–1.47) | 0.74 (0.37–1.48) | |
| Quartile 3 (53.7–74.0) | 1.19 (0.63–2.27) | 0.18 (0.07–0.51) | 0.36 (0.18–0.74) | |
| Quartile 4 (≥74.1) | 0.62 (0.34–1.16) | 0.15 (0.06–0.40) | 0.26 (0.12–0.57) | |
| p-trend | p = 0.18 | p < 0.001 | p ≪0.001 | |
| Erythrocyte folate (nmol/L; 345 cases, 465 controls) | Quartile 1 (≤1488) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Quartile 2 (1489–1878) | 1.48 (0.78–2.83) | 0.59 (0.26–1.33) | 0.54 (0.25–1.14) | |
| Quartile 3 (1879–2486) | 1.13 (0.60–2.14) | 0.62 (0.27–1.43) | 0.16 (0.07–0.37) | |
| Quartile 4 (≥2487) | 1.13 (0.59–2.17) | 0.21 (0.07–0.63) | 0.46 (0.23–0.93) | |
| p-trend | p = 0.97 | p = 0.01 | p = 0.004 | |
| Plasma pyridoxal-5′-phosphate (nmol/L; (347 cases, 468 controls) | Quartile 1 (< 26.0) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Quartile 2 (26.1–45.0) | 1.04 (0.55–1.97) | 0.89 (0.37–2.15) | 0.54 (0.27–1.08) | |
| Quartile 3 (45.1–78.0) | 1.27 (0.68–2.38) | 0.87 (0.37–2.01) | 0.49 (0.23–1.03) | |
| Quartile 4 (≥78.1) | 0.91 (0.49–1.71) | 0.61 (0.25–1.45) | 0.63 (0.30–1.25) | |
| p-trend | p = 0.90 | p = 0.29 | p = 0.14 | |
| Plasma homocysteine (μmol/L; 327 cases, 426 controls) | Quartile 1 (≤3.2) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Quartile 2 (3.21–4.0) | 1.08 (0.59–2.01) | 0.64 (0.26–1.55) | 1.35 (0.61–3.04) | |
| Quartile 3 (4.1–5.0) | 1.47 (0.79–2.73) | 0.47 (0.19–1.19) | 1.35 (0.62–2.95) | |
| Quartile 4 (≥5.1) | 0.89 (0.47–1.67) | 1.18 (0.48–2.88) | 2.23 (1.01–4.91) | |
| p-trend | p = 0.95 | p = 0.90 | p = 0.056 | |
Cases include all oral clefts with the exclusion of known genetic syndromes and chromosomal abnormalities.
Adjusted for maternal age, education, alcohol use, smoking, multivitamin use, and interval between delivery of the index child and maternal blood collection in logistic regression models.
DISCUSSION
In a population-based, case-control study in Utah, certain maternal biomarkers related to folate metabolism were associated with cleft risk. Among our findings, the observation that higher plasma folate concentrations were associated with a reduced cleft risk in a dose-response manner may be the most notable. Findings were similar for erythrocyte folate. Higher PLP concentrations were associated with reduced cleft risk, with statistical significance only in the subgroup with CP-M. Plasma tHcy concentration was not significantly associated with cleft risk in the overall analyses.
The observation of significant case-control differences in blood folate concentrations is unexpected in the era after folic acid fortification of enriched cereal-grain products was allowed to be practiced by food manufacturers starting March 1996 and became mandatory in January 1998 (U.S. Food and Drug Administration, 1996). This fortification program made blood folate concentrations markedly higher and tHcy lower than before the mandate (Pfeiffer et al., 2007). All of the mothers in our study provided blood samples 2 years or more after the mandatory folic acid fortification. Further, multivitamins were used by more than 65% of case and control mothers by the second month of their index pregnancy; this in combination with the high folic acid intake from fortified foods suggests that the majority of our study participants in general had a more than adequate folate intake at the time of their index pregnancy and the later blood collection. Therefore, our finding of significantly lower plasma and erythrocyte folate concentrations in case mothers than in control mothers is puzzling and requires careful scrutiny. Although no clear explanation can be offered, this finding may be due to endogenously altered, folate-dependent, one-carbon metabolism secondary to unidentified, inherited or acquired conditions rather than the result of low folate intake among the case mothers.
The case-control study design was used in this study because the alternative observational study design (i.e., a prospective cohort study) would have been prohibitively expensive. The use of biomarkers based on laboratory assays in retrospective studies of pregnancy outcomes rests on the assumption that the determinants—nutritional intake and acquired and genetically inherited determinants of metabolic function—are relatively constant over a period of years after the index pregnancy. Longitudinal studies lend support to the view that patterns of dietary intake, physical activity, and other behaviors of mothers tend to return to the preconception trajectory after the delivery of a child. Maternal erythrocyte folate levels determined 1 year after delivery in a study in London were correlated with values determined early in pregnancy (Leck et al., 1983). In a study of lifestyle habits from pregnancy through the postpartum period, few women departed from prepregnancy trajectories in three categories in the postpartum period, including weight orientation, diet, and physical activity (Devine et al., 2000). In a prospective study to evaluate the association between preconception and early pregnancy exposures and pregnancy outcomes, the nutrient intakes of women were assessed with a food-frequency questionnaire 3 to 7 years after their index pregnancies. These questionnaires were compared to values determined with the same method during their index pregnancies, and the agreement was comparable to that found in studies of nonpregnant adults over the same time interval. In addition, Bunin et al. (2001) reported that the recall was similar among women with a shorter interval between assessments (<4.5 years) compared to those with a longer interval (≥4.5 years).
The biomarker assays used in this study may provide measures of the collective effects of variation in dietary and supplemental intake of nutrients combined with a variance in metabolism that results from interactions with other nutrients, environmental factors, and genes. This complex chain of events from nutrient consumption, through absorption, storage, utilization, and excretion, also means that observed case-control differences in measured biomarker levels may have many possible explanations other than variance in nutrient intake (Willett, 1990). The use of biomarkers also provides objective data that may reduce the recall bias that can distort recall between case and control mothers in retrospective studies; it also allows for the simultaneous examination of multiple nutrients.
An unexpected finding was that stronger associations among increasing quartiles of plasma and erythrocyte folate and reduced cleft risk, as well as between increasing plasma tHcy quartiles and increased cleft risk, emerged in stratified analyses of longer versus shorter intervals between delivery of the index child and maternal blood collection. This finding appeared to be the result of relatively stable mean concentrations for control mothers and significant declines in mean concentrations for case mothers over these intervals. The emergence of stronger associations between biomarkers of one-carbon metabolism with increasing time since the delivery of the index child suggests the possibility that a disorder of folate-dependent one-carbon metabolism may play a role in the etiology of clefts, and that this disorder becomes more apparent with the passage of time after the affected pregnancy. This conclusion, however, is based on the interpretation of cross-sectional data, and future longitudinal studies will be necessary to verify this observation.
Although the findings of the Utah study could be generalized to other areas in the developed world, they may not be directly applicable to nutritionally impoverished populations in developing countries where no fortification program is practiced, and few prenatal vitamins are available. For example, in our studies in the Philippines where the same methods for sample collection and laboratory analyses were used, we found that the association between blood folate concentration and cleft risk was inconsistent, and this association appeared to be modified by the level of maternal vitamin B6 status (Munger et al., 2004). In the Philippines, inadequate vitamin B6 status was common in women of reproductive age and was independently associated with increased cleft risk (Tamura et al., 2007). In the Utah study, maternal vitamin B6 status, as judged by plasma PLP, appeared to be less strongly associated with cleft risk compared with plasma and erythrocyte folate concentrations. We reported widespread inadequate maternal plasma zinc levels in the Philippines that were strongly associated with cleft risk (Tamura et al., 2005). In contrast, we found generally adequate maternal zinc status and no significant association between zinc status and cleft risk in Utah (Munger et al., 2009).
Strengths of the current study include the statewide ascertainment of case mothers using a birth defects registry with multiple sources of case ascertainment and the random selection of control mothers using all Utah births without clefts from the same time period as the sampling frame. The collection and rapid processing of maternal blood specimens and assays of biomarkers added an objective aspect to the study beyond the collection of interview data alone. An additional strength of the study was the detailed review of each case by a clinical geneticist. This procedure is standard for the Utah Birth Defects Network and can generate the clinical classifications by type of cleft, pattern of other birth defects, chromosomal abnormalities, and known genetic syndromes. Whereas cleft cases with chromosomal abnormalities or known genetic syndromes were excluded from the data analyses, it is possible that abnormalities in one-carbon metabolism may be involved in those cases as well. The participation rates can be considered relatively high and were due to active recruitment through multiple methods including mail, telephone, and face-to-face contacts.
A potential limitation of the design of retrospective case-control studies is recall bias; however, this would not have affected the biomarker levels measured in the maternal blood specimens. A potential bias that may have affected observed biomarker values or other covariates is the differential response rate between cases and controls. More case mothers than controls (73.1% vs. 54.8%) responded to the request by the Utah Department of Health to release their names to study investigators for recruitment; however, no personal information or bio-marker data were available from nonrespondents to allow the evaluation of potential response bias. Once names were released for recruitment, a similar percentage of case and control mothers completed the interview (86.8% vs. 84.8%); of these, a similar percentage provided a blood specimen (71.5% vs. 71.1%). A prospective approach to data collection with enrollment of mothers before pregnancy would be ideal; however, it would have been extremely difficult because of the large sample size required and its high cost. Additional limitations were the small sample size for clefts with multiple birth defects and the exclusion of clefts with known genetic syndromes and chromosomal abnormalities because of the heterogeneity of these cases and their small numbers. It is possible that the biomarker associations observed with the isolated clefts are relevant for these subgroups of clefts, although larger studies will be necessary. Although some may view a biomarker study conducted years after the affected pregnancy as a weakness, we have cited evidence to support the view that nutrient intakes and related metabolic factors are relatively stable. In addition, this study design has revealed a decline in folate status of case mothers relative to control mothers over time, suggesting that biomarker studies after a reasonable time from delivery can unmask biochemical variations in nutritional studies that would be difficult to identify by dietary recall studies or blood sampling immediately after delivery.
The Utah study of maternal biomarkers of folate-dependent one-carbon metabolism provides evidence of the involvement of this metabolic pathway in the risk of clefts. The currently high rates of clefts in Utah and other western states (Gebreab et al., 2008) remain unexplained. Additional mechanistic and population-based studies are needed to explore whether an acquired or inherited disorder of folate metabolism plays a role in the etiology of clefts. The analysis of nutrient-related biomarkers and the evaluation of interactions with candidate gene polymorphisms and lifestyle and behavioral factors will be important in future studies of clefts.
Acknowledgments
Funded by grants 5-RO1-HD39061 and 1R21-DE016877 from the U.S. National Institute of Child Health and Human Development and the U.S. National Institute of Dental and Craniofacial Research (to R.G.M.), grant 1UO1DD000698 from the U.S. Centers for Disease Control and Prevention (R.G.M.) and funding from the Office of the Vice President for Research and the Agricultural Experiment Station of Utah State University.
We thank the Utah families who participated in this study and Merilee Anderson, Sharon Bell, Cara Brewer, Marjorie Carter, Craig Gale, Colleen Mohr, Amy Nance, Dawnya Pearce, Russell Ray, Georgiann Sanborn, Nancy Sassano, Patty Smith, and Nancy West.
References
- Badovinac RL, Werler MM, Williams PL, et al. Folic acid-containing supplement consumption during pregnancy and risk for oral clefts: a meta-analysis. Birth Defects Res A Clin Mol Teratol. 2007;79:8–15. doi: 10.1002/bdra.20315. [DOI] [PubMed] [Google Scholar]
- Been W, Lieuw Kie Song SH. Harelip and cleft palate conditions in chick embryos following local destruction of the cephalic neural crest. A preliminary note. Acta Morphol Neerl Scand. 1978;16:245–255. [PubMed] [Google Scholar]
- Berry RJ, Li Z, Erickson JD, et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Bunin GR, Gyllstrom ME, Brown JE, et al. Recall of diet during a past pregnancy. Am J Epidemiol. 2001;154:1136–1142. doi: 10.1093/aje/154.12.1136. [DOI] [PubMed] [Google Scholar]
- Dai L, Miao L, Zhou GX, et al. The prevalence analysis of cleft palate in Chinese perinatals: 1996–2000. Hua Xi Kou Qiang Yi Xue Za Zhi. 2004;22:35–37. [PubMed] [Google Scholar]
- Devine CM, Bove CF, Olson CM, et al. Continuity and change in women’s weight orientations and lifestyle practices through pregnancy and the postpartum period: the influence of life course trajectories and transitional events. Soc Sci Med. 2000;50:567–582. doi: 10.1016/s0277-9536(99)00314-7. [DOI] [PubMed] [Google Scholar]
- Gebreab SY, Gillies RR, Munger RG, et al. Visualization and interpretation of birth defects data using linked micromap plots. Birth Defects Res A Clin Mol Teratol. 2008;82:110–119. doi: 10.1002/bdra.20419. [DOI] [PubMed] [Google Scholar]
- Annual Report 2008 with data for 2006. Rome: International Clearinghouse for Birth Defects Surveillance and Research; 2008. International Clearinghouse for Birth Defects Surveillance and Research. [Google Scholar]
- Johnson CY, Little J. Folate intake, markers of folate status and oral clefts: is the evidence converging? Int J Epidemiol. 2008;37:1041–1058. doi: 10.1093/ije/dyn098. [DOI] [PubMed] [Google Scholar]
- Lammer EJ, Chen DT, Hoar RM, et al. Retinoic acid embryopathy. N Engl J Med. 1985;313:837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Leck I, Iles CA, Sharman IM, et al. Maternal diet and nutrition during early pregnancy and after delivery in North London. In: Dobbin J, editor. Prevention of spina bifida and other neural tube defects. London: London Academic Press; 1983. [Google Scholar]
- Little J, Gilmour M, Mossey PA, et al. Folate and clefts of the lip and palate: a U.K.-based case-control study: part I: dietary and supplemental folate. Cleft Palate Craniofac J. 2008a;45:420–427. doi: 10.1597/06-150.1. [DOI] [PubMed] [Google Scholar]
- Little J, Gilmour M, Mossey PA, et al. Folate and clefts of the lip and palate: a U.K.-based case-control study: part II: biochemical and genetic analysis. Cleft Palate Craniofac J. 2008b;45:428–438. doi: 10.1597/06-151.1. [DOI] [PubMed] [Google Scholar]
- Mossey PA, Little J, Munger RG, et al. Cleft lip and palate. Lancet. 2009;374:1773–1785. doi: 10.1016/S0140-6736(09)60695-4. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- Munger RG. Maternal nutrition and oral clefts. In: Wyzsynski D, editor. Cleft lip and palate: from origin to treatment. New York: Oxford University Press; 2002. [Google Scholar]
- Munger RG, Sauberlich HE, Corcoran C, et al. Maternal vitamin B-6 and folate status and risk of oral cleft birth defects in the Philippines. Birth Defects Res A Clin Mol Teratol. 2004;70:464–471. doi: 10.1002/bdra.20037. [DOI] [PubMed] [Google Scholar]
- Munger RG, Tamura T, Johnston KE, et al. Plasma zinc concentrations of mothers and the risk of oral clefts in their children in Utah. Birth Defects Res A Clin Mol Teratol. 2009;85:151–155. doi: 10.1002/bdra.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MM, Scott JM, McPartlin JM, et al. The pregnancy-related decrease in fasting plasma homocysteine is not explained by folic acid supplementation, hemodilution, or a decrease in albumin in a longitudinal study. Am J Clin Nutr. 2002;76:614–619. doi: 10.1093/ajcn/76.3.614. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CM, Johnson CL, Jain RB, et al. Trends in blood folate and vitamin B-12 concentrations in the United States, 1988–2004. Am J Clin Nutr. 2007;86:718–727. doi: 10.1093/ajcn/86.3.718. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Carmichael SL, Laurent C, et al. Maternal nutrient intakes and risk of orofacial clefts. Epidemiology. 2006;17:285–291. doi: 10.1097/01.ede.0000208348.30012.35. [DOI] [PubMed] [Google Scholar]
- Tamura T. Microbiological assay of folates. In: Picciano MFSE, Gregory JF III, editors. Folic acid metabolism in health and disease. New York: Wiley-Liss; 1990. pp. 121–137. [Google Scholar]
- Tamura T, Johnston KE, Bergman SM, et al. Homocysteine and folate concentrations in blood from patients treated with hemodialysis. J Am Soc Nephrol. 1996;7:2414–2418. doi: 10.1681/ASN.V7112414. [DOI] [PubMed] [Google Scholar]
- Tamura T, Munger RG, Corcoran C, et al. Plasma zinc concentrations of mothers and the risk of nonsyndromic oral clefts in their children: a case-control study in the Philippines. Birth Defects Res A Clin Mol Teratol. 2005;73:612–616. doi: 10.1002/bdra.20179. [DOI] [PubMed] [Google Scholar]
- Tamura T, Munger RG, Nepomuceno B, et al. Maternal plasma pyridoxal-5′-phosphate concentrations and risk of isolated oral clefts in the Philippines. Birth Defects Res A Clin Mol Teratol. 2007;79:276–280. doi: 10.1002/bdra.20348. [DOI] [PubMed] [Google Scholar]
- Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993–1016. doi: 10.1093/ajcn/83.5.993. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Washington, DC: Federal Register; 1996. pp. 8781–8797. [Google Scholar]
- van Rooij IA, Swinkels DW, Blom HJ, et al. Vitamin and homocysteine status of mothers and infants and the risk of nonsyndromic orofacial clefts. Am J Obstet Gynecol. 2003;189:1155–1160. doi: 10.1067/s0002-9378(03)00592-1. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Lie RT, Solvoll K, et al. Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ. 2007;334:464. doi: 10.1136/bmj.39079.618287.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. New York: Oxford University Press; 1990. [Google Scholar]
- Wong WY, Eskes TK, Kuijpers-Jagtman AM, et al. Nonsyndromic orofacial clefts: association with maternal hyperhomocysteinemia. Teratology. 1999;60:253–257. doi: 10.1002/(SICI)1096-9926(199911)60:5<253::AID-TERA4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]